User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Ethics do not end at the bedside: A commentary about scientific authorship
Sound moral principles are essential in the development of all physicians. Given how heavily each clinical encounter is laden with ethical implications, this is taught early in medical school. The medical student and resident physician must be able to make ethical and moral decisions on a consistent basis.
Speaking as a psychiatrist in training, there is an intimate relationship between psychiatry and moral questions.1 Issues such as determining an individual’s ability to make decisions about their medical care, hospitalizing patients against their will, and involuntarily administering medication are an almost-daily occurrence.2 Physicians, especially those who practice psychiatric medicine, must be ethically grounded to properly make these difficult but common decisions. It is also imperative that residents are given proper guidance in ethical practice in structured didactics and hands-on training.
However, many residents may be unfamiliar with ethics in research, more specifically ethical authorship. While some trainees might have participated in scholarly activities before residency, residency is the time to discover one’s interests, and residents are encouraged to engage in research. Unfortunately, many of the considerations surrounding ethical authorship are not emphasized, and questionable practices are common.3 In this article, I summarize the different faces of unethical authorship, and call for a greater emphasis on ethical authorship in medical residency training programs.
What drives unethical authorship practices
One of the main drivers for the increase in unethical practices is the need to publish to advance one’s academic career. The academic principle of “publish or perish” pressures many faculty researchers.3 The impact of this expectation plays a significant role in potentially unethical authorship practices, and also has increased the number of publications of mediocre quality or fraudulent data.4 This mindset has also seeped into the clinical world because promotions and financial bonuses are incentives for attending physicians to perform scholarly work. Due to these incentives and pressures, a senior academician might compel a junior researcher to include them as a coauthor on the junior researcher’s paper, even when the senior’s contributions to the paper might be limited.5
Most journals have specific criteria for authorship. The International Committee of Medical Journal Editors (ICMJE) has 4 core criteria for authorship: 1) substantial contributions to the conception or design of the work, or the acquisition, analysis, or interpretation of data for the work; 2) drafting the work or revising it critically for important intellectual content; 3) providing final approval of the version to be published, and 4) agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.5,6 One survey found that in certain journals, approximately 15% of authors met full ICMJE authorship criteria, while one-half claimed there were substantial contributions but did not state anything more specific.7
There are several types of authorship abuse.5 Gift authorship is when authorship is awarded to a friend either out of respect or in hopes that friend will return the favor (quid pro quo). Ghost authorship occurs when a third party commissions an author to write or help write a paper (eg, when a pharmaceutical company hires writers to produce a paper about a medication they manufacture) or when legitimate authors are denied recognition on a paper. Honorary authorship occurs when authorship is granted with the hope that the reputation of the honorary author will increase the chances of the paper getting published and possibly boost citations.
While these forms of authorship abuse occur with unsettling frequency, they might not be common among physician trainees who do not engage in full-time research.5 Resident authors might be more likely to experience coercive authorship.
Continue to: Coercive authorship is when...
Coercive authorship is when an individual in a superior position (such as an attending physician) forces their name onto a paper of a junior individual (such as a resident). Kwok8 called this “The White Bull effect,” based on Greek mythology in which Zeus transformed himself into a white bull to seduce Europa. The White Bull represents the predatory nature of the senior individual who exploits ambiguous institutional research regulations to their benefit.8 They stretch out the ICMJE criteria, only superficially satisfying them to justify authorship. In this scenario, the attending physician with promotional incentives notices the work of a resident and demands authorship, given their role as the “supervising” physician (akin to general supervision of a research group). This is not justification for authorship per the ICMJE or any major medical journal criteria. However, a resident with limited research experience may agree to include the attending as a coauthor for a variety of reasons, including fear of a poor performance evaluation or professionalism complaints, or just to maintain a positive working relationship.
Serious implications
While there are countless reasons to be concerned about this behavior, the central issue is the attending physician’s role to train and/or mentor the resident. As previously stated, a physician—especially one practicing psychiatric medicine—must be of morally sound mind. A resident being taught unethical behaviors by their attending physician has dangerous implications. Academic dishonesty does not occur in vacuum. It is likely that dishonest and unethical behavior in research matters can cross over into the clinical arena. One study found that individuals who exhibit dishonest academic behavior are more likely to violate workplace policies.9 Also, these behaviors lead to increased moral disengagement in all areas.10,11 Imagining a morally disengaged attending psychiatrist practicing medicine and training the next generation of psychiatrists is unsettling.
My hope is that residency programs discourage this detrimental conduct in their departments and support those trying to uphold integrity.
1. Scher S, Kozlowska K. Teaching ethics in psychiatry: time to reset. Harv Rev Psychiatry. 2020;28(5):328-333. doi:10.1097/HRP.0000000000000258
2. Allen NG, Khan JS, Alzahri MS, et al. Ethical issues in emergency psychiatry. Emerg Med Clin North Am. 2015;33(4):863-874. doi:10.1016/j.emc.2015.07.012
3. Pfleegor AG, Katz M, Bowers MT. Publish, perish, or salami slice? Authorship ethics in an emerging field. Journal of Business Ethics. 2019;156(1):189-208.
4. Rivera H. Fake peer review and inappropriate authorship are real evils. J Korean Med Sci. 2018;34(2):e6. doi:10.3346/jkms.2019.34.e6
5. Strange K. Authorship: why not just toss a coin? Am J Physiol Cell Physiol. 2008;295(3):C567-C575. doi:10.1152/ajpcell.00208.2008
6. Ali MJ. ICMJE criteria for authorship: why the criticisms are not justified? Graefes Arch Clin Exp Ophthalmol. 2021;259(2):289-290. doi:10.1007/s00417-020-04825-2
7. Malički M, Jerončić A, Marušić M, et al. Why do you think you should be the author on this manuscript? Analysis of open-ended responses of authors in a general medical journal. BMC Med Res Methodol. 2012;12:189. doi:10.1186/1471-2288-12-189
8. Kwok LS. The White Bull effect: abusive coauthorship and publication parasitism. J Med Ethics. 2005;31(9):554-556. doi:10.1136/jme.2004.010553
9. Harding TS, Carpenter DD, Finelli CJ, et al. Does academic dishonesty relate to unethical behavior in professional practice? An exploratory study. Sci Eng Ethics. 2004;10(2):311-324. doi:10.1007/s11948-004-0027-3
10. Shu LL, Gino F. Sweeping dishonesty under the rug: how unethical actions lead to forgetting of moral rules. J Pers Soc Psychol. 2012;102(6):1164-1177. doi:10.1037/a0028381
11. Shu LL, Gino F, Bazerman MH. Dishonest deed, clear conscience: when cheating leads to moral disengagement and motivated forgetting. Pers Soc Psychol Bull. 2011;37(3):330-349. doi:10.1177/0146167211398138
Sound moral principles are essential in the development of all physicians. Given how heavily each clinical encounter is laden with ethical implications, this is taught early in medical school. The medical student and resident physician must be able to make ethical and moral decisions on a consistent basis.
Speaking as a psychiatrist in training, there is an intimate relationship between psychiatry and moral questions.1 Issues such as determining an individual’s ability to make decisions about their medical care, hospitalizing patients against their will, and involuntarily administering medication are an almost-daily occurrence.2 Physicians, especially those who practice psychiatric medicine, must be ethically grounded to properly make these difficult but common decisions. It is also imperative that residents are given proper guidance in ethical practice in structured didactics and hands-on training.
However, many residents may be unfamiliar with ethics in research, more specifically ethical authorship. While some trainees might have participated in scholarly activities before residency, residency is the time to discover one’s interests, and residents are encouraged to engage in research. Unfortunately, many of the considerations surrounding ethical authorship are not emphasized, and questionable practices are common.3 In this article, I summarize the different faces of unethical authorship, and call for a greater emphasis on ethical authorship in medical residency training programs.
What drives unethical authorship practices
One of the main drivers for the increase in unethical practices is the need to publish to advance one’s academic career. The academic principle of “publish or perish” pressures many faculty researchers.3 The impact of this expectation plays a significant role in potentially unethical authorship practices, and also has increased the number of publications of mediocre quality or fraudulent data.4 This mindset has also seeped into the clinical world because promotions and financial bonuses are incentives for attending physicians to perform scholarly work. Due to these incentives and pressures, a senior academician might compel a junior researcher to include them as a coauthor on the junior researcher’s paper, even when the senior’s contributions to the paper might be limited.5
Most journals have specific criteria for authorship. The International Committee of Medical Journal Editors (ICMJE) has 4 core criteria for authorship: 1) substantial contributions to the conception or design of the work, or the acquisition, analysis, or interpretation of data for the work; 2) drafting the work or revising it critically for important intellectual content; 3) providing final approval of the version to be published, and 4) agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.5,6 One survey found that in certain journals, approximately 15% of authors met full ICMJE authorship criteria, while one-half claimed there were substantial contributions but did not state anything more specific.7
There are several types of authorship abuse.5 Gift authorship is when authorship is awarded to a friend either out of respect or in hopes that friend will return the favor (quid pro quo). Ghost authorship occurs when a third party commissions an author to write or help write a paper (eg, when a pharmaceutical company hires writers to produce a paper about a medication they manufacture) or when legitimate authors are denied recognition on a paper. Honorary authorship occurs when authorship is granted with the hope that the reputation of the honorary author will increase the chances of the paper getting published and possibly boost citations.
While these forms of authorship abuse occur with unsettling frequency, they might not be common among physician trainees who do not engage in full-time research.5 Resident authors might be more likely to experience coercive authorship.
Continue to: Coercive authorship is when...
Coercive authorship is when an individual in a superior position (such as an attending physician) forces their name onto a paper of a junior individual (such as a resident). Kwok8 called this “The White Bull effect,” based on Greek mythology in which Zeus transformed himself into a white bull to seduce Europa. The White Bull represents the predatory nature of the senior individual who exploits ambiguous institutional research regulations to their benefit.8 They stretch out the ICMJE criteria, only superficially satisfying them to justify authorship. In this scenario, the attending physician with promotional incentives notices the work of a resident and demands authorship, given their role as the “supervising” physician (akin to general supervision of a research group). This is not justification for authorship per the ICMJE or any major medical journal criteria. However, a resident with limited research experience may agree to include the attending as a coauthor for a variety of reasons, including fear of a poor performance evaluation or professionalism complaints, or just to maintain a positive working relationship.
Serious implications
While there are countless reasons to be concerned about this behavior, the central issue is the attending physician’s role to train and/or mentor the resident. As previously stated, a physician—especially one practicing psychiatric medicine—must be of morally sound mind. A resident being taught unethical behaviors by their attending physician has dangerous implications. Academic dishonesty does not occur in vacuum. It is likely that dishonest and unethical behavior in research matters can cross over into the clinical arena. One study found that individuals who exhibit dishonest academic behavior are more likely to violate workplace policies.9 Also, these behaviors lead to increased moral disengagement in all areas.10,11 Imagining a morally disengaged attending psychiatrist practicing medicine and training the next generation of psychiatrists is unsettling.
My hope is that residency programs discourage this detrimental conduct in their departments and support those trying to uphold integrity.
Sound moral principles are essential in the development of all physicians. Given how heavily each clinical encounter is laden with ethical implications, this is taught early in medical school. The medical student and resident physician must be able to make ethical and moral decisions on a consistent basis.
Speaking as a psychiatrist in training, there is an intimate relationship between psychiatry and moral questions.1 Issues such as determining an individual’s ability to make decisions about their medical care, hospitalizing patients against their will, and involuntarily administering medication are an almost-daily occurrence.2 Physicians, especially those who practice psychiatric medicine, must be ethically grounded to properly make these difficult but common decisions. It is also imperative that residents are given proper guidance in ethical practice in structured didactics and hands-on training.
However, many residents may be unfamiliar with ethics in research, more specifically ethical authorship. While some trainees might have participated in scholarly activities before residency, residency is the time to discover one’s interests, and residents are encouraged to engage in research. Unfortunately, many of the considerations surrounding ethical authorship are not emphasized, and questionable practices are common.3 In this article, I summarize the different faces of unethical authorship, and call for a greater emphasis on ethical authorship in medical residency training programs.
What drives unethical authorship practices
One of the main drivers for the increase in unethical practices is the need to publish to advance one’s academic career. The academic principle of “publish or perish” pressures many faculty researchers.3 The impact of this expectation plays a significant role in potentially unethical authorship practices, and also has increased the number of publications of mediocre quality or fraudulent data.4 This mindset has also seeped into the clinical world because promotions and financial bonuses are incentives for attending physicians to perform scholarly work. Due to these incentives and pressures, a senior academician might compel a junior researcher to include them as a coauthor on the junior researcher’s paper, even when the senior’s contributions to the paper might be limited.5
Most journals have specific criteria for authorship. The International Committee of Medical Journal Editors (ICMJE) has 4 core criteria for authorship: 1) substantial contributions to the conception or design of the work, or the acquisition, analysis, or interpretation of data for the work; 2) drafting the work or revising it critically for important intellectual content; 3) providing final approval of the version to be published, and 4) agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.5,6 One survey found that in certain journals, approximately 15% of authors met full ICMJE authorship criteria, while one-half claimed there were substantial contributions but did not state anything more specific.7
There are several types of authorship abuse.5 Gift authorship is when authorship is awarded to a friend either out of respect or in hopes that friend will return the favor (quid pro quo). Ghost authorship occurs when a third party commissions an author to write or help write a paper (eg, when a pharmaceutical company hires writers to produce a paper about a medication they manufacture) or when legitimate authors are denied recognition on a paper. Honorary authorship occurs when authorship is granted with the hope that the reputation of the honorary author will increase the chances of the paper getting published and possibly boost citations.
While these forms of authorship abuse occur with unsettling frequency, they might not be common among physician trainees who do not engage in full-time research.5 Resident authors might be more likely to experience coercive authorship.
Continue to: Coercive authorship is when...
Coercive authorship is when an individual in a superior position (such as an attending physician) forces their name onto a paper of a junior individual (such as a resident). Kwok8 called this “The White Bull effect,” based on Greek mythology in which Zeus transformed himself into a white bull to seduce Europa. The White Bull represents the predatory nature of the senior individual who exploits ambiguous institutional research regulations to their benefit.8 They stretch out the ICMJE criteria, only superficially satisfying them to justify authorship. In this scenario, the attending physician with promotional incentives notices the work of a resident and demands authorship, given their role as the “supervising” physician (akin to general supervision of a research group). This is not justification for authorship per the ICMJE or any major medical journal criteria. However, a resident with limited research experience may agree to include the attending as a coauthor for a variety of reasons, including fear of a poor performance evaluation or professionalism complaints, or just to maintain a positive working relationship.
Serious implications
While there are countless reasons to be concerned about this behavior, the central issue is the attending physician’s role to train and/or mentor the resident. As previously stated, a physician—especially one practicing psychiatric medicine—must be of morally sound mind. A resident being taught unethical behaviors by their attending physician has dangerous implications. Academic dishonesty does not occur in vacuum. It is likely that dishonest and unethical behavior in research matters can cross over into the clinical arena. One study found that individuals who exhibit dishonest academic behavior are more likely to violate workplace policies.9 Also, these behaviors lead to increased moral disengagement in all areas.10,11 Imagining a morally disengaged attending psychiatrist practicing medicine and training the next generation of psychiatrists is unsettling.
My hope is that residency programs discourage this detrimental conduct in their departments and support those trying to uphold integrity.
1. Scher S, Kozlowska K. Teaching ethics in psychiatry: time to reset. Harv Rev Psychiatry. 2020;28(5):328-333. doi:10.1097/HRP.0000000000000258
2. Allen NG, Khan JS, Alzahri MS, et al. Ethical issues in emergency psychiatry. Emerg Med Clin North Am. 2015;33(4):863-874. doi:10.1016/j.emc.2015.07.012
3. Pfleegor AG, Katz M, Bowers MT. Publish, perish, or salami slice? Authorship ethics in an emerging field. Journal of Business Ethics. 2019;156(1):189-208.
4. Rivera H. Fake peer review and inappropriate authorship are real evils. J Korean Med Sci. 2018;34(2):e6. doi:10.3346/jkms.2019.34.e6
5. Strange K. Authorship: why not just toss a coin? Am J Physiol Cell Physiol. 2008;295(3):C567-C575. doi:10.1152/ajpcell.00208.2008
6. Ali MJ. ICMJE criteria for authorship: why the criticisms are not justified? Graefes Arch Clin Exp Ophthalmol. 2021;259(2):289-290. doi:10.1007/s00417-020-04825-2
7. Malički M, Jerončić A, Marušić M, et al. Why do you think you should be the author on this manuscript? Analysis of open-ended responses of authors in a general medical journal. BMC Med Res Methodol. 2012;12:189. doi:10.1186/1471-2288-12-189
8. Kwok LS. The White Bull effect: abusive coauthorship and publication parasitism. J Med Ethics. 2005;31(9):554-556. doi:10.1136/jme.2004.010553
9. Harding TS, Carpenter DD, Finelli CJ, et al. Does academic dishonesty relate to unethical behavior in professional practice? An exploratory study. Sci Eng Ethics. 2004;10(2):311-324. doi:10.1007/s11948-004-0027-3
10. Shu LL, Gino F. Sweeping dishonesty under the rug: how unethical actions lead to forgetting of moral rules. J Pers Soc Psychol. 2012;102(6):1164-1177. doi:10.1037/a0028381
11. Shu LL, Gino F, Bazerman MH. Dishonest deed, clear conscience: when cheating leads to moral disengagement and motivated forgetting. Pers Soc Psychol Bull. 2011;37(3):330-349. doi:10.1177/0146167211398138
1. Scher S, Kozlowska K. Teaching ethics in psychiatry: time to reset. Harv Rev Psychiatry. 2020;28(5):328-333. doi:10.1097/HRP.0000000000000258
2. Allen NG, Khan JS, Alzahri MS, et al. Ethical issues in emergency psychiatry. Emerg Med Clin North Am. 2015;33(4):863-874. doi:10.1016/j.emc.2015.07.012
3. Pfleegor AG, Katz M, Bowers MT. Publish, perish, or salami slice? Authorship ethics in an emerging field. Journal of Business Ethics. 2019;156(1):189-208.
4. Rivera H. Fake peer review and inappropriate authorship are real evils. J Korean Med Sci. 2018;34(2):e6. doi:10.3346/jkms.2019.34.e6
5. Strange K. Authorship: why not just toss a coin? Am J Physiol Cell Physiol. 2008;295(3):C567-C575. doi:10.1152/ajpcell.00208.2008
6. Ali MJ. ICMJE criteria for authorship: why the criticisms are not justified? Graefes Arch Clin Exp Ophthalmol. 2021;259(2):289-290. doi:10.1007/s00417-020-04825-2
7. Malički M, Jerončić A, Marušić M, et al. Why do you think you should be the author on this manuscript? Analysis of open-ended responses of authors in a general medical journal. BMC Med Res Methodol. 2012;12:189. doi:10.1186/1471-2288-12-189
8. Kwok LS. The White Bull effect: abusive coauthorship and publication parasitism. J Med Ethics. 2005;31(9):554-556. doi:10.1136/jme.2004.010553
9. Harding TS, Carpenter DD, Finelli CJ, et al. Does academic dishonesty relate to unethical behavior in professional practice? An exploratory study. Sci Eng Ethics. 2004;10(2):311-324. doi:10.1007/s11948-004-0027-3
10. Shu LL, Gino F. Sweeping dishonesty under the rug: how unethical actions lead to forgetting of moral rules. J Pers Soc Psychol. 2012;102(6):1164-1177. doi:10.1037/a0028381
11. Shu LL, Gino F, Bazerman MH. Dishonest deed, clear conscience: when cheating leads to moral disengagement and motivated forgetting. Pers Soc Psychol Bull. 2011;37(3):330-349. doi:10.1177/0146167211398138
Adult ADHD: 6 studies of pharmacologic interventions
Attention-deficit/hyperactivity disorder (ADHD) is a developmental disorder that begins in childhood and continues into adulthood. The clinical presentation is characterized by a persistent pattern of inattention, impulsivity, and/or hyperactivity that causes functional interference.1 ADHD affects patients’ interpersonal and professional lives as well as their daily functioning.2 Adults with ADHD may suffer from excessive self-criticism, low self-esteem, and sensitivity to criticism.3 The overall prevalence of adult ADHD is 4.4%.4 ADHD in adults is frequently associated with comorbid psychiatric disorders.5 The diagnosis of ADHD in adults requires the presence of ≥5 symptoms of inattention and hyperactivity/impulsivity that persist for ≥6 months. Patients must have first had such symptoms before age 12; symptoms need to be present in ≥2 settings and interfere with functioning.1
Treatment of ADHD includes pharmacologic and nonpharmacologic interventions. For most patients, pharmacotherapy—specifically stimulant medications—is advised as first-line treatment,6 with adequate trials of methylphenidate and amphetamines before using second-line agents such as nonstimulants. However, despite these medications’ efficacy in randomized controlled trials (RCTs), adherence is low.7 This could be due to inadequate response or adverse effects.8 Guidelines also recommend the use of nonpharmacologic interventions for adults who cannot adhere to or tolerate medication or have an inadequate response.6 Potential nonpharmacologic interventions include transcranial direct current stimulation, mindfulness, psychoeducation, cognitive-behavioral therapy, and chronotherapy.
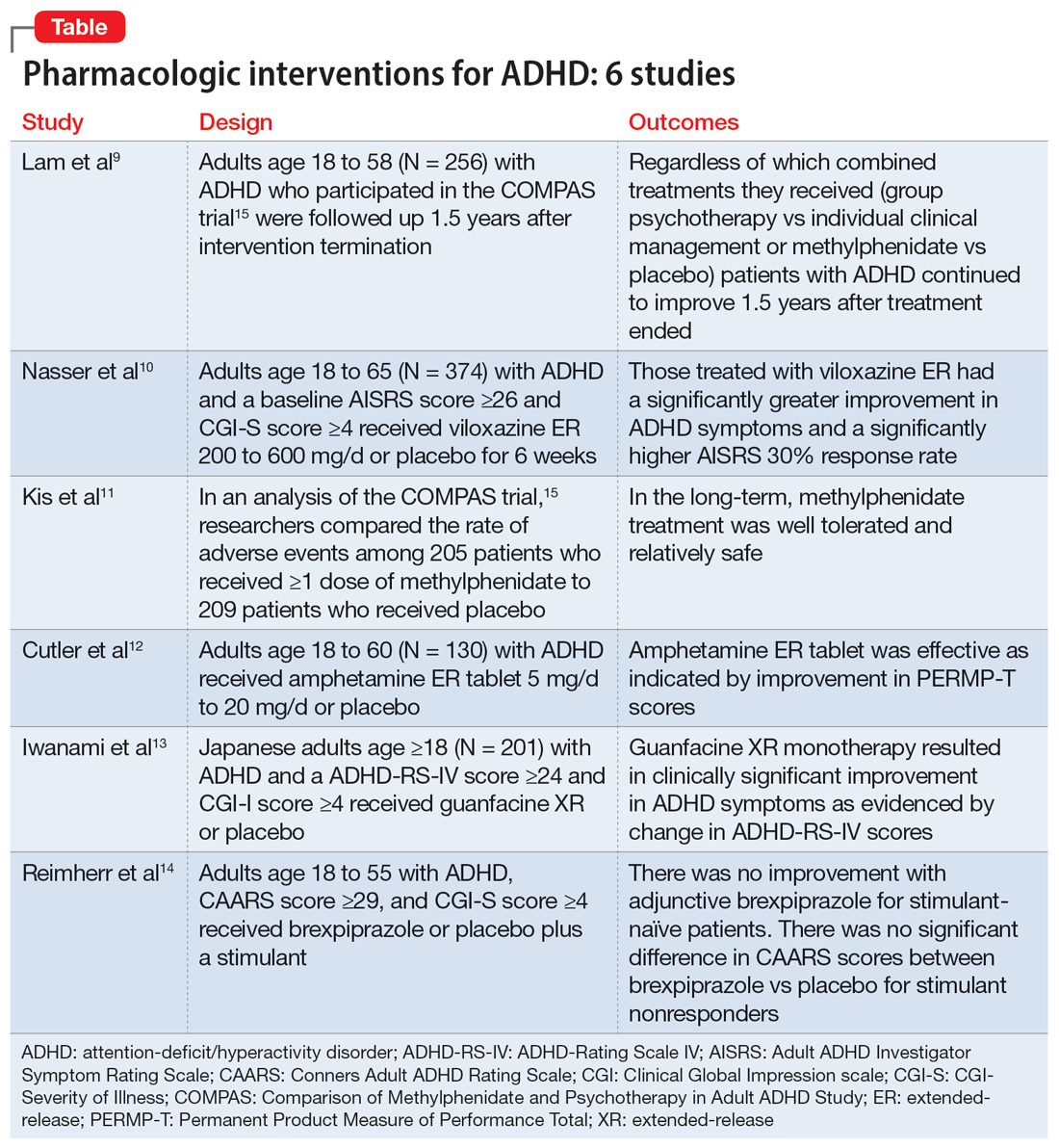
In Part 1 of this 2-part article, we review 6 RCTs of pharmacologic interventions for adult ADHD published within the last 5 years (Table9-14). Part 2 will review nonpharmacologic treatments.
1. Lam AP, Matthies S, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Long-term effects of multimodal treatment on adult attention-deficit/hyperactivity disorder symptoms: follow-up analysis of the COMPAS Trial. JAMA Netw Open. 2019;2(5):e194980. doi:10.1001/jamanetworkopen.2019.4980
The Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) was a multicenter prospective, randomized trial of adults age 18 to 58 with ADHD.15 It compared cognitive-behavioral group psychotherapy (GPT) with individual clinical management (CM), and methylphenidate with placebo. When used in conjunction with methylphenidate, psychological treatments produced better results than placebo. However, studies on the long-term effects of multimodal treatment in ADHD are limited. Lam et al9 performed a follow-up analysis of the COMPAS trial.
Study design
- This observer-masked study involved a follow-up of participants in COMPAS 1.5 years after the interventions were terminated. Of the 433 adults with ADHD who participated in COMPAS, 256 participated in this follow-up.
- The inclusion criteria of COMPAS were age 18 to 58; diagnosis of ADHD according to DSM-IV criteria; chronic course of ADHD symptoms from childhood to adulthood; a Wender Utah Rating Scale short version score ≥30; and no pathological abnormality detected on physical examination.
- The exclusion criteria were having an IQ <85; schizophrenia, bipolar disorder (BD), borderline personality disorder, antisocial personality disorder, suicidal or self-injurious behavior, autism, motor tics, or Tourette syndrome; substance abuse/dependence within 6 months prior to screening; positive drug screening; neurologic diseases, seizures, glaucoma, diabetes, hyperlipidemia, uncontrolled arterial hypertension, angina pectoris, tachycardia arrhythmia, or arterial occlusive disease; previous stroke; current bulimia or anorexia; low weight (body mass index [BMI] <20; pregnancy (current or planned) or breastfeeding; treatment with stimulants or ADHD-specific psychotherapy in the past 6 months; methylphenidate intolerance; treatment with antidepressants, norepinephrine reuptake inhibitors, bupropion, antipsychotics, theophylline, amantadine, anticoagulants derived from coumarin, antacids, or alpha-adrenergic agonists in the 2 weeks prior to baseline; and treatment with fluoxetine or monoamine oxidase inhibitors in the 4 weeks prior to baseline.
- The primary outcome was a change from baseline on the ADHD Index of Conners Adult ADHD Rating Scale (CAARS) score. Secondary outcomes were self-ratings on the Beck Depression Inventory (BDI) and observer-masked ratings of the Clinical Global Impression (CGI) scale and other ADHD rating scale scores, such as the Diagnostic Checklist for the diagnosis of ADHD in adults (ADHD-DC) and subscales of the CAARS.
- COMPAS was open regarding patient and therapist assignment to GPT and CM, but double-masked regarding medication. The statistical analysis focused on the 2x2 comparison of GPT vs CM and methylphenidate vs placebo.
Outcomes
- A total of 251 participants had an assessment with the observer-masked CAARS score. The baseline mean (SD) age was 36.3 (10.1), and approximately one-half (49.8%) of participants were male.
- Overall, 9.2% of patients took methylphenidate >31 days from termination of COMPAS before this study but not at the start of this study. Approximately one-third (31.1%) of patients were taking methylphenidate at follow-up. The mean (SD) daily dosage of methylphenidate was 36 (24.77) mg and 0.46 (0.27) mg/kg of body weight.
- The baseline all-group mean ADHD Index of CAARS score was 20.6. At follow-up, it was 14.7 for the CM arm and 14.2 for the GPT arm (difference not significant, P = .48). The mean score decreased to 13.8 for the methylphenidate arm and to 15.2 for the placebo (significant difference, P = .04).
- Overall, methylphenidate was associated with greater improvement in symptoms than placebo. Patients in the GPT arm had fewer severe symptoms as assessed by the self-reported ADHD Symptoms Total Score compared to the CM arm (P = .04).
- There were no significant differences in self-rating CAARS and observer-rated CAARS subscale scores. Compared to CM, GPT significantly decreased pure hyperactive symptoms on the ADHD-DC (P = .08). No significant differences were observed in BDI scores. The difference between GPT and CM remained significant at follow-up in terms of the CGI evaluation of efficacy (P = .04).
Continue to: Conclusions/limitations
Conclusions/limitations
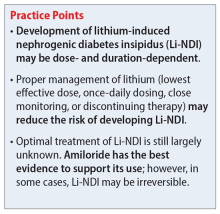
- Regardless of which combined treatments they received, patients with ADHD continued to improve 1.5 years after the 52-week treatment phase ended.
- Patients assigned to methylphenidate performed considerably better on the observer-rated CAARS than patients assigned to placebo.
- Benefits from GPT or CM in addition to methylphenidate therapy lasted 1.5 years. Compared to CM, GPT was not linked to better scores on the CAARS.
- Limitations: Approximately 41% of patients who were recruited did not participate. Daily functioning was measured only by the CGI. There were only marginal differences among the 4 treatments, and the study compared a very regimented approach (GPT) with one that was less focused (CM).
2. Nasser A, Hull JT, Chaturvedi SA, et al. A phase III, randomized, double‐blind, placebo‐controlled trial assessing the efficacy and safety of viloxazine extended‐release capsules in adults with attention‐deficit/hyperactivity disorder. CNS Drugs. 2022;36(8): 897-915. doi:10.1007/s40263-022-00938-w
In 2021, the FDA approved viloxazine extended-release (ER) for treating ADHD in children and adolescents (age 6 to 17). Nasser et al10 reviewed the safety and efficacy of viloxazine ER in adults with ADHD.
Study design
- This phase III, randomized, double-blind, placebo-controlled, multicenter clinical trial included 374 adults with ADHD who received viloxazine ER or placebo.
- Participants were age 18 to 65 and had been given a primary diagnosis of ADHD according to DSM-5 criteria in the last 6 months. Other inclusion criteria were having an Adult ADHD Investigator Symptom Rating Scale (AISRS) total score ≥26 and CGI-Severity of Illness (CGI-S) score ≥4 at baseline, BMI 18 to 35 kg/m2, and being medically healthy.
- Exclusion criteria included having treatment-resistant ADHD, a current diagnosis of any psychiatric disorder other than ADHD, or a history of schizophrenia, schizoaffective disorder, BD, autism, obsessive-compulsive disorder, personality disorder, or posttraumatic stress disorder. Individuals with any significant neurologic disorder, heart condition, arrhythmia, clinically relevant vital sign abnormality, or systemic illness were excluded, as were those with a history (within the past year) or current diagnosis of substance use disorder or a positive drug screen for a drug of abuse. Those with an allergic reaction or intolerance to viloxazine or were breastfeeding, pregnant, or refused to be abstinent or practice birth control were excluded.
- The dosage of viloxazine ER ranged from 200 to 600 mg/d for 6 weeks. This was titrated based on symptom response and adverse effects.
- All individuals received 2 capsules once a day for Week 1 and Week 2. During Week 1 and Week 2, participants in the viloxazine ER group received 200 mg (1 viloxazine ER capsule and 1 placebo capsule) and 400 mg (2 viloxazine ER capsules) of the medication, respectively. Two placebo pills were administered to those in the placebo group. From Week 3 to Week 6, the dose could be titrated or tapered at the investigator’s discretion. Compliance was assessed by comparing the number of pills dispensed vs returned.
- The primary outcome was a change in AISRS score from baselines to Week 6.
- The key secondary outcome was the change in CGI-S score from baseline to Week 6. Scores on the AISRS inattention and hyperactive/impulsivity subscales, Behavioral Regulation Index, Metacognition Index, Behavior Rating Inventory of Executive Function–Adult Version (BRIEF-A), and Generalized Anxiety Disorder-7 item scale (GAD-7) were also evaluated. Also, the rates of 30% and 50% responders on the AISRS (defined as ≥30% or ≥50% reduction from baseline in AISRS total score, respectively), CGI-S scores, and CGI-Improvement (CGI-I) scores were examined.
Outcomes
- Based on change in AISRS total scores, patients who received viloxazine ER had significantly greater improvement in their ADHD symptoms than those taking placebo (P = .0040). Patients in the viloxazine ER group had significantly greater improvement in AISRS hyperactive/impulsive (P = .0380) and inattentive symptoms (P = .0015).
- The decrease in CGI-S score was also significantly greater in the viloxazine ER group than in the placebo group (P = .0023). The viloxazine ER group also had significantly greater improvement in executive function as assessed by the BRIEF-A (P = .0468). The difference in GAD-7 scores between the viloxazine ER group and the placebo group was not significant.
- The viloxazine ER group had a greater AISRS 30% response rate than the placebo group (P = .0395). There were no significant differences between groups in AISRS 50% responder rate or CGI-I responder rate.
- Adverse effects related to viloxazine and occurring in ≥5% of participants included insomnia (14.8%), fatigue (11.6%), nausea, decreased appetite (10.1%), dry mouth (9.0%), and headache (9.0%). The discontinuation rate was 9.0% in the viloxazine ER group vs 4.9% in the placebo group.
Continue to: Conclusions/limitations
Conclusions/limitations
- Compared to placebo, patients treated with viloxazine ER had significantly greater improvements in ADHD symptoms, including both hyperactive/impulsive and inattentive components as well as executive function.
- The viloxazine ER group had a significantly higher AISRS 30% response rate than the placebo group, but there were no significant differences in anxiety symptoms or other measures of response.
- Viloxazine ER was well tolerated and safe.
- Limitations: There was a reduced power to detect differences in treatment due to participants dropping out or discontinuing treatment, a lack of interrater reliability data, and a lack of patient-reported outcome or satisfaction data.
3. Kis B, Lücke C, Abdel-Hamid M, et al. Safety profile of methylphenidate under long-term treatment in adult ADHD patients - results of the COMPAS study. Pharmacopsychiatry. 2020;53(6):263-271. doi:10.1055/a-1207-9851
Kis et al11 analyzed the safety results of COMPAS.15 Details of this trial, including interventions and inclusion/exclusion criteria, are described in the description of Lam et al.9
Study design
- Researchers compared the rate of adverse events (AEs) among 205 patients who received ≥1 dose of methylphenidate with 209 patients who received placebo.
- AEs were documented and analyzed on an “as received” basis during Week 0 to Week 52. Electrocardiogram (ECG) data were recorded at baseline and Week 24. Vital signs were monitored at baseline, every week for the first 12 weeks, then every 4 weeks for the next 52 weeks. Body weight was assessed at Week 6, Week 12, Week 20, Week 28, Week 40, and Week 52. A 12-lead ECG was obtained at baseline and Week 24.
- The sample size was assessed to have 80% power to detect group differences in AEs.
Outcomes
- Overall, 96% of participants in the methylphenidate group and 88% of participants in the placebo group experienced at least 1 AE (difference 8.1%; 95% CI, 2.9% to 13.5%).
- AEs that occurred more frequently with methylphenidate compared to placebo were decreased appetite (22% vs 3.8%); dry mouth (15% vs 4.8%); palpitations (13% vs 3.3%); gastrointestinal (GI) infection (11% vs 4.8%); agitation (11% vs 3.3%); restlessness (10% vs 2.9%); hyperhidrosis, tachycardia, and weight decrease (all 6.3% vs 1.9%); depressive symptoms and influenza (both 4.9% vs 1.0%); and acute tonsillitis (4.4% vs 0.5%). Serious AEs were reported by 7.3% of patients in the methylphenidate group and 4.3% of those in the placebo group, with no difference in frequency (difference 3.0%; 95% CI, 1.6% to 7.9%). The most severe AEs were aggression, depression, somnambulism, and suicidal ideation in the methylphenidate group and car accidents, epicondylitis, and a fall in the placebo group.
- There were no significant differences in AEs between the GPT and CM groups.
- The treatment combinations that included methylphenidate had higher rates of patients experiencing at least 1 AE (CM/methylphenidate 97%, GPT/methylphenidate 96%, CM/placebo 92%, GPT/placebo 84%).
- Overall, 8.8% of patients in the methylphenidate group and 4.8% in the placebo group stopped their medication treatment because of an AE (difference 4.0%; 95% CI, 0.9% to 9.1%). At least 1 dose decrease, increase, or discontinuation was made after an AE in 42% of participants in the placebo group and 69% of those in the methylphenidate group.
- There were no significant differences in clinically pertinent ECG abnormalities between methylphenidate and placebo therapy.
Continue to: Conclusions/limitations
Conclusions/limitations
- AEs were more common in the methylphenidate groups compared to placebo, but there was no significant differences for severe AEs. In the long-term, methylphenidate treatment was well tolerated and relatively safe.
- Limitations: The sample size may have been too small to detect uncommon AEs, all AEs had to be reported and may not have been caused by the treatment, and the original study’s main outcome was efficacy, not safety, which makes this an exploratory analysis of AEs.
4. Cutler AJ, Childress AC, Pardo A, et al. Randomized, double-blind, placebo-controlled, fixed-dose study to evaluate the efficacy and safety of amphetamine extended-release tablets in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2022;83(5):22m14438. doi:10.4088/JCP.22m14438
Once-daily dosing of stimulants, which are commonly used to manage adult ADHD,16 can be beneficial because many patients have schedules that limit taking medication multiple times a day. Cutler et al12 looked at the efficacy and safety of amphetamine extended-release tablet (AMPH ER TAB), which is a 3.2:1 mixture of d- and l-amphetamine released by the LiquiXR drug delivery system. This technology allows for a continuous release following an initial quick onset of action.
Study design
- This parallel-study, double-blind study evaluated adults age 18 to 60 who had a diagnosis of ADHD according to DSM-5 criteria and the Adult ADHD Clinical Diagnostic Scale, normal-range IQ, AISRS score ≥26, and baseline CGI-S score ≥4.
- Women were not lactating or pregnant during the study.
- Exclusion criteria included a history of mental illnesses; chronic medical conditions; clinically significant abnormal ECG or cardiac findings on exam; renal or liver disease; family history of sudden death; significant vital sign findings; uncontrolled hypertension or a resting systolic blood pressure (SBP) >140 mmHg or diastolic blood pressure (DBP) >90 mmHg; recent history of or current alcohol or substance use disorder; use of atomoxetine, monoamine oxidase inhibitors, or tricyclic antidepressants within 14 days of study or the use of other stimulant medications within 1 week of screening; use of GI acidifying agents or urinary acidifying agents within 3 days of screening; answering “yes” to questions 4 or 5 of the Suicidal Ideation section of the Columbia Suicide Severity Rating Scale within 2 years prior to the study; taking another investigational medication within 30 days of screening; allergic to amphetamine or components of the study drug, and a lack of prior response to amphetamine.
- Patients were randomized to receive AMPH ER TAB (n = 65) or placebo (n = 65), taken before 10
am . Participants started at 5 mg/d of the drug/placebo and then entered a 5-week titration period in which the medication was increased by 5 mg/d each week until reaching 20 mg/d, and then continued 20 mg/d for 2 weeks. - The primary outcome was the mean Permanent Product Measure of Performance Total (PERMP-T) score averaged across all time points (0.5-, 1-, 2-, 4-, 8-, 10-, 12-, 13-, and 14-hours postdose) at Visit 5.
- Participants underwent AISRS, CGI-S, and safety evaluations at baseline and at the 5 visits at the end of each treatment week.
Outcomes
- Analyses were completed on participants who received ≥1 dose of the medication and who had ≥1 PERMP-T score at Visit 5.
- Predose PERMP-T scores were similar between the AMPH ER TAB group (259.5) and placebo group (260). The mean postdose PERMP-T score in the AMPH ER TAB group (302.8) was significantly higher (P = .0043) than the placebo group (279.6).
- The PERMP-T scores were significantly different at 0.5-, 1-, 2-, 4-, 8-, and 13-hours postdose but not at 10-, 12-, and 14-hours postdose. The first Visit 5 time point at which the difference between groups was statistically different was at 0.5 hours postdose (P = .01), and the last significant time point was 13 hours (P = .006).
- The improvement in CGI-S scores was significantly greater in the AMPH ER TAB group than the placebo group. The improvement in AISRS scores was significantly greater in the AMPH ER TAB group at Visit 3, Visit 4, and Visit 5. More participants in the AMPH ER TAB group had AEs compared to the placebo group (90% vs 60%). The most common AEs (frequency ≥5% and occurring more in the intervention arm) were decreased appetite, insomnia, dry mouth, irritability, headache, anxiety, nausea, dizziness, and tachycardia.
- The AMPH ER TAB group had nonclinically significant increases in SBP (116.8 to 120.7 mmHg), DBP (74.1 to 77.1 mmHg), and heart rate (73.0 to 81.9 bpm) at Visit 5 compared to baseline.
- No serious AEs occurred. Three participants in the AMPH ER TAB group experienced AEs (increased blood pressure, CNS stimulation, and anxiety) that led them to discontinue the study.
Continue to: Conclusions/limitations
Conclusions/limitations
- AMPH ER TAB reduced symptoms in adults with ADHD as assessed by improvement in PERMP-T scores.
- The safety and tolerability profile of AMPH ER TAB were comparable to other stimulants, with expected rises in blood pressure and heart rate.
- Limitations: Patients were required to be titrated to 20 mg/d of AMPH ER TAB, instead of following a flexible titration based on an individual’s response. Some participants may have had greater improvement at a higher or lower dose. This study did not compare AMPH ER TAB to other stimulants. The 5-week duration of this study limited the ability to evaluate long-term efficacy and tolerability. Patients with a wide range of psychiatric or medical comorbidities were excluded.
5. Iwanami A, Saito K, Fujiwara M, et al. Efficacy and safety of guanfacine extended-release in the treatment of attention-deficit/hyperactivity disorder in adults: results of a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2020;81(3):19m12979. doi:10.4088/JCP.19m12979
Guanfacine extended-release (GXR) is a selective alpha 2A-adrenergic receptor agonist approved for treating ADHD in children and adolescents.17 Iwanami et al13 evaluated the efficacy and safety of GXR for adults.
Study design
- This randomized, double-blinded, placebo-controlled trial enrolled Japanese adults age ≥18 who were diagnosed with ADHD according to DSM-5 criteria and scored ≥24 on the ADHD-Rating Scale IV (ADHD-RS-IV) and ≥4 on CGI- I.
- Exclusion criteria included having anxiety, depression, substance use disorder, tic disorder, BD, personality disorder, schizophrenia, or intellectual disability; a moderate or severe psychiatric disorder requiring treatment other than counseling; seizures; increased risk for suicide; a history of cardiovascular disease, including prolonged QTc/abnormal ECG/abnormal labs, orthostatic hypotension, or continuous bradycardia; or taking medications that affect blood pressure or heart rate.
- Overall, 101 participants were randomized to the GXR group and 100 to the placebo group. Approximately two-thirds of the study population was male. Patients received GXR or placebo once daily at approximately the same time.
- There were 5 phases to the trial. The screening period occurred over 1 to 4 weeks. Part 1 of the treatment period consisted of 5 weeks of medication optimization. Participants were started on GXR 2 mg/d and were required to be receiving a minimum dose of 4 mg/d starting at Week 3. Clinicians were allowed to increase the dose 1 mg/d per week starting at Week 4 based on clinical response to a maximum dosage of 6 mg/d. Part 2 of the treatment period consisted of 5 weeks of maintenance at 4 to 6 mg/d. The tapering period to 2 mg/d occurred over 2 weeks. The follow-up period lasted 1 week.
- Efficacy measurements included the Japanese version of the ADHD-RS-IV and translations of the English-language CAARS, CGI-I, and CGI-S. Participant-reported measures included the Patient Global Impression-Improvement scale (PGI-I), Adult ADHD Quality of Life Questionnaire (AAQoL), and BRIEF-A.
- The primary outcome was the difference in ADHD-RS-IV total score from baseline to the end of the maintenance period (Week 10).
- Safety assessments were completed at Week 5 (end of dose optimization period), Week 10 (end of dose maintenance period), and Week 12 (tapering period).
Outcomes
- The average GXR dose during the maintenance period was 5.07 mg/d.
- Compared to the placebo group, the GXR group had more patients age <30 (47% vs 39%) and fewer patients age ≥40 (17% vs 27%). Baseline ADHD-RS-IV scores in both groups were comparable. At baseline, 51% in the GXR group had a combined inattentive/hyperactive-impulsive presentation and 47% had a predominately inattention presentation, with similar characteristics in the placebo group (49% combined, 49% inattention).
- At Week 10, the least squares mean change from baseline on the ADHD-RS-IV total score was significantly greater in the GXR group than in the placebo group (-11.55 ± 1.10 vs -7.27 ± 1.07; P = .0005), with an effect size of 0.52. There was a greater decrease in the ADHD-RS-IV scores starting at Week 4 and continuing to Week 10 (P < .005).
- There were also significant differences favoring GXR on the ADHD-RS-IV hyperactivity-impulsivity subscale score (P = .0021) and ADHD-RS-IV inattention subscale score (P = .0032).
- There were significant differences in the CAARS total ADHD score (P = .0029) and BRIEF-A scores on the inhibit (P = .0173), initiate (P = .0406), plan/organize (P = .174), and global executive composite index (P = .0404) scales. There was no significant difference in the total AAQoL score (P = .0691), but there was a significant improvement in the AAQoL life productivity subscore (P = .0072).
- At Week 10, there were also significant improvements in the CGI-I scores (P = .0007) and PGI-I scores (P = .0283). The CGI-S scores were similar at all time points.
- Overall, 81.2% of GXR patients reported AEs compared to 62% in the placebo group. There was 1 serious treatment-emergent AE (a suicide attempt) that the authors concluded was unrelated to the study drug. No deaths occurred. The most common AEs (incidence ≥10% in either group) included somnolence, thirst, nasopharyngitis (occurring more in the placebo group), blood pressure decrease, postural dizziness, and constipation. The main AEs leading to discontinuation were somnolence and blood pressure decrease. Overall, 19.8% of patients receiving GXR discontinued treatment due to AEs, compared to 3% in the placebo group.
- Heart rate, blood pressure, and QTc (corrected by the Bazett formula) were decreased in the GXR group at Week 10 while QT and RR intervals increased, and most returned to normal by Week 12.
Continue to: Conclusions/limitations
Conclusions/limitations
- Compared to placebo, GXR monotherapy resulted in clinical improvement in ADHD symptoms, with a moderate effect size.
- The most common AEs were mild to moderate and congruent with known adverse effects of guanfacine. Sedation effects mostly transpired within the first week of medication administration and were transient.
- Limitations: The findings might not be generalizable to non-Japanese patients. The duration of the study was short. Patients with a wide range of psychiatric and medical comorbidities were excluded. Two-thirds of the participants were male, and there was a disparity in participant age in the GXR and placebo groups.
6. Reimherr FW, Gift TE, Steans TA, et al. The use of brexpiprazole combined with a stimulant in adults with treatment-resistant attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2022;42(5):445-453. doi:10.1097/JCP.0000000000001592
While stimulants are a mainstay ADHD treatment, some patients have a partial response or do not respond to amphetamines or methylphenidate. Reimherr et el14 assessed the efficacy and safety of adding brexpiprazole (BXP) to a stimulant.
Study design
- This randomized, double-blinded, placebo-controlled trial recruited 559 stimulant-naive patients and 174 patients who had not responded to previous stimulant therapy.
- Participants were adults age 18 to 55 with a primary diagnosis of ADHD according to DSM-IV-TR criteria and the Conners Adult ADHD Diagnostic Interview. Other inclusion criteria were having a CAARS score ≥29 and a CGI-S score ≥4.
- Exclusion criteria included being at risk for suicide; having current substance abuse or positive alcohol/drug screens; a history of good response to prestudy treatment; a clinically significant medical condition; fasting blood glucose >200 mg/dL or hemoglobin A1C >7%; and hospitalization in past 12 months from a diabetic complication, uncontrolled hypertension, ischemic heart disease, or epilepsy. Further exclusion criteria included a history of psychosis, current MDD or BD, current panic disorder, uncontrolled comorbid psychiatric condition, or clinically significant personality disorder. Investigators excluded any patient with severe DSM-IV axis I or II disorders or abnormal/psychopathological behaviors.
- The trial consisted of 3 segments. Part 1 was screening. If the patient was currently receiving a stimulant but not fully responding, the medication was discontinued for at least 5 half-lives.
- Part 2 (5 weeks) involved administering a stimulant plus a single-blind placebo (597 patients completed this phase). The stimulant was chosen by the investigator, who had the option of using 1 of 2 amphetamine derivatives (mixed amphetamine salts capsules or lisdexamfetamine dimesylate capsules) or 1 of 2 methylphenidate derivatives (methylphenidate hydrochloride ER tabs or dexmethylphenidate HCl ER capsules). If a patient did not respond to a particular stimulant prior to the study, they were given a different stimulant from the list. Patients continued the same stimulant throughout the trial. Patients were monitored for a response, defined as a ≥30% decrease in CAARS score or a CAARS score <24, or a CGI-I score of 1 or 2 at Week 5. Patients who did not show this improvement were categorized as open-label nonresponders.
- Part 3 (6 weeks) involved administering a stimulant plus double-blind BXP vs placebo (stimulant-naive n = 167, stimulant nonresponders n = 68). Nonresponders continued the stimulant (at the same dose reached at the end of Part 2) and added either BXP (n = 155) or continued placebo (n = 80). Patients who responded in Part 2 were continued on the stimulant plus placebo and were not randomized. Patients were started on BXP 0.25 mg/d, and the medication could be titrated to 2 mg/d during the following 3 weeks, depending on the benefit vs AE profile. After the third week, the dose could be decreased but not increased.
- The primary outcome was a change in CAARS score. Secondary measurements included the CGI-S, Wender-Reimherr Adult Attention Deficit Disorder Scale (WRAADDS), Montgomery-Åsberg Depression Rating Scale (MADRS), and BDI.
Outcomes
- Stimulant-naive patients were equally divided among the 4 stimulant groups, and previous nonresponders who continued to not respond in Part 2 were more likely to be given methylphenidate HCl or lisdexamfetamine dimesylate.
- Patients with a history of nonresponse had less response to stimulants in Part 2 compared to stimulant-naive patients, as seen by 27% (n = 167) of stimulant-naive patients entering Part 3 compared to 39% of prior nonresponders (n = 68; P = .0249).
- ADHD improvement with BXP appeared to be greater among pretrial nonresponders.
- For stimulant nonresponders before and during the study, at the end of the double-blind endpoint (Part 3; Week 11), WRAADDS total score was significantly improved in the BXP group compared to the placebo group (P = .013; d = 0.74), with most beneficial effects seen in the hyperactivity/restlessness, emotional dysregulation factor, and impulsivity categories.
- For stimulant nonresponders before and during the study, there was no significant difference at the end of Week 11 on the CAARS (P = .64), MADRS (P = .37), or BDI (P = .73). There was a trend toward significance on the CAARS subscale for hyperactive/impulsive (P = .09).
- For prestudy stimulant-naive patients who did not respond to stimulants in Part 2 and were randomized in Part 3, there was not a significant difference between BXP and placebo at Week 11 as assessed on WRAADDS, CAARS, MADRS, or BDI.
- As assessed on WRAADDS, 50% in the BXP group had a response compared to 41% in the placebo group (Fisher exact = 0.334). Under the emotional dysregulation factor category of the WRAADDS, 64% in the BXP group had a response compared to 41% in the placebo group (Fisher exact = 0.064). The attention factor category showed a 40% improvement in the BXP group compared to 32% in the placebo group (Fisher exact = 0.344).
- There were 2 serious AEs in the BXP group (gall bladder inflammation and diarrhea) and 2 in the placebo group (pneumonia and urinary tract infection). There was no statistically significant difference between groups with regards to common AEs (ie, fatigue, heartburn/nausea/stomachache, weight loss), although there was a trend to significant for insomnia in the BXP group (P = .083).
Conclusions/limitations
- Stimulant-naive patients experienced no improvement with adjunctive BXP.
- For prior stimulant nonresponders, there was no significant difference between BXP vs placebo on the primary outcome of the CAARS score, but there was an improvement as observed by assessment with the WRAADDS.
- The largest change in the WRAADDS occurred in the emotional dysregulation factor compared to the attention factor.
- BXP appeared to be well tolerated.
- Limitations: The WRAADDS was administered without the patients’ significant other/collateral. Raters were not trained in the use of the WRAADDS. Patients with a wide range of psychiatric and medical comorbidities were excluded. Fewer patients were recruited in the prior stimulant nonresponder group.
Bottom Line
Recent randomized controlled trials suggest that methylphenidate, amphetamine extended-release, viloxazine extended-release, and guanfacine extended-release improved symptoms of adult attention-deficit/hyperactivity disorder (ADHD). There were no improvements in ADHD symptoms with adjunctive brexpiprazole.
Related Resources
- Parikh AR, Baker SA. Adult ADHD: pharmacologic treatment in the DSM-5 era. Current Psychiatry. 2016;15(10):18-25.
- Akbar HN. Why we should be scrutinizing the rising prevalence of adult ADHD. Current Psychiatry. 2022; 21(7):e1-e2. doi:10.12788/cp.0268
Drug Brand Names
Amantadine • Gocovri
Amphetamine extended-release tablet • Dyanavel XR
Atomoxetine • Strattera
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Dexmethylphenidate • Focalin
Fluoxetine • Prozac
Guanfacine extended- release • Intuniv
Lisdexamfetamine • Vyvanse
Methylphenidate • Concerta, Methylin
Theophylline • Elixophyllin
Viloxazine • Qelbree
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Harpin V, Mazzone L, Raynaud JP, et al. Long-term outcomes of ADHD: a systematic review of self-esteem and social function. J Atten Disord. 2016;20(4):295-305. doi:10.1177/1087054713486516
3. Beaton DM, Sirois F, Milne E. Experiences of criticism in adults with ADHD: a qualitative study. PLoS One. 2022;17(2):e0263366. doi:10.1371/journal.pone.0263366
4. Attention-deficit/hyperactivity disorder (ADHD). National Institute of Mental Health. Accessed February 9, 2023. https://www.nimh.nih.gov/health/statistics/attention-deficit-hyperactivity-disorder-adhd
5. Katzman MA, Bilkey TS, Chokka PR, et al. Adult ADHD and comorbid disorders: clinical implications of a dimensional approach. BMC Psychiatry. 2017;17(1):302. doi:10.1186/s12888-017-1463-3
6. Attention Deficit Hyperactivity Disorder: Diagnosis and Management. NICE Guideline No. 87. National Institute for Health and Care Excellence (NICE); 2019. Accessed February 9, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493361/
7. Adler LD, Nierenberg AA. Review of medication adherence in children and adults with ADHD. Postgrad Med. 2010;122(1):184-191. doi:10.3810/pgm.2010.01.2112
8. Cunill R, Castells X, Tobias A, et al. Efficacy, safety and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta-analysis and meta-regression in over 9000 patients. Psychopharmacology (Berl). 2016;233(2):187-197. doi:10.1007/s00213-015-4099-3
9. Lam AP, Matthies S, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Long-term effects of multimodal treatment on adult attention-deficit/hyperactivity disorder symptoms: follow-up analysis of the COMPAS Trial. JAMA Netw Open. 2019;2(5):e194980. doi:10.1001/jamanetworkopen.2019.4980
10. Nasser A, Hull JT, Chaturvedi SA, et al. A phase III, randomized, double-blind, placebo-controlled trial assessing the efficacy and safety of viloxazine extended-release capsules in adults with attention-deficit/hyperactivity disorder. CNS Drugs. 2022;36(8):897-915. doi:10.1007/s40263-022-00938-w
11. Kis B, Lücke C, Abdel-Hamid M, et al. Safety profile of methylphenidate under long-term treatment in adult ADHD patients - results of the COMPAS study. Pharmacopsychiatry. 2020;53(6):263-271. doi:10.1055/a-1207-9851
12. Cutler AJ, Childress AC, Pardo A, et al. Randomized, double-blind, placebo-controlled, fixed-dose study to evaluate the efficacy and safety of amphetamine extended-release tablets in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2022;83(5):22m14438. doi:10.4088/JCP.22m14438
13. Iwanami A, Saito K, Fujiwara M, et al. Efficacy and safety of guanfacine extended-release in the treatment of attention-deficit/hyperactivity disorder in adults: results of a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2020;81(3):19m12979. doi:10.4088/JCP.19m12979
14. Reimherr FW, Gift TE, Steans TA, et al. The use of brexpiprazole combined with a stimulant in adults with treatment-resistant attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2022;42(5):445-453. doi:10.1097/JCP.0000000000001592
15. Philipsen A, Jans T, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Effects of group psychotherapy, individual counseling, methylphenidate, and placebo in the treatment of adult attention-deficit/hyperactivity disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(12):1199-1210.
16. McGough JJ. Treatment controversies in adult ADHD. Am J Psychiatry. 2016;173(10):960-966. doi:10.1176/appi.ajp.2016.15091207
17. Cruz MP. Guanfacine extended-release tablets (Intuniv), a nonstimulant selective alpha2a-adrenergic receptor agonist for attention-deficit/hyperactivity disorder. P T. 2010;35(8):448-451.
Attention-deficit/hyperactivity disorder (ADHD) is a developmental disorder that begins in childhood and continues into adulthood. The clinical presentation is characterized by a persistent pattern of inattention, impulsivity, and/or hyperactivity that causes functional interference.1 ADHD affects patients’ interpersonal and professional lives as well as their daily functioning.2 Adults with ADHD may suffer from excessive self-criticism, low self-esteem, and sensitivity to criticism.3 The overall prevalence of adult ADHD is 4.4%.4 ADHD in adults is frequently associated with comorbid psychiatric disorders.5 The diagnosis of ADHD in adults requires the presence of ≥5 symptoms of inattention and hyperactivity/impulsivity that persist for ≥6 months. Patients must have first had such symptoms before age 12; symptoms need to be present in ≥2 settings and interfere with functioning.1
Treatment of ADHD includes pharmacologic and nonpharmacologic interventions. For most patients, pharmacotherapy—specifically stimulant medications—is advised as first-line treatment,6 with adequate trials of methylphenidate and amphetamines before using second-line agents such as nonstimulants. However, despite these medications’ efficacy in randomized controlled trials (RCTs), adherence is low.7 This could be due to inadequate response or adverse effects.8 Guidelines also recommend the use of nonpharmacologic interventions for adults who cannot adhere to or tolerate medication or have an inadequate response.6 Potential nonpharmacologic interventions include transcranial direct current stimulation, mindfulness, psychoeducation, cognitive-behavioral therapy, and chronotherapy.
In Part 1 of this 2-part article, we review 6 RCTs of pharmacologic interventions for adult ADHD published within the last 5 years (Table9-14). Part 2 will review nonpharmacologic treatments.

1. Lam AP, Matthies S, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Long-term effects of multimodal treatment on adult attention-deficit/hyperactivity disorder symptoms: follow-up analysis of the COMPAS Trial. JAMA Netw Open. 2019;2(5):e194980. doi:10.1001/jamanetworkopen.2019.4980
The Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) was a multicenter prospective, randomized trial of adults age 18 to 58 with ADHD.15 It compared cognitive-behavioral group psychotherapy (GPT) with individual clinical management (CM), and methylphenidate with placebo. When used in conjunction with methylphenidate, psychological treatments produced better results than placebo. However, studies on the long-term effects of multimodal treatment in ADHD are limited. Lam et al9 performed a follow-up analysis of the COMPAS trial.
Study design
- This observer-masked study involved a follow-up of participants in COMPAS 1.5 years after the interventions were terminated. Of the 433 adults with ADHD who participated in COMPAS, 256 participated in this follow-up.
- The inclusion criteria of COMPAS were age 18 to 58; diagnosis of ADHD according to DSM-IV criteria; chronic course of ADHD symptoms from childhood to adulthood; a Wender Utah Rating Scale short version score ≥30; and no pathological abnormality detected on physical examination.
- The exclusion criteria were having an IQ <85; schizophrenia, bipolar disorder (BD), borderline personality disorder, antisocial personality disorder, suicidal or self-injurious behavior, autism, motor tics, or Tourette syndrome; substance abuse/dependence within 6 months prior to screening; positive drug screening; neurologic diseases, seizures, glaucoma, diabetes, hyperlipidemia, uncontrolled arterial hypertension, angina pectoris, tachycardia arrhythmia, or arterial occlusive disease; previous stroke; current bulimia or anorexia; low weight (body mass index [BMI] <20; pregnancy (current or planned) or breastfeeding; treatment with stimulants or ADHD-specific psychotherapy in the past 6 months; methylphenidate intolerance; treatment with antidepressants, norepinephrine reuptake inhibitors, bupropion, antipsychotics, theophylline, amantadine, anticoagulants derived from coumarin, antacids, or alpha-adrenergic agonists in the 2 weeks prior to baseline; and treatment with fluoxetine or monoamine oxidase inhibitors in the 4 weeks prior to baseline.
- The primary outcome was a change from baseline on the ADHD Index of Conners Adult ADHD Rating Scale (CAARS) score. Secondary outcomes were self-ratings on the Beck Depression Inventory (BDI) and observer-masked ratings of the Clinical Global Impression (CGI) scale and other ADHD rating scale scores, such as the Diagnostic Checklist for the diagnosis of ADHD in adults (ADHD-DC) and subscales of the CAARS.
- COMPAS was open regarding patient and therapist assignment to GPT and CM, but double-masked regarding medication. The statistical analysis focused on the 2x2 comparison of GPT vs CM and methylphenidate vs placebo.
Outcomes
- A total of 251 participants had an assessment with the observer-masked CAARS score. The baseline mean (SD) age was 36.3 (10.1), and approximately one-half (49.8%) of participants were male.
- Overall, 9.2% of patients took methylphenidate >31 days from termination of COMPAS before this study but not at the start of this study. Approximately one-third (31.1%) of patients were taking methylphenidate at follow-up. The mean (SD) daily dosage of methylphenidate was 36 (24.77) mg and 0.46 (0.27) mg/kg of body weight.
- The baseline all-group mean ADHD Index of CAARS score was 20.6. At follow-up, it was 14.7 for the CM arm and 14.2 for the GPT arm (difference not significant, P = .48). The mean score decreased to 13.8 for the methylphenidate arm and to 15.2 for the placebo (significant difference, P = .04).
- Overall, methylphenidate was associated with greater improvement in symptoms than placebo. Patients in the GPT arm had fewer severe symptoms as assessed by the self-reported ADHD Symptoms Total Score compared to the CM arm (P = .04).
- There were no significant differences in self-rating CAARS and observer-rated CAARS subscale scores. Compared to CM, GPT significantly decreased pure hyperactive symptoms on the ADHD-DC (P = .08). No significant differences were observed in BDI scores. The difference between GPT and CM remained significant at follow-up in terms of the CGI evaluation of efficacy (P = .04).
Continue to: Conclusions/limitations
Conclusions/limitations
- Regardless of which combined treatments they received, patients with ADHD continued to improve 1.5 years after the 52-week treatment phase ended.
- Patients assigned to methylphenidate performed considerably better on the observer-rated CAARS than patients assigned to placebo.
- Benefits from GPT or CM in addition to methylphenidate therapy lasted 1.5 years. Compared to CM, GPT was not linked to better scores on the CAARS.
- Limitations: Approximately 41% of patients who were recruited did not participate. Daily functioning was measured only by the CGI. There were only marginal differences among the 4 treatments, and the study compared a very regimented approach (GPT) with one that was less focused (CM).
2. Nasser A, Hull JT, Chaturvedi SA, et al. A phase III, randomized, double‐blind, placebo‐controlled trial assessing the efficacy and safety of viloxazine extended‐release capsules in adults with attention‐deficit/hyperactivity disorder. CNS Drugs. 2022;36(8): 897-915. doi:10.1007/s40263-022-00938-w
In 2021, the FDA approved viloxazine extended-release (ER) for treating ADHD in children and adolescents (age 6 to 17). Nasser et al10 reviewed the safety and efficacy of viloxazine ER in adults with ADHD.
Study design
- This phase III, randomized, double-blind, placebo-controlled, multicenter clinical trial included 374 adults with ADHD who received viloxazine ER or placebo.
- Participants were age 18 to 65 and had been given a primary diagnosis of ADHD according to DSM-5 criteria in the last 6 months. Other inclusion criteria were having an Adult ADHD Investigator Symptom Rating Scale (AISRS) total score ≥26 and CGI-Severity of Illness (CGI-S) score ≥4 at baseline, BMI 18 to 35 kg/m2, and being medically healthy.
- Exclusion criteria included having treatment-resistant ADHD, a current diagnosis of any psychiatric disorder other than ADHD, or a history of schizophrenia, schizoaffective disorder, BD, autism, obsessive-compulsive disorder, personality disorder, or posttraumatic stress disorder. Individuals with any significant neurologic disorder, heart condition, arrhythmia, clinically relevant vital sign abnormality, or systemic illness were excluded, as were those with a history (within the past year) or current diagnosis of substance use disorder or a positive drug screen for a drug of abuse. Those with an allergic reaction or intolerance to viloxazine or were breastfeeding, pregnant, or refused to be abstinent or practice birth control were excluded.
- The dosage of viloxazine ER ranged from 200 to 600 mg/d for 6 weeks. This was titrated based on symptom response and adverse effects.
- All individuals received 2 capsules once a day for Week 1 and Week 2. During Week 1 and Week 2, participants in the viloxazine ER group received 200 mg (1 viloxazine ER capsule and 1 placebo capsule) and 400 mg (2 viloxazine ER capsules) of the medication, respectively. Two placebo pills were administered to those in the placebo group. From Week 3 to Week 6, the dose could be titrated or tapered at the investigator’s discretion. Compliance was assessed by comparing the number of pills dispensed vs returned.
- The primary outcome was a change in AISRS score from baselines to Week 6.
- The key secondary outcome was the change in CGI-S score from baseline to Week 6. Scores on the AISRS inattention and hyperactive/impulsivity subscales, Behavioral Regulation Index, Metacognition Index, Behavior Rating Inventory of Executive Function–Adult Version (BRIEF-A), and Generalized Anxiety Disorder-7 item scale (GAD-7) were also evaluated. Also, the rates of 30% and 50% responders on the AISRS (defined as ≥30% or ≥50% reduction from baseline in AISRS total score, respectively), CGI-S scores, and CGI-Improvement (CGI-I) scores were examined.
Outcomes
- Based on change in AISRS total scores, patients who received viloxazine ER had significantly greater improvement in their ADHD symptoms than those taking placebo (P = .0040). Patients in the viloxazine ER group had significantly greater improvement in AISRS hyperactive/impulsive (P = .0380) and inattentive symptoms (P = .0015).
- The decrease in CGI-S score was also significantly greater in the viloxazine ER group than in the placebo group (P = .0023). The viloxazine ER group also had significantly greater improvement in executive function as assessed by the BRIEF-A (P = .0468). The difference in GAD-7 scores between the viloxazine ER group and the placebo group was not significant.
- The viloxazine ER group had a greater AISRS 30% response rate than the placebo group (P = .0395). There were no significant differences between groups in AISRS 50% responder rate or CGI-I responder rate.
- Adverse effects related to viloxazine and occurring in ≥5% of participants included insomnia (14.8%), fatigue (11.6%), nausea, decreased appetite (10.1%), dry mouth (9.0%), and headache (9.0%). The discontinuation rate was 9.0% in the viloxazine ER group vs 4.9% in the placebo group.
Continue to: Conclusions/limitations
Conclusions/limitations
- Compared to placebo, patients treated with viloxazine ER had significantly greater improvements in ADHD symptoms, including both hyperactive/impulsive and inattentive components as well as executive function.
- The viloxazine ER group had a significantly higher AISRS 30% response rate than the placebo group, but there were no significant differences in anxiety symptoms or other measures of response.
- Viloxazine ER was well tolerated and safe.
- Limitations: There was a reduced power to detect differences in treatment due to participants dropping out or discontinuing treatment, a lack of interrater reliability data, and a lack of patient-reported outcome or satisfaction data.
3. Kis B, Lücke C, Abdel-Hamid M, et al. Safety profile of methylphenidate under long-term treatment in adult ADHD patients - results of the COMPAS study. Pharmacopsychiatry. 2020;53(6):263-271. doi:10.1055/a-1207-9851
Kis et al11 analyzed the safety results of COMPAS.15 Details of this trial, including interventions and inclusion/exclusion criteria, are described in the description of Lam et al.9
Study design
- Researchers compared the rate of adverse events (AEs) among 205 patients who received ≥1 dose of methylphenidate with 209 patients who received placebo.
- AEs were documented and analyzed on an “as received” basis during Week 0 to Week 52. Electrocardiogram (ECG) data were recorded at baseline and Week 24. Vital signs were monitored at baseline, every week for the first 12 weeks, then every 4 weeks for the next 52 weeks. Body weight was assessed at Week 6, Week 12, Week 20, Week 28, Week 40, and Week 52. A 12-lead ECG was obtained at baseline and Week 24.
- The sample size was assessed to have 80% power to detect group differences in AEs.
Outcomes
- Overall, 96% of participants in the methylphenidate group and 88% of participants in the placebo group experienced at least 1 AE (difference 8.1%; 95% CI, 2.9% to 13.5%).
- AEs that occurred more frequently with methylphenidate compared to placebo were decreased appetite (22% vs 3.8%); dry mouth (15% vs 4.8%); palpitations (13% vs 3.3%); gastrointestinal (GI) infection (11% vs 4.8%); agitation (11% vs 3.3%); restlessness (10% vs 2.9%); hyperhidrosis, tachycardia, and weight decrease (all 6.3% vs 1.9%); depressive symptoms and influenza (both 4.9% vs 1.0%); and acute tonsillitis (4.4% vs 0.5%). Serious AEs were reported by 7.3% of patients in the methylphenidate group and 4.3% of those in the placebo group, with no difference in frequency (difference 3.0%; 95% CI, 1.6% to 7.9%). The most severe AEs were aggression, depression, somnambulism, and suicidal ideation in the methylphenidate group and car accidents, epicondylitis, and a fall in the placebo group.
- There were no significant differences in AEs between the GPT and CM groups.
- The treatment combinations that included methylphenidate had higher rates of patients experiencing at least 1 AE (CM/methylphenidate 97%, GPT/methylphenidate 96%, CM/placebo 92%, GPT/placebo 84%).
- Overall, 8.8% of patients in the methylphenidate group and 4.8% in the placebo group stopped their medication treatment because of an AE (difference 4.0%; 95% CI, 0.9% to 9.1%). At least 1 dose decrease, increase, or discontinuation was made after an AE in 42% of participants in the placebo group and 69% of those in the methylphenidate group.
- There were no significant differences in clinically pertinent ECG abnormalities between methylphenidate and placebo therapy.
Continue to: Conclusions/limitations
Conclusions/limitations
- AEs were more common in the methylphenidate groups compared to placebo, but there was no significant differences for severe AEs. In the long-term, methylphenidate treatment was well tolerated and relatively safe.
- Limitations: The sample size may have been too small to detect uncommon AEs, all AEs had to be reported and may not have been caused by the treatment, and the original study’s main outcome was efficacy, not safety, which makes this an exploratory analysis of AEs.
4. Cutler AJ, Childress AC, Pardo A, et al. Randomized, double-blind, placebo-controlled, fixed-dose study to evaluate the efficacy and safety of amphetamine extended-release tablets in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2022;83(5):22m14438. doi:10.4088/JCP.22m14438
Once-daily dosing of stimulants, which are commonly used to manage adult ADHD,16 can be beneficial because many patients have schedules that limit taking medication multiple times a day. Cutler et al12 looked at the efficacy and safety of amphetamine extended-release tablet (AMPH ER TAB), which is a 3.2:1 mixture of d- and l-amphetamine released by the LiquiXR drug delivery system. This technology allows for a continuous release following an initial quick onset of action.
Study design
- This parallel-study, double-blind study evaluated adults age 18 to 60 who had a diagnosis of ADHD according to DSM-5 criteria and the Adult ADHD Clinical Diagnostic Scale, normal-range IQ, AISRS score ≥26, and baseline CGI-S score ≥4.
- Women were not lactating or pregnant during the study.
- Exclusion criteria included a history of mental illnesses; chronic medical conditions; clinically significant abnormal ECG or cardiac findings on exam; renal or liver disease; family history of sudden death; significant vital sign findings; uncontrolled hypertension or a resting systolic blood pressure (SBP) >140 mmHg or diastolic blood pressure (DBP) >90 mmHg; recent history of or current alcohol or substance use disorder; use of atomoxetine, monoamine oxidase inhibitors, or tricyclic antidepressants within 14 days of study or the use of other stimulant medications within 1 week of screening; use of GI acidifying agents or urinary acidifying agents within 3 days of screening; answering “yes” to questions 4 or 5 of the Suicidal Ideation section of the Columbia Suicide Severity Rating Scale within 2 years prior to the study; taking another investigational medication within 30 days of screening; allergic to amphetamine or components of the study drug, and a lack of prior response to amphetamine.
- Patients were randomized to receive AMPH ER TAB (n = 65) or placebo (n = 65), taken before 10
am . Participants started at 5 mg/d of the drug/placebo and then entered a 5-week titration period in which the medication was increased by 5 mg/d each week until reaching 20 mg/d, and then continued 20 mg/d for 2 weeks. - The primary outcome was the mean Permanent Product Measure of Performance Total (PERMP-T) score averaged across all time points (0.5-, 1-, 2-, 4-, 8-, 10-, 12-, 13-, and 14-hours postdose) at Visit 5.
- Participants underwent AISRS, CGI-S, and safety evaluations at baseline and at the 5 visits at the end of each treatment week.
Outcomes
- Analyses were completed on participants who received ≥1 dose of the medication and who had ≥1 PERMP-T score at Visit 5.
- Predose PERMP-T scores were similar between the AMPH ER TAB group (259.5) and placebo group (260). The mean postdose PERMP-T score in the AMPH ER TAB group (302.8) was significantly higher (P = .0043) than the placebo group (279.6).
- The PERMP-T scores were significantly different at 0.5-, 1-, 2-, 4-, 8-, and 13-hours postdose but not at 10-, 12-, and 14-hours postdose. The first Visit 5 time point at which the difference between groups was statistically different was at 0.5 hours postdose (P = .01), and the last significant time point was 13 hours (P = .006).
- The improvement in CGI-S scores was significantly greater in the AMPH ER TAB group than the placebo group. The improvement in AISRS scores was significantly greater in the AMPH ER TAB group at Visit 3, Visit 4, and Visit 5. More participants in the AMPH ER TAB group had AEs compared to the placebo group (90% vs 60%). The most common AEs (frequency ≥5% and occurring more in the intervention arm) were decreased appetite, insomnia, dry mouth, irritability, headache, anxiety, nausea, dizziness, and tachycardia.
- The AMPH ER TAB group had nonclinically significant increases in SBP (116.8 to 120.7 mmHg), DBP (74.1 to 77.1 mmHg), and heart rate (73.0 to 81.9 bpm) at Visit 5 compared to baseline.
- No serious AEs occurred. Three participants in the AMPH ER TAB group experienced AEs (increased blood pressure, CNS stimulation, and anxiety) that led them to discontinue the study.
Continue to: Conclusions/limitations
Conclusions/limitations
- AMPH ER TAB reduced symptoms in adults with ADHD as assessed by improvement in PERMP-T scores.
- The safety and tolerability profile of AMPH ER TAB were comparable to other stimulants, with expected rises in blood pressure and heart rate.
- Limitations: Patients were required to be titrated to 20 mg/d of AMPH ER TAB, instead of following a flexible titration based on an individual’s response. Some participants may have had greater improvement at a higher or lower dose. This study did not compare AMPH ER TAB to other stimulants. The 5-week duration of this study limited the ability to evaluate long-term efficacy and tolerability. Patients with a wide range of psychiatric or medical comorbidities were excluded.
5. Iwanami A, Saito K, Fujiwara M, et al. Efficacy and safety of guanfacine extended-release in the treatment of attention-deficit/hyperactivity disorder in adults: results of a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2020;81(3):19m12979. doi:10.4088/JCP.19m12979
Guanfacine extended-release (GXR) is a selective alpha 2A-adrenergic receptor agonist approved for treating ADHD in children and adolescents.17 Iwanami et al13 evaluated the efficacy and safety of GXR for adults.
Study design
- This randomized, double-blinded, placebo-controlled trial enrolled Japanese adults age ≥18 who were diagnosed with ADHD according to DSM-5 criteria and scored ≥24 on the ADHD-Rating Scale IV (ADHD-RS-IV) and ≥4 on CGI- I.
- Exclusion criteria included having anxiety, depression, substance use disorder, tic disorder, BD, personality disorder, schizophrenia, or intellectual disability; a moderate or severe psychiatric disorder requiring treatment other than counseling; seizures; increased risk for suicide; a history of cardiovascular disease, including prolonged QTc/abnormal ECG/abnormal labs, orthostatic hypotension, or continuous bradycardia; or taking medications that affect blood pressure or heart rate.
- Overall, 101 participants were randomized to the GXR group and 100 to the placebo group. Approximately two-thirds of the study population was male. Patients received GXR or placebo once daily at approximately the same time.
- There were 5 phases to the trial. The screening period occurred over 1 to 4 weeks. Part 1 of the treatment period consisted of 5 weeks of medication optimization. Participants were started on GXR 2 mg/d and were required to be receiving a minimum dose of 4 mg/d starting at Week 3. Clinicians were allowed to increase the dose 1 mg/d per week starting at Week 4 based on clinical response to a maximum dosage of 6 mg/d. Part 2 of the treatment period consisted of 5 weeks of maintenance at 4 to 6 mg/d. The tapering period to 2 mg/d occurred over 2 weeks. The follow-up period lasted 1 week.
- Efficacy measurements included the Japanese version of the ADHD-RS-IV and translations of the English-language CAARS, CGI-I, and CGI-S. Participant-reported measures included the Patient Global Impression-Improvement scale (PGI-I), Adult ADHD Quality of Life Questionnaire (AAQoL), and BRIEF-A.
- The primary outcome was the difference in ADHD-RS-IV total score from baseline to the end of the maintenance period (Week 10).
- Safety assessments were completed at Week 5 (end of dose optimization period), Week 10 (end of dose maintenance period), and Week 12 (tapering period).
Outcomes
- The average GXR dose during the maintenance period was 5.07 mg/d.
- Compared to the placebo group, the GXR group had more patients age <30 (47% vs 39%) and fewer patients age ≥40 (17% vs 27%). Baseline ADHD-RS-IV scores in both groups were comparable. At baseline, 51% in the GXR group had a combined inattentive/hyperactive-impulsive presentation and 47% had a predominately inattention presentation, with similar characteristics in the placebo group (49% combined, 49% inattention).
- At Week 10, the least squares mean change from baseline on the ADHD-RS-IV total score was significantly greater in the GXR group than in the placebo group (-11.55 ± 1.10 vs -7.27 ± 1.07; P = .0005), with an effect size of 0.52. There was a greater decrease in the ADHD-RS-IV scores starting at Week 4 and continuing to Week 10 (P < .005).
- There were also significant differences favoring GXR on the ADHD-RS-IV hyperactivity-impulsivity subscale score (P = .0021) and ADHD-RS-IV inattention subscale score (P = .0032).
- There were significant differences in the CAARS total ADHD score (P = .0029) and BRIEF-A scores on the inhibit (P = .0173), initiate (P = .0406), plan/organize (P = .174), and global executive composite index (P = .0404) scales. There was no significant difference in the total AAQoL score (P = .0691), but there was a significant improvement in the AAQoL life productivity subscore (P = .0072).
- At Week 10, there were also significant improvements in the CGI-I scores (P = .0007) and PGI-I scores (P = .0283). The CGI-S scores were similar at all time points.
- Overall, 81.2% of GXR patients reported AEs compared to 62% in the placebo group. There was 1 serious treatment-emergent AE (a suicide attempt) that the authors concluded was unrelated to the study drug. No deaths occurred. The most common AEs (incidence ≥10% in either group) included somnolence, thirst, nasopharyngitis (occurring more in the placebo group), blood pressure decrease, postural dizziness, and constipation. The main AEs leading to discontinuation were somnolence and blood pressure decrease. Overall, 19.8% of patients receiving GXR discontinued treatment due to AEs, compared to 3% in the placebo group.
- Heart rate, blood pressure, and QTc (corrected by the Bazett formula) were decreased in the GXR group at Week 10 while QT and RR intervals increased, and most returned to normal by Week 12.
Continue to: Conclusions/limitations
Conclusions/limitations
- Compared to placebo, GXR monotherapy resulted in clinical improvement in ADHD symptoms, with a moderate effect size.
- The most common AEs were mild to moderate and congruent with known adverse effects of guanfacine. Sedation effects mostly transpired within the first week of medication administration and were transient.
- Limitations: The findings might not be generalizable to non-Japanese patients. The duration of the study was short. Patients with a wide range of psychiatric and medical comorbidities were excluded. Two-thirds of the participants were male, and there was a disparity in participant age in the GXR and placebo groups.
6. Reimherr FW, Gift TE, Steans TA, et al. The use of brexpiprazole combined with a stimulant in adults with treatment-resistant attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2022;42(5):445-453. doi:10.1097/JCP.0000000000001592
While stimulants are a mainstay ADHD treatment, some patients have a partial response or do not respond to amphetamines or methylphenidate. Reimherr et el14 assessed the efficacy and safety of adding brexpiprazole (BXP) to a stimulant.
Study design
- This randomized, double-blinded, placebo-controlled trial recruited 559 stimulant-naive patients and 174 patients who had not responded to previous stimulant therapy.
- Participants were adults age 18 to 55 with a primary diagnosis of ADHD according to DSM-IV-TR criteria and the Conners Adult ADHD Diagnostic Interview. Other inclusion criteria were having a CAARS score ≥29 and a CGI-S score ≥4.
- Exclusion criteria included being at risk for suicide; having current substance abuse or positive alcohol/drug screens; a history of good response to prestudy treatment; a clinically significant medical condition; fasting blood glucose >200 mg/dL or hemoglobin A1C >7%; and hospitalization in past 12 months from a diabetic complication, uncontrolled hypertension, ischemic heart disease, or epilepsy. Further exclusion criteria included a history of psychosis, current MDD or BD, current panic disorder, uncontrolled comorbid psychiatric condition, or clinically significant personality disorder. Investigators excluded any patient with severe DSM-IV axis I or II disorders or abnormal/psychopathological behaviors.
- The trial consisted of 3 segments. Part 1 was screening. If the patient was currently receiving a stimulant but not fully responding, the medication was discontinued for at least 5 half-lives.
- Part 2 (5 weeks) involved administering a stimulant plus a single-blind placebo (597 patients completed this phase). The stimulant was chosen by the investigator, who had the option of using 1 of 2 amphetamine derivatives (mixed amphetamine salts capsules or lisdexamfetamine dimesylate capsules) or 1 of 2 methylphenidate derivatives (methylphenidate hydrochloride ER tabs or dexmethylphenidate HCl ER capsules). If a patient did not respond to a particular stimulant prior to the study, they were given a different stimulant from the list. Patients continued the same stimulant throughout the trial. Patients were monitored for a response, defined as a ≥30% decrease in CAARS score or a CAARS score <24, or a CGI-I score of 1 or 2 at Week 5. Patients who did not show this improvement were categorized as open-label nonresponders.
- Part 3 (6 weeks) involved administering a stimulant plus double-blind BXP vs placebo (stimulant-naive n = 167, stimulant nonresponders n = 68). Nonresponders continued the stimulant (at the same dose reached at the end of Part 2) and added either BXP (n = 155) or continued placebo (n = 80). Patients who responded in Part 2 were continued on the stimulant plus placebo and were not randomized. Patients were started on BXP 0.25 mg/d, and the medication could be titrated to 2 mg/d during the following 3 weeks, depending on the benefit vs AE profile. After the third week, the dose could be decreased but not increased.
- The primary outcome was a change in CAARS score. Secondary measurements included the CGI-S, Wender-Reimherr Adult Attention Deficit Disorder Scale (WRAADDS), Montgomery-Åsberg Depression Rating Scale (MADRS), and BDI.
Outcomes
- Stimulant-naive patients were equally divided among the 4 stimulant groups, and previous nonresponders who continued to not respond in Part 2 were more likely to be given methylphenidate HCl or lisdexamfetamine dimesylate.
- Patients with a history of nonresponse had less response to stimulants in Part 2 compared to stimulant-naive patients, as seen by 27% (n = 167) of stimulant-naive patients entering Part 3 compared to 39% of prior nonresponders (n = 68; P = .0249).
- ADHD improvement with BXP appeared to be greater among pretrial nonresponders.
- For stimulant nonresponders before and during the study, at the end of the double-blind endpoint (Part 3; Week 11), WRAADDS total score was significantly improved in the BXP group compared to the placebo group (P = .013; d = 0.74), with most beneficial effects seen in the hyperactivity/restlessness, emotional dysregulation factor, and impulsivity categories.
- For stimulant nonresponders before and during the study, there was no significant difference at the end of Week 11 on the CAARS (P = .64), MADRS (P = .37), or BDI (P = .73). There was a trend toward significance on the CAARS subscale for hyperactive/impulsive (P = .09).
- For prestudy stimulant-naive patients who did not respond to stimulants in Part 2 and were randomized in Part 3, there was not a significant difference between BXP and placebo at Week 11 as assessed on WRAADDS, CAARS, MADRS, or BDI.
- As assessed on WRAADDS, 50% in the BXP group had a response compared to 41% in the placebo group (Fisher exact = 0.334). Under the emotional dysregulation factor category of the WRAADDS, 64% in the BXP group had a response compared to 41% in the placebo group (Fisher exact = 0.064). The attention factor category showed a 40% improvement in the BXP group compared to 32% in the placebo group (Fisher exact = 0.344).
- There were 2 serious AEs in the BXP group (gall bladder inflammation and diarrhea) and 2 in the placebo group (pneumonia and urinary tract infection). There was no statistically significant difference between groups with regards to common AEs (ie, fatigue, heartburn/nausea/stomachache, weight loss), although there was a trend to significant for insomnia in the BXP group (P = .083).
Conclusions/limitations
- Stimulant-naive patients experienced no improvement with adjunctive BXP.
- For prior stimulant nonresponders, there was no significant difference between BXP vs placebo on the primary outcome of the CAARS score, but there was an improvement as observed by assessment with the WRAADDS.
- The largest change in the WRAADDS occurred in the emotional dysregulation factor compared to the attention factor.
- BXP appeared to be well tolerated.
- Limitations: The WRAADDS was administered without the patients’ significant other/collateral. Raters were not trained in the use of the WRAADDS. Patients with a wide range of psychiatric and medical comorbidities were excluded. Fewer patients were recruited in the prior stimulant nonresponder group.
Bottom Line
Recent randomized controlled trials suggest that methylphenidate, amphetamine extended-release, viloxazine extended-release, and guanfacine extended-release improved symptoms of adult attention-deficit/hyperactivity disorder (ADHD). There were no improvements in ADHD symptoms with adjunctive brexpiprazole.
Related Resources
- Parikh AR, Baker SA. Adult ADHD: pharmacologic treatment in the DSM-5 era. Current Psychiatry. 2016;15(10):18-25.
- Akbar HN. Why we should be scrutinizing the rising prevalence of adult ADHD. Current Psychiatry. 2022; 21(7):e1-e2. doi:10.12788/cp.0268
Drug Brand Names
Amantadine • Gocovri
Amphetamine extended-release tablet • Dyanavel XR
Atomoxetine • Strattera
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Dexmethylphenidate • Focalin
Fluoxetine • Prozac
Guanfacine extended- release • Intuniv
Lisdexamfetamine • Vyvanse
Methylphenidate • Concerta, Methylin
Theophylline • Elixophyllin
Viloxazine • Qelbree
Attention-deficit/hyperactivity disorder (ADHD) is a developmental disorder that begins in childhood and continues into adulthood. The clinical presentation is characterized by a persistent pattern of inattention, impulsivity, and/or hyperactivity that causes functional interference.1 ADHD affects patients’ interpersonal and professional lives as well as their daily functioning.2 Adults with ADHD may suffer from excessive self-criticism, low self-esteem, and sensitivity to criticism.3 The overall prevalence of adult ADHD is 4.4%.4 ADHD in adults is frequently associated with comorbid psychiatric disorders.5 The diagnosis of ADHD in adults requires the presence of ≥5 symptoms of inattention and hyperactivity/impulsivity that persist for ≥6 months. Patients must have first had such symptoms before age 12; symptoms need to be present in ≥2 settings and interfere with functioning.1
Treatment of ADHD includes pharmacologic and nonpharmacologic interventions. For most patients, pharmacotherapy—specifically stimulant medications—is advised as first-line treatment,6 with adequate trials of methylphenidate and amphetamines before using second-line agents such as nonstimulants. However, despite these medications’ efficacy in randomized controlled trials (RCTs), adherence is low.7 This could be due to inadequate response or adverse effects.8 Guidelines also recommend the use of nonpharmacologic interventions for adults who cannot adhere to or tolerate medication or have an inadequate response.6 Potential nonpharmacologic interventions include transcranial direct current stimulation, mindfulness, psychoeducation, cognitive-behavioral therapy, and chronotherapy.
In Part 1 of this 2-part article, we review 6 RCTs of pharmacologic interventions for adult ADHD published within the last 5 years (Table9-14). Part 2 will review nonpharmacologic treatments.

1. Lam AP, Matthies S, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Long-term effects of multimodal treatment on adult attention-deficit/hyperactivity disorder symptoms: follow-up analysis of the COMPAS Trial. JAMA Netw Open. 2019;2(5):e194980. doi:10.1001/jamanetworkopen.2019.4980
The Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) was a multicenter prospective, randomized trial of adults age 18 to 58 with ADHD.15 It compared cognitive-behavioral group psychotherapy (GPT) with individual clinical management (CM), and methylphenidate with placebo. When used in conjunction with methylphenidate, psychological treatments produced better results than placebo. However, studies on the long-term effects of multimodal treatment in ADHD are limited. Lam et al9 performed a follow-up analysis of the COMPAS trial.
Study design
- This observer-masked study involved a follow-up of participants in COMPAS 1.5 years after the interventions were terminated. Of the 433 adults with ADHD who participated in COMPAS, 256 participated in this follow-up.
- The inclusion criteria of COMPAS were age 18 to 58; diagnosis of ADHD according to DSM-IV criteria; chronic course of ADHD symptoms from childhood to adulthood; a Wender Utah Rating Scale short version score ≥30; and no pathological abnormality detected on physical examination.
- The exclusion criteria were having an IQ <85; schizophrenia, bipolar disorder (BD), borderline personality disorder, antisocial personality disorder, suicidal or self-injurious behavior, autism, motor tics, or Tourette syndrome; substance abuse/dependence within 6 months prior to screening; positive drug screening; neurologic diseases, seizures, glaucoma, diabetes, hyperlipidemia, uncontrolled arterial hypertension, angina pectoris, tachycardia arrhythmia, or arterial occlusive disease; previous stroke; current bulimia or anorexia; low weight (body mass index [BMI] <20; pregnancy (current or planned) or breastfeeding; treatment with stimulants or ADHD-specific psychotherapy in the past 6 months; methylphenidate intolerance; treatment with antidepressants, norepinephrine reuptake inhibitors, bupropion, antipsychotics, theophylline, amantadine, anticoagulants derived from coumarin, antacids, or alpha-adrenergic agonists in the 2 weeks prior to baseline; and treatment with fluoxetine or monoamine oxidase inhibitors in the 4 weeks prior to baseline.
- The primary outcome was a change from baseline on the ADHD Index of Conners Adult ADHD Rating Scale (CAARS) score. Secondary outcomes were self-ratings on the Beck Depression Inventory (BDI) and observer-masked ratings of the Clinical Global Impression (CGI) scale and other ADHD rating scale scores, such as the Diagnostic Checklist for the diagnosis of ADHD in adults (ADHD-DC) and subscales of the CAARS.
- COMPAS was open regarding patient and therapist assignment to GPT and CM, but double-masked regarding medication. The statistical analysis focused on the 2x2 comparison of GPT vs CM and methylphenidate vs placebo.
Outcomes
- A total of 251 participants had an assessment with the observer-masked CAARS score. The baseline mean (SD) age was 36.3 (10.1), and approximately one-half (49.8%) of participants were male.
- Overall, 9.2% of patients took methylphenidate >31 days from termination of COMPAS before this study but not at the start of this study. Approximately one-third (31.1%) of patients were taking methylphenidate at follow-up. The mean (SD) daily dosage of methylphenidate was 36 (24.77) mg and 0.46 (0.27) mg/kg of body weight.
- The baseline all-group mean ADHD Index of CAARS score was 20.6. At follow-up, it was 14.7 for the CM arm and 14.2 for the GPT arm (difference not significant, P = .48). The mean score decreased to 13.8 for the methylphenidate arm and to 15.2 for the placebo (significant difference, P = .04).
- Overall, methylphenidate was associated with greater improvement in symptoms than placebo. Patients in the GPT arm had fewer severe symptoms as assessed by the self-reported ADHD Symptoms Total Score compared to the CM arm (P = .04).
- There were no significant differences in self-rating CAARS and observer-rated CAARS subscale scores. Compared to CM, GPT significantly decreased pure hyperactive symptoms on the ADHD-DC (P = .08). No significant differences were observed in BDI scores. The difference between GPT and CM remained significant at follow-up in terms of the CGI evaluation of efficacy (P = .04).
Continue to: Conclusions/limitations
Conclusions/limitations
- Regardless of which combined treatments they received, patients with ADHD continued to improve 1.5 years after the 52-week treatment phase ended.
- Patients assigned to methylphenidate performed considerably better on the observer-rated CAARS than patients assigned to placebo.
- Benefits from GPT or CM in addition to methylphenidate therapy lasted 1.5 years. Compared to CM, GPT was not linked to better scores on the CAARS.
- Limitations: Approximately 41% of patients who were recruited did not participate. Daily functioning was measured only by the CGI. There were only marginal differences among the 4 treatments, and the study compared a very regimented approach (GPT) with one that was less focused (CM).
2. Nasser A, Hull JT, Chaturvedi SA, et al. A phase III, randomized, double‐blind, placebo‐controlled trial assessing the efficacy and safety of viloxazine extended‐release capsules in adults with attention‐deficit/hyperactivity disorder. CNS Drugs. 2022;36(8): 897-915. doi:10.1007/s40263-022-00938-w
In 2021, the FDA approved viloxazine extended-release (ER) for treating ADHD in children and adolescents (age 6 to 17). Nasser et al10 reviewed the safety and efficacy of viloxazine ER in adults with ADHD.
Study design
- This phase III, randomized, double-blind, placebo-controlled, multicenter clinical trial included 374 adults with ADHD who received viloxazine ER or placebo.
- Participants were age 18 to 65 and had been given a primary diagnosis of ADHD according to DSM-5 criteria in the last 6 months. Other inclusion criteria were having an Adult ADHD Investigator Symptom Rating Scale (AISRS) total score ≥26 and CGI-Severity of Illness (CGI-S) score ≥4 at baseline, BMI 18 to 35 kg/m2, and being medically healthy.
- Exclusion criteria included having treatment-resistant ADHD, a current diagnosis of any psychiatric disorder other than ADHD, or a history of schizophrenia, schizoaffective disorder, BD, autism, obsessive-compulsive disorder, personality disorder, or posttraumatic stress disorder. Individuals with any significant neurologic disorder, heart condition, arrhythmia, clinically relevant vital sign abnormality, or systemic illness were excluded, as were those with a history (within the past year) or current diagnosis of substance use disorder or a positive drug screen for a drug of abuse. Those with an allergic reaction or intolerance to viloxazine or were breastfeeding, pregnant, or refused to be abstinent or practice birth control were excluded.
- The dosage of viloxazine ER ranged from 200 to 600 mg/d for 6 weeks. This was titrated based on symptom response and adverse effects.
- All individuals received 2 capsules once a day for Week 1 and Week 2. During Week 1 and Week 2, participants in the viloxazine ER group received 200 mg (1 viloxazine ER capsule and 1 placebo capsule) and 400 mg (2 viloxazine ER capsules) of the medication, respectively. Two placebo pills were administered to those in the placebo group. From Week 3 to Week 6, the dose could be titrated or tapered at the investigator’s discretion. Compliance was assessed by comparing the number of pills dispensed vs returned.
- The primary outcome was a change in AISRS score from baselines to Week 6.
- The key secondary outcome was the change in CGI-S score from baseline to Week 6. Scores on the AISRS inattention and hyperactive/impulsivity subscales, Behavioral Regulation Index, Metacognition Index, Behavior Rating Inventory of Executive Function–Adult Version (BRIEF-A), and Generalized Anxiety Disorder-7 item scale (GAD-7) were also evaluated. Also, the rates of 30% and 50% responders on the AISRS (defined as ≥30% or ≥50% reduction from baseline in AISRS total score, respectively), CGI-S scores, and CGI-Improvement (CGI-I) scores were examined.
Outcomes
- Based on change in AISRS total scores, patients who received viloxazine ER had significantly greater improvement in their ADHD symptoms than those taking placebo (P = .0040). Patients in the viloxazine ER group had significantly greater improvement in AISRS hyperactive/impulsive (P = .0380) and inattentive symptoms (P = .0015).
- The decrease in CGI-S score was also significantly greater in the viloxazine ER group than in the placebo group (P = .0023). The viloxazine ER group also had significantly greater improvement in executive function as assessed by the BRIEF-A (P = .0468). The difference in GAD-7 scores between the viloxazine ER group and the placebo group was not significant.
- The viloxazine ER group had a greater AISRS 30% response rate than the placebo group (P = .0395). There were no significant differences between groups in AISRS 50% responder rate or CGI-I responder rate.
- Adverse effects related to viloxazine and occurring in ≥5% of participants included insomnia (14.8%), fatigue (11.6%), nausea, decreased appetite (10.1%), dry mouth (9.0%), and headache (9.0%). The discontinuation rate was 9.0% in the viloxazine ER group vs 4.9% in the placebo group.
Continue to: Conclusions/limitations
Conclusions/limitations
- Compared to placebo, patients treated with viloxazine ER had significantly greater improvements in ADHD symptoms, including both hyperactive/impulsive and inattentive components as well as executive function.
- The viloxazine ER group had a significantly higher AISRS 30% response rate than the placebo group, but there were no significant differences in anxiety symptoms or other measures of response.
- Viloxazine ER was well tolerated and safe.
- Limitations: There was a reduced power to detect differences in treatment due to participants dropping out or discontinuing treatment, a lack of interrater reliability data, and a lack of patient-reported outcome or satisfaction data.
3. Kis B, Lücke C, Abdel-Hamid M, et al. Safety profile of methylphenidate under long-term treatment in adult ADHD patients - results of the COMPAS study. Pharmacopsychiatry. 2020;53(6):263-271. doi:10.1055/a-1207-9851
Kis et al11 analyzed the safety results of COMPAS.15 Details of this trial, including interventions and inclusion/exclusion criteria, are described in the description of Lam et al.9
Study design
- Researchers compared the rate of adverse events (AEs) among 205 patients who received ≥1 dose of methylphenidate with 209 patients who received placebo.
- AEs were documented and analyzed on an “as received” basis during Week 0 to Week 52. Electrocardiogram (ECG) data were recorded at baseline and Week 24. Vital signs were monitored at baseline, every week for the first 12 weeks, then every 4 weeks for the next 52 weeks. Body weight was assessed at Week 6, Week 12, Week 20, Week 28, Week 40, and Week 52. A 12-lead ECG was obtained at baseline and Week 24.
- The sample size was assessed to have 80% power to detect group differences in AEs.
Outcomes
- Overall, 96% of participants in the methylphenidate group and 88% of participants in the placebo group experienced at least 1 AE (difference 8.1%; 95% CI, 2.9% to 13.5%).
- AEs that occurred more frequently with methylphenidate compared to placebo were decreased appetite (22% vs 3.8%); dry mouth (15% vs 4.8%); palpitations (13% vs 3.3%); gastrointestinal (GI) infection (11% vs 4.8%); agitation (11% vs 3.3%); restlessness (10% vs 2.9%); hyperhidrosis, tachycardia, and weight decrease (all 6.3% vs 1.9%); depressive symptoms and influenza (both 4.9% vs 1.0%); and acute tonsillitis (4.4% vs 0.5%). Serious AEs were reported by 7.3% of patients in the methylphenidate group and 4.3% of those in the placebo group, with no difference in frequency (difference 3.0%; 95% CI, 1.6% to 7.9%). The most severe AEs were aggression, depression, somnambulism, and suicidal ideation in the methylphenidate group and car accidents, epicondylitis, and a fall in the placebo group.
- There were no significant differences in AEs between the GPT and CM groups.
- The treatment combinations that included methylphenidate had higher rates of patients experiencing at least 1 AE (CM/methylphenidate 97%, GPT/methylphenidate 96%, CM/placebo 92%, GPT/placebo 84%).
- Overall, 8.8% of patients in the methylphenidate group and 4.8% in the placebo group stopped their medication treatment because of an AE (difference 4.0%; 95% CI, 0.9% to 9.1%). At least 1 dose decrease, increase, or discontinuation was made after an AE in 42% of participants in the placebo group and 69% of those in the methylphenidate group.
- There were no significant differences in clinically pertinent ECG abnormalities between methylphenidate and placebo therapy.
Continue to: Conclusions/limitations
Conclusions/limitations
- AEs were more common in the methylphenidate groups compared to placebo, but there was no significant differences for severe AEs. In the long-term, methylphenidate treatment was well tolerated and relatively safe.
- Limitations: The sample size may have been too small to detect uncommon AEs, all AEs had to be reported and may not have been caused by the treatment, and the original study’s main outcome was efficacy, not safety, which makes this an exploratory analysis of AEs.
4. Cutler AJ, Childress AC, Pardo A, et al. Randomized, double-blind, placebo-controlled, fixed-dose study to evaluate the efficacy and safety of amphetamine extended-release tablets in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2022;83(5):22m14438. doi:10.4088/JCP.22m14438
Once-daily dosing of stimulants, which are commonly used to manage adult ADHD,16 can be beneficial because many patients have schedules that limit taking medication multiple times a day. Cutler et al12 looked at the efficacy and safety of amphetamine extended-release tablet (AMPH ER TAB), which is a 3.2:1 mixture of d- and l-amphetamine released by the LiquiXR drug delivery system. This technology allows for a continuous release following an initial quick onset of action.
Study design
- This parallel-study, double-blind study evaluated adults age 18 to 60 who had a diagnosis of ADHD according to DSM-5 criteria and the Adult ADHD Clinical Diagnostic Scale, normal-range IQ, AISRS score ≥26, and baseline CGI-S score ≥4.
- Women were not lactating or pregnant during the study.
- Exclusion criteria included a history of mental illnesses; chronic medical conditions; clinically significant abnormal ECG or cardiac findings on exam; renal or liver disease; family history of sudden death; significant vital sign findings; uncontrolled hypertension or a resting systolic blood pressure (SBP) >140 mmHg or diastolic blood pressure (DBP) >90 mmHg; recent history of or current alcohol or substance use disorder; use of atomoxetine, monoamine oxidase inhibitors, or tricyclic antidepressants within 14 days of study or the use of other stimulant medications within 1 week of screening; use of GI acidifying agents or urinary acidifying agents within 3 days of screening; answering “yes” to questions 4 or 5 of the Suicidal Ideation section of the Columbia Suicide Severity Rating Scale within 2 years prior to the study; taking another investigational medication within 30 days of screening; allergic to amphetamine or components of the study drug, and a lack of prior response to amphetamine.
- Patients were randomized to receive AMPH ER TAB (n = 65) or placebo (n = 65), taken before 10
am . Participants started at 5 mg/d of the drug/placebo and then entered a 5-week titration period in which the medication was increased by 5 mg/d each week until reaching 20 mg/d, and then continued 20 mg/d for 2 weeks. - The primary outcome was the mean Permanent Product Measure of Performance Total (PERMP-T) score averaged across all time points (0.5-, 1-, 2-, 4-, 8-, 10-, 12-, 13-, and 14-hours postdose) at Visit 5.
- Participants underwent AISRS, CGI-S, and safety evaluations at baseline and at the 5 visits at the end of each treatment week.
Outcomes
- Analyses were completed on participants who received ≥1 dose of the medication and who had ≥1 PERMP-T score at Visit 5.
- Predose PERMP-T scores were similar between the AMPH ER TAB group (259.5) and placebo group (260). The mean postdose PERMP-T score in the AMPH ER TAB group (302.8) was significantly higher (P = .0043) than the placebo group (279.6).
- The PERMP-T scores were significantly different at 0.5-, 1-, 2-, 4-, 8-, and 13-hours postdose but not at 10-, 12-, and 14-hours postdose. The first Visit 5 time point at which the difference between groups was statistically different was at 0.5 hours postdose (P = .01), and the last significant time point was 13 hours (P = .006).
- The improvement in CGI-S scores was significantly greater in the AMPH ER TAB group than the placebo group. The improvement in AISRS scores was significantly greater in the AMPH ER TAB group at Visit 3, Visit 4, and Visit 5. More participants in the AMPH ER TAB group had AEs compared to the placebo group (90% vs 60%). The most common AEs (frequency ≥5% and occurring more in the intervention arm) were decreased appetite, insomnia, dry mouth, irritability, headache, anxiety, nausea, dizziness, and tachycardia.
- The AMPH ER TAB group had nonclinically significant increases in SBP (116.8 to 120.7 mmHg), DBP (74.1 to 77.1 mmHg), and heart rate (73.0 to 81.9 bpm) at Visit 5 compared to baseline.
- No serious AEs occurred. Three participants in the AMPH ER TAB group experienced AEs (increased blood pressure, CNS stimulation, and anxiety) that led them to discontinue the study.
Continue to: Conclusions/limitations
Conclusions/limitations
- AMPH ER TAB reduced symptoms in adults with ADHD as assessed by improvement in PERMP-T scores.
- The safety and tolerability profile of AMPH ER TAB were comparable to other stimulants, with expected rises in blood pressure and heart rate.
- Limitations: Patients were required to be titrated to 20 mg/d of AMPH ER TAB, instead of following a flexible titration based on an individual’s response. Some participants may have had greater improvement at a higher or lower dose. This study did not compare AMPH ER TAB to other stimulants. The 5-week duration of this study limited the ability to evaluate long-term efficacy and tolerability. Patients with a wide range of psychiatric or medical comorbidities were excluded.
5. Iwanami A, Saito K, Fujiwara M, et al. Efficacy and safety of guanfacine extended-release in the treatment of attention-deficit/hyperactivity disorder in adults: results of a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2020;81(3):19m12979. doi:10.4088/JCP.19m12979
Guanfacine extended-release (GXR) is a selective alpha 2A-adrenergic receptor agonist approved for treating ADHD in children and adolescents.17 Iwanami et al13 evaluated the efficacy and safety of GXR for adults.
Study design
- This randomized, double-blinded, placebo-controlled trial enrolled Japanese adults age ≥18 who were diagnosed with ADHD according to DSM-5 criteria and scored ≥24 on the ADHD-Rating Scale IV (ADHD-RS-IV) and ≥4 on CGI- I.
- Exclusion criteria included having anxiety, depression, substance use disorder, tic disorder, BD, personality disorder, schizophrenia, or intellectual disability; a moderate or severe psychiatric disorder requiring treatment other than counseling; seizures; increased risk for suicide; a history of cardiovascular disease, including prolonged QTc/abnormal ECG/abnormal labs, orthostatic hypotension, or continuous bradycardia; or taking medications that affect blood pressure or heart rate.
- Overall, 101 participants were randomized to the GXR group and 100 to the placebo group. Approximately two-thirds of the study population was male. Patients received GXR or placebo once daily at approximately the same time.
- There were 5 phases to the trial. The screening period occurred over 1 to 4 weeks. Part 1 of the treatment period consisted of 5 weeks of medication optimization. Participants were started on GXR 2 mg/d and were required to be receiving a minimum dose of 4 mg/d starting at Week 3. Clinicians were allowed to increase the dose 1 mg/d per week starting at Week 4 based on clinical response to a maximum dosage of 6 mg/d. Part 2 of the treatment period consisted of 5 weeks of maintenance at 4 to 6 mg/d. The tapering period to 2 mg/d occurred over 2 weeks. The follow-up period lasted 1 week.
- Efficacy measurements included the Japanese version of the ADHD-RS-IV and translations of the English-language CAARS, CGI-I, and CGI-S. Participant-reported measures included the Patient Global Impression-Improvement scale (PGI-I), Adult ADHD Quality of Life Questionnaire (AAQoL), and BRIEF-A.
- The primary outcome was the difference in ADHD-RS-IV total score from baseline to the end of the maintenance period (Week 10).
- Safety assessments were completed at Week 5 (end of dose optimization period), Week 10 (end of dose maintenance period), and Week 12 (tapering period).
Outcomes
- The average GXR dose during the maintenance period was 5.07 mg/d.
- Compared to the placebo group, the GXR group had more patients age <30 (47% vs 39%) and fewer patients age ≥40 (17% vs 27%). Baseline ADHD-RS-IV scores in both groups were comparable. At baseline, 51% in the GXR group had a combined inattentive/hyperactive-impulsive presentation and 47% had a predominately inattention presentation, with similar characteristics in the placebo group (49% combined, 49% inattention).
- At Week 10, the least squares mean change from baseline on the ADHD-RS-IV total score was significantly greater in the GXR group than in the placebo group (-11.55 ± 1.10 vs -7.27 ± 1.07; P = .0005), with an effect size of 0.52. There was a greater decrease in the ADHD-RS-IV scores starting at Week 4 and continuing to Week 10 (P < .005).
- There were also significant differences favoring GXR on the ADHD-RS-IV hyperactivity-impulsivity subscale score (P = .0021) and ADHD-RS-IV inattention subscale score (P = .0032).
- There were significant differences in the CAARS total ADHD score (P = .0029) and BRIEF-A scores on the inhibit (P = .0173), initiate (P = .0406), plan/organize (P = .174), and global executive composite index (P = .0404) scales. There was no significant difference in the total AAQoL score (P = .0691), but there was a significant improvement in the AAQoL life productivity subscore (P = .0072).
- At Week 10, there were also significant improvements in the CGI-I scores (P = .0007) and PGI-I scores (P = .0283). The CGI-S scores were similar at all time points.
- Overall, 81.2% of GXR patients reported AEs compared to 62% in the placebo group. There was 1 serious treatment-emergent AE (a suicide attempt) that the authors concluded was unrelated to the study drug. No deaths occurred. The most common AEs (incidence ≥10% in either group) included somnolence, thirst, nasopharyngitis (occurring more in the placebo group), blood pressure decrease, postural dizziness, and constipation. The main AEs leading to discontinuation were somnolence and blood pressure decrease. Overall, 19.8% of patients receiving GXR discontinued treatment due to AEs, compared to 3% in the placebo group.
- Heart rate, blood pressure, and QTc (corrected by the Bazett formula) were decreased in the GXR group at Week 10 while QT and RR intervals increased, and most returned to normal by Week 12.
Continue to: Conclusions/limitations
Conclusions/limitations
- Compared to placebo, GXR monotherapy resulted in clinical improvement in ADHD symptoms, with a moderate effect size.
- The most common AEs were mild to moderate and congruent with known adverse effects of guanfacine. Sedation effects mostly transpired within the first week of medication administration and were transient.
- Limitations: The findings might not be generalizable to non-Japanese patients. The duration of the study was short. Patients with a wide range of psychiatric and medical comorbidities were excluded. Two-thirds of the participants were male, and there was a disparity in participant age in the GXR and placebo groups.
6. Reimherr FW, Gift TE, Steans TA, et al. The use of brexpiprazole combined with a stimulant in adults with treatment-resistant attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2022;42(5):445-453. doi:10.1097/JCP.0000000000001592
While stimulants are a mainstay ADHD treatment, some patients have a partial response or do not respond to amphetamines or methylphenidate. Reimherr et el14 assessed the efficacy and safety of adding brexpiprazole (BXP) to a stimulant.
Study design
- This randomized, double-blinded, placebo-controlled trial recruited 559 stimulant-naive patients and 174 patients who had not responded to previous stimulant therapy.
- Participants were adults age 18 to 55 with a primary diagnosis of ADHD according to DSM-IV-TR criteria and the Conners Adult ADHD Diagnostic Interview. Other inclusion criteria were having a CAARS score ≥29 and a CGI-S score ≥4.
- Exclusion criteria included being at risk for suicide; having current substance abuse or positive alcohol/drug screens; a history of good response to prestudy treatment; a clinically significant medical condition; fasting blood glucose >200 mg/dL or hemoglobin A1C >7%; and hospitalization in past 12 months from a diabetic complication, uncontrolled hypertension, ischemic heart disease, or epilepsy. Further exclusion criteria included a history of psychosis, current MDD or BD, current panic disorder, uncontrolled comorbid psychiatric condition, or clinically significant personality disorder. Investigators excluded any patient with severe DSM-IV axis I or II disorders or abnormal/psychopathological behaviors.
- The trial consisted of 3 segments. Part 1 was screening. If the patient was currently receiving a stimulant but not fully responding, the medication was discontinued for at least 5 half-lives.
- Part 2 (5 weeks) involved administering a stimulant plus a single-blind placebo (597 patients completed this phase). The stimulant was chosen by the investigator, who had the option of using 1 of 2 amphetamine derivatives (mixed amphetamine salts capsules or lisdexamfetamine dimesylate capsules) or 1 of 2 methylphenidate derivatives (methylphenidate hydrochloride ER tabs or dexmethylphenidate HCl ER capsules). If a patient did not respond to a particular stimulant prior to the study, they were given a different stimulant from the list. Patients continued the same stimulant throughout the trial. Patients were monitored for a response, defined as a ≥30% decrease in CAARS score or a CAARS score <24, or a CGI-I score of 1 or 2 at Week 5. Patients who did not show this improvement were categorized as open-label nonresponders.
- Part 3 (6 weeks) involved administering a stimulant plus double-blind BXP vs placebo (stimulant-naive n = 167, stimulant nonresponders n = 68). Nonresponders continued the stimulant (at the same dose reached at the end of Part 2) and added either BXP (n = 155) or continued placebo (n = 80). Patients who responded in Part 2 were continued on the stimulant plus placebo and were not randomized. Patients were started on BXP 0.25 mg/d, and the medication could be titrated to 2 mg/d during the following 3 weeks, depending on the benefit vs AE profile. After the third week, the dose could be decreased but not increased.
- The primary outcome was a change in CAARS score. Secondary measurements included the CGI-S, Wender-Reimherr Adult Attention Deficit Disorder Scale (WRAADDS), Montgomery-Åsberg Depression Rating Scale (MADRS), and BDI.
Outcomes
- Stimulant-naive patients were equally divided among the 4 stimulant groups, and previous nonresponders who continued to not respond in Part 2 were more likely to be given methylphenidate HCl or lisdexamfetamine dimesylate.
- Patients with a history of nonresponse had less response to stimulants in Part 2 compared to stimulant-naive patients, as seen by 27% (n = 167) of stimulant-naive patients entering Part 3 compared to 39% of prior nonresponders (n = 68; P = .0249).
- ADHD improvement with BXP appeared to be greater among pretrial nonresponders.
- For stimulant nonresponders before and during the study, at the end of the double-blind endpoint (Part 3; Week 11), WRAADDS total score was significantly improved in the BXP group compared to the placebo group (P = .013; d = 0.74), with most beneficial effects seen in the hyperactivity/restlessness, emotional dysregulation factor, and impulsivity categories.
- For stimulant nonresponders before and during the study, there was no significant difference at the end of Week 11 on the CAARS (P = .64), MADRS (P = .37), or BDI (P = .73). There was a trend toward significance on the CAARS subscale for hyperactive/impulsive (P = .09).
- For prestudy stimulant-naive patients who did not respond to stimulants in Part 2 and were randomized in Part 3, there was not a significant difference between BXP and placebo at Week 11 as assessed on WRAADDS, CAARS, MADRS, or BDI.
- As assessed on WRAADDS, 50% in the BXP group had a response compared to 41% in the placebo group (Fisher exact = 0.334). Under the emotional dysregulation factor category of the WRAADDS, 64% in the BXP group had a response compared to 41% in the placebo group (Fisher exact = 0.064). The attention factor category showed a 40% improvement in the BXP group compared to 32% in the placebo group (Fisher exact = 0.344).
- There were 2 serious AEs in the BXP group (gall bladder inflammation and diarrhea) and 2 in the placebo group (pneumonia and urinary tract infection). There was no statistically significant difference between groups with regards to common AEs (ie, fatigue, heartburn/nausea/stomachache, weight loss), although there was a trend to significant for insomnia in the BXP group (P = .083).
Conclusions/limitations
- Stimulant-naive patients experienced no improvement with adjunctive BXP.
- For prior stimulant nonresponders, there was no significant difference between BXP vs placebo on the primary outcome of the CAARS score, but there was an improvement as observed by assessment with the WRAADDS.
- The largest change in the WRAADDS occurred in the emotional dysregulation factor compared to the attention factor.
- BXP appeared to be well tolerated.
- Limitations: The WRAADDS was administered without the patients’ significant other/collateral. Raters were not trained in the use of the WRAADDS. Patients with a wide range of psychiatric and medical comorbidities were excluded. Fewer patients were recruited in the prior stimulant nonresponder group.
Bottom Line
Recent randomized controlled trials suggest that methylphenidate, amphetamine extended-release, viloxazine extended-release, and guanfacine extended-release improved symptoms of adult attention-deficit/hyperactivity disorder (ADHD). There were no improvements in ADHD symptoms with adjunctive brexpiprazole.
Related Resources
- Parikh AR, Baker SA. Adult ADHD: pharmacologic treatment in the DSM-5 era. Current Psychiatry. 2016;15(10):18-25.
- Akbar HN. Why we should be scrutinizing the rising prevalence of adult ADHD. Current Psychiatry. 2022; 21(7):e1-e2. doi:10.12788/cp.0268
Drug Brand Names
Amantadine • Gocovri
Amphetamine extended-release tablet • Dyanavel XR
Atomoxetine • Strattera
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Dexmethylphenidate • Focalin
Fluoxetine • Prozac
Guanfacine extended- release • Intuniv
Lisdexamfetamine • Vyvanse
Methylphenidate • Concerta, Methylin
Theophylline • Elixophyllin
Viloxazine • Qelbree
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Harpin V, Mazzone L, Raynaud JP, et al. Long-term outcomes of ADHD: a systematic review of self-esteem and social function. J Atten Disord. 2016;20(4):295-305. doi:10.1177/1087054713486516
3. Beaton DM, Sirois F, Milne E. Experiences of criticism in adults with ADHD: a qualitative study. PLoS One. 2022;17(2):e0263366. doi:10.1371/journal.pone.0263366
4. Attention-deficit/hyperactivity disorder (ADHD). National Institute of Mental Health. Accessed February 9, 2023. https://www.nimh.nih.gov/health/statistics/attention-deficit-hyperactivity-disorder-adhd
5. Katzman MA, Bilkey TS, Chokka PR, et al. Adult ADHD and comorbid disorders: clinical implications of a dimensional approach. BMC Psychiatry. 2017;17(1):302. doi:10.1186/s12888-017-1463-3
6. Attention Deficit Hyperactivity Disorder: Diagnosis and Management. NICE Guideline No. 87. National Institute for Health and Care Excellence (NICE); 2019. Accessed February 9, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493361/
7. Adler LD, Nierenberg AA. Review of medication adherence in children and adults with ADHD. Postgrad Med. 2010;122(1):184-191. doi:10.3810/pgm.2010.01.2112
8. Cunill R, Castells X, Tobias A, et al. Efficacy, safety and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta-analysis and meta-regression in over 9000 patients. Psychopharmacology (Berl). 2016;233(2):187-197. doi:10.1007/s00213-015-4099-3
9. Lam AP, Matthies S, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Long-term effects of multimodal treatment on adult attention-deficit/hyperactivity disorder symptoms: follow-up analysis of the COMPAS Trial. JAMA Netw Open. 2019;2(5):e194980. doi:10.1001/jamanetworkopen.2019.4980
10. Nasser A, Hull JT, Chaturvedi SA, et al. A phase III, randomized, double-blind, placebo-controlled trial assessing the efficacy and safety of viloxazine extended-release capsules in adults with attention-deficit/hyperactivity disorder. CNS Drugs. 2022;36(8):897-915. doi:10.1007/s40263-022-00938-w
11. Kis B, Lücke C, Abdel-Hamid M, et al. Safety profile of methylphenidate under long-term treatment in adult ADHD patients - results of the COMPAS study. Pharmacopsychiatry. 2020;53(6):263-271. doi:10.1055/a-1207-9851
12. Cutler AJ, Childress AC, Pardo A, et al. Randomized, double-blind, placebo-controlled, fixed-dose study to evaluate the efficacy and safety of amphetamine extended-release tablets in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2022;83(5):22m14438. doi:10.4088/JCP.22m14438
13. Iwanami A, Saito K, Fujiwara M, et al. Efficacy and safety of guanfacine extended-release in the treatment of attention-deficit/hyperactivity disorder in adults: results of a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2020;81(3):19m12979. doi:10.4088/JCP.19m12979
14. Reimherr FW, Gift TE, Steans TA, et al. The use of brexpiprazole combined with a stimulant in adults with treatment-resistant attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2022;42(5):445-453. doi:10.1097/JCP.0000000000001592
15. Philipsen A, Jans T, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Effects of group psychotherapy, individual counseling, methylphenidate, and placebo in the treatment of adult attention-deficit/hyperactivity disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(12):1199-1210.
16. McGough JJ. Treatment controversies in adult ADHD. Am J Psychiatry. 2016;173(10):960-966. doi:10.1176/appi.ajp.2016.15091207
17. Cruz MP. Guanfacine extended-release tablets (Intuniv), a nonstimulant selective alpha2a-adrenergic receptor agonist for attention-deficit/hyperactivity disorder. P T. 2010;35(8):448-451.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Harpin V, Mazzone L, Raynaud JP, et al. Long-term outcomes of ADHD: a systematic review of self-esteem and social function. J Atten Disord. 2016;20(4):295-305. doi:10.1177/1087054713486516
3. Beaton DM, Sirois F, Milne E. Experiences of criticism in adults with ADHD: a qualitative study. PLoS One. 2022;17(2):e0263366. doi:10.1371/journal.pone.0263366
4. Attention-deficit/hyperactivity disorder (ADHD). National Institute of Mental Health. Accessed February 9, 2023. https://www.nimh.nih.gov/health/statistics/attention-deficit-hyperactivity-disorder-adhd
5. Katzman MA, Bilkey TS, Chokka PR, et al. Adult ADHD and comorbid disorders: clinical implications of a dimensional approach. BMC Psychiatry. 2017;17(1):302. doi:10.1186/s12888-017-1463-3
6. Attention Deficit Hyperactivity Disorder: Diagnosis and Management. NICE Guideline No. 87. National Institute for Health and Care Excellence (NICE); 2019. Accessed February 9, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493361/
7. Adler LD, Nierenberg AA. Review of medication adherence in children and adults with ADHD. Postgrad Med. 2010;122(1):184-191. doi:10.3810/pgm.2010.01.2112
8. Cunill R, Castells X, Tobias A, et al. Efficacy, safety and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta-analysis and meta-regression in over 9000 patients. Psychopharmacology (Berl). 2016;233(2):187-197. doi:10.1007/s00213-015-4099-3
9. Lam AP, Matthies S, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Long-term effects of multimodal treatment on adult attention-deficit/hyperactivity disorder symptoms: follow-up analysis of the COMPAS Trial. JAMA Netw Open. 2019;2(5):e194980. doi:10.1001/jamanetworkopen.2019.4980
10. Nasser A, Hull JT, Chaturvedi SA, et al. A phase III, randomized, double-blind, placebo-controlled trial assessing the efficacy and safety of viloxazine extended-release capsules in adults with attention-deficit/hyperactivity disorder. CNS Drugs. 2022;36(8):897-915. doi:10.1007/s40263-022-00938-w
11. Kis B, Lücke C, Abdel-Hamid M, et al. Safety profile of methylphenidate under long-term treatment in adult ADHD patients - results of the COMPAS study. Pharmacopsychiatry. 2020;53(6):263-271. doi:10.1055/a-1207-9851
12. Cutler AJ, Childress AC, Pardo A, et al. Randomized, double-blind, placebo-controlled, fixed-dose study to evaluate the efficacy and safety of amphetamine extended-release tablets in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2022;83(5):22m14438. doi:10.4088/JCP.22m14438
13. Iwanami A, Saito K, Fujiwara M, et al. Efficacy and safety of guanfacine extended-release in the treatment of attention-deficit/hyperactivity disorder in adults: results of a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2020;81(3):19m12979. doi:10.4088/JCP.19m12979
14. Reimherr FW, Gift TE, Steans TA, et al. The use of brexpiprazole combined with a stimulant in adults with treatment-resistant attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2022;42(5):445-453. doi:10.1097/JCP.0000000000001592
15. Philipsen A, Jans T, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Effects of group psychotherapy, individual counseling, methylphenidate, and placebo in the treatment of adult attention-deficit/hyperactivity disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(12):1199-1210.
16. McGough JJ. Treatment controversies in adult ADHD. Am J Psychiatry. 2016;173(10):960-966. doi:10.1176/appi.ajp.2016.15091207
17. Cruz MP. Guanfacine extended-release tablets (Intuniv), a nonstimulant selective alpha2a-adrenergic receptor agonist for attention-deficit/hyperactivity disorder. P T. 2010;35(8):448-451.
Transient global amnesia: Psychiatric precipitants, features, and comorbidities
Ms. A, age 48, is a physician’s assistant with no psychiatric history. She presents to the emergency department (ED) with her partner and daughter due to a 15-minute episode of acute-onset memory loss and concern for stroke. In the ED, Ms. A is confused and repeatedly asks, “Where are we?” “How did we get here?” and “What day is it?” Her partner denies Ms. A has focal neurologic deficits or seizures.
Ms. A had only slept 4 hours the night before she came to the ED because she had just learned that her daughter works in the sex industry. According to her daughter, Ms. A was raped by a soldier many years ago. At that time, her perpetrator told Ms. A that he would kill her entire family if she ever told anyone. As a result, she never pursued any psychological or psychiatric treatment.
During the evaluation, Ms. A shares details regarding her social and medical history; however, she does not recall receiving bad news the night before. She asks the interviewer several times how she got to the hospital, and when a cranial nerve exam is performed, she states, “I am not the patient!”
Ms. A’s vital signs and physical exam are unremarkable. Urinalysis is significant for a ketones level of 20 mmol/L (reference range: negative for ketones), and a urine human chorionic gonadotropin test is negative. A neurologic exam does not identify any focal deficits. No imaging is performed.
Transient global amnesia (TGA) describes an episode of anterograde, and possibly retrograde, amnesia that lasts up to 24 hours. On presentation, patients experiencing TGA repeatedly ask, “Where am I?” “What day is it?” and “How did I get here?” However, semantic memory—such as knowledge of the world and autobiographical information—is preserved.1 The first case of TGA was described in 1956, and its diagnostic criteria were most recently modified in 1990 (Table2).
Though TGA is the most common cause of acute-onset amnesia, it is rare, affecting approximately 3 to 10 individuals per 100,000. The average age of onset is 61 to 63, with most cases occurring after age 50. TGA is generally thought to affect males and females equally, though some studies suggest a female predominance.3 In most cases (approximately 90%), there is a precipitating event such as physical or emotional stress, change in temperature, or sexual intercourse.4
In this article, we provide an overview of the classification, presentation, differential diagnosis, workup, and treatment of TGA. While TGA is a neurologic diagnosis, in a subset of patients it can present with psychiatric features resembling conversion disorder. For such patients, we argue that TGA can be considered a neuropsychiatric condition (Box 15-12). This classification may empower emergency psychiatry clinicians and psychotherapists to identify and treat the condition, which is not described by the current psychiatric diagnostic system.
Box 1
Transient global amnesia (TGA) is a neurologic diagnosis. However, in 1956, Bender8 associated the clinical picture of TGA with psychogenic etiology, 2 years before the term was coined. The same year, Courjon et al9 classified TGA as a functional disorder.
As recent literature on TGA has focused on the neuropsychologic mechanism of memory loss, examination of the condition from a psychodynamic standpoint has fallen out of favor. In fact, the earliest discussions of the condition attributed the absence of TGA from literature prior to the 1950s “to erroneous classification of TGA as psychogenic or hysterical amnesia.”10 However, to refer to this condition as purely neurologic—and without any “psychogenic” or functional features— would be reductive.
In a 2019 case report, Espiridion et al6 considered TGA within the same diagnostic realm as—if not actually a form of—dissociative amnesia (DA). They published the case of a 60-year-old woman with a history of posttraumatic stress disorder (PTSD) who experienced an episode of TGA that had manifested as anterograde and retrograde amnesia for 2 days and was precipitated by a psychotherapy session in which she discussed an individual who had assaulted her 5 years earlier. Much like in the case of Ms. A, the report from Espiridion et al6 clearly exemplifies a psychiatric etiology that shares similar context of a stressor unveiling a past memory too unbearable to maintain in consciousness. They concluded that “this case demonstrates anterograde and identity memory impairments likely induced by her PTSD. It is … possible that this presentation may be labeled PTSD-related dissociative amnesia.”6
Considering TGA as a type of DA within a subset of patients represents progression with regards to considering it as a psychiatric disorder. However, a prominent factor distinguishing TGA from DA is that the latter is more commonly associated with loss of personal identity.5 In TGA, memory of autobiographical information typically is preserved.7
Others have argued for a subtype of “emotional arousal–induced TGA”11 or “emotional TGA.”10 We suggest that this “emotional” subtype of TGA, which clearly was affecting Ms. A, shares similarities with functional neurologic symptom disorder, otherwise known as conversion disorder. The psychoanalytic concept that unconscious psychic distress can be “converted” into a neurologic problem is exemplified by Ms. A. Of note, being female and having an emotional stressor are risk factors for conversion disorder. Additionally, migraine— which was not part of Ms. A’s history—is also a risk factor for both TGA and conversion disorder.12 Despite these similarities, however, TGA’s neurophysiological changes on MRI and self-resolving nature still position the disorder as uniquely neuropsychiatric in the term’s purest sense.
Continue to: Differential diagnosis and workup
Differential diagnosis and workup
The differential diagnosis for acute-onset memory loss in the absence of other neurologic or psychiatric features is broad. It includes:
- dissociative amnesia
- ischemic amnesia
- transient epileptic amnesia
- toxic and metabolic amnesia
- posttraumatic amnesia.
Dissociative amnesia (DA), otherwise known as psychogenic amnesia, is “an inability to recall important autobiographical information, usually of a traumatic or stressful nature, that is inconsistent with ordinary forgetting.”13 According to this definition, DA features only retrograde amnesia, as opposed to TGA, which features anterograde amnesia, with possible retrograde amnesia. A subtype of DA—specifically, “continuous amnesia” or “anterograde dissociative amnesia”— is in DSM-5.13 However, the diagnostic criteria are unclear, and no cases have been identified in the literature since 1903, before TGA became a diagnostic entity.5,14 Moreover, patients with DA cannot recall autobiographical information, which is not a feature of TGA. Within DSM-5, TGA is an exclusion criterion for DA.13 Thus, an episode of anterograde amnesia with acute onset best meets criteria for TGA, even if there are substantial psychiatric risk factors.
Ischemic amnesia—including stroke and transient ischemic attack (TIA)—is often the primary concern of patients with TGA and their families upon initial presentation, as was the case with Ms. A.6,15 TIA presenting with isolated, acute-onset amnesia would be highly unusual, because these attacks usually present with focal symptoms including motor deficits, sensory deficits, visual field deficits, and aphasia or dysarthria. A patient with amnesia experiencing a TIA would likely have symptoms lasting from seconds to minutes, which is much shorter than a typical TGA episode.16
Amnesia secondary to stroke may be transient or permanent.7 Amnesia is present in approximately 1% of all strokes and in approximately 19.3% of posterior cerebral artery strokes.7,17 Unlike TIA and TGA, ischemic amnesia would present with MRI findings detectable at symptom onset. TGA does reveal MRI findings, particularly punctate lesions in the CA1 area of the hippocampus; however, these lesions are typically much smaller than those found in stroke, and are not detectable until 12 to 48 hours after episode onset.1,17 MRI findings in ischemic amnesia are typically associated with extrahippocampal lesions.17 Finally, the presence of vascular risk factors such as hyperlipidemia, smoking, diabetes, and hypertension may also favor a diagnosis of stroke or TIA as opposed to TGA, which is not associated with these risk factors.18 Though ischemic amnesia and TGA usually can be differentiated based on history and presentation, MRI with fluid-attenuated inversion recovery and diffusion-weighted imaging may be performed to definitively distinguish stroke from TGA.7
Transient epileptic amnesia (TEA), a focal form of epilepsy within the temporal lobe, should also be considered in patients who present with acute-onset amnesia. Like TGA, TEA may present with simultaneous anterograde and retrograde amnesia accompanied by repetitive questioning.19 Amnesia can be the sole symptom of TEA in up to 24% of cases. However, several key features distinguish TEA from TGA. TEA most often presents with other clinical signs of seizures, such as oral automatisms and/or olfactory hallucinations.20 There is also a significant difference in episode length; TEA episodes last an average of 30 to 60 minutes and tend to occur upon wakening, whereas TGA episodes last an average of 4 to 6 hours and do not preferentially occur at any particular time.1,21 In the interictal period—between seizures—patients with TEA may also experience accelerated long-term forgetting, autobiographical amnesia, and topographical amnesia.19,20 Finally, a diagnosis of TEA also requires recurrent episodes. Recurrence can happen with TGA, but is less frequent.21 Generally, history and presentation can distinguish TEA from TGA. Though there is no formal protocol for TEA workup, Lanzone et al21 recommend 24-hour EEG or EEG sleep monitoring in patients who present with amnesia as well as other clinical manifestations of epileptic phenomenon.
Continue to: Toxic and metabolic
Toxic and metabolic etiologies of amnesia include opioid and cocaine use, general anesthetics,22 and hypoglycemia.7,23 Toxic and metabolic causes of amnesia may mirror TGA in their acute onset as well as anterograde nature. However, these patients will likely present with fluctuating consciousness and/or other neuropsychiatric features, such as pressured speech, delusions, and/or distractability.23 Obtaining a patient’s medical history, including substance use, medication use, and the presence of diabetes,24 is typically sufficient to rule out toxic and metabolic causes.7
Posttraumatic amnesia (PTA) describes transient memory loss that occurs after a traumatic brain injury. Anterograde amnesia is most common, though approximately 20% of patients may also experience retrograde amnesia pertaining to the events near the date of their injury. Unlike TGA, which typically resolves within 24 hours, the recovery time of amnestic symptoms in PTA ranges from minutes to years.7 A distinguishing feature of PTA is the presence of confusion, which often resembles a state of delirium.25 The presentation of PTA can vary immensely with regards to agitation, psychotic symptoms, and the time to resolution of the amnesia. Though TGA can be distinguished from PTA based on a lack of clouding of consciousness, a case of anterograde amnesia warrants inquiry into a potential history of head injuries to rule out a traumatic cause.26
Box 21,3,23,27-33 outlines current theories of the etiology and pathogenesis of TGA.
Box 2
The etiology and pathogenesis of transient global amnesia (TGA) are poorly understood, and TGA remains one of the most enigmatic syndromes in clinical neurology.27 Theories regarding the pathogenesis of TGA are diverse and include vascular, epileptic, migraine, and stress-related etiologies.1,23
Early theories suggested arterial ischemia28 and epileptic phenomena29 as etiologies of TGA. The venous theory posits that TGA stems from jugular venous incompetency, causing venous flow and subsequent venous congestion in the medial temporal lobe, wherein lies the hippocampus. This theory is supported by several studies showing venous valve insufficiency as detected by ultrasonographic evaluation during the Valsalva maneuver in patients with TGA.30 This pathophysiologic mechanism may explain the occurrence of TGA in a specific cluster of cases, including men whose TGA episodes are precipitated by physical stress or the Valsalva maneuver.3 The migraine theory and stress theory share a similar proposed neurophysiologic mechanism.
The migraine theory stems from migraines being a known risk factor for TGA, particularly in middle-aged women.31 The stress theory is based on the known emotional precipitants and psychiatric comorbidities associated with TGA. Notably, both the migraine theory and stress theory implicate the role of excessive glutamate release as well as CNS depression.31,32 Glutamate targets the CA1 region of the hippocampus, which is involved in TGA and is known to have the highest density of N-methyl-D-aspartate receptors among hippocampal regions.33
Given the heterogeneity of the demographics and stressors associated with TGA, multiple mechanisms for the disease process may coexist, leading to a similar clinical picture. In 2006, Quinette et al3 performed a multivariate analysis of variables associated with TGA, including age, sex, medical history, and presentation. They demonstrated 3 “clusters” of TGA pictures: women with anxiety or a personality disorder; men with physical precipitating events; and younger patients (age <56) with a history of migraine. These findings suggest TGA may have unique precipitants corresponding to multiple neurophysiologic mechanisms.
Transient global ischemia: Psychiatric features
Several studies have demonstrated psychiatric precipitants, features, and comorbidities associated with TGA. Of the TGA cases associated with precipitating events, 29% to 50% are associated with an emotional stressor.3,4 Examples of emotional stressors include a quarrel,4 the announcement of a birth or suicide, and a nightmare.15 For Ms. A, learning her daughter worked in the sex industry was an emotional stressor.2
During its acute phase, TGA has been shown to present with mood and anxiety symptoms.34 Moreover, during episodes, patients often demonstrate features of panic attacks, such as dizziness, fainting, choking, palpitations, and paresthesia.3,35
Continue to: Finally, patients with TGA...
Finally, patients with TGA are more likely to have psychiatric comorbidities than those without the condition. In a study of 25 patients who experienced TGA triggered by a precipitant, Inzitari et al4 found a strong association of TGA with phobic personality traits, including agoraphobia and simple phobic attitudes (ie, fear of traveling far from home or the sight of blood). Pantoni et al35 replicated these results in 2005 and found that in comparison to patients with TIA, patients with TGA are more likely to have personal and family histories of psychiatric disease. A 2014 study by Dohring et al36 found that compared to healthy controls, patients with TGA are more likely to have maladaptive coping strategies and stress responses. Patients with TGA tended to exhibit increased feelings of guilt, take more medication, and exhibit more anxiety compared to healthy controls.36
Treatment: Benzodiazepines
There are no published treatment guidelines for TGA. However, in case reports, benzodiazepines (specifically lorazepam37) have been shown to have utility in diagnosing and treating DA. The success of benzodiazepines is attributed to its gamma-aminobutyric acid mechanism, which involves inhibiting activity of the N-methyl-
However, the benzodiazepine midazolam has been identified as a precipitant of TGA. In a case report, Rinehart et al22 identified flumazenil—a benzodiazepine antagonist used primarily to treat retrograde postoperative amnesia—as an antidote. The potentially paradoxical role of benzodiazepines in both the precipitation and treatment of TGA may relate back to the heterogeneity of the etiologies of TGA. Further research comparing the treatment of TGA in patients with stress-induced TGA vs postoperative TGA is needed to better understand the neurochemical basis of TGA and work toward establishing optimal treatment options for different patient demographics.
A generally favorable prognosis
TGA carries a low risk of recurrence. In studies with 3- to 7-year follow-up periods, the recurrence rates ranged from 1.4% to 23.8%.23,35,38
Memory impairments may be present for 5 to 6 months following a TGA episode. The severity of these impairments may range from clinically unnoticeable to the patient meeting the criteria for mild cognitive impairment.23,39 The risk is higher in patients who have had recurrent TGA compared to those patients who have experienced only a single episode.23
Continue to: TGA does not increase...
TGA does not increase the risk of cerebrovascular events. There is controversy regarding a potentially increased risk for dementia as well as epilepsy, though there is insufficient evidence to support these findings.23,40
CASE CONTINUED
Five hours after the onset of Ms. A’s symptoms, the treatment team initiates oral lorazepam 1 mg. One hour after taking lorazepam, Ms. A’s anterograde and retrograde amnesia improve. She cannot recall why she was brought to the hospital but does remember the date and location, which she was not able to do on initial presentation. She feels safe, states a clear plan for self-care, and is discharged in the care of her partner. Though Ms. A’s memory improved soon after she received lorazepam, this improvement also could be attributed to the natural course of time, as TGA tends to resolve on its own within 24 hours.
Bottom Line
Transient global amnesia (TGA) is an episode of anterograde, and possibly retrograde, amnesia that lasts up to 24 hours. It represents an interesting diagnosis at the intersection of psychiatry and neurology. TGA has many established psychiatric risk factors and features—some of which may resemble conversion disorder—but these may only apply to a particular subset of patients, which reflects the heterogeneity of the condition.
Related Resources
- Sparaco M, Pascarella R, Muccio CF, et al. Forgetting the unforgettable: transient global amnesia part I: pathophysiology and etiology. J Clin Med. 2022;11(12): 3373. doi:10.3390/jcm1112337
- Sparaco M, Pascarella R, Muccio CF, et al. Forgetting the unforgettable: transient global amnesia part II: a clinical road map. J Clin Med. 2022;11(14):3940. doi:10.3390/ jcm11143940
Drug Brand Names
Flumazenil • Romazicon
Lorazepam • Ativan
Midazolam • Versed
1. Miller TD, Butler CR. Acute-onset amnesia: transient global amnesia and other causes. Pract Neurol. 2022;22(3):201-208. doi:10.1136/practneurol-2020-002826
2. Hodges JR, Warlow CP. Syndromes of transient amnesia: towards a classification. A study of 153 cases. J Neurol Neurosurg Psychiatry. 1990;53(10):834-843. doi:10.1136/jnnp.53.10.834
3. Quinette P, Guillery-Girard B, Dayan J, et al. What does transient global amnesia really mean? Review of the literature and thorough study of 142 cases. Brain. 2006;129(Pt 7):1640-1658. doi:10.1093/brain/awl105
4. Inzitari D, Pantoni L, Lamassa M, et al. Emotional arousal and phobia in transient global amnesia. Arch Neurol. 1997;54(7):866-873. doi:10.1001/archneur.1997.00550190056015
5. Staniloiu A, Markowitsch HJ. Dissociative amnesia. Lancet Psychiatry. 2014;1(3):226-241. doi:10.1016/S2215-0366(14)70279-2
6. Espiridion ED, Gupta J, Bshara A, et al. Transient global amnesia in a 60-year-old female with post-traumatic stress disorder. Cureus. 2019;11(9):e5792. doi:10.7759/cureus.5792
7. Alessandro L, Ricciardi M, Chaves H, et al. Acute amnestic syndromes. J Neurol Sci. 2020;413:116781. doi:10.1016/j.jns.2020.116781
8. Bender M. Syndrome of isolated episode of confusion with amnesia. J Hillside Hosp. 1956;5:212-215.
9. Courjon J, Guyotat J. Les ictus amnéstiques [Amnesic strokes]. J Med Lyon. 1956;37(882):697-701.
10. Noel A, Quinette P, Hainselin M, et al. The still enigmatic syndrome of transient global amnesia: interactions between neurological and psychopathological factors. Neuropsychol Rev. 2015;25(2):125-133. doi:10.1007/s11065-015-9284-y
11. Merriam AE, Wyszynski B, Betzler T. Emotional arousal-induced transient global amnesia. A clue to the neural transcription of emotion? Psychosomatics. 1992;33(1):109-113. doi:10.1016/S0033-3182(92)72029-5
12. Hallett M, Aybek S, Dworetzky BA, et al. Functional neurological disorder: new subtypes and shared mechanisms. Lancet Neurol. 2022;21(6):537-550. doi:10.1016/S1474-4422(21)00422-1
13. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
14. Bourdon B, Dide M. A case of continuous amnesia with tactile asymbolia, complicated by other troubles. Ann Psychol. 1903;10:84-115.
15. Marinella MA. Transient global amnesia and a father’s worst nightmare. N Engl J Med. 2004;350(8):843-844. doi:10.1056/NEJM200402193500821
16. Amarenco P. Transient ischemic attack. N Engl J Med. 2020;382(20):1933-1941. doi:10.1056/NEJMcp1908837
17. Szabo K, Forster A, Jager T, et al. Hippocampal lesion patterns in acute posterior cerebral artery stroke: clinical and MRI findings. Stroke. 2009;40(6):2042-2045. doi:10.1161/STROKEAHA.108.536144
18. Liampas I, Raptopoulou M, Siokas V, et al. Conventional cardiovascular risk factors in transient global amnesia: systematic review and proposition of a novel hypothesis. Front Neuroendocrinol. 2021;61:100909. doi:10.1016/j.yfrne.2021.100909
19. Zeman A, Butler C. Transient epileptic amnesia. Curr Opin Neurol. 2010;23(6):610-616. doi:10.1097/WCO.0b013e32834027db
20. Baker J, Savage S, Milton F, et al. The syndrome of transient epileptic amnesia: a combined series of 115 cases and literature review. Brain Commun. 2021;3(2):fcab038. doi:10.1093/braincomms/fcab038
21. Lanzone J, Ricci L, Assenza G, et al Transient epileptic and global amnesia: real-life differential diagnosis. Epilepsy Behav. 2018;88:205-211. doi:10.1016/j.yebeh.2018.07.015
22. Rinehart JB, Baker B, Raphael D. Postoperative global amnesia reversed with flumazenil. Neurologist. 2012;18(4):216-218. doi:10.1097/NRL.0b013e31825bbef4
23. Arena JE, Rabinstein AA. Transient global amnesia. Mayo Clin Proc. 2015;90(2):264-272. doi:10.1016/j.mayocp.2014.12.001
24. Holemans X, Dupuis M, Misson N, et al. Reversible amnesia in a type 1 diabetic patient and bilateral hippocampal lesions on magnetic resonance imaging (MRI). Diabet Med. 2001;18(9):761-763. doi:10.1046/j.1464-5491.2001.00481.x
25. Marshman LAG, Jakabek D, Hennessy M, et al. Post-traumatic amnesia. J Clin Neurosci. 2013;20(11):1475-1481. doi:10.1016/j.jocn.2012.11.022
26. Parker TD, Rees R, Rajagopal S, et al. Post-traumatic amnesia. Pract Neurol. 2022;22(2):129-137. doi:10.1136/practneurol-2021-003056
27. You SH, Kim B, Kim BK. Transient global amnesia: signal alteration in 2D/3D T2-FLAIR sequences. Clin Imaging. 2021;78:154-159. doi:10.1016/j.clinimag.2021.03.029
28. Mathew NT, Meyer JS. Pathogenesis and natural history of transient global amnesia. Stroke. 1974;5(3):303-311. doi:10.1161/01.str.5.3.303
29. Fisher CM, Adams RD. Transient global amnesia. Acta Neurol Scand Suppl. 1964;40(SUPPL 9):1-83.
30. Cejas C, Cisneros LF, Lagos R, et al. Internal jugular vein valve incompetence is highly prevalent in transient global amnesia. Stroke. 2010;41(1):67-71. doi:10.1161/STROKEAHA.109.566315
31. Liampas I, Siouras AS, Siokas V, et al. Migraine in transient global amnesia: a meta-analysis of observational studies. J Neurol. 2022;269(1):184-196. doi:10.1007/s00415-020-10363-y
32. Ding X, Peng D. Transient global amnesia: an electrophysiological disorder based on cortical spreading depression-transient global amnesia model. Front Hum Neurosci. 2020;14:602496. doi:10.3389/fnhum.2020.602496
33. Bartsch T, Dohring J, Reuter S, et al. Selective neuronal vulnerability of human hippocampal CA1 neurons: lesion evolution, temporal course, and pattern of hippocampal damage in diffusion-weighted MR imaging. J Cereb Blood Flow Metab. 2015;35(11):1836-1845. doi:10.1038/jcbfm.2015.137
34. Noel A, Quinette P, Guillery-Girard B, et al. Psychopathological factors, memory disorders and transient global amnesia. Br J Psychiatry. 2008;193(2):145-151. doi:10.1192/bjp.bp.107.045716
35. Pantoni L, Bertini E, Lamassa M, et al. Clinical features, risk factors, and prognosis in transient global amnesia: a follow-up study. Eur J Neurol. 2005;12(5):350-356. doi:10.1111/j.1468-1331.2004.00982.x
36. Dohring J, Schmuck A, Bartsch T. Stress-related factors in the emergence of transient global amnesia with hippocampal lesions. Front Behav Neurosci. 2014;8:287. doi:10.3389/fnbeh.2014.00287
37. Jiang S, Gunther S, Hartney K, et al. An intravenous lorazepam infusion for dissociative amnesia: a case report. Psychosomatics. 2020;61(6):814-818. doi:10.1016/j.psym.2020.01.009
38. He S, Ye Z, Yang Q, et al. Transient global amnesia: risk factors, imaging features, and prognosis. Neuropsychiatr Dis Treat. 2021;17:1611-1619. doi:10.2147/NDT.S299168
39. Borroni B, Agosti C, Brambilla C, et al. Is transient global amnesia a risk factor for amnestic mild cognitive impairment? J Neurol. 2004;251(9):1125-1127. doi:10.1007/s00415-004-0497-x
40. Liampas I, Raptopoulou M, Siokas V, et al. The long-term prognosis of transient global amnesia: a systematic review. Rev Neurosci. 2021;32(5):531-543. doi:10.1515/revneuro-2020-0110
Ms. A, age 48, is a physician’s assistant with no psychiatric history. She presents to the emergency department (ED) with her partner and daughter due to a 15-minute episode of acute-onset memory loss and concern for stroke. In the ED, Ms. A is confused and repeatedly asks, “Where are we?” “How did we get here?” and “What day is it?” Her partner denies Ms. A has focal neurologic deficits or seizures.
Ms. A had only slept 4 hours the night before she came to the ED because she had just learned that her daughter works in the sex industry. According to her daughter, Ms. A was raped by a soldier many years ago. At that time, her perpetrator told Ms. A that he would kill her entire family if she ever told anyone. As a result, she never pursued any psychological or psychiatric treatment.
During the evaluation, Ms. A shares details regarding her social and medical history; however, she does not recall receiving bad news the night before. She asks the interviewer several times how she got to the hospital, and when a cranial nerve exam is performed, she states, “I am not the patient!”
Ms. A’s vital signs and physical exam are unremarkable. Urinalysis is significant for a ketones level of 20 mmol/L (reference range: negative for ketones), and a urine human chorionic gonadotropin test is negative. A neurologic exam does not identify any focal deficits. No imaging is performed.
Transient global amnesia (TGA) describes an episode of anterograde, and possibly retrograde, amnesia that lasts up to 24 hours. On presentation, patients experiencing TGA repeatedly ask, “Where am I?” “What day is it?” and “How did I get here?” However, semantic memory—such as knowledge of the world and autobiographical information—is preserved.1 The first case of TGA was described in 1956, and its diagnostic criteria were most recently modified in 1990 (Table2).
Though TGA is the most common cause of acute-onset amnesia, it is rare, affecting approximately 3 to 10 individuals per 100,000. The average age of onset is 61 to 63, with most cases occurring after age 50. TGA is generally thought to affect males and females equally, though some studies suggest a female predominance.3 In most cases (approximately 90%), there is a precipitating event such as physical or emotional stress, change in temperature, or sexual intercourse.4
In this article, we provide an overview of the classification, presentation, differential diagnosis, workup, and treatment of TGA. While TGA is a neurologic diagnosis, in a subset of patients it can present with psychiatric features resembling conversion disorder. For such patients, we argue that TGA can be considered a neuropsychiatric condition (Box 15-12). This classification may empower emergency psychiatry clinicians and psychotherapists to identify and treat the condition, which is not described by the current psychiatric diagnostic system.
Box 1
Transient global amnesia (TGA) is a neurologic diagnosis. However, in 1956, Bender8 associated the clinical picture of TGA with psychogenic etiology, 2 years before the term was coined. The same year, Courjon et al9 classified TGA as a functional disorder.
As recent literature on TGA has focused on the neuropsychologic mechanism of memory loss, examination of the condition from a psychodynamic standpoint has fallen out of favor. In fact, the earliest discussions of the condition attributed the absence of TGA from literature prior to the 1950s “to erroneous classification of TGA as psychogenic or hysterical amnesia.”10 However, to refer to this condition as purely neurologic—and without any “psychogenic” or functional features— would be reductive.
In a 2019 case report, Espiridion et al6 considered TGA within the same diagnostic realm as—if not actually a form of—dissociative amnesia (DA). They published the case of a 60-year-old woman with a history of posttraumatic stress disorder (PTSD) who experienced an episode of TGA that had manifested as anterograde and retrograde amnesia for 2 days and was precipitated by a psychotherapy session in which she discussed an individual who had assaulted her 5 years earlier. Much like in the case of Ms. A, the report from Espiridion et al6 clearly exemplifies a psychiatric etiology that shares similar context of a stressor unveiling a past memory too unbearable to maintain in consciousness. They concluded that “this case demonstrates anterograde and identity memory impairments likely induced by her PTSD. It is … possible that this presentation may be labeled PTSD-related dissociative amnesia.”6
Considering TGA as a type of DA within a subset of patients represents progression with regards to considering it as a psychiatric disorder. However, a prominent factor distinguishing TGA from DA is that the latter is more commonly associated with loss of personal identity.5 In TGA, memory of autobiographical information typically is preserved.7
Others have argued for a subtype of “emotional arousal–induced TGA”11 or “emotional TGA.”10 We suggest that this “emotional” subtype of TGA, which clearly was affecting Ms. A, shares similarities with functional neurologic symptom disorder, otherwise known as conversion disorder. The psychoanalytic concept that unconscious psychic distress can be “converted” into a neurologic problem is exemplified by Ms. A. Of note, being female and having an emotional stressor are risk factors for conversion disorder. Additionally, migraine— which was not part of Ms. A’s history—is also a risk factor for both TGA and conversion disorder.12 Despite these similarities, however, TGA’s neurophysiological changes on MRI and self-resolving nature still position the disorder as uniquely neuropsychiatric in the term’s purest sense.
Continue to: Differential diagnosis and workup
Differential diagnosis and workup
The differential diagnosis for acute-onset memory loss in the absence of other neurologic or psychiatric features is broad. It includes:
- dissociative amnesia
- ischemic amnesia
- transient epileptic amnesia
- toxic and metabolic amnesia
- posttraumatic amnesia.
Dissociative amnesia (DA), otherwise known as psychogenic amnesia, is “an inability to recall important autobiographical information, usually of a traumatic or stressful nature, that is inconsistent with ordinary forgetting.”13 According to this definition, DA features only retrograde amnesia, as opposed to TGA, which features anterograde amnesia, with possible retrograde amnesia. A subtype of DA—specifically, “continuous amnesia” or “anterograde dissociative amnesia”— is in DSM-5.13 However, the diagnostic criteria are unclear, and no cases have been identified in the literature since 1903, before TGA became a diagnostic entity.5,14 Moreover, patients with DA cannot recall autobiographical information, which is not a feature of TGA. Within DSM-5, TGA is an exclusion criterion for DA.13 Thus, an episode of anterograde amnesia with acute onset best meets criteria for TGA, even if there are substantial psychiatric risk factors.
Ischemic amnesia—including stroke and transient ischemic attack (TIA)—is often the primary concern of patients with TGA and their families upon initial presentation, as was the case with Ms. A.6,15 TIA presenting with isolated, acute-onset amnesia would be highly unusual, because these attacks usually present with focal symptoms including motor deficits, sensory deficits, visual field deficits, and aphasia or dysarthria. A patient with amnesia experiencing a TIA would likely have symptoms lasting from seconds to minutes, which is much shorter than a typical TGA episode.16
Amnesia secondary to stroke may be transient or permanent.7 Amnesia is present in approximately 1% of all strokes and in approximately 19.3% of posterior cerebral artery strokes.7,17 Unlike TIA and TGA, ischemic amnesia would present with MRI findings detectable at symptom onset. TGA does reveal MRI findings, particularly punctate lesions in the CA1 area of the hippocampus; however, these lesions are typically much smaller than those found in stroke, and are not detectable until 12 to 48 hours after episode onset.1,17 MRI findings in ischemic amnesia are typically associated with extrahippocampal lesions.17 Finally, the presence of vascular risk factors such as hyperlipidemia, smoking, diabetes, and hypertension may also favor a diagnosis of stroke or TIA as opposed to TGA, which is not associated with these risk factors.18 Though ischemic amnesia and TGA usually can be differentiated based on history and presentation, MRI with fluid-attenuated inversion recovery and diffusion-weighted imaging may be performed to definitively distinguish stroke from TGA.7
Transient epileptic amnesia (TEA), a focal form of epilepsy within the temporal lobe, should also be considered in patients who present with acute-onset amnesia. Like TGA, TEA may present with simultaneous anterograde and retrograde amnesia accompanied by repetitive questioning.19 Amnesia can be the sole symptom of TEA in up to 24% of cases. However, several key features distinguish TEA from TGA. TEA most often presents with other clinical signs of seizures, such as oral automatisms and/or olfactory hallucinations.20 There is also a significant difference in episode length; TEA episodes last an average of 30 to 60 minutes and tend to occur upon wakening, whereas TGA episodes last an average of 4 to 6 hours and do not preferentially occur at any particular time.1,21 In the interictal period—between seizures—patients with TEA may also experience accelerated long-term forgetting, autobiographical amnesia, and topographical amnesia.19,20 Finally, a diagnosis of TEA also requires recurrent episodes. Recurrence can happen with TGA, but is less frequent.21 Generally, history and presentation can distinguish TEA from TGA. Though there is no formal protocol for TEA workup, Lanzone et al21 recommend 24-hour EEG or EEG sleep monitoring in patients who present with amnesia as well as other clinical manifestations of epileptic phenomenon.
Continue to: Toxic and metabolic
Toxic and metabolic etiologies of amnesia include opioid and cocaine use, general anesthetics,22 and hypoglycemia.7,23 Toxic and metabolic causes of amnesia may mirror TGA in their acute onset as well as anterograde nature. However, these patients will likely present with fluctuating consciousness and/or other neuropsychiatric features, such as pressured speech, delusions, and/or distractability.23 Obtaining a patient’s medical history, including substance use, medication use, and the presence of diabetes,24 is typically sufficient to rule out toxic and metabolic causes.7
Posttraumatic amnesia (PTA) describes transient memory loss that occurs after a traumatic brain injury. Anterograde amnesia is most common, though approximately 20% of patients may also experience retrograde amnesia pertaining to the events near the date of their injury. Unlike TGA, which typically resolves within 24 hours, the recovery time of amnestic symptoms in PTA ranges from minutes to years.7 A distinguishing feature of PTA is the presence of confusion, which often resembles a state of delirium.25 The presentation of PTA can vary immensely with regards to agitation, psychotic symptoms, and the time to resolution of the amnesia. Though TGA can be distinguished from PTA based on a lack of clouding of consciousness, a case of anterograde amnesia warrants inquiry into a potential history of head injuries to rule out a traumatic cause.26
Box 21,3,23,27-33 outlines current theories of the etiology and pathogenesis of TGA.
Box 2
The etiology and pathogenesis of transient global amnesia (TGA) are poorly understood, and TGA remains one of the most enigmatic syndromes in clinical neurology.27 Theories regarding the pathogenesis of TGA are diverse and include vascular, epileptic, migraine, and stress-related etiologies.1,23
Early theories suggested arterial ischemia28 and epileptic phenomena29 as etiologies of TGA. The venous theory posits that TGA stems from jugular venous incompetency, causing venous flow and subsequent venous congestion in the medial temporal lobe, wherein lies the hippocampus. This theory is supported by several studies showing venous valve insufficiency as detected by ultrasonographic evaluation during the Valsalva maneuver in patients with TGA.30 This pathophysiologic mechanism may explain the occurrence of TGA in a specific cluster of cases, including men whose TGA episodes are precipitated by physical stress or the Valsalva maneuver.3 The migraine theory and stress theory share a similar proposed neurophysiologic mechanism.
The migraine theory stems from migraines being a known risk factor for TGA, particularly in middle-aged women.31 The stress theory is based on the known emotional precipitants and psychiatric comorbidities associated with TGA. Notably, both the migraine theory and stress theory implicate the role of excessive glutamate release as well as CNS depression.31,32 Glutamate targets the CA1 region of the hippocampus, which is involved in TGA and is known to have the highest density of N-methyl-D-aspartate receptors among hippocampal regions.33
Given the heterogeneity of the demographics and stressors associated with TGA, multiple mechanisms for the disease process may coexist, leading to a similar clinical picture. In 2006, Quinette et al3 performed a multivariate analysis of variables associated with TGA, including age, sex, medical history, and presentation. They demonstrated 3 “clusters” of TGA pictures: women with anxiety or a personality disorder; men with physical precipitating events; and younger patients (age <56) with a history of migraine. These findings suggest TGA may have unique precipitants corresponding to multiple neurophysiologic mechanisms.
Transient global ischemia: Psychiatric features
Several studies have demonstrated psychiatric precipitants, features, and comorbidities associated with TGA. Of the TGA cases associated with precipitating events, 29% to 50% are associated with an emotional stressor.3,4 Examples of emotional stressors include a quarrel,4 the announcement of a birth or suicide, and a nightmare.15 For Ms. A, learning her daughter worked in the sex industry was an emotional stressor.2
During its acute phase, TGA has been shown to present with mood and anxiety symptoms.34 Moreover, during episodes, patients often demonstrate features of panic attacks, such as dizziness, fainting, choking, palpitations, and paresthesia.3,35
Continue to: Finally, patients with TGA...
Finally, patients with TGA are more likely to have psychiatric comorbidities than those without the condition. In a study of 25 patients who experienced TGA triggered by a precipitant, Inzitari et al4 found a strong association of TGA with phobic personality traits, including agoraphobia and simple phobic attitudes (ie, fear of traveling far from home or the sight of blood). Pantoni et al35 replicated these results in 2005 and found that in comparison to patients with TIA, patients with TGA are more likely to have personal and family histories of psychiatric disease. A 2014 study by Dohring et al36 found that compared to healthy controls, patients with TGA are more likely to have maladaptive coping strategies and stress responses. Patients with TGA tended to exhibit increased feelings of guilt, take more medication, and exhibit more anxiety compared to healthy controls.36
Treatment: Benzodiazepines
There are no published treatment guidelines for TGA. However, in case reports, benzodiazepines (specifically lorazepam37) have been shown to have utility in diagnosing and treating DA. The success of benzodiazepines is attributed to its gamma-aminobutyric acid mechanism, which involves inhibiting activity of the N-methyl-
However, the benzodiazepine midazolam has been identified as a precipitant of TGA. In a case report, Rinehart et al22 identified flumazenil—a benzodiazepine antagonist used primarily to treat retrograde postoperative amnesia—as an antidote. The potentially paradoxical role of benzodiazepines in both the precipitation and treatment of TGA may relate back to the heterogeneity of the etiologies of TGA. Further research comparing the treatment of TGA in patients with stress-induced TGA vs postoperative TGA is needed to better understand the neurochemical basis of TGA and work toward establishing optimal treatment options for different patient demographics.
A generally favorable prognosis
TGA carries a low risk of recurrence. In studies with 3- to 7-year follow-up periods, the recurrence rates ranged from 1.4% to 23.8%.23,35,38
Memory impairments may be present for 5 to 6 months following a TGA episode. The severity of these impairments may range from clinically unnoticeable to the patient meeting the criteria for mild cognitive impairment.23,39 The risk is higher in patients who have had recurrent TGA compared to those patients who have experienced only a single episode.23
Continue to: TGA does not increase...
TGA does not increase the risk of cerebrovascular events. There is controversy regarding a potentially increased risk for dementia as well as epilepsy, though there is insufficient evidence to support these findings.23,40
CASE CONTINUED
Five hours after the onset of Ms. A’s symptoms, the treatment team initiates oral lorazepam 1 mg. One hour after taking lorazepam, Ms. A’s anterograde and retrograde amnesia improve. She cannot recall why she was brought to the hospital but does remember the date and location, which she was not able to do on initial presentation. She feels safe, states a clear plan for self-care, and is discharged in the care of her partner. Though Ms. A’s memory improved soon after she received lorazepam, this improvement also could be attributed to the natural course of time, as TGA tends to resolve on its own within 24 hours.
Bottom Line
Transient global amnesia (TGA) is an episode of anterograde, and possibly retrograde, amnesia that lasts up to 24 hours. It represents an interesting diagnosis at the intersection of psychiatry and neurology. TGA has many established psychiatric risk factors and features—some of which may resemble conversion disorder—but these may only apply to a particular subset of patients, which reflects the heterogeneity of the condition.
Related Resources
- Sparaco M, Pascarella R, Muccio CF, et al. Forgetting the unforgettable: transient global amnesia part I: pathophysiology and etiology. J Clin Med. 2022;11(12): 3373. doi:10.3390/jcm1112337
- Sparaco M, Pascarella R, Muccio CF, et al. Forgetting the unforgettable: transient global amnesia part II: a clinical road map. J Clin Med. 2022;11(14):3940. doi:10.3390/ jcm11143940
Drug Brand Names
Flumazenil • Romazicon
Lorazepam • Ativan
Midazolam • Versed
Ms. A, age 48, is a physician’s assistant with no psychiatric history. She presents to the emergency department (ED) with her partner and daughter due to a 15-minute episode of acute-onset memory loss and concern for stroke. In the ED, Ms. A is confused and repeatedly asks, “Where are we?” “How did we get here?” and “What day is it?” Her partner denies Ms. A has focal neurologic deficits or seizures.
Ms. A had only slept 4 hours the night before she came to the ED because she had just learned that her daughter works in the sex industry. According to her daughter, Ms. A was raped by a soldier many years ago. At that time, her perpetrator told Ms. A that he would kill her entire family if she ever told anyone. As a result, she never pursued any psychological or psychiatric treatment.
During the evaluation, Ms. A shares details regarding her social and medical history; however, she does not recall receiving bad news the night before. She asks the interviewer several times how she got to the hospital, and when a cranial nerve exam is performed, she states, “I am not the patient!”
Ms. A’s vital signs and physical exam are unremarkable. Urinalysis is significant for a ketones level of 20 mmol/L (reference range: negative for ketones), and a urine human chorionic gonadotropin test is negative. A neurologic exam does not identify any focal deficits. No imaging is performed.
Transient global amnesia (TGA) describes an episode of anterograde, and possibly retrograde, amnesia that lasts up to 24 hours. On presentation, patients experiencing TGA repeatedly ask, “Where am I?” “What day is it?” and “How did I get here?” However, semantic memory—such as knowledge of the world and autobiographical information—is preserved.1 The first case of TGA was described in 1956, and its diagnostic criteria were most recently modified in 1990 (Table2).
Though TGA is the most common cause of acute-onset amnesia, it is rare, affecting approximately 3 to 10 individuals per 100,000. The average age of onset is 61 to 63, with most cases occurring after age 50. TGA is generally thought to affect males and females equally, though some studies suggest a female predominance.3 In most cases (approximately 90%), there is a precipitating event such as physical or emotional stress, change in temperature, or sexual intercourse.4
In this article, we provide an overview of the classification, presentation, differential diagnosis, workup, and treatment of TGA. While TGA is a neurologic diagnosis, in a subset of patients it can present with psychiatric features resembling conversion disorder. For such patients, we argue that TGA can be considered a neuropsychiatric condition (Box 15-12). This classification may empower emergency psychiatry clinicians and psychotherapists to identify and treat the condition, which is not described by the current psychiatric diagnostic system.
Box 1
Transient global amnesia (TGA) is a neurologic diagnosis. However, in 1956, Bender8 associated the clinical picture of TGA with psychogenic etiology, 2 years before the term was coined. The same year, Courjon et al9 classified TGA as a functional disorder.
As recent literature on TGA has focused on the neuropsychologic mechanism of memory loss, examination of the condition from a psychodynamic standpoint has fallen out of favor. In fact, the earliest discussions of the condition attributed the absence of TGA from literature prior to the 1950s “to erroneous classification of TGA as psychogenic or hysterical amnesia.”10 However, to refer to this condition as purely neurologic—and without any “psychogenic” or functional features— would be reductive.
In a 2019 case report, Espiridion et al6 considered TGA within the same diagnostic realm as—if not actually a form of—dissociative amnesia (DA). They published the case of a 60-year-old woman with a history of posttraumatic stress disorder (PTSD) who experienced an episode of TGA that had manifested as anterograde and retrograde amnesia for 2 days and was precipitated by a psychotherapy session in which she discussed an individual who had assaulted her 5 years earlier. Much like in the case of Ms. A, the report from Espiridion et al6 clearly exemplifies a psychiatric etiology that shares similar context of a stressor unveiling a past memory too unbearable to maintain in consciousness. They concluded that “this case demonstrates anterograde and identity memory impairments likely induced by her PTSD. It is … possible that this presentation may be labeled PTSD-related dissociative amnesia.”6
Considering TGA as a type of DA within a subset of patients represents progression with regards to considering it as a psychiatric disorder. However, a prominent factor distinguishing TGA from DA is that the latter is more commonly associated with loss of personal identity.5 In TGA, memory of autobiographical information typically is preserved.7
Others have argued for a subtype of “emotional arousal–induced TGA”11 or “emotional TGA.”10 We suggest that this “emotional” subtype of TGA, which clearly was affecting Ms. A, shares similarities with functional neurologic symptom disorder, otherwise known as conversion disorder. The psychoanalytic concept that unconscious psychic distress can be “converted” into a neurologic problem is exemplified by Ms. A. Of note, being female and having an emotional stressor are risk factors for conversion disorder. Additionally, migraine— which was not part of Ms. A’s history—is also a risk factor for both TGA and conversion disorder.12 Despite these similarities, however, TGA’s neurophysiological changes on MRI and self-resolving nature still position the disorder as uniquely neuropsychiatric in the term’s purest sense.
Continue to: Differential diagnosis and workup
Differential diagnosis and workup
The differential diagnosis for acute-onset memory loss in the absence of other neurologic or psychiatric features is broad. It includes:
- dissociative amnesia
- ischemic amnesia
- transient epileptic amnesia
- toxic and metabolic amnesia
- posttraumatic amnesia.
Dissociative amnesia (DA), otherwise known as psychogenic amnesia, is “an inability to recall important autobiographical information, usually of a traumatic or stressful nature, that is inconsistent with ordinary forgetting.”13 According to this definition, DA features only retrograde amnesia, as opposed to TGA, which features anterograde amnesia, with possible retrograde amnesia. A subtype of DA—specifically, “continuous amnesia” or “anterograde dissociative amnesia”— is in DSM-5.13 However, the diagnostic criteria are unclear, and no cases have been identified in the literature since 1903, before TGA became a diagnostic entity.5,14 Moreover, patients with DA cannot recall autobiographical information, which is not a feature of TGA. Within DSM-5, TGA is an exclusion criterion for DA.13 Thus, an episode of anterograde amnesia with acute onset best meets criteria for TGA, even if there are substantial psychiatric risk factors.
Ischemic amnesia—including stroke and transient ischemic attack (TIA)—is often the primary concern of patients with TGA and their families upon initial presentation, as was the case with Ms. A.6,15 TIA presenting with isolated, acute-onset amnesia would be highly unusual, because these attacks usually present with focal symptoms including motor deficits, sensory deficits, visual field deficits, and aphasia or dysarthria. A patient with amnesia experiencing a TIA would likely have symptoms lasting from seconds to minutes, which is much shorter than a typical TGA episode.16
Amnesia secondary to stroke may be transient or permanent.7 Amnesia is present in approximately 1% of all strokes and in approximately 19.3% of posterior cerebral artery strokes.7,17 Unlike TIA and TGA, ischemic amnesia would present with MRI findings detectable at symptom onset. TGA does reveal MRI findings, particularly punctate lesions in the CA1 area of the hippocampus; however, these lesions are typically much smaller than those found in stroke, and are not detectable until 12 to 48 hours after episode onset.1,17 MRI findings in ischemic amnesia are typically associated with extrahippocampal lesions.17 Finally, the presence of vascular risk factors such as hyperlipidemia, smoking, diabetes, and hypertension may also favor a diagnosis of stroke or TIA as opposed to TGA, which is not associated with these risk factors.18 Though ischemic amnesia and TGA usually can be differentiated based on history and presentation, MRI with fluid-attenuated inversion recovery and diffusion-weighted imaging may be performed to definitively distinguish stroke from TGA.7
Transient epileptic amnesia (TEA), a focal form of epilepsy within the temporal lobe, should also be considered in patients who present with acute-onset amnesia. Like TGA, TEA may present with simultaneous anterograde and retrograde amnesia accompanied by repetitive questioning.19 Amnesia can be the sole symptom of TEA in up to 24% of cases. However, several key features distinguish TEA from TGA. TEA most often presents with other clinical signs of seizures, such as oral automatisms and/or olfactory hallucinations.20 There is also a significant difference in episode length; TEA episodes last an average of 30 to 60 minutes and tend to occur upon wakening, whereas TGA episodes last an average of 4 to 6 hours and do not preferentially occur at any particular time.1,21 In the interictal period—between seizures—patients with TEA may also experience accelerated long-term forgetting, autobiographical amnesia, and topographical amnesia.19,20 Finally, a diagnosis of TEA also requires recurrent episodes. Recurrence can happen with TGA, but is less frequent.21 Generally, history and presentation can distinguish TEA from TGA. Though there is no formal protocol for TEA workup, Lanzone et al21 recommend 24-hour EEG or EEG sleep monitoring in patients who present with amnesia as well as other clinical manifestations of epileptic phenomenon.
Continue to: Toxic and metabolic
Toxic and metabolic etiologies of amnesia include opioid and cocaine use, general anesthetics,22 and hypoglycemia.7,23 Toxic and metabolic causes of amnesia may mirror TGA in their acute onset as well as anterograde nature. However, these patients will likely present with fluctuating consciousness and/or other neuropsychiatric features, such as pressured speech, delusions, and/or distractability.23 Obtaining a patient’s medical history, including substance use, medication use, and the presence of diabetes,24 is typically sufficient to rule out toxic and metabolic causes.7
Posttraumatic amnesia (PTA) describes transient memory loss that occurs after a traumatic brain injury. Anterograde amnesia is most common, though approximately 20% of patients may also experience retrograde amnesia pertaining to the events near the date of their injury. Unlike TGA, which typically resolves within 24 hours, the recovery time of amnestic symptoms in PTA ranges from minutes to years.7 A distinguishing feature of PTA is the presence of confusion, which often resembles a state of delirium.25 The presentation of PTA can vary immensely with regards to agitation, psychotic symptoms, and the time to resolution of the amnesia. Though TGA can be distinguished from PTA based on a lack of clouding of consciousness, a case of anterograde amnesia warrants inquiry into a potential history of head injuries to rule out a traumatic cause.26
Box 21,3,23,27-33 outlines current theories of the etiology and pathogenesis of TGA.
Box 2
The etiology and pathogenesis of transient global amnesia (TGA) are poorly understood, and TGA remains one of the most enigmatic syndromes in clinical neurology.27 Theories regarding the pathogenesis of TGA are diverse and include vascular, epileptic, migraine, and stress-related etiologies.1,23
Early theories suggested arterial ischemia28 and epileptic phenomena29 as etiologies of TGA. The venous theory posits that TGA stems from jugular venous incompetency, causing venous flow and subsequent venous congestion in the medial temporal lobe, wherein lies the hippocampus. This theory is supported by several studies showing venous valve insufficiency as detected by ultrasonographic evaluation during the Valsalva maneuver in patients with TGA.30 This pathophysiologic mechanism may explain the occurrence of TGA in a specific cluster of cases, including men whose TGA episodes are precipitated by physical stress or the Valsalva maneuver.3 The migraine theory and stress theory share a similar proposed neurophysiologic mechanism.
The migraine theory stems from migraines being a known risk factor for TGA, particularly in middle-aged women.31 The stress theory is based on the known emotional precipitants and psychiatric comorbidities associated with TGA. Notably, both the migraine theory and stress theory implicate the role of excessive glutamate release as well as CNS depression.31,32 Glutamate targets the CA1 region of the hippocampus, which is involved in TGA and is known to have the highest density of N-methyl-D-aspartate receptors among hippocampal regions.33
Given the heterogeneity of the demographics and stressors associated with TGA, multiple mechanisms for the disease process may coexist, leading to a similar clinical picture. In 2006, Quinette et al3 performed a multivariate analysis of variables associated with TGA, including age, sex, medical history, and presentation. They demonstrated 3 “clusters” of TGA pictures: women with anxiety or a personality disorder; men with physical precipitating events; and younger patients (age <56) with a history of migraine. These findings suggest TGA may have unique precipitants corresponding to multiple neurophysiologic mechanisms.
Transient global ischemia: Psychiatric features
Several studies have demonstrated psychiatric precipitants, features, and comorbidities associated with TGA. Of the TGA cases associated with precipitating events, 29% to 50% are associated with an emotional stressor.3,4 Examples of emotional stressors include a quarrel,4 the announcement of a birth or suicide, and a nightmare.15 For Ms. A, learning her daughter worked in the sex industry was an emotional stressor.2
During its acute phase, TGA has been shown to present with mood and anxiety symptoms.34 Moreover, during episodes, patients often demonstrate features of panic attacks, such as dizziness, fainting, choking, palpitations, and paresthesia.3,35
Continue to: Finally, patients with TGA...
Finally, patients with TGA are more likely to have psychiatric comorbidities than those without the condition. In a study of 25 patients who experienced TGA triggered by a precipitant, Inzitari et al4 found a strong association of TGA with phobic personality traits, including agoraphobia and simple phobic attitudes (ie, fear of traveling far from home or the sight of blood). Pantoni et al35 replicated these results in 2005 and found that in comparison to patients with TIA, patients with TGA are more likely to have personal and family histories of psychiatric disease. A 2014 study by Dohring et al36 found that compared to healthy controls, patients with TGA are more likely to have maladaptive coping strategies and stress responses. Patients with TGA tended to exhibit increased feelings of guilt, take more medication, and exhibit more anxiety compared to healthy controls.36
Treatment: Benzodiazepines
There are no published treatment guidelines for TGA. However, in case reports, benzodiazepines (specifically lorazepam37) have been shown to have utility in diagnosing and treating DA. The success of benzodiazepines is attributed to its gamma-aminobutyric acid mechanism, which involves inhibiting activity of the N-methyl-
However, the benzodiazepine midazolam has been identified as a precipitant of TGA. In a case report, Rinehart et al22 identified flumazenil—a benzodiazepine antagonist used primarily to treat retrograde postoperative amnesia—as an antidote. The potentially paradoxical role of benzodiazepines in both the precipitation and treatment of TGA may relate back to the heterogeneity of the etiologies of TGA. Further research comparing the treatment of TGA in patients with stress-induced TGA vs postoperative TGA is needed to better understand the neurochemical basis of TGA and work toward establishing optimal treatment options for different patient demographics.
A generally favorable prognosis
TGA carries a low risk of recurrence. In studies with 3- to 7-year follow-up periods, the recurrence rates ranged from 1.4% to 23.8%.23,35,38
Memory impairments may be present for 5 to 6 months following a TGA episode. The severity of these impairments may range from clinically unnoticeable to the patient meeting the criteria for mild cognitive impairment.23,39 The risk is higher in patients who have had recurrent TGA compared to those patients who have experienced only a single episode.23
Continue to: TGA does not increase...
TGA does not increase the risk of cerebrovascular events. There is controversy regarding a potentially increased risk for dementia as well as epilepsy, though there is insufficient evidence to support these findings.23,40
CASE CONTINUED
Five hours after the onset of Ms. A’s symptoms, the treatment team initiates oral lorazepam 1 mg. One hour after taking lorazepam, Ms. A’s anterograde and retrograde amnesia improve. She cannot recall why she was brought to the hospital but does remember the date and location, which she was not able to do on initial presentation. She feels safe, states a clear plan for self-care, and is discharged in the care of her partner. Though Ms. A’s memory improved soon after she received lorazepam, this improvement also could be attributed to the natural course of time, as TGA tends to resolve on its own within 24 hours.
Bottom Line
Transient global amnesia (TGA) is an episode of anterograde, and possibly retrograde, amnesia that lasts up to 24 hours. It represents an interesting diagnosis at the intersection of psychiatry and neurology. TGA has many established psychiatric risk factors and features—some of which may resemble conversion disorder—but these may only apply to a particular subset of patients, which reflects the heterogeneity of the condition.
Related Resources
- Sparaco M, Pascarella R, Muccio CF, et al. Forgetting the unforgettable: transient global amnesia part I: pathophysiology and etiology. J Clin Med. 2022;11(12): 3373. doi:10.3390/jcm1112337
- Sparaco M, Pascarella R, Muccio CF, et al. Forgetting the unforgettable: transient global amnesia part II: a clinical road map. J Clin Med. 2022;11(14):3940. doi:10.3390/ jcm11143940
Drug Brand Names
Flumazenil • Romazicon
Lorazepam • Ativan
Midazolam • Versed
1. Miller TD, Butler CR. Acute-onset amnesia: transient global amnesia and other causes. Pract Neurol. 2022;22(3):201-208. doi:10.1136/practneurol-2020-002826
2. Hodges JR, Warlow CP. Syndromes of transient amnesia: towards a classification. A study of 153 cases. J Neurol Neurosurg Psychiatry. 1990;53(10):834-843. doi:10.1136/jnnp.53.10.834
3. Quinette P, Guillery-Girard B, Dayan J, et al. What does transient global amnesia really mean? Review of the literature and thorough study of 142 cases. Brain. 2006;129(Pt 7):1640-1658. doi:10.1093/brain/awl105
4. Inzitari D, Pantoni L, Lamassa M, et al. Emotional arousal and phobia in transient global amnesia. Arch Neurol. 1997;54(7):866-873. doi:10.1001/archneur.1997.00550190056015
5. Staniloiu A, Markowitsch HJ. Dissociative amnesia. Lancet Psychiatry. 2014;1(3):226-241. doi:10.1016/S2215-0366(14)70279-2
6. Espiridion ED, Gupta J, Bshara A, et al. Transient global amnesia in a 60-year-old female with post-traumatic stress disorder. Cureus. 2019;11(9):e5792. doi:10.7759/cureus.5792
7. Alessandro L, Ricciardi M, Chaves H, et al. Acute amnestic syndromes. J Neurol Sci. 2020;413:116781. doi:10.1016/j.jns.2020.116781
8. Bender M. Syndrome of isolated episode of confusion with amnesia. J Hillside Hosp. 1956;5:212-215.
9. Courjon J, Guyotat J. Les ictus amnéstiques [Amnesic strokes]. J Med Lyon. 1956;37(882):697-701.
10. Noel A, Quinette P, Hainselin M, et al. The still enigmatic syndrome of transient global amnesia: interactions between neurological and psychopathological factors. Neuropsychol Rev. 2015;25(2):125-133. doi:10.1007/s11065-015-9284-y
11. Merriam AE, Wyszynski B, Betzler T. Emotional arousal-induced transient global amnesia. A clue to the neural transcription of emotion? Psychosomatics. 1992;33(1):109-113. doi:10.1016/S0033-3182(92)72029-5
12. Hallett M, Aybek S, Dworetzky BA, et al. Functional neurological disorder: new subtypes and shared mechanisms. Lancet Neurol. 2022;21(6):537-550. doi:10.1016/S1474-4422(21)00422-1
13. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
14. Bourdon B, Dide M. A case of continuous amnesia with tactile asymbolia, complicated by other troubles. Ann Psychol. 1903;10:84-115.
15. Marinella MA. Transient global amnesia and a father’s worst nightmare. N Engl J Med. 2004;350(8):843-844. doi:10.1056/NEJM200402193500821
16. Amarenco P. Transient ischemic attack. N Engl J Med. 2020;382(20):1933-1941. doi:10.1056/NEJMcp1908837
17. Szabo K, Forster A, Jager T, et al. Hippocampal lesion patterns in acute posterior cerebral artery stroke: clinical and MRI findings. Stroke. 2009;40(6):2042-2045. doi:10.1161/STROKEAHA.108.536144
18. Liampas I, Raptopoulou M, Siokas V, et al. Conventional cardiovascular risk factors in transient global amnesia: systematic review and proposition of a novel hypothesis. Front Neuroendocrinol. 2021;61:100909. doi:10.1016/j.yfrne.2021.100909
19. Zeman A, Butler C. Transient epileptic amnesia. Curr Opin Neurol. 2010;23(6):610-616. doi:10.1097/WCO.0b013e32834027db
20. Baker J, Savage S, Milton F, et al. The syndrome of transient epileptic amnesia: a combined series of 115 cases and literature review. Brain Commun. 2021;3(2):fcab038. doi:10.1093/braincomms/fcab038
21. Lanzone J, Ricci L, Assenza G, et al Transient epileptic and global amnesia: real-life differential diagnosis. Epilepsy Behav. 2018;88:205-211. doi:10.1016/j.yebeh.2018.07.015
22. Rinehart JB, Baker B, Raphael D. Postoperative global amnesia reversed with flumazenil. Neurologist. 2012;18(4):216-218. doi:10.1097/NRL.0b013e31825bbef4
23. Arena JE, Rabinstein AA. Transient global amnesia. Mayo Clin Proc. 2015;90(2):264-272. doi:10.1016/j.mayocp.2014.12.001
24. Holemans X, Dupuis M, Misson N, et al. Reversible amnesia in a type 1 diabetic patient and bilateral hippocampal lesions on magnetic resonance imaging (MRI). Diabet Med. 2001;18(9):761-763. doi:10.1046/j.1464-5491.2001.00481.x
25. Marshman LAG, Jakabek D, Hennessy M, et al. Post-traumatic amnesia. J Clin Neurosci. 2013;20(11):1475-1481. doi:10.1016/j.jocn.2012.11.022
26. Parker TD, Rees R, Rajagopal S, et al. Post-traumatic amnesia. Pract Neurol. 2022;22(2):129-137. doi:10.1136/practneurol-2021-003056
27. You SH, Kim B, Kim BK. Transient global amnesia: signal alteration in 2D/3D T2-FLAIR sequences. Clin Imaging. 2021;78:154-159. doi:10.1016/j.clinimag.2021.03.029
28. Mathew NT, Meyer JS. Pathogenesis and natural history of transient global amnesia. Stroke. 1974;5(3):303-311. doi:10.1161/01.str.5.3.303
29. Fisher CM, Adams RD. Transient global amnesia. Acta Neurol Scand Suppl. 1964;40(SUPPL 9):1-83.
30. Cejas C, Cisneros LF, Lagos R, et al. Internal jugular vein valve incompetence is highly prevalent in transient global amnesia. Stroke. 2010;41(1):67-71. doi:10.1161/STROKEAHA.109.566315
31. Liampas I, Siouras AS, Siokas V, et al. Migraine in transient global amnesia: a meta-analysis of observational studies. J Neurol. 2022;269(1):184-196. doi:10.1007/s00415-020-10363-y
32. Ding X, Peng D. Transient global amnesia: an electrophysiological disorder based on cortical spreading depression-transient global amnesia model. Front Hum Neurosci. 2020;14:602496. doi:10.3389/fnhum.2020.602496
33. Bartsch T, Dohring J, Reuter S, et al. Selective neuronal vulnerability of human hippocampal CA1 neurons: lesion evolution, temporal course, and pattern of hippocampal damage in diffusion-weighted MR imaging. J Cereb Blood Flow Metab. 2015;35(11):1836-1845. doi:10.1038/jcbfm.2015.137
34. Noel A, Quinette P, Guillery-Girard B, et al. Psychopathological factors, memory disorders and transient global amnesia. Br J Psychiatry. 2008;193(2):145-151. doi:10.1192/bjp.bp.107.045716
35. Pantoni L, Bertini E, Lamassa M, et al. Clinical features, risk factors, and prognosis in transient global amnesia: a follow-up study. Eur J Neurol. 2005;12(5):350-356. doi:10.1111/j.1468-1331.2004.00982.x
36. Dohring J, Schmuck A, Bartsch T. Stress-related factors in the emergence of transient global amnesia with hippocampal lesions. Front Behav Neurosci. 2014;8:287. doi:10.3389/fnbeh.2014.00287
37. Jiang S, Gunther S, Hartney K, et al. An intravenous lorazepam infusion for dissociative amnesia: a case report. Psychosomatics. 2020;61(6):814-818. doi:10.1016/j.psym.2020.01.009
38. He S, Ye Z, Yang Q, et al. Transient global amnesia: risk factors, imaging features, and prognosis. Neuropsychiatr Dis Treat. 2021;17:1611-1619. doi:10.2147/NDT.S299168
39. Borroni B, Agosti C, Brambilla C, et al. Is transient global amnesia a risk factor for amnestic mild cognitive impairment? J Neurol. 2004;251(9):1125-1127. doi:10.1007/s00415-004-0497-x
40. Liampas I, Raptopoulou M, Siokas V, et al. The long-term prognosis of transient global amnesia: a systematic review. Rev Neurosci. 2021;32(5):531-543. doi:10.1515/revneuro-2020-0110
1. Miller TD, Butler CR. Acute-onset amnesia: transient global amnesia and other causes. Pract Neurol. 2022;22(3):201-208. doi:10.1136/practneurol-2020-002826
2. Hodges JR, Warlow CP. Syndromes of transient amnesia: towards a classification. A study of 153 cases. J Neurol Neurosurg Psychiatry. 1990;53(10):834-843. doi:10.1136/jnnp.53.10.834
3. Quinette P, Guillery-Girard B, Dayan J, et al. What does transient global amnesia really mean? Review of the literature and thorough study of 142 cases. Brain. 2006;129(Pt 7):1640-1658. doi:10.1093/brain/awl105
4. Inzitari D, Pantoni L, Lamassa M, et al. Emotional arousal and phobia in transient global amnesia. Arch Neurol. 1997;54(7):866-873. doi:10.1001/archneur.1997.00550190056015
5. Staniloiu A, Markowitsch HJ. Dissociative amnesia. Lancet Psychiatry. 2014;1(3):226-241. doi:10.1016/S2215-0366(14)70279-2
6. Espiridion ED, Gupta J, Bshara A, et al. Transient global amnesia in a 60-year-old female with post-traumatic stress disorder. Cureus. 2019;11(9):e5792. doi:10.7759/cureus.5792
7. Alessandro L, Ricciardi M, Chaves H, et al. Acute amnestic syndromes. J Neurol Sci. 2020;413:116781. doi:10.1016/j.jns.2020.116781
8. Bender M. Syndrome of isolated episode of confusion with amnesia. J Hillside Hosp. 1956;5:212-215.
9. Courjon J, Guyotat J. Les ictus amnéstiques [Amnesic strokes]. J Med Lyon. 1956;37(882):697-701.
10. Noel A, Quinette P, Hainselin M, et al. The still enigmatic syndrome of transient global amnesia: interactions between neurological and psychopathological factors. Neuropsychol Rev. 2015;25(2):125-133. doi:10.1007/s11065-015-9284-y
11. Merriam AE, Wyszynski B, Betzler T. Emotional arousal-induced transient global amnesia. A clue to the neural transcription of emotion? Psychosomatics. 1992;33(1):109-113. doi:10.1016/S0033-3182(92)72029-5
12. Hallett M, Aybek S, Dworetzky BA, et al. Functional neurological disorder: new subtypes and shared mechanisms. Lancet Neurol. 2022;21(6):537-550. doi:10.1016/S1474-4422(21)00422-1
13. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
14. Bourdon B, Dide M. A case of continuous amnesia with tactile asymbolia, complicated by other troubles. Ann Psychol. 1903;10:84-115.
15. Marinella MA. Transient global amnesia and a father’s worst nightmare. N Engl J Med. 2004;350(8):843-844. doi:10.1056/NEJM200402193500821
16. Amarenco P. Transient ischemic attack. N Engl J Med. 2020;382(20):1933-1941. doi:10.1056/NEJMcp1908837
17. Szabo K, Forster A, Jager T, et al. Hippocampal lesion patterns in acute posterior cerebral artery stroke: clinical and MRI findings. Stroke. 2009;40(6):2042-2045. doi:10.1161/STROKEAHA.108.536144
18. Liampas I, Raptopoulou M, Siokas V, et al. Conventional cardiovascular risk factors in transient global amnesia: systematic review and proposition of a novel hypothesis. Front Neuroendocrinol. 2021;61:100909. doi:10.1016/j.yfrne.2021.100909
19. Zeman A, Butler C. Transient epileptic amnesia. Curr Opin Neurol. 2010;23(6):610-616. doi:10.1097/WCO.0b013e32834027db
20. Baker J, Savage S, Milton F, et al. The syndrome of transient epileptic amnesia: a combined series of 115 cases and literature review. Brain Commun. 2021;3(2):fcab038. doi:10.1093/braincomms/fcab038
21. Lanzone J, Ricci L, Assenza G, et al Transient epileptic and global amnesia: real-life differential diagnosis. Epilepsy Behav. 2018;88:205-211. doi:10.1016/j.yebeh.2018.07.015
22. Rinehart JB, Baker B, Raphael D. Postoperative global amnesia reversed with flumazenil. Neurologist. 2012;18(4):216-218. doi:10.1097/NRL.0b013e31825bbef4
23. Arena JE, Rabinstein AA. Transient global amnesia. Mayo Clin Proc. 2015;90(2):264-272. doi:10.1016/j.mayocp.2014.12.001
24. Holemans X, Dupuis M, Misson N, et al. Reversible amnesia in a type 1 diabetic patient and bilateral hippocampal lesions on magnetic resonance imaging (MRI). Diabet Med. 2001;18(9):761-763. doi:10.1046/j.1464-5491.2001.00481.x
25. Marshman LAG, Jakabek D, Hennessy M, et al. Post-traumatic amnesia. J Clin Neurosci. 2013;20(11):1475-1481. doi:10.1016/j.jocn.2012.11.022
26. Parker TD, Rees R, Rajagopal S, et al. Post-traumatic amnesia. Pract Neurol. 2022;22(2):129-137. doi:10.1136/practneurol-2021-003056
27. You SH, Kim B, Kim BK. Transient global amnesia: signal alteration in 2D/3D T2-FLAIR sequences. Clin Imaging. 2021;78:154-159. doi:10.1016/j.clinimag.2021.03.029
28. Mathew NT, Meyer JS. Pathogenesis and natural history of transient global amnesia. Stroke. 1974;5(3):303-311. doi:10.1161/01.str.5.3.303
29. Fisher CM, Adams RD. Transient global amnesia. Acta Neurol Scand Suppl. 1964;40(SUPPL 9):1-83.
30. Cejas C, Cisneros LF, Lagos R, et al. Internal jugular vein valve incompetence is highly prevalent in transient global amnesia. Stroke. 2010;41(1):67-71. doi:10.1161/STROKEAHA.109.566315
31. Liampas I, Siouras AS, Siokas V, et al. Migraine in transient global amnesia: a meta-analysis of observational studies. J Neurol. 2022;269(1):184-196. doi:10.1007/s00415-020-10363-y
32. Ding X, Peng D. Transient global amnesia: an electrophysiological disorder based on cortical spreading depression-transient global amnesia model. Front Hum Neurosci. 2020;14:602496. doi:10.3389/fnhum.2020.602496
33. Bartsch T, Dohring J, Reuter S, et al. Selective neuronal vulnerability of human hippocampal CA1 neurons: lesion evolution, temporal course, and pattern of hippocampal damage in diffusion-weighted MR imaging. J Cereb Blood Flow Metab. 2015;35(11):1836-1845. doi:10.1038/jcbfm.2015.137
34. Noel A, Quinette P, Guillery-Girard B, et al. Psychopathological factors, memory disorders and transient global amnesia. Br J Psychiatry. 2008;193(2):145-151. doi:10.1192/bjp.bp.107.045716
35. Pantoni L, Bertini E, Lamassa M, et al. Clinical features, risk factors, and prognosis in transient global amnesia: a follow-up study. Eur J Neurol. 2005;12(5):350-356. doi:10.1111/j.1468-1331.2004.00982.x
36. Dohring J, Schmuck A, Bartsch T. Stress-related factors in the emergence of transient global amnesia with hippocampal lesions. Front Behav Neurosci. 2014;8:287. doi:10.3389/fnbeh.2014.00287
37. Jiang S, Gunther S, Hartney K, et al. An intravenous lorazepam infusion for dissociative amnesia: a case report. Psychosomatics. 2020;61(6):814-818. doi:10.1016/j.psym.2020.01.009
38. He S, Ye Z, Yang Q, et al. Transient global amnesia: risk factors, imaging features, and prognosis. Neuropsychiatr Dis Treat. 2021;17:1611-1619. doi:10.2147/NDT.S299168
39. Borroni B, Agosti C, Brambilla C, et al. Is transient global amnesia a risk factor for amnestic mild cognitive impairment? J Neurol. 2004;251(9):1125-1127. doi:10.1007/s00415-004-0497-x
40. Liampas I, Raptopoulou M, Siokas V, et al. The long-term prognosis of transient global amnesia: a systematic review. Rev Neurosci. 2021;32(5):531-543. doi:10.1515/revneuro-2020-0110
For artificial intelligence, the future is finally here
We are currently on the verge of yet another societal “revolution” that will exert an unprecedented impact on our lives. It may surpass prior seismic cultural breakthroughs like the internet, smartphones, and social media. Artificial intelligence (AI) has been fermenting for several decades, gathering steam to become equivalent (and eventually superior) to human intelligence. The escalation of AI sophistication will be jarring and perhaps change human life in completely unpredictable ways.
Composing thoughts into words and coherent sentences has always been a uniquely human attribute among all living organisms. Now, that sublime feature of the human mind is being simulated, thanks to advances in AI software, ironically created by the human mind itself! On November 30, 2022, Open AI introduced ChatGPT (generative pre-trained transformer), which can generate an article on any topic a user requests. Within a few weeks, it was used by more than 100 million people. ChatGPT is taking the world by storm because it is a harbinger (some pessimists may label it an omen) of how human existence will be radically impacted in the future. Such AI breakthroughs to surpass human intelligence are, ironically, the product of the advanced human brain, which I previously described as concurrently a triumph and a blunder by evolution.1
How we got here, and what’s next
ChatGPT is a large language model based on neural networks.2 It generates realistic text responses to a wide range of questions by mimicking the pattern of language in gargantuan online databases. One Hong Kong–based, AI-powered drug discovery company (Insilico Medicine) declared it published articles generated by AI tools, even before ChatGPT became available. This indicates how AI can be misused in scientific publications and may be hard to detect as a new form of plagiarism.3
The roots of AI date back to the 1950s, when Alan Turing, now considered the father of AI, published a seminal article about creating a machine to “imitate the brain” and to “mimic the behavior of the human.”4 The term “artificial intelligence” was coined in 1989 by McCarthy,5 who defined it as “the science of engineering for making intelligent machines.” Since then, several subsets of AI have been developed:
- Machine learning: The study of computer algorithms to generate hypotheses
- Deep learning: A type of machine learning algorithm that uses multiple layers to progressively extract higher-level features from raw input. (Both machine learning and deep learning are used in the burgeoning fields of computational psychiatry6 and neuroscience research7)
- Expert knowledge system: A computer-based system that mimics human decision-making ability
- Neural networks: An interconnected group of artificial neurons that uses a math or computer model for information processing
- Predictive analytics: An algorithm to predict future outcomes based on historical data.
These subsets of AI have been used to identify psychiatric disorders using neuroimaging data8 and to classify brain disorders.9 There are many potential uses of AI in psychiatry.10,11 My first experience with AI was 13 years ago, when we conducted a project to distinguish fake suicide notes from genuine ones.12 AI was more successful in correctly identifying fake notes (78% correctly detected) than senior psychiatric residents (49%) or even faculty (53%).
AI will dramatically change how humans interact with the world and may lead to enhanced creativity and new explorations and forays into novel, previously unknown horizons. It is expected to significantly boost the global economy by many trillions of dollars over the next decade. Major high-tech companies are vigorously competing to develop their own AI tools like ChatGPT (Microsoft invested $10 billion in Open AI). Google, which owns DeepMind (an AI lab that invented the T in GPT) developed its own chatbot called Bard. Amazon has invested heavily in Stability AI by giving its founder and CEO Emad Mostaque 4,000 Nvidia AI chips to assemble the world’s largest supercomputer (1 year ago, Stability had only 32 AI chips!). Apple recently integrated Stable Diffusion into its latest operating system. Chinese tech giants Alibaba and Baidu also announced their own chatbots to be released soon.
Other competitors include Cohere, Hugging Face, Midjourney, GitHub Copilot, Game Changer, Jasper, and Anthropic, which released Claude as its chatbot at a lower cost than ChatGPT. Open AI also developed Dall-E2 in April 2022, which can generate very realistic images from text, one of which recently won an award at an art competition.
Continue to: One of the major...
One of the major concerns about these AI developments is that chatbots can make errors or disseminate misinformation and even enunciate racist or misogynist statements. The greatest worry is that the ultimate implicit goal of AI is what is called artificial general intelligence (AGI), which can think and learn better than humans. Some fear AGI may wipe out humans as a species, a grave outcome indeed. That’s why AI developers intend to align AGI with “basic human values” in the event it surpasses human intelligence. Some are even calling for a transition from AI to AW (artificial wisdom), with built-in empathy, kindness, self-reflection, acceptance of diverse perspectives, and promotion of prosocial ideas.13
Creating content: artificial intelligence vs human authors
ChatGPT has opened a Pandora’s box of concern about potential plagiarism by students or scientific authors. This has prompted several journals,14 including
- Anosognosia in schizophrenia (Box 1); based on “Is anosognosia a delusion, a negative symptom, or a cognitive deficit?”
Current Psychiatry , January 2022, p. 6-8,14. doi: 10.12788/cp.0210 - Benefits of metformin in psychiatry (Box 2); based on “Beyond diabetes: The beneficial uses of metformin in psychiatry,”
Current Psychiatry , March 2022, p. 7-9. doi: 10.12788/cp.0225 - Epidemic of intellectual constipation (Box 3); based on “From debate to stalemate and hate: An epidemic of intellectual constipation,”
Current Psychiatry , January 2023, p. 3-4. doi: 10.12788/cp.0321 - SWOT analysis of psychiatry (Box 4); based on “Contemporary psychiatry: A SWOT analysis,”
Current Psychiatry , January 2023, p. 16-19,27. doi: 10.12788/cp.0320.
These ChatGPT articles are verbatim as generated, without any copyediting, fact-checking, or addition of reference citations. One can easily notice that while factually acceptable, the ChatGPT articles tend to be rather synthetic, simple, bland, and lacking creative language or going beyond the facts to express personal viewpoints. However, I have no doubt that future versions of ChatGPT or other chatbots will evolve into far more sophisticated products and may match what a human can compose in terms of quality and complexity.
AI has arrived with a bang. Going forward, I predict it will continue to surprise us, with elements of “future shock.” The credit (or blame) belongs to the boundless creativity of human intelligence that is seeking to invent an intelligence higher than its own. Caveat emptor!
Box 1
Anosognosia is a condition in which a person is unaware or unable to recognize their own illness or disability. In schizophrenia, anosognosia can be present in up to 50% of cases and is characterized by a lack of insight into the presence and severity of symptoms.
People with anosognosia in schizophrenia may not believe that they have a mental illness, may deny the reality of their symptoms, or may attribute them to external causes. This can make it difficult for them to comply with treatment, as they may not see the need for medication or other interventions.
Anosognosia is thought to be caused by structural and functional changes in the brain, particularly in areas associated with self-awareness and insight. It is often more severe in people with more severe forms of schizophrenia and may be associated with poorer treatment outcomes.
Effective treatment for anosognosia in schizophrenia typically involves a combination of medication and therapy, as well as strategies for improving insight and awareness of symptoms. Family members and caregivers can also play an important role in helping people with schizophrenia to recognize the presence and impact of their illness, and encouraging them to engage in treatment.
a Based on “Is anosognosia a delusion, a negative symptom, or a cognitive deficit?” Current Psychiatry, January 2022, p. 6-8,14. doi: 10.12788/cp.0210
Box 2
Metformin is a medication commonly used in the treatment of type 2 diabetes. However, there is growing interest in its potential use in psychiatry, particularly in the treatment of certain mental health conditions. Some potential benefits of metformin in psychiatry include:
1. Treatment of mood disorders: Studies have suggested that metformin may be helpful in the treatment of mood disorders, such as depression and bipolar disorder. One proposed mechanism is that metformin may help to regulate glucose metabolism and insulin sensitivity, which in turn may help to stabilize mood.
2. Treatment of schizophrenia: Some studies have suggested that metformin may be helpful in reducing symptoms of schizophrenia, particularly negative symptoms such as social withdrawal and apathy. One proposed mechanism is that metformin may improve brain energy metabolism, which may in turn improve cognitive function and reduce symptoms.
3. Weight management: Metformin has been shown to be effective in weight management, which can be particularly important for people with certain mental health conditions, such as bipolar disorder, where weight gain can be a side effect of medication.
4. Neuroprotection: Metformin has been shown to have neuroprotective effects, which may be beneficial for people with certain mental health conditions, such as Alzheimer’s disease and Parkinson’s disease.
It is important to note that while there is some evidence for the potential benefits of metformin in psychiatry, further research is needed to fully understand its effectiveness and safety in this context. People with mental health conditions should not start taking metformin without first consulting with their healthcare provider.
a Based on “Beyond diabetes: The beneficial uses of metformin in psychiatry,” Current Psychiatry, March 2022, p. 7-9. doi: 10.12788/cp.0225
Box 3
The term “epidemic of intellectual constipation” refers to a phenomenon in which individuals, groups, or even entire societies are seen as lacking the ability to think critically, creatively, or expansively. It can be characterized by a lack of curiosity, a narrow range of interests, and a reluctance to engage with new or challenging ideas.
The cause of this phenomenon can be attributed to a variety of factors, including cultural, social, and educational influences. For example, some argue that a narrow and standardized educational system, as well as a lack of exposure to diverse perspectives, can limit creativity and critical thinking skills. Additionally, the prevalence of social media and other forms of digital media may encourage a focus on immediate gratification and quick information, rather than more in-depth and meaningful engagement with information.
The consequences of this epidemic can be far-reaching and can impact personal, social, and political aspects of life. For instance, a lack of critical thinking skills can lead to the spread of misinformation, while a narrow range of interests can limit personal growth and the ability to connect with others.
To combat the epidemic of intellectual constipation, it’s important to foster critical thinking skills, encourage exposure to diverse perspectives, and encourage a lifelong love of learning. This can be achieved through education, community engagement, and personal effort.
In conclusion, the “epidemic of intellectual constipation” refers to a phenomenon in which individuals, groups, or entire societies lack the ability to think critically, creatively, and expansively. To combat this epidemic, it’s important to foster critical thinking skills, encourage exposure to diverse perspectives, and encourage a lifelong love of learning.
a Based on “From debate to stalemate and hate: An epidemic of intellectual constipation,” Current Psychiatry, January 2023, p. 3-4. doi: 10.12788/cp.0321
Box 4
SWOT analysis is a tool used to assess the strengths, weaknesses, opportunities, and threats of a particular entity or industry. In this case, we will conduct a SWOT analysis of psychiatry as a field of medicine.
Strengths
• Psychiatry plays a crucial role in addressing mental health issues and improving the quality of life of individuals who struggle with them.
• Psychiatry has made significant advances in understanding the biological and psychological underpinnings of mental illness, which has led to the development of more effective treatments.
• There is growing public awareness and acceptance of the importance of mental health, which has led to increased demand for psychiatric services.
• The field of psychiatry attracts many talented and compassionate professionals who are dedicated to helping those in need.
Weaknesses
• There is still a significant stigma associated with mental illness, which can deter individuals from seeking treatment.
• The effectiveness of some psychiatric treatments can be limited, particularly in cases of severe mental illness.
• The shortage of mental health professionals in many areas means that access to psychiatric services may be limited, particularly for individuals with lower incomes.
• The cost of psychiatric treatment can be prohibitively expensive, particularly for individuals without insurance coverage.
Opportunities
• New advances in neuroscience and technology, such as brain imaging and genetic testing, hold promise for improving the accuracy of psychiatric diagnoses and developing more targeted treatments.
• Telepsychiatry and other online mental health services offer new ways to increase access to care and reach individuals in underserved areas.
• Greater collaboration with primary care physicians and other healthcare professionals could improve the integration of mental health services into routine healthcare.
• The ongoing destigmatization of mental illness could lead to greater public acceptance and support for mental health services.
Threats
• The ongoing COVID-19 pandemic has increased the prevalence of mental health issues and placed additional strain on mental health services.
• The shortage of mental health professionals, particularly in certain areas of the country, could worsen in the coming years.
• Changes in healthcare policy, such as cuts to mental health funding or changes to insurance coverage, could limit access to care.
• Misinformation and stigma surrounding mental illness could continue to deter individuals from seeking treatment.
a Based on “Contemporary psychiatry: A SWOT analysis,” Current Psychiatry, January 2023, p. 16-19,27. doi: 10.12788/cp.0320
1. Nasrallah HA. Is evolution’s greatest triumph its worst blunder? Current Psychiatry. 2022;21(11):5-11. doi: 10.12788/cp.0301
2. Macpherson T, Churchland A, Sejnowski T, et al. Natural and artificial intelligence: a brief introduction to the interplay between AI and neuroscience research. Neural Netw. 2021;144:603-613.
3. Dehbouche N. Plagiarism in the age of massive Generative Pre-trained Transformers (GPT-3): “The best time to act was yesterday. The next best time is now.” Ethics Sci Environ Polit. 2021;21:17-23.
4. Turing AM. Computing machinery and intelligence. Mind. 1950;59(236):433-460.
5. McCarthy J. Artificial intelligence, logic, and formulising common sense. In: Richard H. Thomason, ed. Philosophical Logic and Artificial Intelligence. Kluwer Academic Publishing; 1989:161-190.
6. Koppe G, Meyer-Lindenberg A, Durstewitz D. Deep learning for small and big data in psychiatry. Neuropsychopharmacology. 2021;46(1):176-190.
7. Dabney W, Kurth-Nelson Z, Uchida N, et al. A distributional code for value in dopamine-based reinforcement learning. Nature. 2020;577(7792):671-675.
8. Zhou Z, Wu TC, Wang B, et al. Machine learning methods in psychiatry: a brief introduction. Gen Psychiatr. 2020;33(1):e100171.
9. Sun J, Cao R, Zhou M, et al. A hybrid deep neural network for classification of schizophrenia using EEG Data. Sci Rep. 2021;11(1):4706.
10. Kalenderian H, Nasrallah HA. Artificial intelligence in psychiatry. Current Psychiatry. 2019;18(8):33-38.
11. Ray A, Bhardwaj A, Malik YK, et al. Artificial intelligence and psychiatry: an overview. Asian J Psychiatr. 2022;70:103021.
12. Pestian E, Nasrallah HA, Matykiewicz P, et al. Suicide note classification using natural language processing: a content analysis. Biomed Inform Insights. 2010(3):19-28.
13. Chen Y, Wei Z, Gou H, et al. How far is brain-inspired artificial intelligence away from brain? Frontiers Neurosci. 2022;16:1096737.
14. Tools such as ChatGPT threaten transparent science; here are our ground rules for their use. Nature. 2023;613(7945):612. doi:10.1038/d41586-023-00191-1
We are currently on the verge of yet another societal “revolution” that will exert an unprecedented impact on our lives. It may surpass prior seismic cultural breakthroughs like the internet, smartphones, and social media. Artificial intelligence (AI) has been fermenting for several decades, gathering steam to become equivalent (and eventually superior) to human intelligence. The escalation of AI sophistication will be jarring and perhaps change human life in completely unpredictable ways.
Composing thoughts into words and coherent sentences has always been a uniquely human attribute among all living organisms. Now, that sublime feature of the human mind is being simulated, thanks to advances in AI software, ironically created by the human mind itself! On November 30, 2022, Open AI introduced ChatGPT (generative pre-trained transformer), which can generate an article on any topic a user requests. Within a few weeks, it was used by more than 100 million people. ChatGPT is taking the world by storm because it is a harbinger (some pessimists may label it an omen) of how human existence will be radically impacted in the future. Such AI breakthroughs to surpass human intelligence are, ironically, the product of the advanced human brain, which I previously described as concurrently a triumph and a blunder by evolution.1
How we got here, and what’s next
ChatGPT is a large language model based on neural networks.2 It generates realistic text responses to a wide range of questions by mimicking the pattern of language in gargantuan online databases. One Hong Kong–based, AI-powered drug discovery company (Insilico Medicine) declared it published articles generated by AI tools, even before ChatGPT became available. This indicates how AI can be misused in scientific publications and may be hard to detect as a new form of plagiarism.3
The roots of AI date back to the 1950s, when Alan Turing, now considered the father of AI, published a seminal article about creating a machine to “imitate the brain” and to “mimic the behavior of the human.”4 The term “artificial intelligence” was coined in 1989 by McCarthy,5 who defined it as “the science of engineering for making intelligent machines.” Since then, several subsets of AI have been developed:
- Machine learning: The study of computer algorithms to generate hypotheses
- Deep learning: A type of machine learning algorithm that uses multiple layers to progressively extract higher-level features from raw input. (Both machine learning and deep learning are used in the burgeoning fields of computational psychiatry6 and neuroscience research7)
- Expert knowledge system: A computer-based system that mimics human decision-making ability
- Neural networks: An interconnected group of artificial neurons that uses a math or computer model for information processing
- Predictive analytics: An algorithm to predict future outcomes based on historical data.
These subsets of AI have been used to identify psychiatric disorders using neuroimaging data8 and to classify brain disorders.9 There are many potential uses of AI in psychiatry.10,11 My first experience with AI was 13 years ago, when we conducted a project to distinguish fake suicide notes from genuine ones.12 AI was more successful in correctly identifying fake notes (78% correctly detected) than senior psychiatric residents (49%) or even faculty (53%).
AI will dramatically change how humans interact with the world and may lead to enhanced creativity and new explorations and forays into novel, previously unknown horizons. It is expected to significantly boost the global economy by many trillions of dollars over the next decade. Major high-tech companies are vigorously competing to develop their own AI tools like ChatGPT (Microsoft invested $10 billion in Open AI). Google, which owns DeepMind (an AI lab that invented the T in GPT) developed its own chatbot called Bard. Amazon has invested heavily in Stability AI by giving its founder and CEO Emad Mostaque 4,000 Nvidia AI chips to assemble the world’s largest supercomputer (1 year ago, Stability had only 32 AI chips!). Apple recently integrated Stable Diffusion into its latest operating system. Chinese tech giants Alibaba and Baidu also announced their own chatbots to be released soon.
Other competitors include Cohere, Hugging Face, Midjourney, GitHub Copilot, Game Changer, Jasper, and Anthropic, which released Claude as its chatbot at a lower cost than ChatGPT. Open AI also developed Dall-E2 in April 2022, which can generate very realistic images from text, one of which recently won an award at an art competition.
Continue to: One of the major...
One of the major concerns about these AI developments is that chatbots can make errors or disseminate misinformation and even enunciate racist or misogynist statements. The greatest worry is that the ultimate implicit goal of AI is what is called artificial general intelligence (AGI), which can think and learn better than humans. Some fear AGI may wipe out humans as a species, a grave outcome indeed. That’s why AI developers intend to align AGI with “basic human values” in the event it surpasses human intelligence. Some are even calling for a transition from AI to AW (artificial wisdom), with built-in empathy, kindness, self-reflection, acceptance of diverse perspectives, and promotion of prosocial ideas.13
Creating content: artificial intelligence vs human authors
ChatGPT has opened a Pandora’s box of concern about potential plagiarism by students or scientific authors. This has prompted several journals,14 including
- Anosognosia in schizophrenia (Box 1); based on “Is anosognosia a delusion, a negative symptom, or a cognitive deficit?”
Current Psychiatry , January 2022, p. 6-8,14. doi: 10.12788/cp.0210 - Benefits of metformin in psychiatry (Box 2); based on “Beyond diabetes: The beneficial uses of metformin in psychiatry,”
Current Psychiatry , March 2022, p. 7-9. doi: 10.12788/cp.0225 - Epidemic of intellectual constipation (Box 3); based on “From debate to stalemate and hate: An epidemic of intellectual constipation,”
Current Psychiatry , January 2023, p. 3-4. doi: 10.12788/cp.0321 - SWOT analysis of psychiatry (Box 4); based on “Contemporary psychiatry: A SWOT analysis,”
Current Psychiatry , January 2023, p. 16-19,27. doi: 10.12788/cp.0320.
These ChatGPT articles are verbatim as generated, without any copyediting, fact-checking, or addition of reference citations. One can easily notice that while factually acceptable, the ChatGPT articles tend to be rather synthetic, simple, bland, and lacking creative language or going beyond the facts to express personal viewpoints. However, I have no doubt that future versions of ChatGPT or other chatbots will evolve into far more sophisticated products and may match what a human can compose in terms of quality and complexity.
AI has arrived with a bang. Going forward, I predict it will continue to surprise us, with elements of “future shock.” The credit (or blame) belongs to the boundless creativity of human intelligence that is seeking to invent an intelligence higher than its own. Caveat emptor!
Box 1
Anosognosia is a condition in which a person is unaware or unable to recognize their own illness or disability. In schizophrenia, anosognosia can be present in up to 50% of cases and is characterized by a lack of insight into the presence and severity of symptoms.
People with anosognosia in schizophrenia may not believe that they have a mental illness, may deny the reality of their symptoms, or may attribute them to external causes. This can make it difficult for them to comply with treatment, as they may not see the need for medication or other interventions.
Anosognosia is thought to be caused by structural and functional changes in the brain, particularly in areas associated with self-awareness and insight. It is often more severe in people with more severe forms of schizophrenia and may be associated with poorer treatment outcomes.
Effective treatment for anosognosia in schizophrenia typically involves a combination of medication and therapy, as well as strategies for improving insight and awareness of symptoms. Family members and caregivers can also play an important role in helping people with schizophrenia to recognize the presence and impact of their illness, and encouraging them to engage in treatment.
a Based on “Is anosognosia a delusion, a negative symptom, or a cognitive deficit?” Current Psychiatry, January 2022, p. 6-8,14. doi: 10.12788/cp.0210
Box 2
Metformin is a medication commonly used in the treatment of type 2 diabetes. However, there is growing interest in its potential use in psychiatry, particularly in the treatment of certain mental health conditions. Some potential benefits of metformin in psychiatry include:
1. Treatment of mood disorders: Studies have suggested that metformin may be helpful in the treatment of mood disorders, such as depression and bipolar disorder. One proposed mechanism is that metformin may help to regulate glucose metabolism and insulin sensitivity, which in turn may help to stabilize mood.
2. Treatment of schizophrenia: Some studies have suggested that metformin may be helpful in reducing symptoms of schizophrenia, particularly negative symptoms such as social withdrawal and apathy. One proposed mechanism is that metformin may improve brain energy metabolism, which may in turn improve cognitive function and reduce symptoms.
3. Weight management: Metformin has been shown to be effective in weight management, which can be particularly important for people with certain mental health conditions, such as bipolar disorder, where weight gain can be a side effect of medication.
4. Neuroprotection: Metformin has been shown to have neuroprotective effects, which may be beneficial for people with certain mental health conditions, such as Alzheimer’s disease and Parkinson’s disease.
It is important to note that while there is some evidence for the potential benefits of metformin in psychiatry, further research is needed to fully understand its effectiveness and safety in this context. People with mental health conditions should not start taking metformin without first consulting with their healthcare provider.
a Based on “Beyond diabetes: The beneficial uses of metformin in psychiatry,” Current Psychiatry, March 2022, p. 7-9. doi: 10.12788/cp.0225
Box 3
The term “epidemic of intellectual constipation” refers to a phenomenon in which individuals, groups, or even entire societies are seen as lacking the ability to think critically, creatively, or expansively. It can be characterized by a lack of curiosity, a narrow range of interests, and a reluctance to engage with new or challenging ideas.
The cause of this phenomenon can be attributed to a variety of factors, including cultural, social, and educational influences. For example, some argue that a narrow and standardized educational system, as well as a lack of exposure to diverse perspectives, can limit creativity and critical thinking skills. Additionally, the prevalence of social media and other forms of digital media may encourage a focus on immediate gratification and quick information, rather than more in-depth and meaningful engagement with information.
The consequences of this epidemic can be far-reaching and can impact personal, social, and political aspects of life. For instance, a lack of critical thinking skills can lead to the spread of misinformation, while a narrow range of interests can limit personal growth and the ability to connect with others.
To combat the epidemic of intellectual constipation, it’s important to foster critical thinking skills, encourage exposure to diverse perspectives, and encourage a lifelong love of learning. This can be achieved through education, community engagement, and personal effort.
In conclusion, the “epidemic of intellectual constipation” refers to a phenomenon in which individuals, groups, or entire societies lack the ability to think critically, creatively, and expansively. To combat this epidemic, it’s important to foster critical thinking skills, encourage exposure to diverse perspectives, and encourage a lifelong love of learning.
a Based on “From debate to stalemate and hate: An epidemic of intellectual constipation,” Current Psychiatry, January 2023, p. 3-4. doi: 10.12788/cp.0321
Box 4
SWOT analysis is a tool used to assess the strengths, weaknesses, opportunities, and threats of a particular entity or industry. In this case, we will conduct a SWOT analysis of psychiatry as a field of medicine.
Strengths
• Psychiatry plays a crucial role in addressing mental health issues and improving the quality of life of individuals who struggle with them.
• Psychiatry has made significant advances in understanding the biological and psychological underpinnings of mental illness, which has led to the development of more effective treatments.
• There is growing public awareness and acceptance of the importance of mental health, which has led to increased demand for psychiatric services.
• The field of psychiatry attracts many talented and compassionate professionals who are dedicated to helping those in need.
Weaknesses
• There is still a significant stigma associated with mental illness, which can deter individuals from seeking treatment.
• The effectiveness of some psychiatric treatments can be limited, particularly in cases of severe mental illness.
• The shortage of mental health professionals in many areas means that access to psychiatric services may be limited, particularly for individuals with lower incomes.
• The cost of psychiatric treatment can be prohibitively expensive, particularly for individuals without insurance coverage.
Opportunities
• New advances in neuroscience and technology, such as brain imaging and genetic testing, hold promise for improving the accuracy of psychiatric diagnoses and developing more targeted treatments.
• Telepsychiatry and other online mental health services offer new ways to increase access to care and reach individuals in underserved areas.
• Greater collaboration with primary care physicians and other healthcare professionals could improve the integration of mental health services into routine healthcare.
• The ongoing destigmatization of mental illness could lead to greater public acceptance and support for mental health services.
Threats
• The ongoing COVID-19 pandemic has increased the prevalence of mental health issues and placed additional strain on mental health services.
• The shortage of mental health professionals, particularly in certain areas of the country, could worsen in the coming years.
• Changes in healthcare policy, such as cuts to mental health funding or changes to insurance coverage, could limit access to care.
• Misinformation and stigma surrounding mental illness could continue to deter individuals from seeking treatment.
a Based on “Contemporary psychiatry: A SWOT analysis,” Current Psychiatry, January 2023, p. 16-19,27. doi: 10.12788/cp.0320
We are currently on the verge of yet another societal “revolution” that will exert an unprecedented impact on our lives. It may surpass prior seismic cultural breakthroughs like the internet, smartphones, and social media. Artificial intelligence (AI) has been fermenting for several decades, gathering steam to become equivalent (and eventually superior) to human intelligence. The escalation of AI sophistication will be jarring and perhaps change human life in completely unpredictable ways.
Composing thoughts into words and coherent sentences has always been a uniquely human attribute among all living organisms. Now, that sublime feature of the human mind is being simulated, thanks to advances in AI software, ironically created by the human mind itself! On November 30, 2022, Open AI introduced ChatGPT (generative pre-trained transformer), which can generate an article on any topic a user requests. Within a few weeks, it was used by more than 100 million people. ChatGPT is taking the world by storm because it is a harbinger (some pessimists may label it an omen) of how human existence will be radically impacted in the future. Such AI breakthroughs to surpass human intelligence are, ironically, the product of the advanced human brain, which I previously described as concurrently a triumph and a blunder by evolution.1
How we got here, and what’s next
ChatGPT is a large language model based on neural networks.2 It generates realistic text responses to a wide range of questions by mimicking the pattern of language in gargantuan online databases. One Hong Kong–based, AI-powered drug discovery company (Insilico Medicine) declared it published articles generated by AI tools, even before ChatGPT became available. This indicates how AI can be misused in scientific publications and may be hard to detect as a new form of plagiarism.3
The roots of AI date back to the 1950s, when Alan Turing, now considered the father of AI, published a seminal article about creating a machine to “imitate the brain” and to “mimic the behavior of the human.”4 The term “artificial intelligence” was coined in 1989 by McCarthy,5 who defined it as “the science of engineering for making intelligent machines.” Since then, several subsets of AI have been developed:
- Machine learning: The study of computer algorithms to generate hypotheses
- Deep learning: A type of machine learning algorithm that uses multiple layers to progressively extract higher-level features from raw input. (Both machine learning and deep learning are used in the burgeoning fields of computational psychiatry6 and neuroscience research7)
- Expert knowledge system: A computer-based system that mimics human decision-making ability
- Neural networks: An interconnected group of artificial neurons that uses a math or computer model for information processing
- Predictive analytics: An algorithm to predict future outcomes based on historical data.
These subsets of AI have been used to identify psychiatric disorders using neuroimaging data8 and to classify brain disorders.9 There are many potential uses of AI in psychiatry.10,11 My first experience with AI was 13 years ago, when we conducted a project to distinguish fake suicide notes from genuine ones.12 AI was more successful in correctly identifying fake notes (78% correctly detected) than senior psychiatric residents (49%) or even faculty (53%).
AI will dramatically change how humans interact with the world and may lead to enhanced creativity and new explorations and forays into novel, previously unknown horizons. It is expected to significantly boost the global economy by many trillions of dollars over the next decade. Major high-tech companies are vigorously competing to develop their own AI tools like ChatGPT (Microsoft invested $10 billion in Open AI). Google, which owns DeepMind (an AI lab that invented the T in GPT) developed its own chatbot called Bard. Amazon has invested heavily in Stability AI by giving its founder and CEO Emad Mostaque 4,000 Nvidia AI chips to assemble the world’s largest supercomputer (1 year ago, Stability had only 32 AI chips!). Apple recently integrated Stable Diffusion into its latest operating system. Chinese tech giants Alibaba and Baidu also announced their own chatbots to be released soon.
Other competitors include Cohere, Hugging Face, Midjourney, GitHub Copilot, Game Changer, Jasper, and Anthropic, which released Claude as its chatbot at a lower cost than ChatGPT. Open AI also developed Dall-E2 in April 2022, which can generate very realistic images from text, one of which recently won an award at an art competition.
Continue to: One of the major...
One of the major concerns about these AI developments is that chatbots can make errors or disseminate misinformation and even enunciate racist or misogynist statements. The greatest worry is that the ultimate implicit goal of AI is what is called artificial general intelligence (AGI), which can think and learn better than humans. Some fear AGI may wipe out humans as a species, a grave outcome indeed. That’s why AI developers intend to align AGI with “basic human values” in the event it surpasses human intelligence. Some are even calling for a transition from AI to AW (artificial wisdom), with built-in empathy, kindness, self-reflection, acceptance of diverse perspectives, and promotion of prosocial ideas.13
Creating content: artificial intelligence vs human authors
ChatGPT has opened a Pandora’s box of concern about potential plagiarism by students or scientific authors. This has prompted several journals,14 including
- Anosognosia in schizophrenia (Box 1); based on “Is anosognosia a delusion, a negative symptom, or a cognitive deficit?”
Current Psychiatry , January 2022, p. 6-8,14. doi: 10.12788/cp.0210 - Benefits of metformin in psychiatry (Box 2); based on “Beyond diabetes: The beneficial uses of metformin in psychiatry,”
Current Psychiatry , March 2022, p. 7-9. doi: 10.12788/cp.0225 - Epidemic of intellectual constipation (Box 3); based on “From debate to stalemate and hate: An epidemic of intellectual constipation,”
Current Psychiatry , January 2023, p. 3-4. doi: 10.12788/cp.0321 - SWOT analysis of psychiatry (Box 4); based on “Contemporary psychiatry: A SWOT analysis,”
Current Psychiatry , January 2023, p. 16-19,27. doi: 10.12788/cp.0320.
These ChatGPT articles are verbatim as generated, without any copyediting, fact-checking, or addition of reference citations. One can easily notice that while factually acceptable, the ChatGPT articles tend to be rather synthetic, simple, bland, and lacking creative language or going beyond the facts to express personal viewpoints. However, I have no doubt that future versions of ChatGPT or other chatbots will evolve into far more sophisticated products and may match what a human can compose in terms of quality and complexity.
AI has arrived with a bang. Going forward, I predict it will continue to surprise us, with elements of “future shock.” The credit (or blame) belongs to the boundless creativity of human intelligence that is seeking to invent an intelligence higher than its own. Caveat emptor!
Box 1
Anosognosia is a condition in which a person is unaware or unable to recognize their own illness or disability. In schizophrenia, anosognosia can be present in up to 50% of cases and is characterized by a lack of insight into the presence and severity of symptoms.
People with anosognosia in schizophrenia may not believe that they have a mental illness, may deny the reality of their symptoms, or may attribute them to external causes. This can make it difficult for them to comply with treatment, as they may not see the need for medication or other interventions.
Anosognosia is thought to be caused by structural and functional changes in the brain, particularly in areas associated with self-awareness and insight. It is often more severe in people with more severe forms of schizophrenia and may be associated with poorer treatment outcomes.
Effective treatment for anosognosia in schizophrenia typically involves a combination of medication and therapy, as well as strategies for improving insight and awareness of symptoms. Family members and caregivers can also play an important role in helping people with schizophrenia to recognize the presence and impact of their illness, and encouraging them to engage in treatment.
a Based on “Is anosognosia a delusion, a negative symptom, or a cognitive deficit?” Current Psychiatry, January 2022, p. 6-8,14. doi: 10.12788/cp.0210
Box 2
Metformin is a medication commonly used in the treatment of type 2 diabetes. However, there is growing interest in its potential use in psychiatry, particularly in the treatment of certain mental health conditions. Some potential benefits of metformin in psychiatry include:
1. Treatment of mood disorders: Studies have suggested that metformin may be helpful in the treatment of mood disorders, such as depression and bipolar disorder. One proposed mechanism is that metformin may help to regulate glucose metabolism and insulin sensitivity, which in turn may help to stabilize mood.
2. Treatment of schizophrenia: Some studies have suggested that metformin may be helpful in reducing symptoms of schizophrenia, particularly negative symptoms such as social withdrawal and apathy. One proposed mechanism is that metformin may improve brain energy metabolism, which may in turn improve cognitive function and reduce symptoms.
3. Weight management: Metformin has been shown to be effective in weight management, which can be particularly important for people with certain mental health conditions, such as bipolar disorder, where weight gain can be a side effect of medication.
4. Neuroprotection: Metformin has been shown to have neuroprotective effects, which may be beneficial for people with certain mental health conditions, such as Alzheimer’s disease and Parkinson’s disease.
It is important to note that while there is some evidence for the potential benefits of metformin in psychiatry, further research is needed to fully understand its effectiveness and safety in this context. People with mental health conditions should not start taking metformin without first consulting with their healthcare provider.
a Based on “Beyond diabetes: The beneficial uses of metformin in psychiatry,” Current Psychiatry, March 2022, p. 7-9. doi: 10.12788/cp.0225
Box 3
The term “epidemic of intellectual constipation” refers to a phenomenon in which individuals, groups, or even entire societies are seen as lacking the ability to think critically, creatively, or expansively. It can be characterized by a lack of curiosity, a narrow range of interests, and a reluctance to engage with new or challenging ideas.
The cause of this phenomenon can be attributed to a variety of factors, including cultural, social, and educational influences. For example, some argue that a narrow and standardized educational system, as well as a lack of exposure to diverse perspectives, can limit creativity and critical thinking skills. Additionally, the prevalence of social media and other forms of digital media may encourage a focus on immediate gratification and quick information, rather than more in-depth and meaningful engagement with information.
The consequences of this epidemic can be far-reaching and can impact personal, social, and political aspects of life. For instance, a lack of critical thinking skills can lead to the spread of misinformation, while a narrow range of interests can limit personal growth and the ability to connect with others.
To combat the epidemic of intellectual constipation, it’s important to foster critical thinking skills, encourage exposure to diverse perspectives, and encourage a lifelong love of learning. This can be achieved through education, community engagement, and personal effort.
In conclusion, the “epidemic of intellectual constipation” refers to a phenomenon in which individuals, groups, or entire societies lack the ability to think critically, creatively, and expansively. To combat this epidemic, it’s important to foster critical thinking skills, encourage exposure to diverse perspectives, and encourage a lifelong love of learning.
a Based on “From debate to stalemate and hate: An epidemic of intellectual constipation,” Current Psychiatry, January 2023, p. 3-4. doi: 10.12788/cp.0321
Box 4
SWOT analysis is a tool used to assess the strengths, weaknesses, opportunities, and threats of a particular entity or industry. In this case, we will conduct a SWOT analysis of psychiatry as a field of medicine.
Strengths
• Psychiatry plays a crucial role in addressing mental health issues and improving the quality of life of individuals who struggle with them.
• Psychiatry has made significant advances in understanding the biological and psychological underpinnings of mental illness, which has led to the development of more effective treatments.
• There is growing public awareness and acceptance of the importance of mental health, which has led to increased demand for psychiatric services.
• The field of psychiatry attracts many talented and compassionate professionals who are dedicated to helping those in need.
Weaknesses
• There is still a significant stigma associated with mental illness, which can deter individuals from seeking treatment.
• The effectiveness of some psychiatric treatments can be limited, particularly in cases of severe mental illness.
• The shortage of mental health professionals in many areas means that access to psychiatric services may be limited, particularly for individuals with lower incomes.
• The cost of psychiatric treatment can be prohibitively expensive, particularly for individuals without insurance coverage.
Opportunities
• New advances in neuroscience and technology, such as brain imaging and genetic testing, hold promise for improving the accuracy of psychiatric diagnoses and developing more targeted treatments.
• Telepsychiatry and other online mental health services offer new ways to increase access to care and reach individuals in underserved areas.
• Greater collaboration with primary care physicians and other healthcare professionals could improve the integration of mental health services into routine healthcare.
• The ongoing destigmatization of mental illness could lead to greater public acceptance and support for mental health services.
Threats
• The ongoing COVID-19 pandemic has increased the prevalence of mental health issues and placed additional strain on mental health services.
• The shortage of mental health professionals, particularly in certain areas of the country, could worsen in the coming years.
• Changes in healthcare policy, such as cuts to mental health funding or changes to insurance coverage, could limit access to care.
• Misinformation and stigma surrounding mental illness could continue to deter individuals from seeking treatment.
a Based on “Contemporary psychiatry: A SWOT analysis,” Current Psychiatry, January 2023, p. 16-19,27. doi: 10.12788/cp.0320
1. Nasrallah HA. Is evolution’s greatest triumph its worst blunder? Current Psychiatry. 2022;21(11):5-11. doi: 10.12788/cp.0301
2. Macpherson T, Churchland A, Sejnowski T, et al. Natural and artificial intelligence: a brief introduction to the interplay between AI and neuroscience research. Neural Netw. 2021;144:603-613.
3. Dehbouche N. Plagiarism in the age of massive Generative Pre-trained Transformers (GPT-3): “The best time to act was yesterday. The next best time is now.” Ethics Sci Environ Polit. 2021;21:17-23.
4. Turing AM. Computing machinery and intelligence. Mind. 1950;59(236):433-460.
5. McCarthy J. Artificial intelligence, logic, and formulising common sense. In: Richard H. Thomason, ed. Philosophical Logic and Artificial Intelligence. Kluwer Academic Publishing; 1989:161-190.
6. Koppe G, Meyer-Lindenberg A, Durstewitz D. Deep learning for small and big data in psychiatry. Neuropsychopharmacology. 2021;46(1):176-190.
7. Dabney W, Kurth-Nelson Z, Uchida N, et al. A distributional code for value in dopamine-based reinforcement learning. Nature. 2020;577(7792):671-675.
8. Zhou Z, Wu TC, Wang B, et al. Machine learning methods in psychiatry: a brief introduction. Gen Psychiatr. 2020;33(1):e100171.
9. Sun J, Cao R, Zhou M, et al. A hybrid deep neural network for classification of schizophrenia using EEG Data. Sci Rep. 2021;11(1):4706.
10. Kalenderian H, Nasrallah HA. Artificial intelligence in psychiatry. Current Psychiatry. 2019;18(8):33-38.
11. Ray A, Bhardwaj A, Malik YK, et al. Artificial intelligence and psychiatry: an overview. Asian J Psychiatr. 2022;70:103021.
12. Pestian E, Nasrallah HA, Matykiewicz P, et al. Suicide note classification using natural language processing: a content analysis. Biomed Inform Insights. 2010(3):19-28.
13. Chen Y, Wei Z, Gou H, et al. How far is brain-inspired artificial intelligence away from brain? Frontiers Neurosci. 2022;16:1096737.
14. Tools such as ChatGPT threaten transparent science; here are our ground rules for their use. Nature. 2023;613(7945):612. doi:10.1038/d41586-023-00191-1
1. Nasrallah HA. Is evolution’s greatest triumph its worst blunder? Current Psychiatry. 2022;21(11):5-11. doi: 10.12788/cp.0301
2. Macpherson T, Churchland A, Sejnowski T, et al. Natural and artificial intelligence: a brief introduction to the interplay between AI and neuroscience research. Neural Netw. 2021;144:603-613.
3. Dehbouche N. Plagiarism in the age of massive Generative Pre-trained Transformers (GPT-3): “The best time to act was yesterday. The next best time is now.” Ethics Sci Environ Polit. 2021;21:17-23.
4. Turing AM. Computing machinery and intelligence. Mind. 1950;59(236):433-460.
5. McCarthy J. Artificial intelligence, logic, and formulising common sense. In: Richard H. Thomason, ed. Philosophical Logic and Artificial Intelligence. Kluwer Academic Publishing; 1989:161-190.
6. Koppe G, Meyer-Lindenberg A, Durstewitz D. Deep learning for small and big data in psychiatry. Neuropsychopharmacology. 2021;46(1):176-190.
7. Dabney W, Kurth-Nelson Z, Uchida N, et al. A distributional code for value in dopamine-based reinforcement learning. Nature. 2020;577(7792):671-675.
8. Zhou Z, Wu TC, Wang B, et al. Machine learning methods in psychiatry: a brief introduction. Gen Psychiatr. 2020;33(1):e100171.
9. Sun J, Cao R, Zhou M, et al. A hybrid deep neural network for classification of schizophrenia using EEG Data. Sci Rep. 2021;11(1):4706.
10. Kalenderian H, Nasrallah HA. Artificial intelligence in psychiatry. Current Psychiatry. 2019;18(8):33-38.
11. Ray A, Bhardwaj A, Malik YK, et al. Artificial intelligence and psychiatry: an overview. Asian J Psychiatr. 2022;70:103021.
12. Pestian E, Nasrallah HA, Matykiewicz P, et al. Suicide note classification using natural language processing: a content analysis. Biomed Inform Insights. 2010(3):19-28.
13. Chen Y, Wei Z, Gou H, et al. How far is brain-inspired artificial intelligence away from brain? Frontiers Neurosci. 2022;16:1096737.
14. Tools such as ChatGPT threaten transparent science; here are our ground rules for their use. Nature. 2023;613(7945):612. doi:10.1038/d41586-023-00191-1
A ‘guest editorial’ … generated by ChatGPT?
The artificial intelligence (AI) program ChatGPT (generative pre-trained transformer) was introduced to the public by Open AI on November 30, 2022. It has been described as a game-changer for society, culture, literature, arts, and science, with a potential impact that rivals that of the internet and social media.
ChatGPT can produce human-like essays in response to queries. Some researchers used ChatGPT to generate abstracts based on the titles of articles published in 5 high-impact journals. These abstracts were so “real” that they escaped detection by expert peer reviewers in 32% of cases.1 In addition, several medical/science articles were published that included ChatGPT as a bylined author.2
Like other journals,3-7
- Nonhuman AI technologies do not qualify for named authorship.
- Authors should be transparent about the use of AI technologies to aid in writing content. Authors should disclose which tool was used and how it was applied in the article submitted to the journal.
- Human authors are responsible for the accuracy of work compiled by an AI tool, including the absence of plagiarism. Proper referencing of statements is necessary.
- Images created by AI tools are not permitted for publication.
Because the overwhelming majority of articles published in
Can AI generate an acceptable ‘guest editorial?’
In addition to creating formulaic writing, some of the concerns about using AI in scientific publishing is that the results might be outdated, incorrect, or lacking appropriate references, and that it can produce “plausible-sounding but incorrect or nonsensical” answers to questions.3 To test this, I asked ChatGPT to write an article on the same topic as 4 of my articles published in
Box
There are many myths and misconceptions surrounding psychiatry, the branch of medicine that deals with the diagnosis, treatment, and prevention of mental illness. Some of the most common myths include:
1. Psychiatry is not a real medical specialty: This is a false myth. Psychiatry is a recognized medical specialty, and psychiatrists are licensed medical doctors who have undergone extensive training in the diagnosis and treatment of mental illness.
2. Mental illness is not a real illness: Mental illness is a real and biologically based medical condition, just like physical illness. Research has shown that mental illness is caused by a combination of genetic, environmental, and lifestyle factors.
3. Antidepressants are not effective: Antidepressants have been shown to be effective in treating depression and other mental illnesses. While they are not a cure, they can provide significant symptom relief and improve quality of life for many people.
4. Psychiatric drugs are dangerous and have serious side effects: While all drugs have potential side effects, psychiatric medications have been proven to be safe and effective for many people. The risks and benefits of taking any medication should be carefully considered and discussed with a healthcare professional.
5. Therapy is not effective: Therapy, also known as psychotherapy, has been shown to be effective in treating a wide range of mental illnesses, including depression, anxiety, and post-traumatic stress disorder (PTSD).
6. People with mental illness are violent: People with mental illness are no more likely to be violent than the general population. In fact, people with mental illness are more likely to be victims of violence than perpetrators.
It is important to dispel these myths and misconceptions about psychiatry, as they can prevent people from seeking the help they need and can also contribute to stigma and discrimination towards those with mental illness.
In conclusion, there are many myths and misconceptions surrounding psychiatry. Dispelling these myths and increasing public understanding about mental illness and its treatment can help reduce stigma and encourage more people to seek the help they need.
What do you think? I encourage you to send me your take on this AI-generated “guest editorial,” and whether you deem its quality to be similar to that of an article authored by a human psychiatrist.
1. Else H. Abstracts written by ChatGPT fool scientists. Nature. 2023;613(7944):423. doi: 10.1038/d41586-023-00056-7
2. Stokel-Walker C. ChatGPT listed as author on research papers: many scientists disapprove. Nature. 2023;613(7945):620-621. doi:10.1038/d41586-023-00107-z
3. Flanagin A, Bibbins-Domingo K, Berkwits M, et al. Nonhuman “authors” and implications for the integrity of scientific publication and medical knowledge. JAMA. 2023;329(8):637-639. doi:10.1001/jama.2023.1344
4. Tools such as ChatGPT threaten transparent science; here are our ground rules for their use. Nature. 2023;613(7945):612. doi:10.1038/d41586-023-00191-1
5. Thorp HH. ChatGPT is fun, but not an author. Science. 2023;379(6630):313. doi:10.1126/science.adg7879
6. PNAS. The PNAS journals outline their policies for ChatGPT and generative AI. February 21, 2023. Accessed March 9, 2023. https://www.pnas.org/post/update/pnas-policy-for-chatgpt-generative-ai
7. Marušic’ A. JoGH policy on the use of artificial intelligence in scholarly manuscripts. J Glob Health. 2023;13:01002. doi:10.7189/jogh.13.01002
The artificial intelligence (AI) program ChatGPT (generative pre-trained transformer) was introduced to the public by Open AI on November 30, 2022. It has been described as a game-changer for society, culture, literature, arts, and science, with a potential impact that rivals that of the internet and social media.
ChatGPT can produce human-like essays in response to queries. Some researchers used ChatGPT to generate abstracts based on the titles of articles published in 5 high-impact journals. These abstracts were so “real” that they escaped detection by expert peer reviewers in 32% of cases.1 In addition, several medical/science articles were published that included ChatGPT as a bylined author.2
Like other journals,3-7
- Nonhuman AI technologies do not qualify for named authorship.
- Authors should be transparent about the use of AI technologies to aid in writing content. Authors should disclose which tool was used and how it was applied in the article submitted to the journal.
- Human authors are responsible for the accuracy of work compiled by an AI tool, including the absence of plagiarism. Proper referencing of statements is necessary.
- Images created by AI tools are not permitted for publication.
Because the overwhelming majority of articles published in
Can AI generate an acceptable ‘guest editorial?’
In addition to creating formulaic writing, some of the concerns about using AI in scientific publishing is that the results might be outdated, incorrect, or lacking appropriate references, and that it can produce “plausible-sounding but incorrect or nonsensical” answers to questions.3 To test this, I asked ChatGPT to write an article on the same topic as 4 of my articles published in
Box
There are many myths and misconceptions surrounding psychiatry, the branch of medicine that deals with the diagnosis, treatment, and prevention of mental illness. Some of the most common myths include:
1. Psychiatry is not a real medical specialty: This is a false myth. Psychiatry is a recognized medical specialty, and psychiatrists are licensed medical doctors who have undergone extensive training in the diagnosis and treatment of mental illness.
2. Mental illness is not a real illness: Mental illness is a real and biologically based medical condition, just like physical illness. Research has shown that mental illness is caused by a combination of genetic, environmental, and lifestyle factors.
3. Antidepressants are not effective: Antidepressants have been shown to be effective in treating depression and other mental illnesses. While they are not a cure, they can provide significant symptom relief and improve quality of life for many people.
4. Psychiatric drugs are dangerous and have serious side effects: While all drugs have potential side effects, psychiatric medications have been proven to be safe and effective for many people. The risks and benefits of taking any medication should be carefully considered and discussed with a healthcare professional.
5. Therapy is not effective: Therapy, also known as psychotherapy, has been shown to be effective in treating a wide range of mental illnesses, including depression, anxiety, and post-traumatic stress disorder (PTSD).
6. People with mental illness are violent: People with mental illness are no more likely to be violent than the general population. In fact, people with mental illness are more likely to be victims of violence than perpetrators.
It is important to dispel these myths and misconceptions about psychiatry, as they can prevent people from seeking the help they need and can also contribute to stigma and discrimination towards those with mental illness.
In conclusion, there are many myths and misconceptions surrounding psychiatry. Dispelling these myths and increasing public understanding about mental illness and its treatment can help reduce stigma and encourage more people to seek the help they need.
What do you think? I encourage you to send me your take on this AI-generated “guest editorial,” and whether you deem its quality to be similar to that of an article authored by a human psychiatrist.
The artificial intelligence (AI) program ChatGPT (generative pre-trained transformer) was introduced to the public by Open AI on November 30, 2022. It has been described as a game-changer for society, culture, literature, arts, and science, with a potential impact that rivals that of the internet and social media.
ChatGPT can produce human-like essays in response to queries. Some researchers used ChatGPT to generate abstracts based on the titles of articles published in 5 high-impact journals. These abstracts were so “real” that they escaped detection by expert peer reviewers in 32% of cases.1 In addition, several medical/science articles were published that included ChatGPT as a bylined author.2
Like other journals,3-7
- Nonhuman AI technologies do not qualify for named authorship.
- Authors should be transparent about the use of AI technologies to aid in writing content. Authors should disclose which tool was used and how it was applied in the article submitted to the journal.
- Human authors are responsible for the accuracy of work compiled by an AI tool, including the absence of plagiarism. Proper referencing of statements is necessary.
- Images created by AI tools are not permitted for publication.
Because the overwhelming majority of articles published in
Can AI generate an acceptable ‘guest editorial?’
In addition to creating formulaic writing, some of the concerns about using AI in scientific publishing is that the results might be outdated, incorrect, or lacking appropriate references, and that it can produce “plausible-sounding but incorrect or nonsensical” answers to questions.3 To test this, I asked ChatGPT to write an article on the same topic as 4 of my articles published in
Box
There are many myths and misconceptions surrounding psychiatry, the branch of medicine that deals with the diagnosis, treatment, and prevention of mental illness. Some of the most common myths include:
1. Psychiatry is not a real medical specialty: This is a false myth. Psychiatry is a recognized medical specialty, and psychiatrists are licensed medical doctors who have undergone extensive training in the diagnosis and treatment of mental illness.
2. Mental illness is not a real illness: Mental illness is a real and biologically based medical condition, just like physical illness. Research has shown that mental illness is caused by a combination of genetic, environmental, and lifestyle factors.
3. Antidepressants are not effective: Antidepressants have been shown to be effective in treating depression and other mental illnesses. While they are not a cure, they can provide significant symptom relief and improve quality of life for many people.
4. Psychiatric drugs are dangerous and have serious side effects: While all drugs have potential side effects, psychiatric medications have been proven to be safe and effective for many people. The risks and benefits of taking any medication should be carefully considered and discussed with a healthcare professional.
5. Therapy is not effective: Therapy, also known as psychotherapy, has been shown to be effective in treating a wide range of mental illnesses, including depression, anxiety, and post-traumatic stress disorder (PTSD).
6. People with mental illness are violent: People with mental illness are no more likely to be violent than the general population. In fact, people with mental illness are more likely to be victims of violence than perpetrators.
It is important to dispel these myths and misconceptions about psychiatry, as they can prevent people from seeking the help they need and can also contribute to stigma and discrimination towards those with mental illness.
In conclusion, there are many myths and misconceptions surrounding psychiatry. Dispelling these myths and increasing public understanding about mental illness and its treatment can help reduce stigma and encourage more people to seek the help they need.
What do you think? I encourage you to send me your take on this AI-generated “guest editorial,” and whether you deem its quality to be similar to that of an article authored by a human psychiatrist.
1. Else H. Abstracts written by ChatGPT fool scientists. Nature. 2023;613(7944):423. doi: 10.1038/d41586-023-00056-7
2. Stokel-Walker C. ChatGPT listed as author on research papers: many scientists disapprove. Nature. 2023;613(7945):620-621. doi:10.1038/d41586-023-00107-z
3. Flanagin A, Bibbins-Domingo K, Berkwits M, et al. Nonhuman “authors” and implications for the integrity of scientific publication and medical knowledge. JAMA. 2023;329(8):637-639. doi:10.1001/jama.2023.1344
4. Tools such as ChatGPT threaten transparent science; here are our ground rules for their use. Nature. 2023;613(7945):612. doi:10.1038/d41586-023-00191-1
5. Thorp HH. ChatGPT is fun, but not an author. Science. 2023;379(6630):313. doi:10.1126/science.adg7879
6. PNAS. The PNAS journals outline their policies for ChatGPT and generative AI. February 21, 2023. Accessed March 9, 2023. https://www.pnas.org/post/update/pnas-policy-for-chatgpt-generative-ai
7. Marušic’ A. JoGH policy on the use of artificial intelligence in scholarly manuscripts. J Glob Health. 2023;13:01002. doi:10.7189/jogh.13.01002
1. Else H. Abstracts written by ChatGPT fool scientists. Nature. 2023;613(7944):423. doi: 10.1038/d41586-023-00056-7
2. Stokel-Walker C. ChatGPT listed as author on research papers: many scientists disapprove. Nature. 2023;613(7945):620-621. doi:10.1038/d41586-023-00107-z
3. Flanagin A, Bibbins-Domingo K, Berkwits M, et al. Nonhuman “authors” and implications for the integrity of scientific publication and medical knowledge. JAMA. 2023;329(8):637-639. doi:10.1001/jama.2023.1344
4. Tools such as ChatGPT threaten transparent science; here are our ground rules for their use. Nature. 2023;613(7945):612. doi:10.1038/d41586-023-00191-1
5. Thorp HH. ChatGPT is fun, but not an author. Science. 2023;379(6630):313. doi:10.1126/science.adg7879
6. PNAS. The PNAS journals outline their policies for ChatGPT and generative AI. February 21, 2023. Accessed March 9, 2023. https://www.pnas.org/post/update/pnas-policy-for-chatgpt-generative-ai
7. Marušic’ A. JoGH policy on the use of artificial intelligence in scholarly manuscripts. J Glob Health. 2023;13:01002. doi:10.7189/jogh.13.01002
Lithium-induced diabetes insipidus: Pathophysiology and treatment
Ms. V, age 58, presents to the emergency department after falling in the middle of the night while walking to the bathroom. Her medical history includes bipolar I disorder (BDI). According to her granddaughter, Ms. V has been stable on lithium 600 mg twice daily for 1 to 2 years. Her laboratory workup shows a serum creatinine level of 0.93 mg/dL (reference range 0.6 to 1.2 mg/dL), high sodium (154 mEq/L; reference range 135 to 145 mEq/L), and a lithium level of 0.9 mEq/L (therapeutic range 0.6 to 1.2 mEq/L). On Day 2 of admission, Ms. V’s sodium level remains high (152 mEq/L), her urine output is 5 L/d (normal output <2 L/d), and her serum osmolality is high (326 mmol/kg; reference range 275 to 295 mmol/kg).
After additional questioning, Ms. V says for the past 3 weeks she has been urinating approximately 4 times per night and experiencing excessive thirst. Given her laboratory values and physical presentation, a desmopressin challenge test is performed and confirms a diagnosis of lithium-induced nephrogenic diabetes insipidus (Li-NDI). Nephrogenic diabetes insipidus (NDI) occurs when the kidneys become unresponsive to the action of antidiuretic hormone (ADH; also known as vasopressin).1 The most common cause of NDI is lithium. The prevalence varies from 50% to 73% with long-term lithium use.1,2 It is important to recognize the homeostatic regulation of water prior to understanding Li-NDI. The excretion of water is regulated by ADH. ADH binds to the vasopressin receptors on the basolateral membrane of the collecting duct cells. This stimulates Gs protein and adenylate cyclase, which subsequently increase intracellular cyclic adenosine monophosphate (cAMP).1 Eventually, this leads to the activation of protein kinase A and phosphorylation of aquaporin 2 (AQP2) water channels. The AQP2 channels redistribute from storage vesicles to the apical membrane and the membrane becomes permeable to water, allowing for reabsorption.1,3
In Li-NDI, lithium enters the cells of the collecting duct through the epithelial sodium channel (ENaC).1,4 There, lithium inhibits the action of ADH, glycogen synthase kinase-3 (GSK-3) activity, and the generation of cAMP.1,4 It also induces cyclooxygenase-2 expression in renal interstitial cells and the production of prostaglandin E2 (PGE2).1,5-8 Lithium may also reduce the amount of AQP2 water channels in the apical membrane of the collecting duct. 1,3 Additionally, polymorphisms of the GSK-3 beta gene can occur, which may be related to differences in the extent of the lithium-induced renal concentrating defect among patients who take lithium.9
Symptoms of Li-NDI include polyuria (ie, urine production >3 L/day) and polydipsia.1 More than 40% of patients with symptomatic Li-NDI experience a significant interference with their daily routine and occupational activities, and may be at risk for severe dehydration with concurrent electrolyte disturbances, resulting in lithium toxicity.1,2 This could especially impact older adults, who may have a diminished thirst sensation and insufficient fluid intake (ie, psychological decompensation, decreased mobility).1,2
Li-NDI is reversible early in treatment; however, it may become irreversible over time.1 The degree of reversibility depends on the stage of kidney damage (ie, functional vs morphological) and/or duration of lithium treatment.7 Even with the discontinuation of lithium, symptoms may persist. Imaging can be used to identify the extent of kidney damage, but given the inconsistent data regarding the reversibility of Li-NDI, it would be difficult to predict if symptoms will resolve.8
Establishing the diagnosis
A physical examination and laboratory workup are the first steps in diagnosing and determining the underlying cause of NDI. Table 110 outlines common laboratory abnormalities associated with NDI. Additionally, serum sodium levels can be used to determine water balance; hypernatremia is often seen in cases of NDI.10 Water deprivation tests are useful for diagnosing diabetes insipidus and allow for differentiation of nephrogenic vs central diabetes insipidus.10 Once the patient is water-deprived for ≥4 hours, a single 5-unit dose of subcutaneous desmopressin may be administered. In Li-NDI, the urine often remains dilute with urine osmolality levels <200 mmol/kg, even after administration of exogenous arginine vasopressin.10
Several treatment options
In many cases, Li-NDI symptoms can be reduced by using the lowest effective dose of lithium, switching to a once-daily formulation, or discontinuing therapy. Some patients may find relief from certain diuretics, such as amiloride. Thiazide diuretics can also be used but may require a ≥50% reduction in lithium dose. Nonsteroid anti-inflammatory drugs, such as indomethacin, in combination with diuretics, have been found to be effective by increasing the concentration of urine.1,2Table 21,2,10 summarizes potential treatment options.
Continue to: Amiloride has the most...
Amiloride has the most supporting evidence in the treatment of Li-NDI. A potassium-sparing diuretic, amiloride works by blocking the ENaC in the distal and collecting duct. Blocking the ENaC inhibits uptake of lithium into the principal cells of the collecting duct within the kidney. Research has shown that amiloride can be effective in treating existing Li-NDI, but there is a lack of evidence supporting its preventative effects.1
Thiazide diuretics work by blocking the sodium-chloride cotransporter in the distal tubules of the kidney. They also upregulate the AQP2 water channels.1 Research has shown that sodium replacement counteracts the antidiuretic effect of thiazide diuretics; limitations in dietary sodium intake may be necessary for treatment efficacy.1
Within the kidneys, PGE2 inhibits adenyl cyclase and diminishes water permeability.10 This causes water to be excreted in urine rather than be reabsorbed.10 Indomethacin blocks PGE2 activity and increases water reabsorption in the collecting ducts, and sodium reabsorption in the thick ascending loop of Henle.10 This mechanism can lead to increased lithium reabsorption, which may precipitate toxicity. Research has shown increases in lithium levels by as much as 59% in addition to the risk of causing acute renal failure, especially in older adults.10 Due to these risks, indomethacin should not be considered a first-line treatment for Li-NDI.
Overall, several medications have shown benefits in the treatment of Li-NDI, with amiloride having the most data. There are currently no medications with sufficient evidence to support prophylactic use.
CASE CONTINUED
Ms. V’s treatment team initiates amiloride 5 mg/d. They increase the dose to 10 mg/d after 2 days, and Ms. V’s hypernatremia resolves as her serum sodium normalizes to 142 mEq/L. Her urinary output also decreases to <3 L/d. Throughout treatment, Ms. V continues taking lithium carbonate to prevent destabilization of her BDI. The team subsequently discharges her, and she has been stable for the past 6 months.
Related Resources
- Andreasen A, Ellingrod V. Lithium-induced diabetes insipidus: prevention and management. Current Psychiatry. 2013;12(7):42-45.
- Zhang P, Gandhi H, Kassis N. Lithium-induced nephropathy; one medication with multiple side effects: a case report. BMC Nephrol. 2022;23(1):309. doi:10.1186/s12882-022-02934-0
Drug Brand Names
Amiloride • Midamor
Desmopressin • DDAVP
Hydrochlorothiazide • Microzide
Indomethacin • Indocin, Tivorbex
Lithium • Eskalith, Lithobid
1. Schoot TS, Molmans THJ, Grootens KP, et al. Systematic review and practical guideline from the prevention and management of renal side effects of lithium therapy. Eur Neuropsychopharmacol. 2020;31:16-32.
2. Lithium induced diabetes insipidus. DiabetesInsipidus.org. Accessed June 7, 2022. https://diabetesinsipidus.org/lithium-induced-diabetes-insipidus
3. Rej S, Segal M, Low NC, et al. The McGill geriatric lithium-induced diabetes insipidus clinical study (McGLIDICS). Can J Psychiatry. 2014;59(6):327-334.
4. Christensen BM, Zuber AM, Loffing J, et al. alphaENaC-mediated lithium absorption promotes nephrogenic diabetes insipidus. J Am Soc Nephrol. 2011;22(2):253-261.
5. Bendz H, Aurell M, Balldin J, et al. Kidney damage in long-term lithium patients: a cross sectional study of patients with 15 years or more on lithium. Nephrol Dial Transplant. 1994;9(9):1250-1254.
6. Bendz H. Kidney function in a selected lithium population. A prospective, controlled, lithium-withdrawal study. Acta Psychiatr Scand. 1985;72(5):451-463.
7. Azab AN, Shnaider A, Osher Y, et al. Lithium nephrotoxicity. Int J Bipolar Disord. 2015;3(1):28.
8. Garofeanu CG, Weir M, Rosas-Arellano MP, et al. Causes of reversible nephrogenic diabetes insipidus: a systematic review. Am J Kidney Dis. 2005;45(4):626-637.
9. Bucht G, Whalin A. Renal concentrating capacity in long-term lithium treatment and after withdrawal of lithium. Acta Med Scand. 1980;207(4):309-314.
10. Finch CK, Brooks TWA, Yam P, et al. Management and treatment of lithium-induced nephrogenic diabetes insipidus. Therapy. 2005;2(4):669-675. doi:10.1586/14750708.2.4.669
Ms. V, age 58, presents to the emergency department after falling in the middle of the night while walking to the bathroom. Her medical history includes bipolar I disorder (BDI). According to her granddaughter, Ms. V has been stable on lithium 600 mg twice daily for 1 to 2 years. Her laboratory workup shows a serum creatinine level of 0.93 mg/dL (reference range 0.6 to 1.2 mg/dL), high sodium (154 mEq/L; reference range 135 to 145 mEq/L), and a lithium level of 0.9 mEq/L (therapeutic range 0.6 to 1.2 mEq/L). On Day 2 of admission, Ms. V’s sodium level remains high (152 mEq/L), her urine output is 5 L/d (normal output <2 L/d), and her serum osmolality is high (326 mmol/kg; reference range 275 to 295 mmol/kg).
After additional questioning, Ms. V says for the past 3 weeks she has been urinating approximately 4 times per night and experiencing excessive thirst. Given her laboratory values and physical presentation, a desmopressin challenge test is performed and confirms a diagnosis of lithium-induced nephrogenic diabetes insipidus (Li-NDI). Nephrogenic diabetes insipidus (NDI) occurs when the kidneys become unresponsive to the action of antidiuretic hormone (ADH; also known as vasopressin).1 The most common cause of NDI is lithium. The prevalence varies from 50% to 73% with long-term lithium use.1,2 It is important to recognize the homeostatic regulation of water prior to understanding Li-NDI. The excretion of water is regulated by ADH. ADH binds to the vasopressin receptors on the basolateral membrane of the collecting duct cells. This stimulates Gs protein and adenylate cyclase, which subsequently increase intracellular cyclic adenosine monophosphate (cAMP).1 Eventually, this leads to the activation of protein kinase A and phosphorylation of aquaporin 2 (AQP2) water channels. The AQP2 channels redistribute from storage vesicles to the apical membrane and the membrane becomes permeable to water, allowing for reabsorption.1,3
In Li-NDI, lithium enters the cells of the collecting duct through the epithelial sodium channel (ENaC).1,4 There, lithium inhibits the action of ADH, glycogen synthase kinase-3 (GSK-3) activity, and the generation of cAMP.1,4 It also induces cyclooxygenase-2 expression in renal interstitial cells and the production of prostaglandin E2 (PGE2).1,5-8 Lithium may also reduce the amount of AQP2 water channels in the apical membrane of the collecting duct. 1,3 Additionally, polymorphisms of the GSK-3 beta gene can occur, which may be related to differences in the extent of the lithium-induced renal concentrating defect among patients who take lithium.9
Symptoms of Li-NDI include polyuria (ie, urine production >3 L/day) and polydipsia.1 More than 40% of patients with symptomatic Li-NDI experience a significant interference with their daily routine and occupational activities, and may be at risk for severe dehydration with concurrent electrolyte disturbances, resulting in lithium toxicity.1,2 This could especially impact older adults, who may have a diminished thirst sensation and insufficient fluid intake (ie, psychological decompensation, decreased mobility).1,2
Li-NDI is reversible early in treatment; however, it may become irreversible over time.1 The degree of reversibility depends on the stage of kidney damage (ie, functional vs morphological) and/or duration of lithium treatment.7 Even with the discontinuation of lithium, symptoms may persist. Imaging can be used to identify the extent of kidney damage, but given the inconsistent data regarding the reversibility of Li-NDI, it would be difficult to predict if symptoms will resolve.8
Establishing the diagnosis
A physical examination and laboratory workup are the first steps in diagnosing and determining the underlying cause of NDI. Table 110 outlines common laboratory abnormalities associated with NDI. Additionally, serum sodium levels can be used to determine water balance; hypernatremia is often seen in cases of NDI.10 Water deprivation tests are useful for diagnosing diabetes insipidus and allow for differentiation of nephrogenic vs central diabetes insipidus.10 Once the patient is water-deprived for ≥4 hours, a single 5-unit dose of subcutaneous desmopressin may be administered. In Li-NDI, the urine often remains dilute with urine osmolality levels <200 mmol/kg, even after administration of exogenous arginine vasopressin.10

Several treatment options
In many cases, Li-NDI symptoms can be reduced by using the lowest effective dose of lithium, switching to a once-daily formulation, or discontinuing therapy. Some patients may find relief from certain diuretics, such as amiloride. Thiazide diuretics can also be used but may require a ≥50% reduction in lithium dose. Nonsteroid anti-inflammatory drugs, such as indomethacin, in combination with diuretics, have been found to be effective by increasing the concentration of urine.1,2Table 21,2,10 summarizes potential treatment options.

Continue to: Amiloride has the most...
Amiloride has the most supporting evidence in the treatment of Li-NDI. A potassium-sparing diuretic, amiloride works by blocking the ENaC in the distal and collecting duct. Blocking the ENaC inhibits uptake of lithium into the principal cells of the collecting duct within the kidney. Research has shown that amiloride can be effective in treating existing Li-NDI, but there is a lack of evidence supporting its preventative effects.1
Thiazide diuretics work by blocking the sodium-chloride cotransporter in the distal tubules of the kidney. They also upregulate the AQP2 water channels.1 Research has shown that sodium replacement counteracts the antidiuretic effect of thiazide diuretics; limitations in dietary sodium intake may be necessary for treatment efficacy.1
Within the kidneys, PGE2 inhibits adenyl cyclase and diminishes water permeability.10 This causes water to be excreted in urine rather than be reabsorbed.10 Indomethacin blocks PGE2 activity and increases water reabsorption in the collecting ducts, and sodium reabsorption in the thick ascending loop of Henle.10 This mechanism can lead to increased lithium reabsorption, which may precipitate toxicity. Research has shown increases in lithium levels by as much as 59% in addition to the risk of causing acute renal failure, especially in older adults.10 Due to these risks, indomethacin should not be considered a first-line treatment for Li-NDI.
Overall, several medications have shown benefits in the treatment of Li-NDI, with amiloride having the most data. There are currently no medications with sufficient evidence to support prophylactic use.
CASE CONTINUED
Ms. V’s treatment team initiates amiloride 5 mg/d. They increase the dose to 10 mg/d after 2 days, and Ms. V’s hypernatremia resolves as her serum sodium normalizes to 142 mEq/L. Her urinary output also decreases to <3 L/d. Throughout treatment, Ms. V continues taking lithium carbonate to prevent destabilization of her BDI. The team subsequently discharges her, and she has been stable for the past 6 months.
Related Resources
- Andreasen A, Ellingrod V. Lithium-induced diabetes insipidus: prevention and management. Current Psychiatry. 2013;12(7):42-45.
- Zhang P, Gandhi H, Kassis N. Lithium-induced nephropathy; one medication with multiple side effects: a case report. BMC Nephrol. 2022;23(1):309. doi:10.1186/s12882-022-02934-0
Drug Brand Names
Amiloride • Midamor
Desmopressin • DDAVP
Hydrochlorothiazide • Microzide
Indomethacin • Indocin, Tivorbex
Lithium • Eskalith, Lithobid
Ms. V, age 58, presents to the emergency department after falling in the middle of the night while walking to the bathroom. Her medical history includes bipolar I disorder (BDI). According to her granddaughter, Ms. V has been stable on lithium 600 mg twice daily for 1 to 2 years. Her laboratory workup shows a serum creatinine level of 0.93 mg/dL (reference range 0.6 to 1.2 mg/dL), high sodium (154 mEq/L; reference range 135 to 145 mEq/L), and a lithium level of 0.9 mEq/L (therapeutic range 0.6 to 1.2 mEq/L). On Day 2 of admission, Ms. V’s sodium level remains high (152 mEq/L), her urine output is 5 L/d (normal output <2 L/d), and her serum osmolality is high (326 mmol/kg; reference range 275 to 295 mmol/kg).
After additional questioning, Ms. V says for the past 3 weeks she has been urinating approximately 4 times per night and experiencing excessive thirst. Given her laboratory values and physical presentation, a desmopressin challenge test is performed and confirms a diagnosis of lithium-induced nephrogenic diabetes insipidus (Li-NDI). Nephrogenic diabetes insipidus (NDI) occurs when the kidneys become unresponsive to the action of antidiuretic hormone (ADH; also known as vasopressin).1 The most common cause of NDI is lithium. The prevalence varies from 50% to 73% with long-term lithium use.1,2 It is important to recognize the homeostatic regulation of water prior to understanding Li-NDI. The excretion of water is regulated by ADH. ADH binds to the vasopressin receptors on the basolateral membrane of the collecting duct cells. This stimulates Gs protein and adenylate cyclase, which subsequently increase intracellular cyclic adenosine monophosphate (cAMP).1 Eventually, this leads to the activation of protein kinase A and phosphorylation of aquaporin 2 (AQP2) water channels. The AQP2 channels redistribute from storage vesicles to the apical membrane and the membrane becomes permeable to water, allowing for reabsorption.1,3
In Li-NDI, lithium enters the cells of the collecting duct through the epithelial sodium channel (ENaC).1,4 There, lithium inhibits the action of ADH, glycogen synthase kinase-3 (GSK-3) activity, and the generation of cAMP.1,4 It also induces cyclooxygenase-2 expression in renal interstitial cells and the production of prostaglandin E2 (PGE2).1,5-8 Lithium may also reduce the amount of AQP2 water channels in the apical membrane of the collecting duct. 1,3 Additionally, polymorphisms of the GSK-3 beta gene can occur, which may be related to differences in the extent of the lithium-induced renal concentrating defect among patients who take lithium.9
Symptoms of Li-NDI include polyuria (ie, urine production >3 L/day) and polydipsia.1 More than 40% of patients with symptomatic Li-NDI experience a significant interference with their daily routine and occupational activities, and may be at risk for severe dehydration with concurrent electrolyte disturbances, resulting in lithium toxicity.1,2 This could especially impact older adults, who may have a diminished thirst sensation and insufficient fluid intake (ie, psychological decompensation, decreased mobility).1,2
Li-NDI is reversible early in treatment; however, it may become irreversible over time.1 The degree of reversibility depends on the stage of kidney damage (ie, functional vs morphological) and/or duration of lithium treatment.7 Even with the discontinuation of lithium, symptoms may persist. Imaging can be used to identify the extent of kidney damage, but given the inconsistent data regarding the reversibility of Li-NDI, it would be difficult to predict if symptoms will resolve.8
Establishing the diagnosis
A physical examination and laboratory workup are the first steps in diagnosing and determining the underlying cause of NDI. Table 110 outlines common laboratory abnormalities associated with NDI. Additionally, serum sodium levels can be used to determine water balance; hypernatremia is often seen in cases of NDI.10 Water deprivation tests are useful for diagnosing diabetes insipidus and allow for differentiation of nephrogenic vs central diabetes insipidus.10 Once the patient is water-deprived for ≥4 hours, a single 5-unit dose of subcutaneous desmopressin may be administered. In Li-NDI, the urine often remains dilute with urine osmolality levels <200 mmol/kg, even after administration of exogenous arginine vasopressin.10

Several treatment options
In many cases, Li-NDI symptoms can be reduced by using the lowest effective dose of lithium, switching to a once-daily formulation, or discontinuing therapy. Some patients may find relief from certain diuretics, such as amiloride. Thiazide diuretics can also be used but may require a ≥50% reduction in lithium dose. Nonsteroid anti-inflammatory drugs, such as indomethacin, in combination with diuretics, have been found to be effective by increasing the concentration of urine.1,2Table 21,2,10 summarizes potential treatment options.

Continue to: Amiloride has the most...
Amiloride has the most supporting evidence in the treatment of Li-NDI. A potassium-sparing diuretic, amiloride works by blocking the ENaC in the distal and collecting duct. Blocking the ENaC inhibits uptake of lithium into the principal cells of the collecting duct within the kidney. Research has shown that amiloride can be effective in treating existing Li-NDI, but there is a lack of evidence supporting its preventative effects.1
Thiazide diuretics work by blocking the sodium-chloride cotransporter in the distal tubules of the kidney. They also upregulate the AQP2 water channels.1 Research has shown that sodium replacement counteracts the antidiuretic effect of thiazide diuretics; limitations in dietary sodium intake may be necessary for treatment efficacy.1
Within the kidneys, PGE2 inhibits adenyl cyclase and diminishes water permeability.10 This causes water to be excreted in urine rather than be reabsorbed.10 Indomethacin blocks PGE2 activity and increases water reabsorption in the collecting ducts, and sodium reabsorption in the thick ascending loop of Henle.10 This mechanism can lead to increased lithium reabsorption, which may precipitate toxicity. Research has shown increases in lithium levels by as much as 59% in addition to the risk of causing acute renal failure, especially in older adults.10 Due to these risks, indomethacin should not be considered a first-line treatment for Li-NDI.
Overall, several medications have shown benefits in the treatment of Li-NDI, with amiloride having the most data. There are currently no medications with sufficient evidence to support prophylactic use.
CASE CONTINUED
Ms. V’s treatment team initiates amiloride 5 mg/d. They increase the dose to 10 mg/d after 2 days, and Ms. V’s hypernatremia resolves as her serum sodium normalizes to 142 mEq/L. Her urinary output also decreases to <3 L/d. Throughout treatment, Ms. V continues taking lithium carbonate to prevent destabilization of her BDI. The team subsequently discharges her, and she has been stable for the past 6 months.
Related Resources
- Andreasen A, Ellingrod V. Lithium-induced diabetes insipidus: prevention and management. Current Psychiatry. 2013;12(7):42-45.
- Zhang P, Gandhi H, Kassis N. Lithium-induced nephropathy; one medication with multiple side effects: a case report. BMC Nephrol. 2022;23(1):309. doi:10.1186/s12882-022-02934-0
Drug Brand Names
Amiloride • Midamor
Desmopressin • DDAVP
Hydrochlorothiazide • Microzide
Indomethacin • Indocin, Tivorbex
Lithium • Eskalith, Lithobid
1. Schoot TS, Molmans THJ, Grootens KP, et al. Systematic review and practical guideline from the prevention and management of renal side effects of lithium therapy. Eur Neuropsychopharmacol. 2020;31:16-32.
2. Lithium induced diabetes insipidus. DiabetesInsipidus.org. Accessed June 7, 2022. https://diabetesinsipidus.org/lithium-induced-diabetes-insipidus
3. Rej S, Segal M, Low NC, et al. The McGill geriatric lithium-induced diabetes insipidus clinical study (McGLIDICS). Can J Psychiatry. 2014;59(6):327-334.
4. Christensen BM, Zuber AM, Loffing J, et al. alphaENaC-mediated lithium absorption promotes nephrogenic diabetes insipidus. J Am Soc Nephrol. 2011;22(2):253-261.
5. Bendz H, Aurell M, Balldin J, et al. Kidney damage in long-term lithium patients: a cross sectional study of patients with 15 years or more on lithium. Nephrol Dial Transplant. 1994;9(9):1250-1254.
6. Bendz H. Kidney function in a selected lithium population. A prospective, controlled, lithium-withdrawal study. Acta Psychiatr Scand. 1985;72(5):451-463.
7. Azab AN, Shnaider A, Osher Y, et al. Lithium nephrotoxicity. Int J Bipolar Disord. 2015;3(1):28.
8. Garofeanu CG, Weir M, Rosas-Arellano MP, et al. Causes of reversible nephrogenic diabetes insipidus: a systematic review. Am J Kidney Dis. 2005;45(4):626-637.
9. Bucht G, Whalin A. Renal concentrating capacity in long-term lithium treatment and after withdrawal of lithium. Acta Med Scand. 1980;207(4):309-314.
10. Finch CK, Brooks TWA, Yam P, et al. Management and treatment of lithium-induced nephrogenic diabetes insipidus. Therapy. 2005;2(4):669-675. doi:10.1586/14750708.2.4.669
1. Schoot TS, Molmans THJ, Grootens KP, et al. Systematic review and practical guideline from the prevention and management of renal side effects of lithium therapy. Eur Neuropsychopharmacol. 2020;31:16-32.
2. Lithium induced diabetes insipidus. DiabetesInsipidus.org. Accessed June 7, 2022. https://diabetesinsipidus.org/lithium-induced-diabetes-insipidus
3. Rej S, Segal M, Low NC, et al. The McGill geriatric lithium-induced diabetes insipidus clinical study (McGLIDICS). Can J Psychiatry. 2014;59(6):327-334.
4. Christensen BM, Zuber AM, Loffing J, et al. alphaENaC-mediated lithium absorption promotes nephrogenic diabetes insipidus. J Am Soc Nephrol. 2011;22(2):253-261.
5. Bendz H, Aurell M, Balldin J, et al. Kidney damage in long-term lithium patients: a cross sectional study of patients with 15 years or more on lithium. Nephrol Dial Transplant. 1994;9(9):1250-1254.
6. Bendz H. Kidney function in a selected lithium population. A prospective, controlled, lithium-withdrawal study. Acta Psychiatr Scand. 1985;72(5):451-463.
7. Azab AN, Shnaider A, Osher Y, et al. Lithium nephrotoxicity. Int J Bipolar Disord. 2015;3(1):28.
8. Garofeanu CG, Weir M, Rosas-Arellano MP, et al. Causes of reversible nephrogenic diabetes insipidus: a systematic review. Am J Kidney Dis. 2005;45(4):626-637.
9. Bucht G, Whalin A. Renal concentrating capacity in long-term lithium treatment and after withdrawal of lithium. Acta Med Scand. 1980;207(4):309-314.
10. Finch CK, Brooks TWA, Yam P, et al. Management and treatment of lithium-induced nephrogenic diabetes insipidus. Therapy. 2005;2(4):669-675. doi:10.1586/14750708.2.4.669
When a patient with chronic alcohol use abruptly stops drinking
CASE A difficult withdrawal
Three days after he stops drinking alcohol, Mr. G, age 49, presents to a detoxification center with his wife, who drove him there because she was concerned about his condition. She says her husband had been drinking alcohol every night for as long as she can remember. Despite numerous admissions to rehabilitation centers, Mr. G usually would resume drinking soon after he was discharged. Three days ago, Mr. G’s wife had told him she “could not take it anymore,” so he got rid of all his alcohol and stopped drinking. Mr. G’s wife felt he was doing fine the first day, but his condition increasingly worsened the second and third days. The triage nurse who attempts to interview Mr. G finds him tremulous, vomiting, and sweating. She notices that he seems preoccupied with pulling at his shirt, appearing to pick at things that are not there.
HISTORY Untreated depression, other comorbidities
Mr. G’s wife says he has never been psychiatrically hospitalized or exhibited suicidal behavior. Mr. G previously received care from a psychiatrist, who diagnosed him with major depressive disorder (MDD) and prescribed an antidepressant, though his wife cannot recall which specific medication. She shares it has been “a long time” since Mr. G has taken the antidepressant and the last time he received treatment for his MDD was 5 years ago. Mr. G’s wife says her husband had once abstained from alcohol use for >6 months following one of his stints at a rehabilitation center. She is not able to share many other details about Mr. G’s previous stays at rehabilitation centers, but says he always had “a rough time.”
She says Mr. G had been drinking an average of 10 drinks each night, usually within 4 hours. He has no history of nicotine or illicit substance use and has held a corporate job for the last 18 years. Several years ago, a physician had diagnosed Mr. G with hypertension and high cholesterol, but he did not follow up for treatment. Mr. G’s wife also recalls a physician told her husband he had a fatty liver. His family history includes heart disease and cancer.
[polldaddy:12041618]
The author’s observations
The treatment team observed several elements of alcohol withdrawal and classified Mr. G as a priority patient. If the team had completed the Clinical Institute Withdrawal Assessment for Alcohol–Revised scale (CIWA-Ar) (Table 11), Mr. G would score ≥10. While the protocol for initiating treatment for patients experiencing alcohol withdrawal varies by institution, patients with moderate to severe scores on the CIWA-Ar when experiencing withdrawal typically are managed with pharmacotherapy to address their symptoms.1 Given the timeline of his last drink as reported by his wife, Mr. G is on the brink of experiencing a cascade of symptoms concerning for delirium tremens (DTs).2Table 22 provides a timeline and symptoms related to alcohol withdrawal. To prevent further exacerbation of symptoms, which could lead to DTs, Mr. G’s treatment team will likely initiate a benzodiazepine, using either scheduled or symptom-driven dosing.3
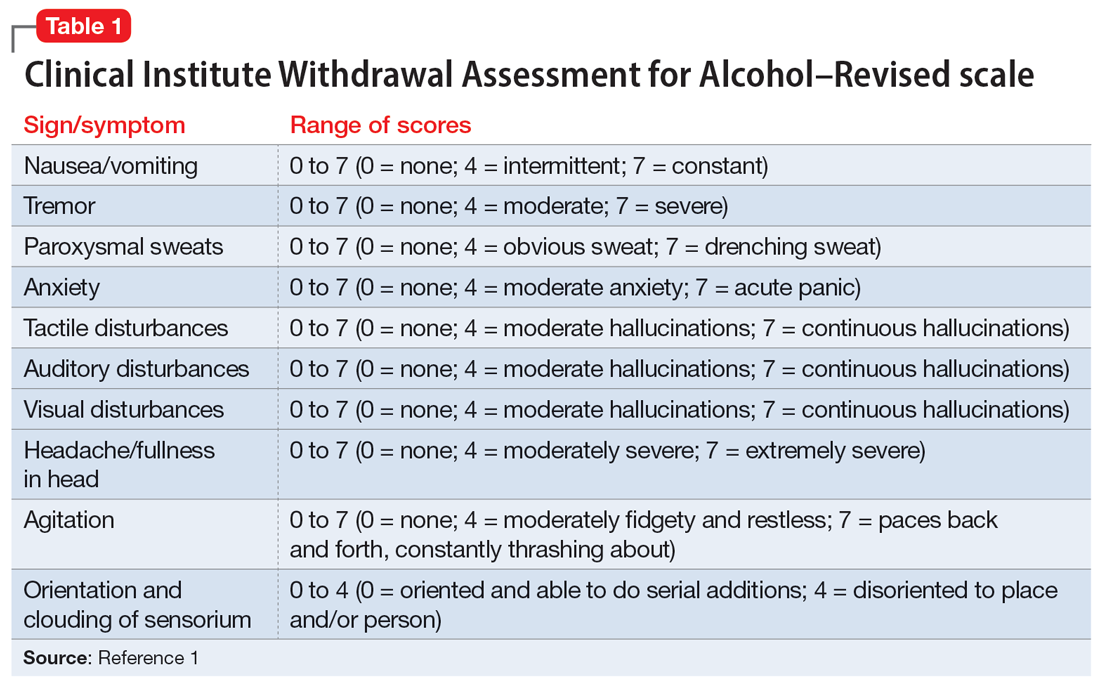
Two neurotransmitters that play a role in DTs are glutamate (excitatory) and GABA (inhibitory). In a normal state, the competing actions of these neurotransmitters balance each other. Acute alcohol intake causes a shift in the excitatory and inhibitory levels, with more inhibition taking place, thus causing disequilibrium. If chronic alcohol use continues, the amount of GABA inhibition reduction is related to downregulation of receptors.2,4 Excitation increases by way of upregulation of the N-methyl-
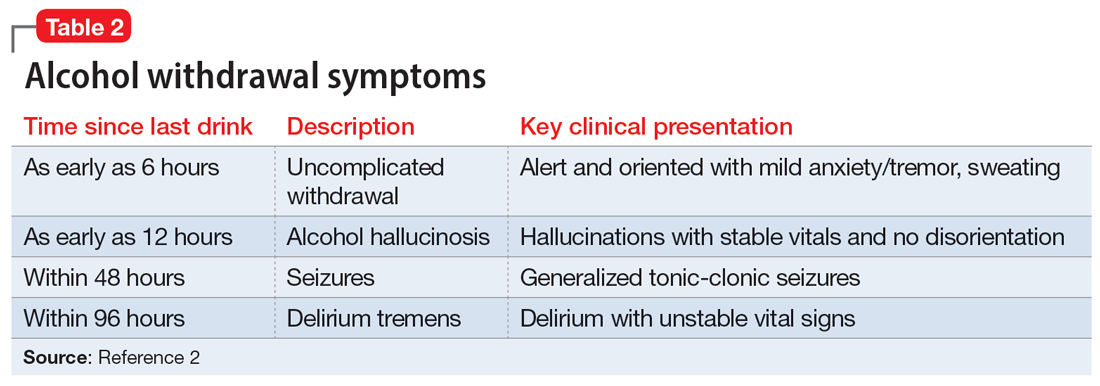
If alcohol is suddenly removed following chronic use, there is unchecked glutamate excitation related to a blunted GABA state. This added increase in the excitation of glutamate leads to withdrawal symptoms.2,4Table 32,4,5 depicts the neurotransmitter equilibrium of GABA and glutamate relative to alcohol use.
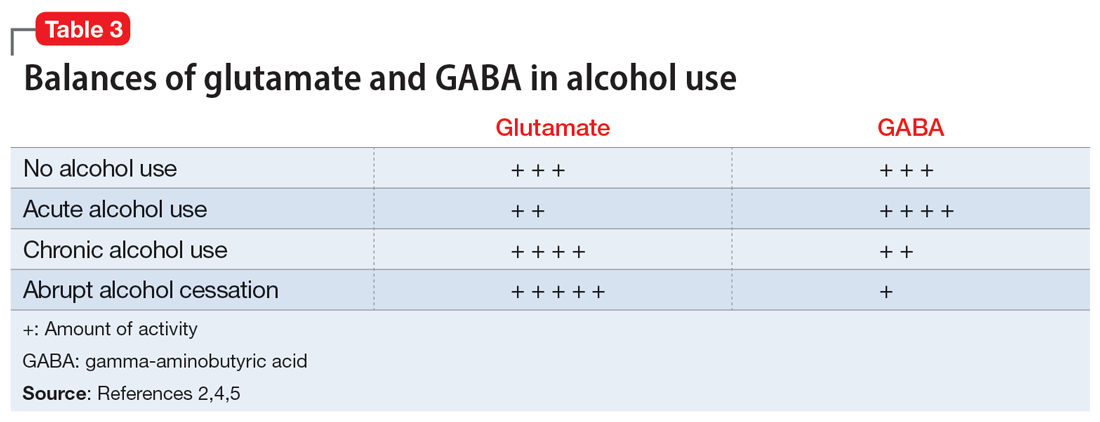
EVALUATION Bleeding gums and bruising
The treatment team admits Mr. G to the triage bay and contacts the addiction psychiatrist. The physician orders laboratory tests to assess nutritional deficits and electrolyte abnormalities. Mr. G is also placed on routine assessments with symptom-triggered therapy. An assessment reveals bleeding gums and bruises, which are believed to be a result of thrombocytopenia (low blood platelet count).
[polldaddy:12041627]
Continue to: The author's observations
The author’s observations
Though regular clinical assessment of PEth varies, it is considered to have high sensitivity and specificity to detect alcohol use.6 When ethanol is present, the phospholipase D enzyme acts upon phosphatidylcholine, forming a direct biomarker, PEth, on the surface of the red blood cell.6,7 PEth’s half-life ranges from 4.5 to 12 days,6 and it can be detected in blood for 3 to 4 weeks after alcohol ingestion.6,7 A PEth value <20 ng/mL indicates light or no alcohol consumption; 20 to 199 ng/mL indicates significant consumption; and >200 ng/mL indicates heavy consumption.7 Since Mr. G has a history of chronic alcohol use, his PEth level is expected to be >200 ng/mL.
AST/ALT and MCV are indirect biomarkers, meaning the tests are not alcohol-specific and the role of alcohol is instead observed by the damage to the body with excessive use over time.7 The expected AST:ALT ratio is 2:1. This is related to 3 mechanisms. The first is a decrease in ALT usually relative to B6 deficiency in individuals with alcohol use disorder (AUD). Another mechanism is related to alcohol’s propensity to affect mitochondria, which is a source for AST. Additionally, AST is also found in higher proportions in the kidneys, heart, and muscles.8
An MCV <100 fL would be within the normal range (80 to 100 fL) for red blood cells. While the reasons for an enlarged red blood cell (or macrocyte) are extensive, alcohol can be a factor once other causes are excluded. Additional laboratory tests and a peripheral blood smear test can help in this investigation.Alcohol disrupts the complete maturation of red blood cells.9,10 If the cause of the macrocyte is alcohol-related and alcohol use is terminated, those enlarged cells can resolve in an average of 3 months.9
Vitamin B1 levels >200 nmol/L would be within normal range (74 to 222 nmol/L). Mr. G’s chronic alcohol use would likely cause him to be vitamin B1–deficient. The deficiency is usually related to diet, malabsorption, and the cells’ impaired ability to utilize vitamin B1. A consequence of vitamin B1 deficiency is Wernicke-Korsakoff syndrome.11
Due to his chronic alcohol use, Mr. G’s magnesium stores most likely would be below normal range (1.7 to 2.2 mg/dL). Acting as a diuretic, alcohol depletes magnesium and other electrolytes. The intracellular shift that occurs to balance the deficit causes the body to use its normal stores of magnesium, which leads to further magnesium depletion. Other common causes include nutritional deficiency and decreased gastrointestinal absorption.12 The bleeding the physician suspected was a result of drinking likely occurred through direct and indirect mechanisms that affect platelets.9,13 Platelets can show improvement 1 week after drinking cessation. Some evidence suggests the risk of seizure or DTs increases significantly with a platelet count <119,000 µL per unit of blood.13
Continue to: TREATMENT Pharmacotherapy for alcohol use disorder
TREATMENT Pharmacotherapy for alcohol use disorder
As Mr. G’s condition starts to stabilize, he discusses treatment options for AUD with his physician. At the end of the discussion, Mr. G expresses an interest in starting a medication. The doctor reviews his laboratory results and available treatment options.
[polldaddy:12041630]
The author’s observations
Of the 3 FDA-approved medications for treating AUD (disulfiram, acamprosate, and naltrexone), naltrexone has been shown to decrease heavy drinking days5,14 and comes in oral and injectable forms. Reducing drinking is achieved by reducing the rewarding effects of alcohol5,14 and alcohol cravings.5 Disulfiram often has poor adherence, and like acamprosate it may be more helpful for maintenance of abstinence.Neither topiramate nor gabapentin are FDA-approved for AUD but may be used for their affects on GABA.5 Gabapentin may also help patients experiencing alcohol withdrawal syndrome.5,15 Mr. G did not have any concomitant medications or comorbid medical conditions, but these factors as well as any renal or hepatic dysfunction must be considered before initiating any medications.
OUTCOME Improved well-being
Mr. G’s treatment team initiates oral naltrexone 50 mg/d, which he tolerates well without complications. He stops drinking entirely and expresses an interest in transitioning to an injectable form of naltrexone in the future. In addition to taking medication, Mr. G wants to participate in psychotherapy. Mr. G thanks his team for the care he received in the hospital, telling them, “You all saved my life.” As he discusses his past issues with alcohol, Mr. G asks his physician how he could get involved to make changes to reduce excessive alcohol consumption in his community (Box5,15-21).
Box
Alcohol use disorder is undertreated5,15-17 and excessive alcohol use accounts for 1 in 5 deaths in individuals within Mr. G’s age range.18 An April 2011 report from the Community Preventive Services Task Force19 did not recommend privatization of retail alcohol sales as an intervention to reduce excessive alcohol consumption, because it would instead lead to an increase in alcohol consumption per capita, a known gateway to excessive alcohol consumption.20
The Task Force was established in 1996 by the US Department of Health and Human Services. Its objective is to identify scientifically proven interventions to save lives, increase lifespans, and improve quality of life. Recommendations are based on systematic reviews to inform lawmakers, health departments, and other organizations and agencies.21 The Task Force’s recommendations were divided into interventions that have strong evidence, sufficient evidence, or insufficient evidence. If Mr. G wanted to have the greatest impact in his efforts to reduce excessive alcohol consumption in his community, the strongest evidence supporting change focuses on electronic screening and brief intervention, maintaining limits on days of alcohol sale, increasing taxes on alcohol, and establishing dram shop liability (laws that hold retail establishments that sell alcohol liable for the injuries or harms caused by their intoxicated or underage customers).19
Bottom Line
Patients experiencing alcohol withdrawal can present with several layers of complexity. Failure to achieve acute stabilization may be life-threatening. After providing critical care, promptly start alcohol use disorder treatment for patients who expresses a desire to change.
Related Resources
- American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management. https://www.asam.org/quality-care/clinical-guidelines/alcohol-withdrawal-management-guideline
- American Psychiatric Association. The American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. American Psychiatric Association Publishing; 2018.
Drug Brand Names
Acamprosate • Campral
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone (injection) • Vivitrol
Naltrexone (oral) • ReVia
Topiramate • Topamax
1. Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353-1357.
2. Trevisan LA, Boutros N, Petrakis IL, et al. Complications of alcohol withdrawal: pathophysiological insights. Alcohol Health Res World. 1998;22(1):61-66.
3. Holleck JL, Merchant N, Gunderson CG. Symptom-triggered therapy for alcohol withdrawal syndrome: a systematic review and meta-analysis of randomized controlled trials. J Gen Intern Med. 2019;34(6):1018-1024.
4. Clapp P, Bhave SV, Hoffman PL. How adaptation of the brain to alcohol leads to dependence: a pharmacological perspective. Alcohol Res Health. 2008;31(4):310-339.
5. Burnette EM, Nieto SJ, Grodin EN, et al. Novel agents for the pharmacological treatment of alcohol use disorder. Drugs. 2022;82(3):251-274.
6. Selim R, Zhou Y, Rupp LB, et al. Availability of PEth testing is associated with reduced eligibility for liver transplant among patients with alcohol-related liver disease. Clin Transplant. 2022;36(5):e14595.
7. Ulwelling W, Smith K. The PEth blood test in the security environment: what it is; why it is important; and interpretative guidelines. J Forensic Sci. 2018;63(6):1634-1640.
8. Botros M, Sikaris KA. The de ritis ratio: the test of time. Clin Biochem Rev. 2013;34(3):117-130.
9. Ballard HS. The hematological complications of alcoholism. Alcohol Health Res World. 1997;21(1):42-52.
10. Kaferle J, Strzoda CE. Evaluation of macrocytosis. Am Fam Physician. 2009;79(3):203-208.
11. Martin PR, Singleton CK, Hiller-Sturmhöfel S. The role of thiamine deficiency in alcoholic brain disease. Alcohol Res Health. 2003;27(2):134-142.
12. Palmer BF, Clegg DJ. Electrolyte disturbances in patients with chronic alcohol-use disorder. N Engl J Med. 2017;377(14):1368-1377.
13. Silczuk A, Habrat B. Alcohol-induced thrombocytopenia: current review. Alcohol. 2020;86:9-16. doi:10.1016/j.alcohol.2020.02.166
14. Pettinati HM, Rabinowitz AR. New pharmacotherapies for treating the neurobiology of alcohol and drug addiction. Psychiatry (Edgmont). 2006;3(5):14-16.
15. Anton RF, Latham P, Voronin K, et al. Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: a randomized clinical trial. JAMA Intern Med. 2020;180(5):728-736.
16. Chockalingam L, Burnham EL, Jolley SE. Medication prescribing for alcohol use disorders during alcohol-related encounters in a Colorado regional healthcare system. Alcoholism Clin Exp Res. 2022;46(6):1094-1102.
17. Mintz CM, Hartz SM, Fisher SL, et al. A cascade of care for alcohol use disorder: using 2015-2019 National Survey on Drug Use and Health data to identify gaps in past 12-month care. Alcohol Clin Exp Res. 2021;45(6):1276-1286.
18. Esser MB, Leung G, Sherk A, et al. Estimated deaths attributable to excessive alcohol use among US adults aged 20 to 64 years, 2015 to 2019. JAMA Netw Open. 2022;5(11):e2239485. doi:10.1001/jamanet workopen.2022.39485
19. The Community Guide. CPSTF Findings for Excessive Alcohol Consumption. Updated June 27, 2022. Accessed December 1, 2022. https://www.thecommunityguide.org/pages/task-force-findings-excessive-alcohol-consumption.html
20. The Community Guide. Alcohol Excessive Consumption: Privatization of Retail Alcohol Sales. Updated June 27, 2022. Accessed December 1, 2022. https://www.thecommunityguide.org/findings/alcohol-excessive-consumption-privatization-retail-alcohol-sales.html
21. The Community Guide. What is the CPSTF? Updated June 27, 2022. Accessed December 1, 2022. https://www.thecommunityguide.org/pages/what-is-the-cpstf.html
CASE A difficult withdrawal
Three days after he stops drinking alcohol, Mr. G, age 49, presents to a detoxification center with his wife, who drove him there because she was concerned about his condition. She says her husband had been drinking alcohol every night for as long as she can remember. Despite numerous admissions to rehabilitation centers, Mr. G usually would resume drinking soon after he was discharged. Three days ago, Mr. G’s wife had told him she “could not take it anymore,” so he got rid of all his alcohol and stopped drinking. Mr. G’s wife felt he was doing fine the first day, but his condition increasingly worsened the second and third days. The triage nurse who attempts to interview Mr. G finds him tremulous, vomiting, and sweating. She notices that he seems preoccupied with pulling at his shirt, appearing to pick at things that are not there.
HISTORY Untreated depression, other comorbidities
Mr. G’s wife says he has never been psychiatrically hospitalized or exhibited suicidal behavior. Mr. G previously received care from a psychiatrist, who diagnosed him with major depressive disorder (MDD) and prescribed an antidepressant, though his wife cannot recall which specific medication. She shares it has been “a long time” since Mr. G has taken the antidepressant and the last time he received treatment for his MDD was 5 years ago. Mr. G’s wife says her husband had once abstained from alcohol use for >6 months following one of his stints at a rehabilitation center. She is not able to share many other details about Mr. G’s previous stays at rehabilitation centers, but says he always had “a rough time.”
She says Mr. G had been drinking an average of 10 drinks each night, usually within 4 hours. He has no history of nicotine or illicit substance use and has held a corporate job for the last 18 years. Several years ago, a physician had diagnosed Mr. G with hypertension and high cholesterol, but he did not follow up for treatment. Mr. G’s wife also recalls a physician told her husband he had a fatty liver. His family history includes heart disease and cancer.
[polldaddy:12041618]
The author’s observations
The treatment team observed several elements of alcohol withdrawal and classified Mr. G as a priority patient. If the team had completed the Clinical Institute Withdrawal Assessment for Alcohol–Revised scale (CIWA-Ar) (Table 11), Mr. G would score ≥10. While the protocol for initiating treatment for patients experiencing alcohol withdrawal varies by institution, patients with moderate to severe scores on the CIWA-Ar when experiencing withdrawal typically are managed with pharmacotherapy to address their symptoms.1 Given the timeline of his last drink as reported by his wife, Mr. G is on the brink of experiencing a cascade of symptoms concerning for delirium tremens (DTs).2Table 22 provides a timeline and symptoms related to alcohol withdrawal. To prevent further exacerbation of symptoms, which could lead to DTs, Mr. G’s treatment team will likely initiate a benzodiazepine, using either scheduled or symptom-driven dosing.3

Two neurotransmitters that play a role in DTs are glutamate (excitatory) and GABA (inhibitory). In a normal state, the competing actions of these neurotransmitters balance each other. Acute alcohol intake causes a shift in the excitatory and inhibitory levels, with more inhibition taking place, thus causing disequilibrium. If chronic alcohol use continues, the amount of GABA inhibition reduction is related to downregulation of receptors.2,4 Excitation increases by way of upregulation of the N-methyl-

If alcohol is suddenly removed following chronic use, there is unchecked glutamate excitation related to a blunted GABA state. This added increase in the excitation of glutamate leads to withdrawal symptoms.2,4Table 32,4,5 depicts the neurotransmitter equilibrium of GABA and glutamate relative to alcohol use.

EVALUATION Bleeding gums and bruising
The treatment team admits Mr. G to the triage bay and contacts the addiction psychiatrist. The physician orders laboratory tests to assess nutritional deficits and electrolyte abnormalities. Mr. G is also placed on routine assessments with symptom-triggered therapy. An assessment reveals bleeding gums and bruises, which are believed to be a result of thrombocytopenia (low blood platelet count).
[polldaddy:12041627]
Continue to: The author's observations
The author’s observations
Though regular clinical assessment of PEth varies, it is considered to have high sensitivity and specificity to detect alcohol use.6 When ethanol is present, the phospholipase D enzyme acts upon phosphatidylcholine, forming a direct biomarker, PEth, on the surface of the red blood cell.6,7 PEth’s half-life ranges from 4.5 to 12 days,6 and it can be detected in blood for 3 to 4 weeks after alcohol ingestion.6,7 A PEth value <20 ng/mL indicates light or no alcohol consumption; 20 to 199 ng/mL indicates significant consumption; and >200 ng/mL indicates heavy consumption.7 Since Mr. G has a history of chronic alcohol use, his PEth level is expected to be >200 ng/mL.
AST/ALT and MCV are indirect biomarkers, meaning the tests are not alcohol-specific and the role of alcohol is instead observed by the damage to the body with excessive use over time.7 The expected AST:ALT ratio is 2:1. This is related to 3 mechanisms. The first is a decrease in ALT usually relative to B6 deficiency in individuals with alcohol use disorder (AUD). Another mechanism is related to alcohol’s propensity to affect mitochondria, which is a source for AST. Additionally, AST is also found in higher proportions in the kidneys, heart, and muscles.8
An MCV <100 fL would be within the normal range (80 to 100 fL) for red blood cells. While the reasons for an enlarged red blood cell (or macrocyte) are extensive, alcohol can be a factor once other causes are excluded. Additional laboratory tests and a peripheral blood smear test can help in this investigation.Alcohol disrupts the complete maturation of red blood cells.9,10 If the cause of the macrocyte is alcohol-related and alcohol use is terminated, those enlarged cells can resolve in an average of 3 months.9
Vitamin B1 levels >200 nmol/L would be within normal range (74 to 222 nmol/L). Mr. G’s chronic alcohol use would likely cause him to be vitamin B1–deficient. The deficiency is usually related to diet, malabsorption, and the cells’ impaired ability to utilize vitamin B1. A consequence of vitamin B1 deficiency is Wernicke-Korsakoff syndrome.11
Due to his chronic alcohol use, Mr. G’s magnesium stores most likely would be below normal range (1.7 to 2.2 mg/dL). Acting as a diuretic, alcohol depletes magnesium and other electrolytes. The intracellular shift that occurs to balance the deficit causes the body to use its normal stores of magnesium, which leads to further magnesium depletion. Other common causes include nutritional deficiency and decreased gastrointestinal absorption.12 The bleeding the physician suspected was a result of drinking likely occurred through direct and indirect mechanisms that affect platelets.9,13 Platelets can show improvement 1 week after drinking cessation. Some evidence suggests the risk of seizure or DTs increases significantly with a platelet count <119,000 µL per unit of blood.13
Continue to: TREATMENT Pharmacotherapy for alcohol use disorder
TREATMENT Pharmacotherapy for alcohol use disorder
As Mr. G’s condition starts to stabilize, he discusses treatment options for AUD with his physician. At the end of the discussion, Mr. G expresses an interest in starting a medication. The doctor reviews his laboratory results and available treatment options.
[polldaddy:12041630]
The author’s observations
Of the 3 FDA-approved medications for treating AUD (disulfiram, acamprosate, and naltrexone), naltrexone has been shown to decrease heavy drinking days5,14 and comes in oral and injectable forms. Reducing drinking is achieved by reducing the rewarding effects of alcohol5,14 and alcohol cravings.5 Disulfiram often has poor adherence, and like acamprosate it may be more helpful for maintenance of abstinence.Neither topiramate nor gabapentin are FDA-approved for AUD but may be used for their affects on GABA.5 Gabapentin may also help patients experiencing alcohol withdrawal syndrome.5,15 Mr. G did not have any concomitant medications or comorbid medical conditions, but these factors as well as any renal or hepatic dysfunction must be considered before initiating any medications.
OUTCOME Improved well-being
Mr. G’s treatment team initiates oral naltrexone 50 mg/d, which he tolerates well without complications. He stops drinking entirely and expresses an interest in transitioning to an injectable form of naltrexone in the future. In addition to taking medication, Mr. G wants to participate in psychotherapy. Mr. G thanks his team for the care he received in the hospital, telling them, “You all saved my life.” As he discusses his past issues with alcohol, Mr. G asks his physician how he could get involved to make changes to reduce excessive alcohol consumption in his community (Box5,15-21).
Box
Alcohol use disorder is undertreated5,15-17 and excessive alcohol use accounts for 1 in 5 deaths in individuals within Mr. G’s age range.18 An April 2011 report from the Community Preventive Services Task Force19 did not recommend privatization of retail alcohol sales as an intervention to reduce excessive alcohol consumption, because it would instead lead to an increase in alcohol consumption per capita, a known gateway to excessive alcohol consumption.20
The Task Force was established in 1996 by the US Department of Health and Human Services. Its objective is to identify scientifically proven interventions to save lives, increase lifespans, and improve quality of life. Recommendations are based on systematic reviews to inform lawmakers, health departments, and other organizations and agencies.21 The Task Force’s recommendations were divided into interventions that have strong evidence, sufficient evidence, or insufficient evidence. If Mr. G wanted to have the greatest impact in his efforts to reduce excessive alcohol consumption in his community, the strongest evidence supporting change focuses on electronic screening and brief intervention, maintaining limits on days of alcohol sale, increasing taxes on alcohol, and establishing dram shop liability (laws that hold retail establishments that sell alcohol liable for the injuries or harms caused by their intoxicated or underage customers).19
Bottom Line
Patients experiencing alcohol withdrawal can present with several layers of complexity. Failure to achieve acute stabilization may be life-threatening. After providing critical care, promptly start alcohol use disorder treatment for patients who expresses a desire to change.
Related Resources
- American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management. https://www.asam.org/quality-care/clinical-guidelines/alcohol-withdrawal-management-guideline
- American Psychiatric Association. The American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. American Psychiatric Association Publishing; 2018.
Drug Brand Names
Acamprosate • Campral
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone (injection) • Vivitrol
Naltrexone (oral) • ReVia
Topiramate • Topamax
CASE A difficult withdrawal
Three days after he stops drinking alcohol, Mr. G, age 49, presents to a detoxification center with his wife, who drove him there because she was concerned about his condition. She says her husband had been drinking alcohol every night for as long as she can remember. Despite numerous admissions to rehabilitation centers, Mr. G usually would resume drinking soon after he was discharged. Three days ago, Mr. G’s wife had told him she “could not take it anymore,” so he got rid of all his alcohol and stopped drinking. Mr. G’s wife felt he was doing fine the first day, but his condition increasingly worsened the second and third days. The triage nurse who attempts to interview Mr. G finds him tremulous, vomiting, and sweating. She notices that he seems preoccupied with pulling at his shirt, appearing to pick at things that are not there.
HISTORY Untreated depression, other comorbidities
Mr. G’s wife says he has never been psychiatrically hospitalized or exhibited suicidal behavior. Mr. G previously received care from a psychiatrist, who diagnosed him with major depressive disorder (MDD) and prescribed an antidepressant, though his wife cannot recall which specific medication. She shares it has been “a long time” since Mr. G has taken the antidepressant and the last time he received treatment for his MDD was 5 years ago. Mr. G’s wife says her husband had once abstained from alcohol use for >6 months following one of his stints at a rehabilitation center. She is not able to share many other details about Mr. G’s previous stays at rehabilitation centers, but says he always had “a rough time.”
She says Mr. G had been drinking an average of 10 drinks each night, usually within 4 hours. He has no history of nicotine or illicit substance use and has held a corporate job for the last 18 years. Several years ago, a physician had diagnosed Mr. G with hypertension and high cholesterol, but he did not follow up for treatment. Mr. G’s wife also recalls a physician told her husband he had a fatty liver. His family history includes heart disease and cancer.
[polldaddy:12041618]
The author’s observations
The treatment team observed several elements of alcohol withdrawal and classified Mr. G as a priority patient. If the team had completed the Clinical Institute Withdrawal Assessment for Alcohol–Revised scale (CIWA-Ar) (Table 11), Mr. G would score ≥10. While the protocol for initiating treatment for patients experiencing alcohol withdrawal varies by institution, patients with moderate to severe scores on the CIWA-Ar when experiencing withdrawal typically are managed with pharmacotherapy to address their symptoms.1 Given the timeline of his last drink as reported by his wife, Mr. G is on the brink of experiencing a cascade of symptoms concerning for delirium tremens (DTs).2Table 22 provides a timeline and symptoms related to alcohol withdrawal. To prevent further exacerbation of symptoms, which could lead to DTs, Mr. G’s treatment team will likely initiate a benzodiazepine, using either scheduled or symptom-driven dosing.3

Two neurotransmitters that play a role in DTs are glutamate (excitatory) and GABA (inhibitory). In a normal state, the competing actions of these neurotransmitters balance each other. Acute alcohol intake causes a shift in the excitatory and inhibitory levels, with more inhibition taking place, thus causing disequilibrium. If chronic alcohol use continues, the amount of GABA inhibition reduction is related to downregulation of receptors.2,4 Excitation increases by way of upregulation of the N-methyl-

If alcohol is suddenly removed following chronic use, there is unchecked glutamate excitation related to a blunted GABA state. This added increase in the excitation of glutamate leads to withdrawal symptoms.2,4Table 32,4,5 depicts the neurotransmitter equilibrium of GABA and glutamate relative to alcohol use.

EVALUATION Bleeding gums and bruising
The treatment team admits Mr. G to the triage bay and contacts the addiction psychiatrist. The physician orders laboratory tests to assess nutritional deficits and electrolyte abnormalities. Mr. G is also placed on routine assessments with symptom-triggered therapy. An assessment reveals bleeding gums and bruises, which are believed to be a result of thrombocytopenia (low blood platelet count).
[polldaddy:12041627]
Continue to: The author's observations
The author’s observations
Though regular clinical assessment of PEth varies, it is considered to have high sensitivity and specificity to detect alcohol use.6 When ethanol is present, the phospholipase D enzyme acts upon phosphatidylcholine, forming a direct biomarker, PEth, on the surface of the red blood cell.6,7 PEth’s half-life ranges from 4.5 to 12 days,6 and it can be detected in blood for 3 to 4 weeks after alcohol ingestion.6,7 A PEth value <20 ng/mL indicates light or no alcohol consumption; 20 to 199 ng/mL indicates significant consumption; and >200 ng/mL indicates heavy consumption.7 Since Mr. G has a history of chronic alcohol use, his PEth level is expected to be >200 ng/mL.
AST/ALT and MCV are indirect biomarkers, meaning the tests are not alcohol-specific and the role of alcohol is instead observed by the damage to the body with excessive use over time.7 The expected AST:ALT ratio is 2:1. This is related to 3 mechanisms. The first is a decrease in ALT usually relative to B6 deficiency in individuals with alcohol use disorder (AUD). Another mechanism is related to alcohol’s propensity to affect mitochondria, which is a source for AST. Additionally, AST is also found in higher proportions in the kidneys, heart, and muscles.8
An MCV <100 fL would be within the normal range (80 to 100 fL) for red blood cells. While the reasons for an enlarged red blood cell (or macrocyte) are extensive, alcohol can be a factor once other causes are excluded. Additional laboratory tests and a peripheral blood smear test can help in this investigation.Alcohol disrupts the complete maturation of red blood cells.9,10 If the cause of the macrocyte is alcohol-related and alcohol use is terminated, those enlarged cells can resolve in an average of 3 months.9
Vitamin B1 levels >200 nmol/L would be within normal range (74 to 222 nmol/L). Mr. G’s chronic alcohol use would likely cause him to be vitamin B1–deficient. The deficiency is usually related to diet, malabsorption, and the cells’ impaired ability to utilize vitamin B1. A consequence of vitamin B1 deficiency is Wernicke-Korsakoff syndrome.11
Due to his chronic alcohol use, Mr. G’s magnesium stores most likely would be below normal range (1.7 to 2.2 mg/dL). Acting as a diuretic, alcohol depletes magnesium and other electrolytes. The intracellular shift that occurs to balance the deficit causes the body to use its normal stores of magnesium, which leads to further magnesium depletion. Other common causes include nutritional deficiency and decreased gastrointestinal absorption.12 The bleeding the physician suspected was a result of drinking likely occurred through direct and indirect mechanisms that affect platelets.9,13 Platelets can show improvement 1 week after drinking cessation. Some evidence suggests the risk of seizure or DTs increases significantly with a platelet count <119,000 µL per unit of blood.13
Continue to: TREATMENT Pharmacotherapy for alcohol use disorder
TREATMENT Pharmacotherapy for alcohol use disorder
As Mr. G’s condition starts to stabilize, he discusses treatment options for AUD with his physician. At the end of the discussion, Mr. G expresses an interest in starting a medication. The doctor reviews his laboratory results and available treatment options.
[polldaddy:12041630]
The author’s observations
Of the 3 FDA-approved medications for treating AUD (disulfiram, acamprosate, and naltrexone), naltrexone has been shown to decrease heavy drinking days5,14 and comes in oral and injectable forms. Reducing drinking is achieved by reducing the rewarding effects of alcohol5,14 and alcohol cravings.5 Disulfiram often has poor adherence, and like acamprosate it may be more helpful for maintenance of abstinence.Neither topiramate nor gabapentin are FDA-approved for AUD but may be used for their affects on GABA.5 Gabapentin may also help patients experiencing alcohol withdrawal syndrome.5,15 Mr. G did not have any concomitant medications or comorbid medical conditions, but these factors as well as any renal or hepatic dysfunction must be considered before initiating any medications.
OUTCOME Improved well-being
Mr. G’s treatment team initiates oral naltrexone 50 mg/d, which he tolerates well without complications. He stops drinking entirely and expresses an interest in transitioning to an injectable form of naltrexone in the future. In addition to taking medication, Mr. G wants to participate in psychotherapy. Mr. G thanks his team for the care he received in the hospital, telling them, “You all saved my life.” As he discusses his past issues with alcohol, Mr. G asks his physician how he could get involved to make changes to reduce excessive alcohol consumption in his community (Box5,15-21).
Box
Alcohol use disorder is undertreated5,15-17 and excessive alcohol use accounts for 1 in 5 deaths in individuals within Mr. G’s age range.18 An April 2011 report from the Community Preventive Services Task Force19 did not recommend privatization of retail alcohol sales as an intervention to reduce excessive alcohol consumption, because it would instead lead to an increase in alcohol consumption per capita, a known gateway to excessive alcohol consumption.20
The Task Force was established in 1996 by the US Department of Health and Human Services. Its objective is to identify scientifically proven interventions to save lives, increase lifespans, and improve quality of life. Recommendations are based on systematic reviews to inform lawmakers, health departments, and other organizations and agencies.21 The Task Force’s recommendations were divided into interventions that have strong evidence, sufficient evidence, or insufficient evidence. If Mr. G wanted to have the greatest impact in his efforts to reduce excessive alcohol consumption in his community, the strongest evidence supporting change focuses on electronic screening and brief intervention, maintaining limits on days of alcohol sale, increasing taxes on alcohol, and establishing dram shop liability (laws that hold retail establishments that sell alcohol liable for the injuries or harms caused by their intoxicated or underage customers).19
Bottom Line
Patients experiencing alcohol withdrawal can present with several layers of complexity. Failure to achieve acute stabilization may be life-threatening. After providing critical care, promptly start alcohol use disorder treatment for patients who expresses a desire to change.
Related Resources
- American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management. https://www.asam.org/quality-care/clinical-guidelines/alcohol-withdrawal-management-guideline
- American Psychiatric Association. The American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. American Psychiatric Association Publishing; 2018.
Drug Brand Names
Acamprosate • Campral
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone (injection) • Vivitrol
Naltrexone (oral) • ReVia
Topiramate • Topamax
1. Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353-1357.
2. Trevisan LA, Boutros N, Petrakis IL, et al. Complications of alcohol withdrawal: pathophysiological insights. Alcohol Health Res World. 1998;22(1):61-66.
3. Holleck JL, Merchant N, Gunderson CG. Symptom-triggered therapy for alcohol withdrawal syndrome: a systematic review and meta-analysis of randomized controlled trials. J Gen Intern Med. 2019;34(6):1018-1024.
4. Clapp P, Bhave SV, Hoffman PL. How adaptation of the brain to alcohol leads to dependence: a pharmacological perspective. Alcohol Res Health. 2008;31(4):310-339.
5. Burnette EM, Nieto SJ, Grodin EN, et al. Novel agents for the pharmacological treatment of alcohol use disorder. Drugs. 2022;82(3):251-274.
6. Selim R, Zhou Y, Rupp LB, et al. Availability of PEth testing is associated with reduced eligibility for liver transplant among patients with alcohol-related liver disease. Clin Transplant. 2022;36(5):e14595.
7. Ulwelling W, Smith K. The PEth blood test in the security environment: what it is; why it is important; and interpretative guidelines. J Forensic Sci. 2018;63(6):1634-1640.
8. Botros M, Sikaris KA. The de ritis ratio: the test of time. Clin Biochem Rev. 2013;34(3):117-130.
9. Ballard HS. The hematological complications of alcoholism. Alcohol Health Res World. 1997;21(1):42-52.
10. Kaferle J, Strzoda CE. Evaluation of macrocytosis. Am Fam Physician. 2009;79(3):203-208.
11. Martin PR, Singleton CK, Hiller-Sturmhöfel S. The role of thiamine deficiency in alcoholic brain disease. Alcohol Res Health. 2003;27(2):134-142.
12. Palmer BF, Clegg DJ. Electrolyte disturbances in patients with chronic alcohol-use disorder. N Engl J Med. 2017;377(14):1368-1377.
13. Silczuk A, Habrat B. Alcohol-induced thrombocytopenia: current review. Alcohol. 2020;86:9-16. doi:10.1016/j.alcohol.2020.02.166
14. Pettinati HM, Rabinowitz AR. New pharmacotherapies for treating the neurobiology of alcohol and drug addiction. Psychiatry (Edgmont). 2006;3(5):14-16.
15. Anton RF, Latham P, Voronin K, et al. Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: a randomized clinical trial. JAMA Intern Med. 2020;180(5):728-736.
16. Chockalingam L, Burnham EL, Jolley SE. Medication prescribing for alcohol use disorders during alcohol-related encounters in a Colorado regional healthcare system. Alcoholism Clin Exp Res. 2022;46(6):1094-1102.
17. Mintz CM, Hartz SM, Fisher SL, et al. A cascade of care for alcohol use disorder: using 2015-2019 National Survey on Drug Use and Health data to identify gaps in past 12-month care. Alcohol Clin Exp Res. 2021;45(6):1276-1286.
18. Esser MB, Leung G, Sherk A, et al. Estimated deaths attributable to excessive alcohol use among US adults aged 20 to 64 years, 2015 to 2019. JAMA Netw Open. 2022;5(11):e2239485. doi:10.1001/jamanet workopen.2022.39485
19. The Community Guide. CPSTF Findings for Excessive Alcohol Consumption. Updated June 27, 2022. Accessed December 1, 2022. https://www.thecommunityguide.org/pages/task-force-findings-excessive-alcohol-consumption.html
20. The Community Guide. Alcohol Excessive Consumption: Privatization of Retail Alcohol Sales. Updated June 27, 2022. Accessed December 1, 2022. https://www.thecommunityguide.org/findings/alcohol-excessive-consumption-privatization-retail-alcohol-sales.html
21. The Community Guide. What is the CPSTF? Updated June 27, 2022. Accessed December 1, 2022. https://www.thecommunityguide.org/pages/what-is-the-cpstf.html
1. Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353-1357.
2. Trevisan LA, Boutros N, Petrakis IL, et al. Complications of alcohol withdrawal: pathophysiological insights. Alcohol Health Res World. 1998;22(1):61-66.
3. Holleck JL, Merchant N, Gunderson CG. Symptom-triggered therapy for alcohol withdrawal syndrome: a systematic review and meta-analysis of randomized controlled trials. J Gen Intern Med. 2019;34(6):1018-1024.
4. Clapp P, Bhave SV, Hoffman PL. How adaptation of the brain to alcohol leads to dependence: a pharmacological perspective. Alcohol Res Health. 2008;31(4):310-339.
5. Burnette EM, Nieto SJ, Grodin EN, et al. Novel agents for the pharmacological treatment of alcohol use disorder. Drugs. 2022;82(3):251-274.
6. Selim R, Zhou Y, Rupp LB, et al. Availability of PEth testing is associated with reduced eligibility for liver transplant among patients with alcohol-related liver disease. Clin Transplant. 2022;36(5):e14595.
7. Ulwelling W, Smith K. The PEth blood test in the security environment: what it is; why it is important; and interpretative guidelines. J Forensic Sci. 2018;63(6):1634-1640.
8. Botros M, Sikaris KA. The de ritis ratio: the test of time. Clin Biochem Rev. 2013;34(3):117-130.
9. Ballard HS. The hematological complications of alcoholism. Alcohol Health Res World. 1997;21(1):42-52.
10. Kaferle J, Strzoda CE. Evaluation of macrocytosis. Am Fam Physician. 2009;79(3):203-208.
11. Martin PR, Singleton CK, Hiller-Sturmhöfel S. The role of thiamine deficiency in alcoholic brain disease. Alcohol Res Health. 2003;27(2):134-142.
12. Palmer BF, Clegg DJ. Electrolyte disturbances in patients with chronic alcohol-use disorder. N Engl J Med. 2017;377(14):1368-1377.
13. Silczuk A, Habrat B. Alcohol-induced thrombocytopenia: current review. Alcohol. 2020;86:9-16. doi:10.1016/j.alcohol.2020.02.166
14. Pettinati HM, Rabinowitz AR. New pharmacotherapies for treating the neurobiology of alcohol and drug addiction. Psychiatry (Edgmont). 2006;3(5):14-16.
15. Anton RF, Latham P, Voronin K, et al. Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: a randomized clinical trial. JAMA Intern Med. 2020;180(5):728-736.
16. Chockalingam L, Burnham EL, Jolley SE. Medication prescribing for alcohol use disorders during alcohol-related encounters in a Colorado regional healthcare system. Alcoholism Clin Exp Res. 2022;46(6):1094-1102.
17. Mintz CM, Hartz SM, Fisher SL, et al. A cascade of care for alcohol use disorder: using 2015-2019 National Survey on Drug Use and Health data to identify gaps in past 12-month care. Alcohol Clin Exp Res. 2021;45(6):1276-1286.
18. Esser MB, Leung G, Sherk A, et al. Estimated deaths attributable to excessive alcohol use among US adults aged 20 to 64 years, 2015 to 2019. JAMA Netw Open. 2022;5(11):e2239485. doi:10.1001/jamanet workopen.2022.39485
19. The Community Guide. CPSTF Findings for Excessive Alcohol Consumption. Updated June 27, 2022. Accessed December 1, 2022. https://www.thecommunityguide.org/pages/task-force-findings-excessive-alcohol-consumption.html
20. The Community Guide. Alcohol Excessive Consumption: Privatization of Retail Alcohol Sales. Updated June 27, 2022. Accessed December 1, 2022. https://www.thecommunityguide.org/findings/alcohol-excessive-consumption-privatization-retail-alcohol-sales.html
21. The Community Guide. What is the CPSTF? Updated June 27, 2022. Accessed December 1, 2022. https://www.thecommunityguide.org/pages/what-is-the-cpstf.html
The importance of diversity in psychiatry
In a sea of blonde hair and blue eyes, my black hair and brown eyes stood out. At the time, I was a medical student and one of the few people of color rotating through the inpatient child psychiatric unit. While I was aware I looked “different,” I discovered that my young patients had an unbridled curiosity about such differences. Common questions I received included “Where are you from? Why are your eyes so small? Is it because you eat rice?” Their questions were never of malicious intent, but rather due to my patient’s unfamiliarity with the Asian-American community and with Black, Indigenous, and people of color (BIPOC) communities in general.
Therefore, it came as no surprise that my BIPOC patients could keenly detect similarities. I could see their eyes widen, a spark of recognition, surprise, or even perhaps relief, when they saw my dark hair or the color of my skin. For members of minority racial/ethnic groups in a predominantly White society, there is a special kinship with other underrepresented BIPOC individuals. We are a community; our shared experiences of discrimination and disadvantages bind us together.
Perhaps it was because of our similarities that my BIPOC patients felt comfortable sharing their most intimate secrets: struggling with social anxiety due to language barriers in school, feeling anxious about balancing their familial expectations vs being “American,” or wishing they were dead due to the color of their skin. It hurt to hear this from my patients. My BIPOC patients’ narratives shared a common theme of fear. Fear that others wouldn’t understand their experiences. Fear that no one would understand their pain. When I reflect upon my own experiences with racism, from microaggressions to outright threats, I am reminded of my own fears, loneliness, and pain. It is these experiences that fuel every BIPOC medical student, resident, and physician to provide culturally sensitive care to patients and promote greater mental health for the BIPOC community.
Why diversity matters
Diversity is important in health care. Our patients come from various backgrounds and cultural experiences. A 2019 survey recruited participants who self-identified with >1 race or as a member of an interracial family relationship, to evaluate their preferences in clinicians.1 Through thematic evaluation of participants’ responses, researchers noted that participants expressed a preference for clinicians who identified as a person of color.1 Participants desired clinicians who were culturally sensitive, who could connect and empathize with their experiences as people of color.1 Ultimately, by having a diverse array of clinicians, health care systems ensure that medical professionals can make important connections with patients due to shared experiences.
I remember talking to a mother about her daughter’s suicide attempt. During our conversation, the mother began to shake her head. “She doesn’t have depression,” she exclaimed. “She needs to snap out of it.” As I listened to her, I was reminded of my own grandmother.
My grandmother struggled with depression throughout her life, yet she was adamant she was “fine.” For my grandmother, her insistence that she did not have depression was rooted in shame. In our community, depression was not viewed as a disease, but rather a moral failing. My patient’s mother shared a similar attitude towards depression, believing her daughter was struggling due to her lack of willpower.
As the only person of color on the treatment team, I understood the importance of helping others on the team to also understand the mother’s perspective—doing so changed the dynamics of the relationship between the team and the family. Rather than having an antagonistic view of the mother who seemed to be callous of her daughter’s needs, the team viewed her differently; she was now understood as a mother who was overwhelmed and lacked an understanding of the disease. This changed the treatment team’s focus. The first step was to educate the family about depression, before providing therapeutic and medication treatments.
To fully understand the patient, the physician must place the story in the correct context, recognizing how the intersectionality of race, sexuality, socioeconomic status, and culture impact mental health. I am now a resident, and as a physician, my primary goal is to be an advocate for patients. To improve patient care, we must continue to find ways to improve diversity in the field of psychiatry. One crucial way is for clinicians to share their stories and be vulnerable with our colleagues, as our patients are with us. Through sharing our personal narratives, we further honor and encourage greater diversity.
1. Snyder CR, Truitt AR. Exploring the provider preferences of multiracial patients. J Patient Exp. 2020;7(4):479-483. doi:10.1177/2374373519851694
In a sea of blonde hair and blue eyes, my black hair and brown eyes stood out. At the time, I was a medical student and one of the few people of color rotating through the inpatient child psychiatric unit. While I was aware I looked “different,” I discovered that my young patients had an unbridled curiosity about such differences. Common questions I received included “Where are you from? Why are your eyes so small? Is it because you eat rice?” Their questions were never of malicious intent, but rather due to my patient’s unfamiliarity with the Asian-American community and with Black, Indigenous, and people of color (BIPOC) communities in general.
Therefore, it came as no surprise that my BIPOC patients could keenly detect similarities. I could see their eyes widen, a spark of recognition, surprise, or even perhaps relief, when they saw my dark hair or the color of my skin. For members of minority racial/ethnic groups in a predominantly White society, there is a special kinship with other underrepresented BIPOC individuals. We are a community; our shared experiences of discrimination and disadvantages bind us together.
Perhaps it was because of our similarities that my BIPOC patients felt comfortable sharing their most intimate secrets: struggling with social anxiety due to language barriers in school, feeling anxious about balancing their familial expectations vs being “American,” or wishing they were dead due to the color of their skin. It hurt to hear this from my patients. My BIPOC patients’ narratives shared a common theme of fear. Fear that others wouldn’t understand their experiences. Fear that no one would understand their pain. When I reflect upon my own experiences with racism, from microaggressions to outright threats, I am reminded of my own fears, loneliness, and pain. It is these experiences that fuel every BIPOC medical student, resident, and physician to provide culturally sensitive care to patients and promote greater mental health for the BIPOC community.
Why diversity matters
Diversity is important in health care. Our patients come from various backgrounds and cultural experiences. A 2019 survey recruited participants who self-identified with >1 race or as a member of an interracial family relationship, to evaluate their preferences in clinicians.1 Through thematic evaluation of participants’ responses, researchers noted that participants expressed a preference for clinicians who identified as a person of color.1 Participants desired clinicians who were culturally sensitive, who could connect and empathize with their experiences as people of color.1 Ultimately, by having a diverse array of clinicians, health care systems ensure that medical professionals can make important connections with patients due to shared experiences.
I remember talking to a mother about her daughter’s suicide attempt. During our conversation, the mother began to shake her head. “She doesn’t have depression,” she exclaimed. “She needs to snap out of it.” As I listened to her, I was reminded of my own grandmother.
My grandmother struggled with depression throughout her life, yet she was adamant she was “fine.” For my grandmother, her insistence that she did not have depression was rooted in shame. In our community, depression was not viewed as a disease, but rather a moral failing. My patient’s mother shared a similar attitude towards depression, believing her daughter was struggling due to her lack of willpower.
As the only person of color on the treatment team, I understood the importance of helping others on the team to also understand the mother’s perspective—doing so changed the dynamics of the relationship between the team and the family. Rather than having an antagonistic view of the mother who seemed to be callous of her daughter’s needs, the team viewed her differently; she was now understood as a mother who was overwhelmed and lacked an understanding of the disease. This changed the treatment team’s focus. The first step was to educate the family about depression, before providing therapeutic and medication treatments.
To fully understand the patient, the physician must place the story in the correct context, recognizing how the intersectionality of race, sexuality, socioeconomic status, and culture impact mental health. I am now a resident, and as a physician, my primary goal is to be an advocate for patients. To improve patient care, we must continue to find ways to improve diversity in the field of psychiatry. One crucial way is for clinicians to share their stories and be vulnerable with our colleagues, as our patients are with us. Through sharing our personal narratives, we further honor and encourage greater diversity.
In a sea of blonde hair and blue eyes, my black hair and brown eyes stood out. At the time, I was a medical student and one of the few people of color rotating through the inpatient child psychiatric unit. While I was aware I looked “different,” I discovered that my young patients had an unbridled curiosity about such differences. Common questions I received included “Where are you from? Why are your eyes so small? Is it because you eat rice?” Their questions were never of malicious intent, but rather due to my patient’s unfamiliarity with the Asian-American community and with Black, Indigenous, and people of color (BIPOC) communities in general.
Therefore, it came as no surprise that my BIPOC patients could keenly detect similarities. I could see their eyes widen, a spark of recognition, surprise, or even perhaps relief, when they saw my dark hair or the color of my skin. For members of minority racial/ethnic groups in a predominantly White society, there is a special kinship with other underrepresented BIPOC individuals. We are a community; our shared experiences of discrimination and disadvantages bind us together.
Perhaps it was because of our similarities that my BIPOC patients felt comfortable sharing their most intimate secrets: struggling with social anxiety due to language barriers in school, feeling anxious about balancing their familial expectations vs being “American,” or wishing they were dead due to the color of their skin. It hurt to hear this from my patients. My BIPOC patients’ narratives shared a common theme of fear. Fear that others wouldn’t understand their experiences. Fear that no one would understand their pain. When I reflect upon my own experiences with racism, from microaggressions to outright threats, I am reminded of my own fears, loneliness, and pain. It is these experiences that fuel every BIPOC medical student, resident, and physician to provide culturally sensitive care to patients and promote greater mental health for the BIPOC community.
Why diversity matters
Diversity is important in health care. Our patients come from various backgrounds and cultural experiences. A 2019 survey recruited participants who self-identified with >1 race or as a member of an interracial family relationship, to evaluate their preferences in clinicians.1 Through thematic evaluation of participants’ responses, researchers noted that participants expressed a preference for clinicians who identified as a person of color.1 Participants desired clinicians who were culturally sensitive, who could connect and empathize with their experiences as people of color.1 Ultimately, by having a diverse array of clinicians, health care systems ensure that medical professionals can make important connections with patients due to shared experiences.
I remember talking to a mother about her daughter’s suicide attempt. During our conversation, the mother began to shake her head. “She doesn’t have depression,” she exclaimed. “She needs to snap out of it.” As I listened to her, I was reminded of my own grandmother.
My grandmother struggled with depression throughout her life, yet she was adamant she was “fine.” For my grandmother, her insistence that she did not have depression was rooted in shame. In our community, depression was not viewed as a disease, but rather a moral failing. My patient’s mother shared a similar attitude towards depression, believing her daughter was struggling due to her lack of willpower.
As the only person of color on the treatment team, I understood the importance of helping others on the team to also understand the mother’s perspective—doing so changed the dynamics of the relationship between the team and the family. Rather than having an antagonistic view of the mother who seemed to be callous of her daughter’s needs, the team viewed her differently; she was now understood as a mother who was overwhelmed and lacked an understanding of the disease. This changed the treatment team’s focus. The first step was to educate the family about depression, before providing therapeutic and medication treatments.
To fully understand the patient, the physician must place the story in the correct context, recognizing how the intersectionality of race, sexuality, socioeconomic status, and culture impact mental health. I am now a resident, and as a physician, my primary goal is to be an advocate for patients. To improve patient care, we must continue to find ways to improve diversity in the field of psychiatry. One crucial way is for clinicians to share their stories and be vulnerable with our colleagues, as our patients are with us. Through sharing our personal narratives, we further honor and encourage greater diversity.
1. Snyder CR, Truitt AR. Exploring the provider preferences of multiracial patients. J Patient Exp. 2020;7(4):479-483. doi:10.1177/2374373519851694
1. Snyder CR, Truitt AR. Exploring the provider preferences of multiracial patients. J Patient Exp. 2020;7(4):479-483. doi:10.1177/2374373519851694
Clonidine: Off-label uses in pediatric patients
Clonidine is a centrally acting alpha-2 agonist originally developed for treating hypertension. It is believed to work by stimulating alpha-2 receptors in various areas of the brain. It is nonselective, binding alpha-2A, -2B, and -2C receptors, and mediates inattentiveness, hyperactivity, impulsivity, sedation, and hypotension.1 Clonidine is available as immediate-release (IR), extended-release, and patch formulations, with typical doses ranging from 0.1 to 0.4 mg/d. The most common adverse effects are anticholinergic, such as sedation, dry mouth, and constipation. Since clonidine is effective at lowering blood pressure, the main safety concern is the possibility of rebound hypertension if abruptly stopped, which necessitates a short taper period.1
In child and adolescent psychiatry, the only FDA-approved use of clonidine is for treating attention-deficit/hyperactivity disorder (ADHD). Yet this medication has been increasingly used off-label for several common psychiatric ailments in pediatric patients. In this article, we discuss potential uses of clonidine in child and adolescent psychiatry; except for ADHD, all uses we describe are off-label.
ADHD. Clonidine is effective both as a monotherapy and as an adjunctive therapy to stimulants for pediatric ADHD. When used alone, clonidine is better suited for patients who have hyperactivity as their primary concern, whereas stimulants may be better suited for patients with inattentive subtypes. It also can help reduce sleep disturbances associated with the use of stimulants, especially insomnia.1
Tics/Tourette syndrome. Clonidine is a first-line treatment for tics in Tourette syndrome, demonstrating high efficacy with limited or no adverse effects. Furthermore, ADHD is the most common comorbid condition in patients with dystonic tics, which makes clonidine useful for simultaneously treating both conditions.2
Insomnia. Currently, there are no FDA-approved medications for treating sleep disorders in children and adolescents. However, clonidine is among the most used medications for childhood sleep difficulties, second only to antihistamines. The IR formulation is often preferred for this indication due to increased sedation.3
Posttraumatic stress disorder (PTSD). Research has shown clonidine can help reduce hyperarousal symptoms, address sleep difficulties, and reduce PTSD trauma nightmares, anxiety, and irritability.4
Substance detoxification. Clonidine successfully suppresses opiate withdrawal signs and symptoms by reducing sympathetic overactivity. It can help with alcohol withdrawal and smoking cessation.2
Antipsychotic-induced akathisia. Controlled trials have shown that clonidine significantly reduces akathisia associated with the use of antipsychotics.2
Sialorrhea. Due to its anticholinergic effects, clonidine can effectively reduce antipsychotic-induced hypersalivation.2
Behavioral disturbances. Due to its sedative and anti-impulsive properties, clonidine can be used to address broadly defined behavioral issues, including anxiety-related behaviors, aggression, and agitation, although there is a lack of proven efficacy.1,2,4
1. Stahl SM, Grady MM, Muntner N. Stahl’s Essential Psychopharmacology: Prescriber’s Guide: Children and Adolescents. Cambridge University Press; 2019.
2. Naguy A. Clonidine use in psychiatry: panacea or panache. Pharmacology. 2016;98(1-2):87-92. doi:10.1159/000446441
3. Jang YJ, Choi H, Han TS, et al. Effectiveness of clonidine in child and adolescent sleep disorders. Psychiatry Investig. 2022;19(9):738-747. doi:10.30773/pi.2022.0117
4. Bajor LA, Balsara C, Osser DN. An evidence-based approach to psychopharmacology for posttraumatic stress disorder (PTSD) - 2022 update. Psychiatry Res. 2022;317:114840. doi:10.1016/j.psychres.2022.114840
Clonidine is a centrally acting alpha-2 agonist originally developed for treating hypertension. It is believed to work by stimulating alpha-2 receptors in various areas of the brain. It is nonselective, binding alpha-2A, -2B, and -2C receptors, and mediates inattentiveness, hyperactivity, impulsivity, sedation, and hypotension.1 Clonidine is available as immediate-release (IR), extended-release, and patch formulations, with typical doses ranging from 0.1 to 0.4 mg/d. The most common adverse effects are anticholinergic, such as sedation, dry mouth, and constipation. Since clonidine is effective at lowering blood pressure, the main safety concern is the possibility of rebound hypertension if abruptly stopped, which necessitates a short taper period.1
In child and adolescent psychiatry, the only FDA-approved use of clonidine is for treating attention-deficit/hyperactivity disorder (ADHD). Yet this medication has been increasingly used off-label for several common psychiatric ailments in pediatric patients. In this article, we discuss potential uses of clonidine in child and adolescent psychiatry; except for ADHD, all uses we describe are off-label.
ADHD. Clonidine is effective both as a monotherapy and as an adjunctive therapy to stimulants for pediatric ADHD. When used alone, clonidine is better suited for patients who have hyperactivity as their primary concern, whereas stimulants may be better suited for patients with inattentive subtypes. It also can help reduce sleep disturbances associated with the use of stimulants, especially insomnia.1
Tics/Tourette syndrome. Clonidine is a first-line treatment for tics in Tourette syndrome, demonstrating high efficacy with limited or no adverse effects. Furthermore, ADHD is the most common comorbid condition in patients with dystonic tics, which makes clonidine useful for simultaneously treating both conditions.2
Insomnia. Currently, there are no FDA-approved medications for treating sleep disorders in children and adolescents. However, clonidine is among the most used medications for childhood sleep difficulties, second only to antihistamines. The IR formulation is often preferred for this indication due to increased sedation.3
Posttraumatic stress disorder (PTSD). Research has shown clonidine can help reduce hyperarousal symptoms, address sleep difficulties, and reduce PTSD trauma nightmares, anxiety, and irritability.4
Substance detoxification. Clonidine successfully suppresses opiate withdrawal signs and symptoms by reducing sympathetic overactivity. It can help with alcohol withdrawal and smoking cessation.2
Antipsychotic-induced akathisia. Controlled trials have shown that clonidine significantly reduces akathisia associated with the use of antipsychotics.2
Sialorrhea. Due to its anticholinergic effects, clonidine can effectively reduce antipsychotic-induced hypersalivation.2
Behavioral disturbances. Due to its sedative and anti-impulsive properties, clonidine can be used to address broadly defined behavioral issues, including anxiety-related behaviors, aggression, and agitation, although there is a lack of proven efficacy.1,2,4
Clonidine is a centrally acting alpha-2 agonist originally developed for treating hypertension. It is believed to work by stimulating alpha-2 receptors in various areas of the brain. It is nonselective, binding alpha-2A, -2B, and -2C receptors, and mediates inattentiveness, hyperactivity, impulsivity, sedation, and hypotension.1 Clonidine is available as immediate-release (IR), extended-release, and patch formulations, with typical doses ranging from 0.1 to 0.4 mg/d. The most common adverse effects are anticholinergic, such as sedation, dry mouth, and constipation. Since clonidine is effective at lowering blood pressure, the main safety concern is the possibility of rebound hypertension if abruptly stopped, which necessitates a short taper period.1
In child and adolescent psychiatry, the only FDA-approved use of clonidine is for treating attention-deficit/hyperactivity disorder (ADHD). Yet this medication has been increasingly used off-label for several common psychiatric ailments in pediatric patients. In this article, we discuss potential uses of clonidine in child and adolescent psychiatry; except for ADHD, all uses we describe are off-label.
ADHD. Clonidine is effective both as a monotherapy and as an adjunctive therapy to stimulants for pediatric ADHD. When used alone, clonidine is better suited for patients who have hyperactivity as their primary concern, whereas stimulants may be better suited for patients with inattentive subtypes. It also can help reduce sleep disturbances associated with the use of stimulants, especially insomnia.1
Tics/Tourette syndrome. Clonidine is a first-line treatment for tics in Tourette syndrome, demonstrating high efficacy with limited or no adverse effects. Furthermore, ADHD is the most common comorbid condition in patients with dystonic tics, which makes clonidine useful for simultaneously treating both conditions.2
Insomnia. Currently, there are no FDA-approved medications for treating sleep disorders in children and adolescents. However, clonidine is among the most used medications for childhood sleep difficulties, second only to antihistamines. The IR formulation is often preferred for this indication due to increased sedation.3
Posttraumatic stress disorder (PTSD). Research has shown clonidine can help reduce hyperarousal symptoms, address sleep difficulties, and reduce PTSD trauma nightmares, anxiety, and irritability.4
Substance detoxification. Clonidine successfully suppresses opiate withdrawal signs and symptoms by reducing sympathetic overactivity. It can help with alcohol withdrawal and smoking cessation.2
Antipsychotic-induced akathisia. Controlled trials have shown that clonidine significantly reduces akathisia associated with the use of antipsychotics.2
Sialorrhea. Due to its anticholinergic effects, clonidine can effectively reduce antipsychotic-induced hypersalivation.2
Behavioral disturbances. Due to its sedative and anti-impulsive properties, clonidine can be used to address broadly defined behavioral issues, including anxiety-related behaviors, aggression, and agitation, although there is a lack of proven efficacy.1,2,4
1. Stahl SM, Grady MM, Muntner N. Stahl’s Essential Psychopharmacology: Prescriber’s Guide: Children and Adolescents. Cambridge University Press; 2019.
2. Naguy A. Clonidine use in psychiatry: panacea or panache. Pharmacology. 2016;98(1-2):87-92. doi:10.1159/000446441
3. Jang YJ, Choi H, Han TS, et al. Effectiveness of clonidine in child and adolescent sleep disorders. Psychiatry Investig. 2022;19(9):738-747. doi:10.30773/pi.2022.0117
4. Bajor LA, Balsara C, Osser DN. An evidence-based approach to psychopharmacology for posttraumatic stress disorder (PTSD) - 2022 update. Psychiatry Res. 2022;317:114840. doi:10.1016/j.psychres.2022.114840
1. Stahl SM, Grady MM, Muntner N. Stahl’s Essential Psychopharmacology: Prescriber’s Guide: Children and Adolescents. Cambridge University Press; 2019.
2. Naguy A. Clonidine use in psychiatry: panacea or panache. Pharmacology. 2016;98(1-2):87-92. doi:10.1159/000446441
3. Jang YJ, Choi H, Han TS, et al. Effectiveness of clonidine in child and adolescent sleep disorders. Psychiatry Investig. 2022;19(9):738-747. doi:10.30773/pi.2022.0117
4. Bajor LA, Balsara C, Osser DN. An evidence-based approach to psychopharmacology for posttraumatic stress disorder (PTSD) - 2022 update. Psychiatry Res. 2022;317:114840. doi:10.1016/j.psychres.2022.114840