From the Cosmetic Dermatology Archives
Cosmetic Corner: Dermatologists Weigh in on Face Washes
Leading dermatologists offered their recommendations on the top face washes. See what they’re recommending to their patients and why.
Dr. Draelos is from Dermatology Consulting Services, High Point, North Carolina. Dr. Fowler is from Dermatology Specialists, PSC, Louisville, Kentucky. Dr. Larsen is from Portland Dermatology Clinic, Oregon. Ms. Hornby, Dr. Walters, and Dr. Appa are from Johnson & Johnson Consumer Inc, Skillman, New Jersey.
These studies were supported by Johnson & Johnson Consumer Inc. Dr. Draelos received a research grant from Johnson & Johnson Consumer Inc. Dr. Fowler has served on the advisory board for and has received research grants from Johnson & Johnson Consumer Inc. Dr. Larsen reports no conflict of interest. Ms. Hornby, Dr. Walters, and Dr. Appa are or were employees of Johnson & Johnson Consumer Inc at the time these studies were carried out. Dr. Walters also is an inventor on patents owned by Johnson & Johnson Consumer Inc.
Correspondence: Sidney Hornby, MS, Johnson & Johnson Consumer Inc, 199 Grandview Rd, Skillman, NJ 08558 (shornby@its.jnj.com).

There were no reported differences in participant-reported cleanser effectiveness for the fragranced versus the fragrance-free cleanser either at baseline or weeks 1 or 3 (data not shown).
Study 2 Assessment
A total of 153 women aged 25 to 54 years with sensitive skin were enrolled in the study. Seventy-three participants were randomized to receive the fragranced test cleanser and 80 were randomized to receive the benchmark fragrance-free cleanser.
At week 3, there were no differences between the fragranced test cleanser and the benchmark cleanser in any of the clinician-assessed skin parameters (Figure 2). Of the parameters assessed, itching, irritation, and desquamation were the most improved from baseline in both treatment groups. Similar results were observed at week 1 (data not shown).
There were no apparent differences in subjective self-assessment of skin irritation between the test and benchmark cleansers at week 1 (15.7% vs 13.0%) or week 3 (24.3% vs 12.3%). When asked to respond to a series of 8 statements related to cleanser effectiveness, most participants either agreed strongly or agreed somewhat with the statements (Figure 3). There were no statistically significant differences between treatment groups, and responses to all statements indicated that participants were as satisfied with the test cleanser as they were with the benchmark cleanser.
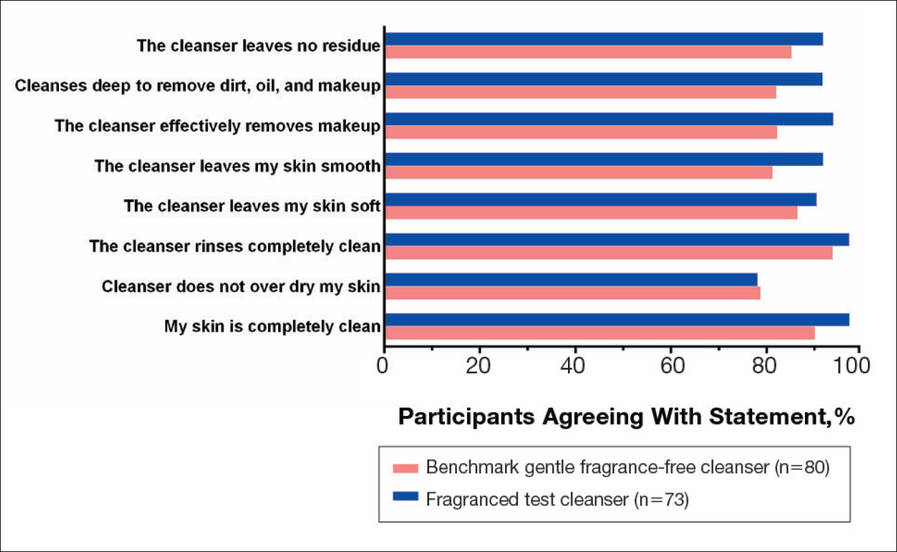
Figure 3. Self-assessment of cleanser effectiveness after 3 weeks. Participants selected from the following responses: agree strongly, agree somewhat, neither, disagree somewhat, and disagree strongly. Percentage of participants agreeing with statement indicates those who responded agree strongly and agree somewhat.
Comment
Consumers value cleansing, fragrance, viscosity, and foaming attributes in skin care products very highly.3,4,10 Fragrances are added to personal care products to positively affect consumers’ perception of product performance and to add emotional benefits by implying social or economic prestige to the use of a product.19 In one study, shampoo formulations that varied only in the added fragrance received different consumer evaluations for cleansing effectiveness and foaming.4
Although mild nonfoaming cleansers can be effective, adult consumers generally use cleansers that foam10,16 and often judge the performance of a cleansing product based on its foaming properties.3,10 Mild cleansers with HMPs maintain the ability to foam while also reducing the likelihood of skin irritation.16 One study showed that a mild, fragrance-free, foaming cleanser containing HMPs was as effective, well tolerated, and nonirritating in patients with sensitive skin as a benchmark nonfoaming gentle cleanser.20
Results from study 1 presented here show that fragranced and fragrance-free formulations of a mild, HMP-containing cleanser are equally efficacious and well tolerated in a small sample of participants with clinically diagnosed fragrance sensitivity. Skin tolerance attributes improved with both cleansers over a 3-week period, particularly global disease severity, irritation, and erythema. These results suggest that a fragrance free of common allergens and irritating essential oils could be introduced into a mild foaming cleanser containing HMPs without causing adverse reactions, even in patients who are fragrance sensitive.
Although the populations of studies 1 and 2 both included female participants with sensitive skin, they were not identical. While study 1 assessed a limited number of participants with clinically diagnosed fragrance sensitivity, study 2 was larger and included a broader range of participants with clinically diagnosed skin sensitivity, which could include fragrance sensitivity. The well-chosen fragrance of the test cleanser containing HMPs was well tolerated; however, this does not imply that any other fragrances added to this cleanser formulation would be as well tolerated.
Conclusion
The current studies indicate that a gentle fragranced foaming cleanser with HMPs was well tolerated in a small population of participants with clinically diagnosed fragrance sensitivity. In a larger population of female participants with sensitive skin, the gentle fragranced foaming cleanser with HMPs was as effective as a leading dermatologist-recommended, fragrance-free, gentle, nonfoaming cleanser. The gentle, HMP-containing, foaming cleanser with a fragrance that does not contain common allergens and irritating essential oils offers a new cleansing option for adults with sensitive skin who may prefer to use a fragranced and foaming product.
Acknowledgments—The authors are grateful to the patients and clinicians who participated in these studies. Editorial and medical writing support was provided by Tove Anderson, PhD, and Alex Loeb, PhD, both from Evidence Scientific Solutions, Inc, Philadelphia, Pennsylvania, and was funded by Johnson & Johnson Consumer Inc.
Leading dermatologists offered their recommendations on the top face washes. See what they’re recommending to their patients and why.

Leading dermatologists offered their recommendations on the top face washes. See what they’re recommending to their patients and why.
