COPENHAGEN – An oral small molecule with a novel mechanism of action for treatment of moderate to severe plaque psoriasis is being developed as a potential first-line systemic treatment in view of its highly favorable safety profile.
The investigational drug, known for now as CF101, is a first-in-class agonist of the A3 adenosine receptor. This cell surface receptor is upregulated in the pathologic cells of certain inflammatory diseases, but has little or no expression in normal cells. This high degree of specificity accounts for its safety, which in a recent phase II/III trial was essentially indistinguishable from placebo, making it an attractive potential alternative to methotrexate or biologics as a starting point in systemic therapy, Pnina Fishman, Ph.D., said at the annual congress of the European Academy of Dermatology and Venereology.
Planning is underway for a pivotal phase III trial of CF101 in psoriasis, which is also being organized for CF101 in rheumatoid arthritis on the strength of favorable phase II findings, according to Dr. Fishman, CEO of Can-Fite BioPharma, an Israeli biotech company that is developing the drug.
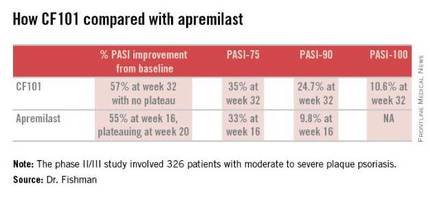
The phase II/III psoriasis trial was a 326-patient, double-blind, placebo-controlled study. It showed efficacy comparable to and in some respects better than that reported in the phase III ESTEEM-1 study of the oral phosphodiesterase 4 inhibitor apremilast (J Am Acad Dermatol. 2015 Jul;73:37-49).
Moreover, it appears that the twice-daily 2-mg dosing of CF101 studied in the phase II/III trial was suboptimal in light of the observed linear increase in Psoriasis Area and Severity Index (PASI)-75, -90, and -100 response rates over time. Those response rates rose steadily until the study’s end at week 32 with no evidence of a plateau. In contrast, in ESTEEM-1, the improvement with apremilast (Otezla) leveled off starting at about week 20, she observed.
In the recent phase II/III trial, the PASI-75 rate for CF101 at week 12 – the prespecified primary endpoint – was not significantly better than placebo was. However, the PASI-75 response rate continued to climb such that by week 32, it was 35.3%, similar to the 33.1% PASI-75 response seen at 16 weeks in ESTEEM-1. PASI improved by an average of 57% from baseline to week 32 with CF101 with no plateau in sight, and by 55% with apremilast at week 16, with a leveling off at week 20. This is why the upcoming phase III trial will employ a higher dose of CF101 than twice-daily 2-mg dose used in the phase II/III study and will run longer. The goal is to achieve higher PASI response rates faster than obtainable with 2 mg BID, Dr. Fishman explained.
She added that the PASI-90 response data in the phase II/III trial bode particularly well for the future of CF101 as a first-line systemic agent. At week 32, this stringent outcome measure was achieved by 26.9% of participants who hadn’t previously been on methotrexate or a biologic and by 13.7% of those who had. And as was the case for the PASI-75 results, the PASI-90 response increased in linear fashion out to 32 weeks with no plateau. In ESTEEM-1, the PASI-90 rate was 9.8% at week 16.
No treatment-related adverse events were seen in the phase II/III CF101 study, Dr. Fishman reported.
The study was sponsored by Can-Fite BioPharma and presented by Dr. Fishman, who is the company’s CEO.