COPENHAGEN – Treatment breaks due to adverse events in patients taking vismodegib for advanced basal cell carcinoma don’t appear to compromise the oral hedgehog pathway inhibitor’s efficacy; in fact, they might even enhance it, according to a prespecified interim analysis of the STEVIE trial.
STEVIE is an ongoing phase II, long-term, open-label international study designed primarily to assess the safety of vismodegib (Erivedge) in a situation similar to routine clinical practice. Efficacy and impact on quality of life are secondary endpoints. Although STEVIE has enrolled 1,227 patients, a prespecified interim analysis was conducted in the first 499 followed for at least 12 months, of whom 468 had locally advanced basal cell carcinoma (BCC) and 31 had metastatic BCC, explained Dr. Johan Hansson, an oncologist at the Karolinska Institute in Stockholm.
The drug was dosed at 150 mg once daily continuously in 28-day cycles until disease progression, intolerable toxicity, or study withdrawal. Safety follow-up was conducted at 1, 3, 5, 9, and 12 months. In an earlier report, the complete and partial response rates were 34% and 33%, respectively, in patients with locally advanced BCC, and 7% and 31% in those with metastatic disease (Lancet Oncol. 2015 Jun;16[6]:729-36).
Dr. Hansson presented new data on efficacy outcomes broken down according to treatment breaks, as well as quality of life results, at the annual congress of the European Academy of Dermatology and Venereology.
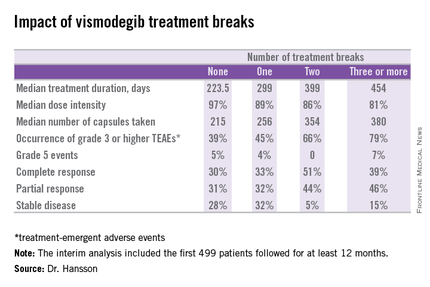
Twenty-six percent of patients had one or more treatment breaks. Seventy-six patients had one, 41 had two, and 14 had three or more. The median duration of the breaks was 22 days. The two most frequent reasons for treatment breaks were intolerable adverse events in 53% of cases, and lesser adverse events in 23%.
Close to 100% of STEVIE participants had treatment-emergent adverse events. The most common were muscle spasms, alopecia, altered sense of smell, and weight loss.
Although the number of patients with treatment breaks was relatively small, the response rates were higher in patients with more treatment breaks. So was median treatment duration as well as the median number of capsules taken.
Median progression-free survival was 19.8 months in patients with no treatment breaks, was 19.0 months in those with one, and hasn’t yet been reached in patients with two or more breaks.
In interpreting these findings, Dr. Hansson said, “We have to remember that although intriguing, these are tentative results from an exploratory analysis of subgroups in an ongoing study and should be interpreted with caution.”
The oncologist added, however, based upon these promising results he and his coinvestigators plan to look further into the concept of deliberate intermittent dosing of vismodegib.
Quality of life was assessed using the Skindex-16 questionnaire at baseline, again after two and seven 28-day cycles of vismodegib, and at 12 months. Three domains were examined: emotion, function, and symptoms.
A clinically meaningful improvement – defined as a 10-point or greater reduction from baseline – was seen in the emotion domain at all time points in patients with locally advanced BCC, with median improvements of 14.3 points after two cycles and 23.8 points after seven cycles and at the 12-month mark. Clinically meaningful improvement in symptom scores on the Skindex-16 were noted in patients aged 65 and older, in women, and in those with BCCs in locations other than the head or neck. However, no clinically meaningful improvement in the domain of function was seen at any time in patients with locally advanced BCC.
Patients with metastatic BCC didn’t show significant improvement in any of the three quality of life domains at any time point, added Dr. Hansson.
The STEVIE trial is sponsored by F. Hoffmann–La Roche/Genentech. Dr. Hansson reported receiving research grants from and serving as a consultant to Bristol-Myers Squibb, GlaxoSmithKline, Merck, Novartis, and Roche.