To the Editor:
An 85-year-old woman with a history of hypertension, hyperlipidemia, stroke, hypothyroidism, chronic obstructive pulmonary disease, and chronic myeloproliferative disorder presented to our clinic for evaluation of brown lesions on the hands and discoloration of the fingernails and toenails of 4 months’ duration. Six months prior to visiting our clinic she was admitted to the hospital for a pulmonary embolism. On admission she was noted to have a platelet count of more than 2 million/μL (reference range, 150,000–350,000/μL). She received urgent plasmapheresis and started hydroxyurea 500 mg twice daily, which she continued as an outpatient.
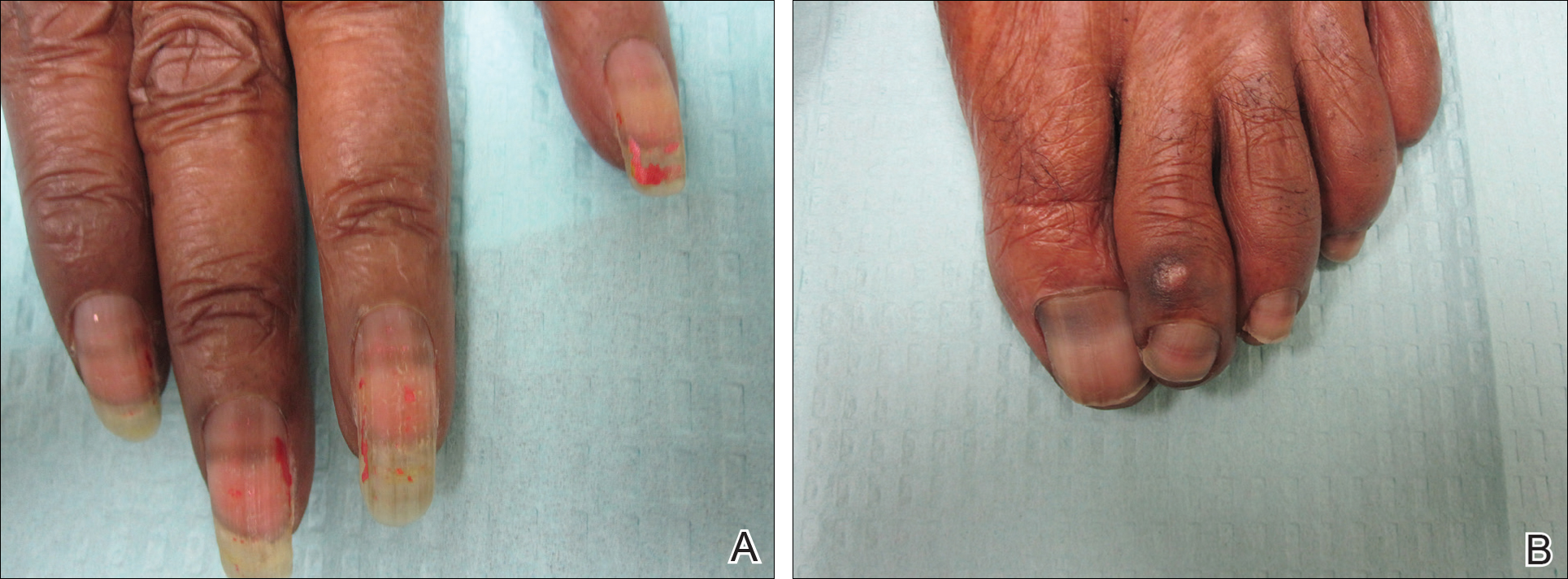
On physical examination at our clinic she had diffusely scattered red and brown macules on the bilateral palms and transverse hyperpigmented bands of various intensities on all fingernails and toenails (Figure). Her platelet count was 372,000/μL, white blood cell count was 5200/μL (reference range, 4500–11,000/μL), hemoglobin was 12.6 g/dL (reference range, 14.0–17.5 g/dL), hematocrit was 39.0% (reference range, 41%–50%), and mean corpuscular volume was 87.5 fL per red cell (reference range, 80–96 fL per red cell).
The patient was diagnosed with hydroxyurea-induced nail hyperpigmentation and was counseled on the benign nature of the condition. Three months later her platelet count decreased to below 100,000/μL, and hydroxyurea was discontinued. She noticed considerable improvement in the lesions on the hands and nails with the cessation of hydroxyurea.
Hydroxyurea is a cytostatic agent that has been used for more than 40 years in the treatment of myeloproliferative disorders including chronic myelogenous leukemia, polycythemia vera, essential thrombocythemia, and sickle cell anemia.1 It inhibits ribonucleoside diphosphate reductase and promotes cell death in the S phase of the cell cycle.1-3
Several adverse cutaneous reactions have been associated with hydroxyurea including increased pigmentation, hyperkeratosis, skin atrophy, xerosis, lichenoid eruptions, palmoplantar keratoderma, cutaneous vasculitis, alopecia, chronic leg ulcers, cutaneous carcinomas, and melanonychia.3,4
Hydroxyurea-induced melanonychia most often occurs after several months of therapy but has been reported to occur as early as 4 months and as late as 5 years after initiating the drug.1,4-6 The prevalence of melanonychia in the general population has been estimated at 1% and is thought to increase to approximately 4% in patients treated with hydroxyurea.1,2,6,7 The prevalence of affected individuals increases with age; it is more common in females as well as black and Hispanic patients.2
Multiple patterns of hydroxyurea-induced melanonychia have been described, including longitudinal bands, transverse bands, and diffuse hyperpigmentation.1-3,6 By far the most common pattern described in the literature is longitudinal banding1-3,8; transverse bands are more rare. Although there are sporadic case reports linking the transverse bands with hydroxyurea, these bands occur more frequently with systemic chemotherapy such as doxorubicin and cyclosphosphamide.1,6
The exact pathogenesis of hydroxyurea-induced melanonychia remains unclear, though it is thought to result from focal melanogenesis in the nail bed or matrix followed by deposition of melanin granules on the nail plate.5,8 When these melanocytes are activated, melanosomes filled with melanin are transferred to differentiating matrix cells, which migrate distally as they become nail plate oncocytes, resulting in a visible band of pigmentation in the nail plate.2 There also may be a genetic and photosensitivity component.1,2
Prior case series have described spontaneous remission of nail hyperpigmentation following discontinuation of hydroxyurea therapy.1 In many patients, however, the chronic nature of the myeloproliferative disorder and lack of alternative treatments make a therapeutic change difficult. Although the melanonychia itself is benign, it may precede the appearance of more serious mucocutaneous side effects, such as skin ulceration or development of cutaneous carcinomas, so careful monitoring should be performed.2
Our patient presented with melanonychia that was transverse, polydactylic, monochromic, stable in size and shape, and associated with palmar hyperpigmentation. Of note, the pigmentation remitted over time along with discontinuation of the drug. Although this presentation did not warrant a nail matrix biopsy, it should be noted that patients with single nail melanonychia suspicious for melanoma should have a biopsy, even with concomitant use of hydroxyurea.2 Although transverse melanonychia most commonly is associated with other systemic chemotherapeutics, in the absence of such medications hydroxyurea was the likely culprit in our patient. The palmar hyperpigmentation, which has previously been reported with hydroxyurea use, further solidifies the diagnosis.