WAIKOLOA, HAWAII – The highly selective interleukin-17A subunit inhibitor in the long-term extension phase of the randomized, controlled UNCOVER-3 (NCT01646177) trial, Craig L. Leonardi, MD, reported at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
However, the strict inclusion and exclusion criteria employed in randomized trials such as this raise questions about the broader applicability of the results in real-world clinical practice. So separately at the Hawaii seminar, Dr. Leonardi presented a single-center retrospective observational cohort study of the rapidity and duration of response to ixekizumab in his own clinical practice after the biologic received Food and Drug Administration marketing approval. Those results, too, were impressive and, in his view, highly generalizable.
“It is expected that this study cohort is generally representative of patients who are routinely seen at dermatology referral practices in the U.S.,” commented Dr. Leonardi, of Saint Louis University.
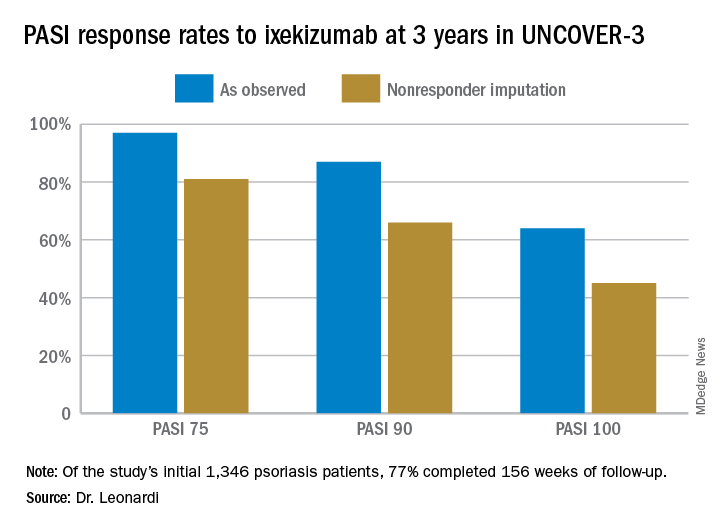
UNCOVER-3 included 1,346 psoriasis patients initially randomized 2:2:2:1 to double-blind subcutaneous ixekizumab (Taltz) at 80 mg either every 2 weeks or every 4 weeks after a 160-mg loading dose; subcutaneous etanercept at 50 mg twice weekly; or placebo for 12 weeks, followed by a switch to ixekizumab at 80 mg every 4 weeks from week 12 out to 3 years. The long-term efficacy analysis was restricted to patients who received the biologic according to what ultimately became the approved dosing schedule: a 160-mg loading dose, followed by 80 mg every 2 weeks through week 12, then 80 mg every 4 weeks. The safety analysis, in contrast, included everybody.
Dr. Leonardi presented the efficacy data using several different statistical methodologies, thereby providing an instructive lesson regarding the importance of examining the fine print when viewing clinical trial results. At one extreme is the as-observed analysis. Under this methodology, if a patient dropped out of UNCOVER-3 at, for example, week 11, the last measurement of treatment response, recorded at week 8, is carried forward by investigators and assumed to be valid for the rest of the study. Since week 8 may have been the last time the patient was doing well on the drug, the as-observed analysis can create a distorted overly favorable picture of the drug’s performance.