Evidence of human exposure to mercury dates as far back as the Egyptians in 1500 bc . 1 The ancient Chinese believed mercury could prolong life, heal bones, and maintain vitality. 2 Western medicine has utilized mercury in diuretics, laxatives, antibacterial agents, and antiseptics. 3 Health effects caused by chronic mercury exposure became increasingly apparent in the 1800s after hat makers who had inhaled mercuric nitrate vapors began to present with a host of neurologic symptoms, which is where the p hrase "mad as a hatter" was derived. 4,5 In 1889, French neurologist Jean-Martin Charcot attributed rapid tremors to mercury poisoning. 6 By 1940, Kinnier Wilson 7 further characterized the effects of mercury, describing mercury-induced cognitive impairments. In the 1960s, Japanese researchers correlated elevated urinary mercury levels with an outbreak of Minamata disease, a condition characterized by tremors, sensory loss, ataxia, and visual constrictions. 8 The World Health Organization considers mercury to be one of the top 10 chemicals of major public health concern. 9
Mercury release in the environment primarily is a function of human activity, including coal-fired power plants, residential heating, and mining.9,10 Mercury from these sources is commonly found in the sediment of lakes and bays, where it is enzymatically converted to methylmercury by aquatic microorganisms; subsequent food chain biomagnification results in elevated mercury levels in apex predators. Substantial release of mercury into the environment also can be attributed to health care facilities from their use of thermometers containing 0.5 to 3 g of elemental mercury,11 blood pressure monitors, and medical waste incinerators.5
Mercury has been reported as the second most common cause of heavy metal poisoning after lead.12 Standards from the US Food and Drug Administration dictate that methylmercury levels in fish and wheat products must not exceed 1 ppm.13 Most plant and animal food sources contain methylmercury at levels between 0.0001 and 0.01 ppm; mercury concentrations are especially high in tuna, averaging 0.4 ppm, while larger predatory fish contain levels in excess of 1 ppm.14 The use of mercury-containing cosmetic products also presents a substantial exposure risk to consumers.5,10 In one study, 3.3% of skin-lightening creams and soaps purchased within the United States contained concentrations of mercury exceeding 1000 ppm.15
We describe a case of mercury toxicity resulting from intentional injection of liquid mercury into the right antecubital fossa in a suicide attempt.
Case Report
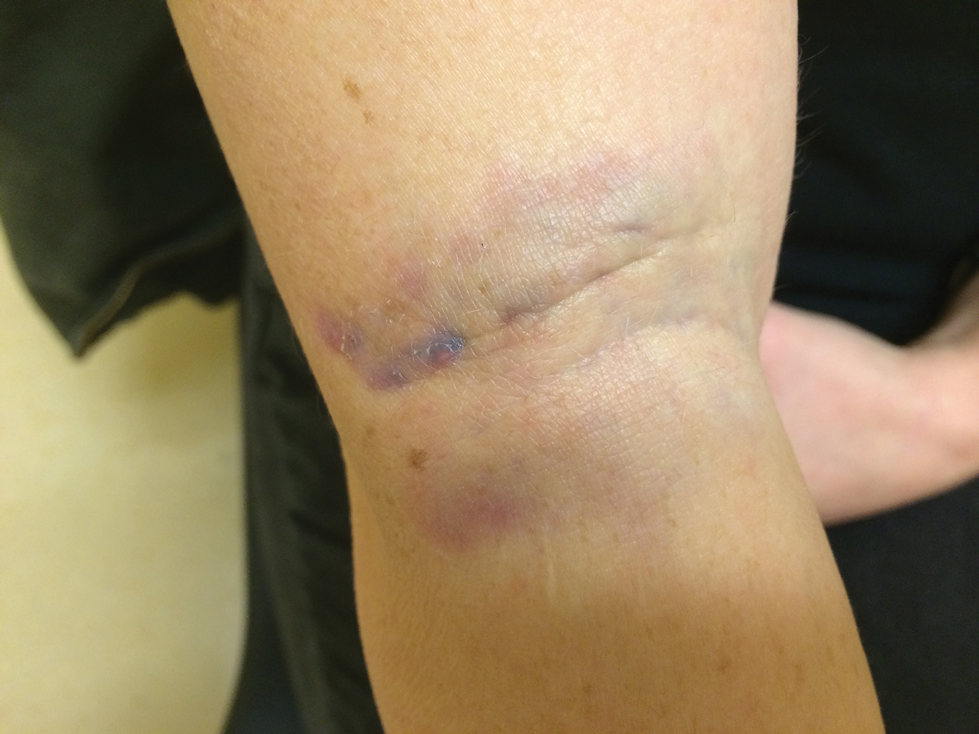
A 31-year-old woman presented to the family practice center for evaluation of a firm stained area on the skin of the right arm. She reported increasing anxiety, depression, tremors, irritability, and difficulty concentrating over the last 6 months. She denied headache and joint or muscle pain. Four years earlier, she had broken apart a thermometer and injected approximately 0.7 mL of its contents into the right arm in a suicide attempt. She intended to inject the thermometer’s contents directly into a vein, but the material instead entered the surrounding tissue. She denied notable pain or itching overlying the injection site. Her medications included aripiprazole and buspirone. She noted that she smoked half a pack of cigarettes per day and had a history of methamphetamine abuse. She was homeless and unemployed. Physical examination revealed an anxious tremulous woman with an erythematous to bluish gray, firm plaque on the right antecubital fossa (Figure 1). There were no notable tremors and no gait disturbance.
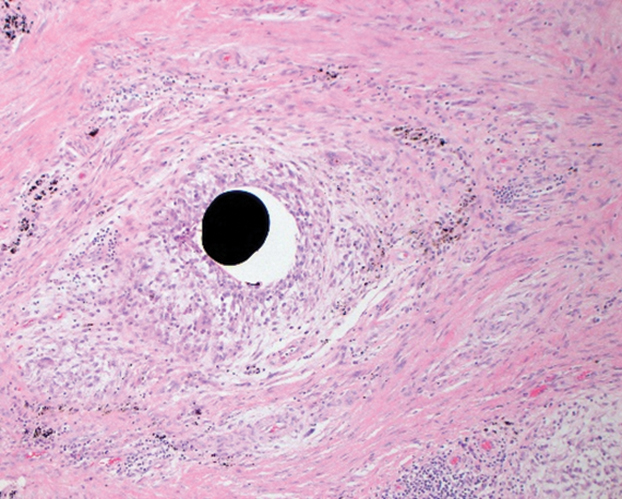
Her blood mercury level was greater than 100 µg/L and urine mercury was 477 µg/g (reference ranges, 1–8 μg/L and 4–5 μg/L, respectively). A radiograph of the right elbow area revealed scattered punctate foci of increased density within or overlying the anterolateral elbow soft tissues. She was diagnosed with mercury granuloma causing chronic mercury elevation. She underwent excision of the granuloma (Figure 2) with endovascular surgery via an elliptical incision. The patient was subsequently lost to follow-up.
Comment
Elemental mercury is a silver liquid at room temperature that spontaneously evaporates to form mercury vapor, an invisible, odorless, toxic gas. Accidental cutaneous exposure typically is safely managed by washing exposed skin with soap and water,16 though there is a potential risk for systemic absorption, especially when the skin is inflamed. When metallic mercury is subcutaneously injected, it is advised to promptly excise all subcutaneous areas containing mercury, regardless of any symptoms of systemic toxicity. Patients should subsequently be monitored for signs of both central nervous system (CNS) and renal deficits, undergo chelation therapy when systemic effects are apparent, and finally receive psychiatric consultation and treatment when necessary.17