To the Editor:
A 44-year-old woman presented with a low-grade fever (temperature, 38.0 °C) and painful acral lesions of 1 week’s duration. She had a history of hepatitis C viral infection and intravenous (IV) drug use, as well as polymicrobial infective endocarditis that involved the tricuspid and aortic valves; pathogenic organisms were identified via blood culture as Enterococcus faecalis, Serratia species, Streptococcus viridans, and Candida albicans. The patient had received a mechanical aortic valve and bioprosthetic tricuspid valve replacement 5 months prior with warfarin therapy and had completed a postsurgical 6-week course of high-dose micafungin. She reported that she had developed painful, violaceous, thin papules on the plantar surface of the left foot 2 weeks prior to presentation. Her symptoms improved with a short systemic steroid taper; however, within a week she developed new tender, erythematous, thin papules on the plantar surface of the right foot and the palmar surface of the left hand with associated lower extremity swelling. She denied other symptoms, including fever, chills, neurologic symptoms, shortness of breath, chest pain, nausea, vomiting, hematuria, and hematochezia. Due to worsening cutaneous findings, the patient presented to the emergency department, prompting hospital admission for empiric antibacterial therapy with vancomycin and piperacillin-tazobactam for suspected infectious endocarditis. Dermatology was consulted after 1 day of antibacterial therapy without improvement to determine the etiology of the patient’s skin findings.
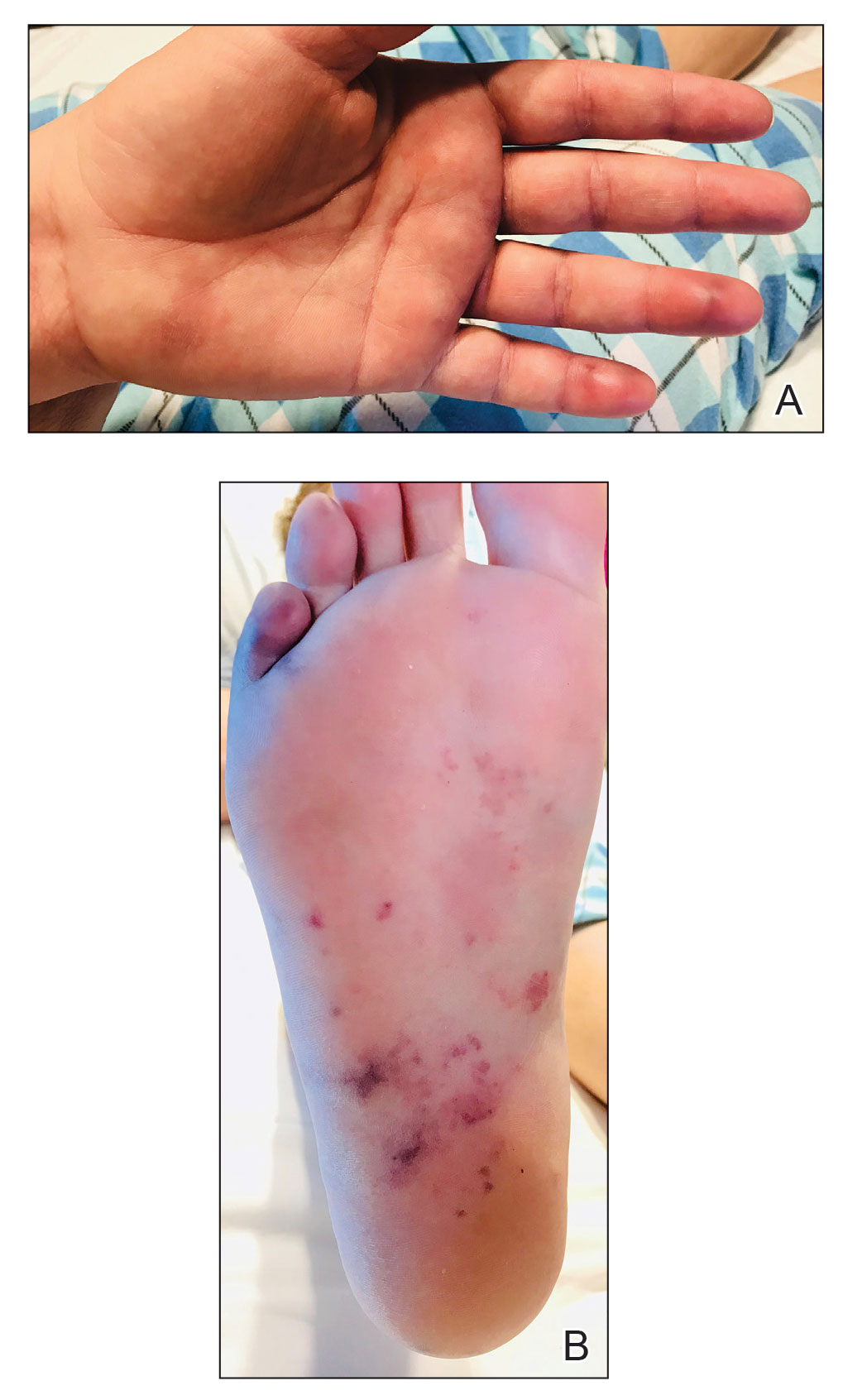
Physical examination revealed the patient was afebrile with partially blanching violaceous to purpuric, tender, edematous papules on the left fourth and fifth finger pads, as well as scattered, painful, purpuric patches with stellate borders on the right plantar foot (Figure 1). Laboratory test results revealed mild anemia (hemoglobin, 11.9 g/dL [reference range, 12.0–15.0 g/dL], mild neutrophilia (neutrophils, 8.4×109/L [reference range, 1.9–7.9×109/L], elevated acute-phase reactants (erythrocyte sedimentation rate, 71 mm/h [reference range, 0–20 mm/h]; C-reactive protein, 5.7 mg/dL [reference range, 0.0–0.5 mg/dL]), and positive hepatitis C virus antibody with an undetectable viral load. At the time of dermatologic evaluation, admission blood cultures and transthoracic echocardiogram were negative. Additionally, a transesophageal echocardiogram, limited by artifact from the mechanical aortic valve, was equivocal for valvular pathology. Subsequent ophthalmologic evaluation was negative for lesions associated with endocarditis, such as retinal hemorrhages.
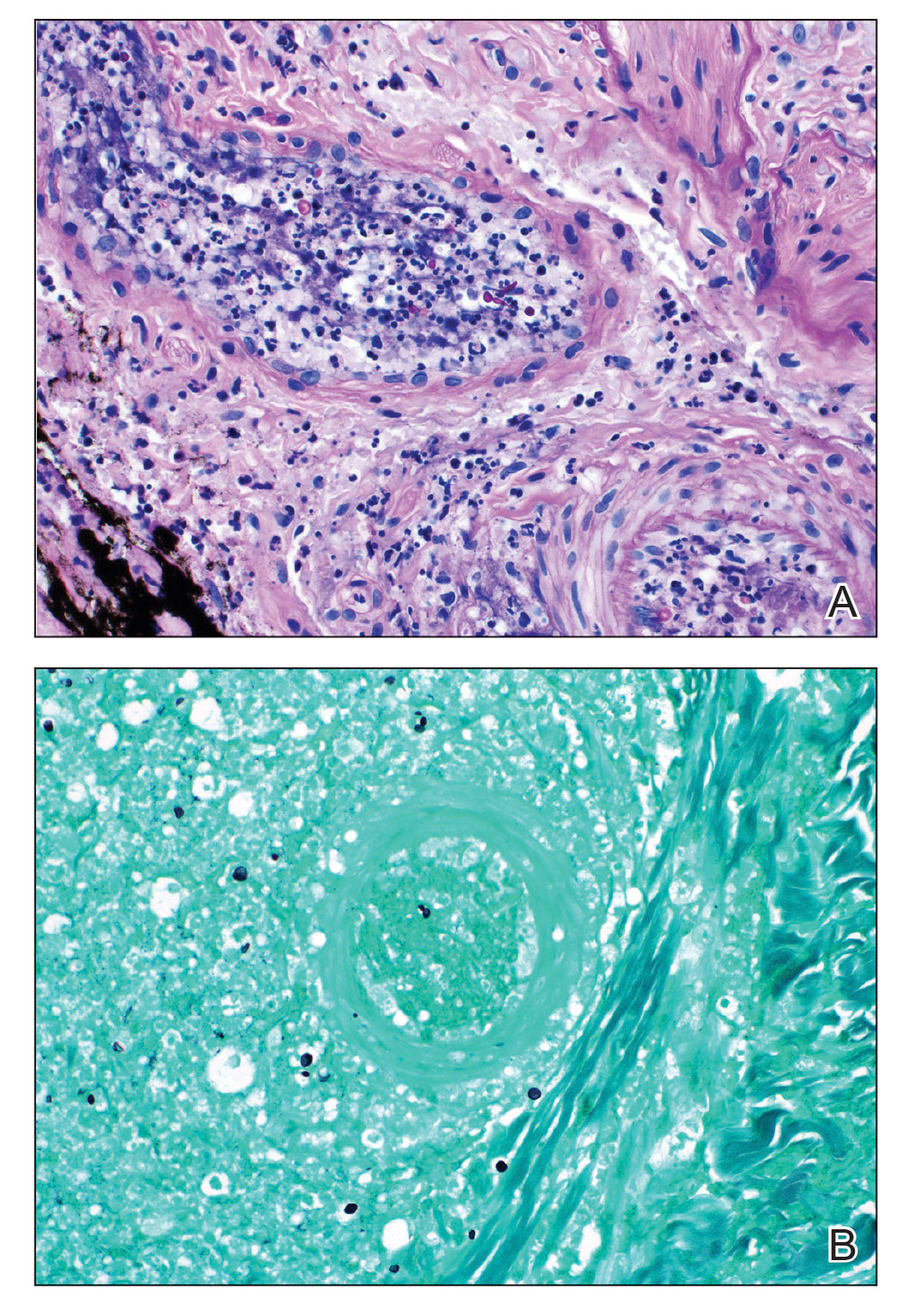
Punch biopsies of the left fourth finger pad were submitted for histopathologic analysis and tissue cultures. Histopathology demonstrated deep dermal perivascular neutrophilic inflammation with multiple intravascular thrombi, perivascular fibrin, and karyorrhectic debris (Figure 2). Periodic acid–Schiff and Grocott-Gomori methenamine-silver stains revealed fungal spores with rare pseudohyphae within the thrombosed vascular spaces and the perivascular dermis, consistent with fungal septic emboli (Figure 3).
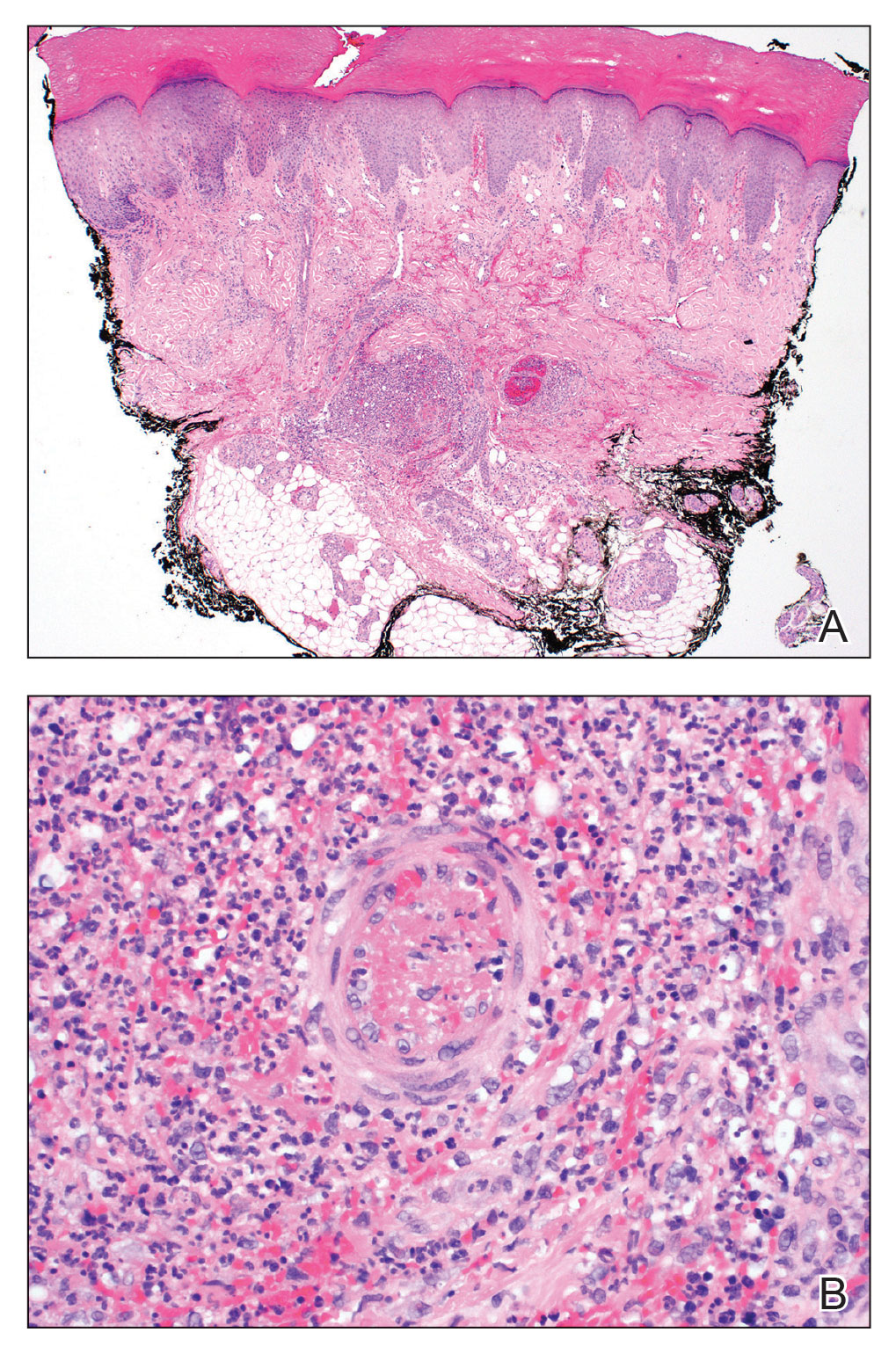
FIGURE 2. A, A punch biopsy of the left fourth finger pad revealed multiple intravascular microthrombi with edema and a dense perivascular neutrophilic infiltrate (H&E, original magnification ×40). B, Higher power showed a thrombus with surrounding fibrin deposition and a dense perivascular neutrophilic infiltrate (H&E, original magnification ×100).
Empiric systemic antifungal coverage composed of IV liposomal amphotericin B and oral flucytosine was initiated, and the patient’s tender acral papules rapidly improved. Within 48 hours of biopsy, skin tissue culture confirmed the presence of C albicans. Four days after the preliminary dermatopathology report, confirmatory blood cultures resulted with pansensitive C albicans. Final tissue and blood cultures were negative for bacteria including mycobacteria. In addition to a 6-week course of IV amphotericin B and flucytosine, repeat surgical intervention was considered, and lifelong suppressive antifungal oral therapy was recommended. Unfortunately, the patient did not present for follow-up. Three months later, she presented to the emergency department with peritonitis; in the operating room, she was found to have ischemia of the entirety of the small and large intestines and died shortly thereafter.
Fungal endocarditis is rare, tending to develop in patient populations with particular risk factors such as immune compromise, structural heart defects or prosthetic valves, and IV drug use. Candida infective endocarditis (CIE) represents less than 2% of infective endocarditis cases and carries a high mortality rate (30%–80%).1-3 Diagnosis may be challenging, as the clinical presentation varies widely. Although some patients may present with classic features of infective endocarditis, including fever, cardiac murmurs, and positive blood cultures, many cases of infective endocarditis present with nonspecific symptoms, raising a broad clinical differential diagnosis. Delay in diagnosis, which is seen in 82% of patients with fungal endocarditis, may be attributed to the slow progression of symptoms, inconclusive cardiac imaging, or negative blood cultures seen in almost one-third of cases.2,3 The feared complication of systemic embolization via infective endocarditis may occur in up to one-half of cases, with the highest rates associated with staphylococcal or fungal pathogens.2 The risk for embolization in fungal endocarditis is independent of the size of the cardiac valve vegetations; accordingly, sequelae of embolic complications may arise despite negative cardiac imaging.4 Embolic complications, which typically are seen within the first 2 to 4 weeks of treatment, may serve as the presenting feature of endocarditis and may even occur after completion of antimicrobial therapy.
Detection of cutaneous manifestations of infective endocarditis, including Janeway lesions, Osler nodes, and splinter hemorrhages, may allow for earlier diagnosis. Despite eponymous recognition, Janeway lesions and Osler nodes are relatively uncommon manifestations of infective endocarditis and may be found in only 5% to 15% of cases.5 Biopsies of suspected Janeway lesions and Osler nodes may allow for recognition of relevant vascular pathology, identification of the causative pathogen, and strong support for the diagnosis of infective endocarditis.4-7
The initial photomicrograph of corresponding Janeway lesion histopathology was published by Kerr in 1955 and revealed dermal microabscesses posited to be secondary to bacterial emboli.8,9 Additional cases through the years have reported overlapping histopathologic features of Janeway lesions and Osler nodes, with the latter often defined by the presence of vasculitis.4 Although there appears to be ongoing debate and overlap between the 2 integumentary findings, a general consensus on differentiation takes into account both the clinical signs and symptoms as well as the histopathologic findings.10,11