HONOLULU – , Anthony J. Mancini, MD, said during a presentation at the Hawaii Dermatology Seminar provided by MedscapeLIVE!
In April 2021, spinosad topical suspension 0.9%, was approved by the Food and Drug Administration for treating scabies infestations in adult and pediatric patients 4 years of age and older – a first-in-class drug and the first new scabicide approved in 31 years. It was also approved for treating head lice in adults and children aged 6 months of age and older.
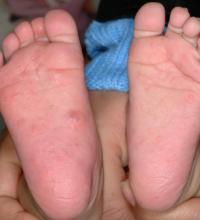
“Scabies has been described as the worst itch one can experience,” said Dr. Mancini, professor of pediatrics and dermatology at Northwestern University, Chicago. “It’s a hallmark of the disease, it can persist for weeks, it’s most intense at night, and patients report various sensations. It’s believed to be a both type I and type IV hypersensitivity reaction.”
The microscopic scabies mite burrows into the upper layer of the skin where it lives and lays its eggs. Besides intense itching, the classic presentation consists of a skin rash composed of inflammatory papules, linear burrows and crusted papules (especially on the hands, feet, and groin), and at times, larger red nodules. “Scabies nodules can persist for many months,” he said.
The Global Burden of Disease Study 2015 cited scabies as having the greatest burden of disease in tropical regions, especially among children, adolescents, and the elderly. The greatest burden of disability-adjusted life years (DALYs) occurred in East and Southeast Asia, Oceana, and tropical South America, but in North America, there was a 24% increase in the DALY rate between 1990 and 2015.
In addition, the World Health Organization designated scabies as a neglected tropical disease in 2017 and included it in its 10-year road map for neglected tropical diseases 2021-2030 with goals of promoting disease awareness and encouraging research and achieving global control.
“In our country, we typically see scabies treated successfully without complications, but there can be complications, especially in underdeveloped areas, like Staph aureus and Group A beta-hemolytic streptococcal infections,” which can be fatal, said Dr. Mancini, who is also head of pediatric dermatology at Ann & Robert H. Lurie Children’s Hospital of Chicago.
Permethrin 5% cream is typically offered as first-line topical therapy in the United States for the treatment of scabies. However, in vitro studies and small investigator-initiated in vivo studies have reported that efficacy appears to be decreasing. In one of the trials, Italian researchers enrolled 155 patients who were treated with permethrin 5% for 8 hours for 2 consecutive days and repeated the treatment 5 days later . Following the course of permethrin, only 34 responded, 96 failed treatment, and 25 were lost to follow-up.
“The study authors concluded that mite resistance to permethrin 5% seems to be increasing, following a path like other ectoparasite resistance,” said Dr. Mancini, who was not involved with the study. “We may even be seeing more ivermectin resistance in some geographic locations, as well.”
According to new scabicide efficacy criteria established by the FDA in 2016, complete cure is now defined as meeting both clinical and confirmatory criteria. A clinical cure means that all signs and symptoms of scabies have completely resolved, including burrows, inflammatory/noninflammatory lesions, and pruritus. A confirmatory cure means there is an absence of mites, eggs, scybala (feces), and burrows via microscopy or dermoscopy.
Enter spinosad, which is derived from a naturally occurring soil microorganism known as Saccharopolyspora spinosa and is composed of two active molecules: spinosyn A and spinosyn D. According to Dr. Mancini, spinosad’s mechanism of action is unique from other medications used to treat ectoparasites. It activates nicotinic and GABA-gated sodium channels, leads to sodium influx in the insect nerves, hyperexcitation, then paralysis and death. Cross-resistance to other insecticides has not been reported, he added, and there is no known evidence of resistance to its active compound.
Approval of the drug was based on data from two phase 3 randomized clinical trials involving 551 index cases and household contacts. In the intent-to-treat population, with the two trials combined, complete cure was achieved in 78.1% of the spinosad-treated group, compared with 39.6% in the vehicle group (P < .0001), clinical cure was achieved in 79.6% of the spinosad group, compared with 41.2% in the vehicle group (P < .001), and microscopic cure occurred in 85.9% of the spinosad group, compared with 52.6% in the vehicle group (P < .001).
Of the 306 participants in the study, the only adverse events reported by more than one patient each included abdominal pain, back pain, cough, headache, neck pain, and decreased weight in two patients each (0.8%), which investigators believed were not attributable to the study drug. Adverse events that investigators considered to be potentially related to the study drug included burning sensation in two participants (0.7%) and dry skin in another (0.3%). In clinical trials reported in the prescribing information, adverse events occurring in greater than 1% of subjects included application-site irritation (3% spinosad vs. 0% vehicle) and dry skin (2% spinosad vs. 0% vehicle).
“Spinosad met the FDA’s new stringent criteria, with all signs and symptoms of scabies completely resolved and confirmed via microscopy or dermoscopy,” said Dr. Mancini, who was not involved in the trials. “The patented formulation drives the active compound to the stratum corneum, where mites live and breed. It’s a single full-body application, without any resistance observed to date. This is an exciting newer option for treating our scabies patients.”
In an interview at the meeting, John S. Barbieri, MD, MBA, of the department of dermatology, Brigham and Women’s Hospital, Boston, said that, while he has no clinical experience with spinosad for scabies, he welcomes a new option for the condition. “The fact that it has a different mechanism of action than permethrin is a good thing,” he said.
Dr. Mancini disclosed that he is a consultant or an adviser for ParaPRO, the manufacturer of spinosad, and Cassiopea, Castle Creek, Novan, Novartis, and Verrica. He was not involved in clinical trials of spinosad. Dr. Barbieri disclosed that he receives consulting fees from Dexcel.
Medscape and this news organization are owned by the same parent company.