Results
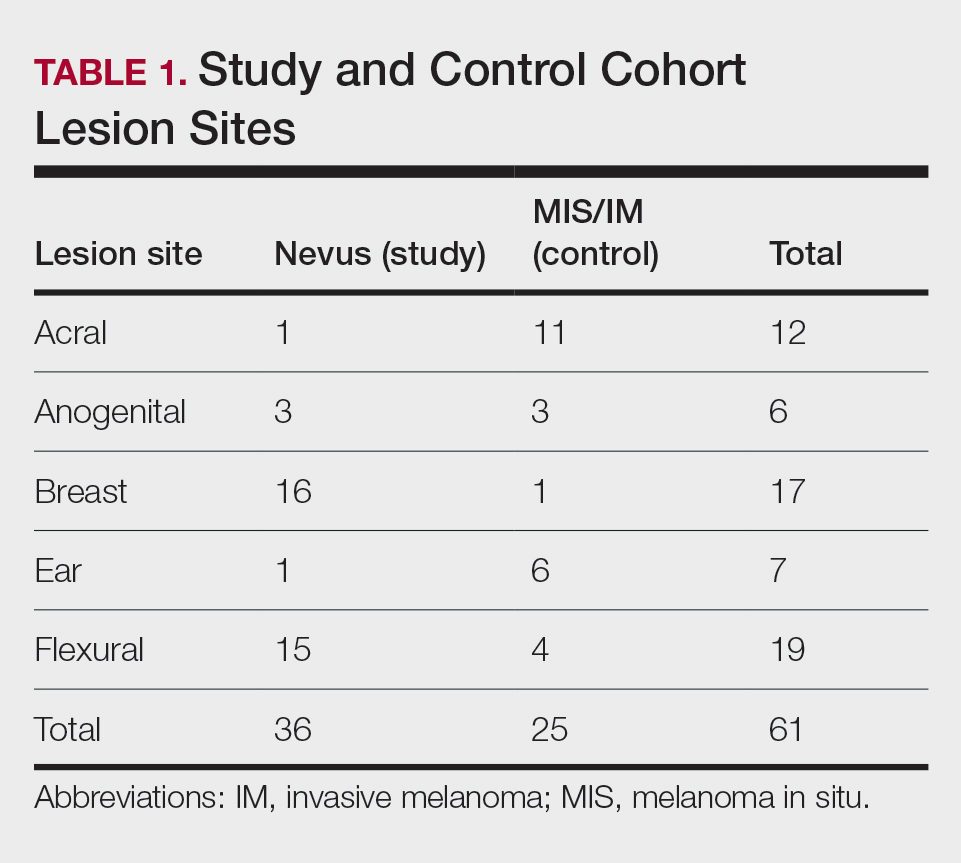
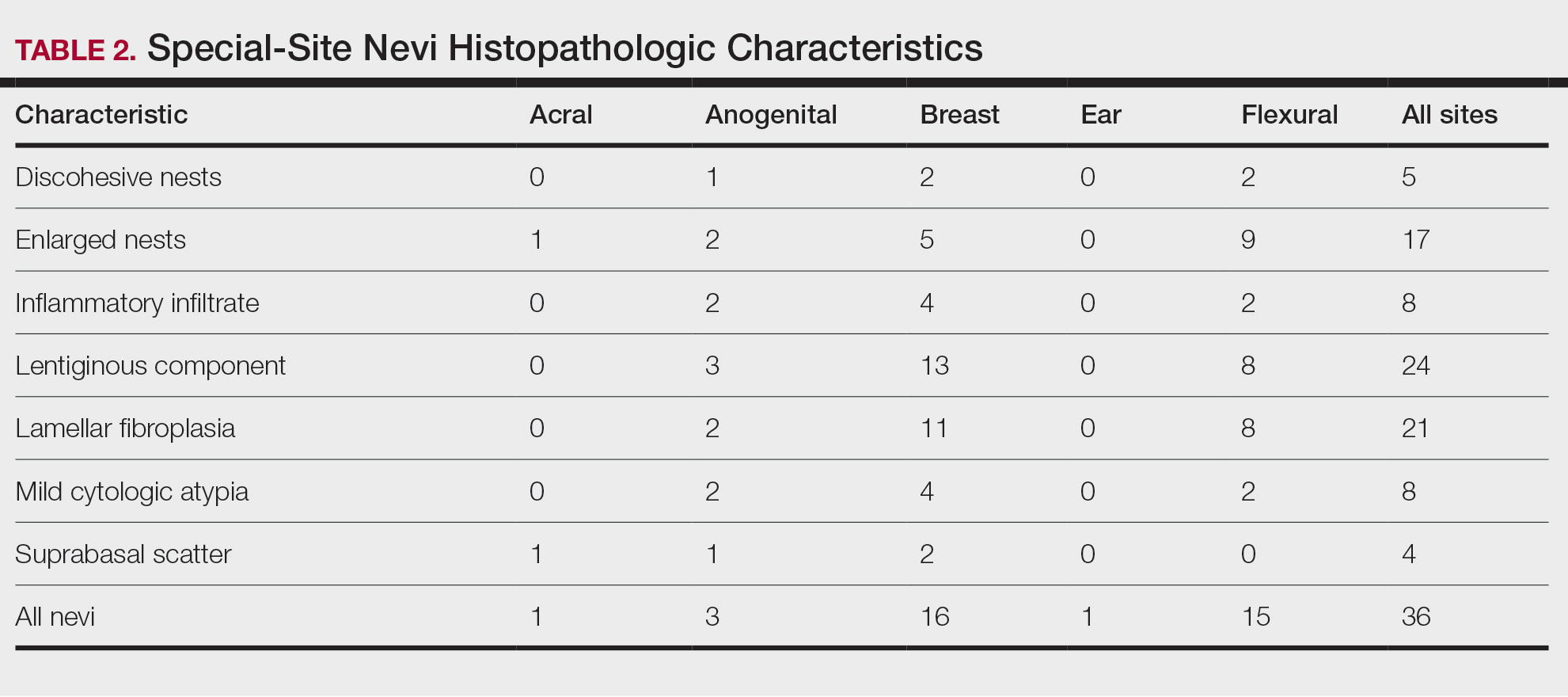
Our study cohort included 36 special-site nevi, and the control cohort comprised 25 melanoma in situ (MIS) or invasive melanoma (IM) lesions occurring at special sites. Table 1 provides a breakdown of the study and control cohorts by lesion site. Table 2 details the results of our microscopic examination, describing frequency of various characteristics of special-site nevi stratified by site.
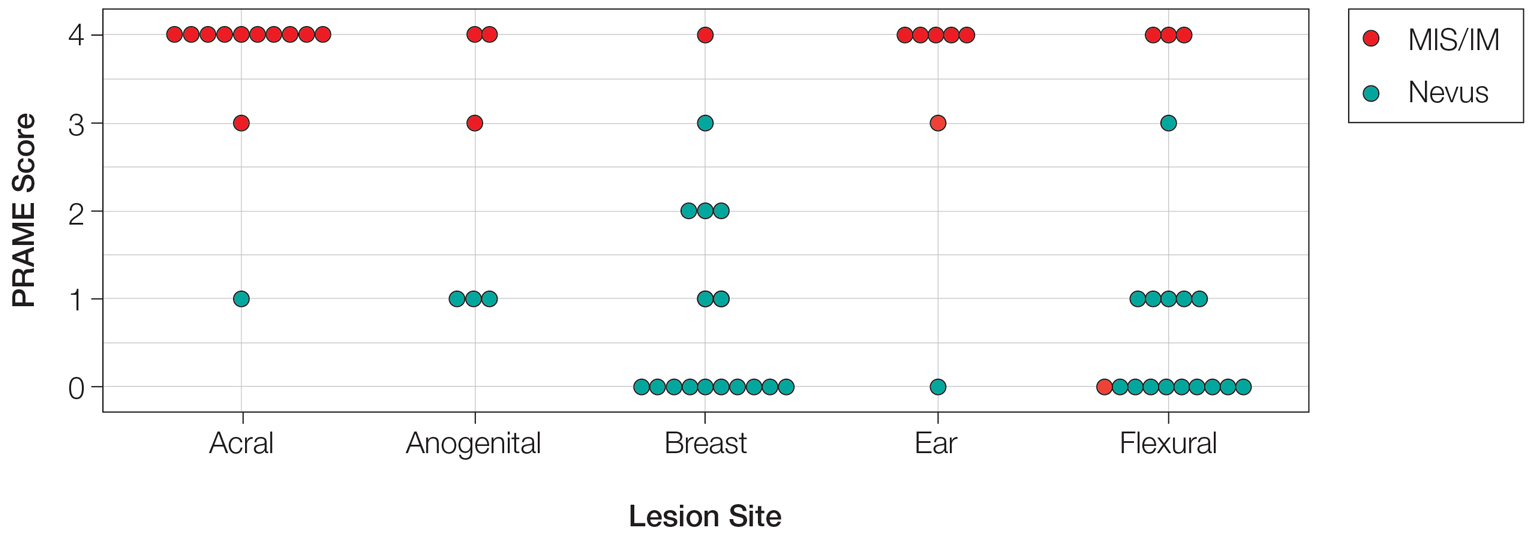
Of the 36 special-site nevi in our cohort, 20 (56%) had no staining (0) for PRAME, 11 (31%) demonstrated 1+ PRAME expression, 3 (8%) demonstrated 2+ PRAME expression, and 2 (6%) demonstrated 3+ PRAME expression. No nevi showed 4+ expression. In the control cohort, 24 of 25 (96%) MIS and IM showed 3+ or 4+ expression, with 21 (84%) demonstrating diffuse/4+ expression. One control case (4%) demonstrated 0 PRAME expression. These data are summarized in Table 3 and Figure 1. There is a significant difference in diffuse (4+) PRAME expression between special-site nevi and MIS/IM occurring at special sites (P=1.039×10-12).
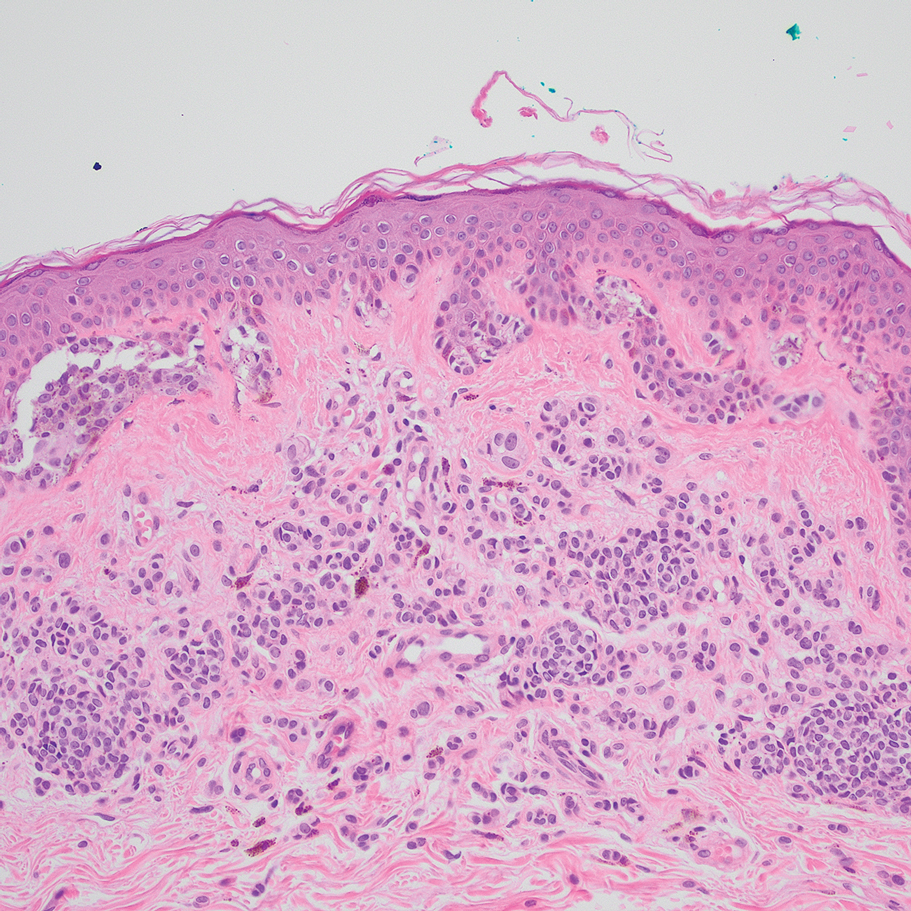
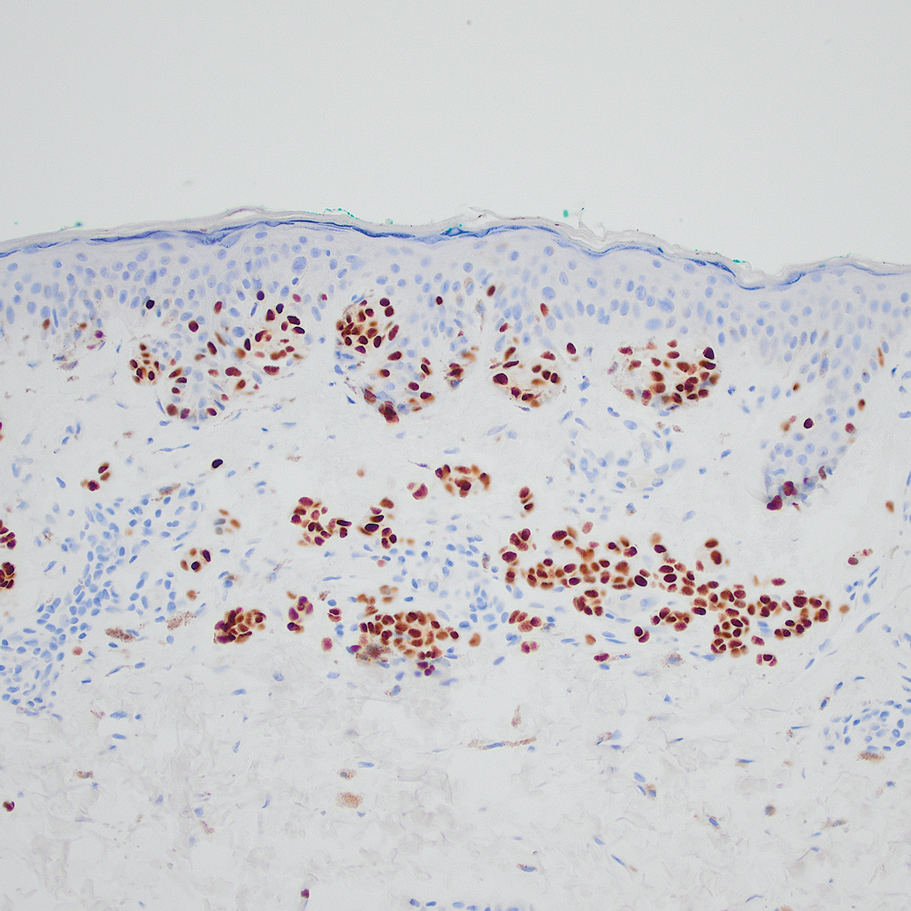
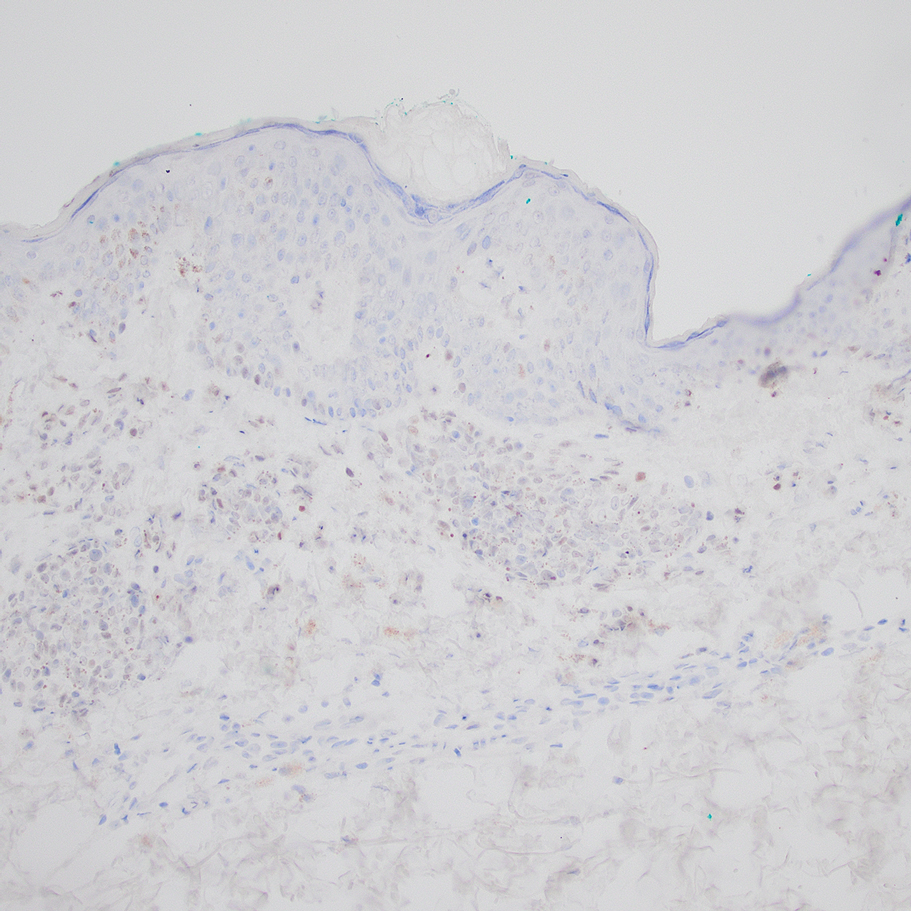
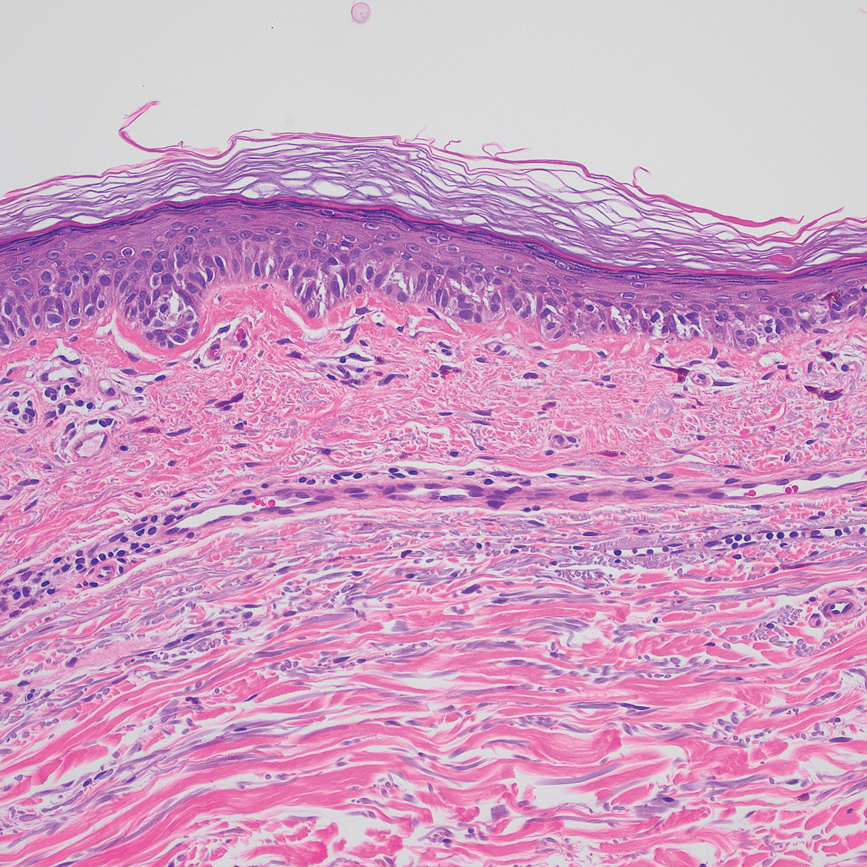
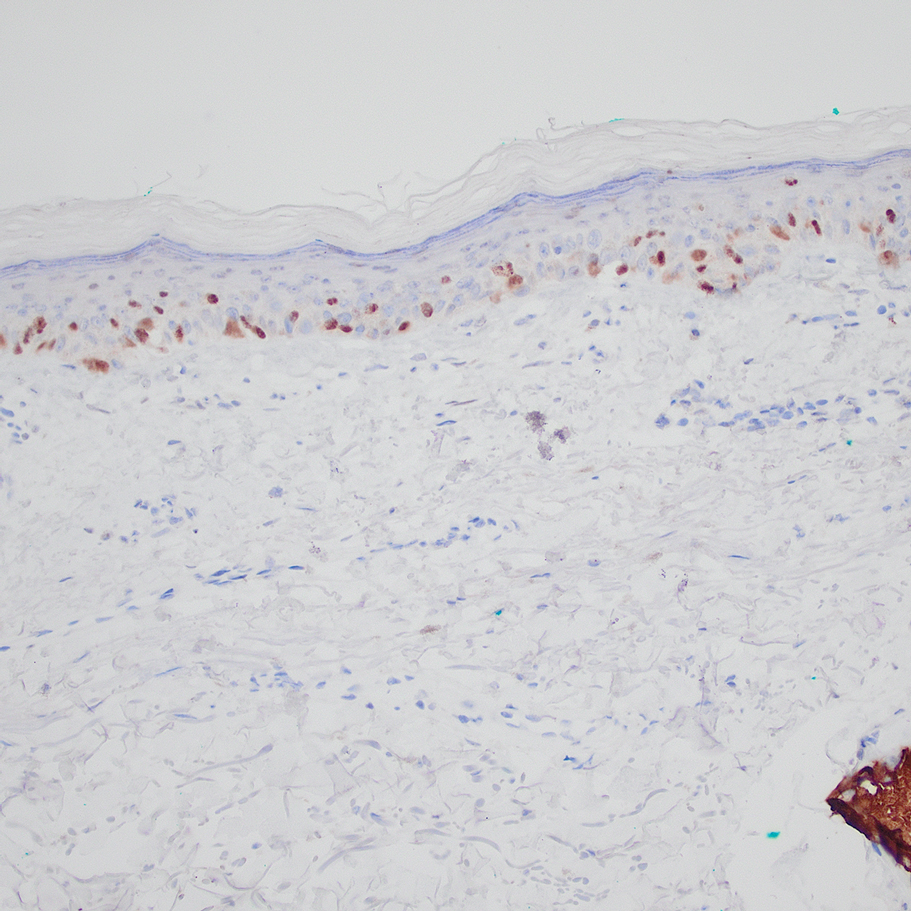
Based on our cohort, a positivity threshold of 3+ for PRAME expression for the diagnosis of melanoma in a special-site lesion would have a sensitivity of 96% and a specificity of 94%, while a positivity threshold of 4+ for PRAME expression would have a sensitivity of 84% and a specificity of 100%. Figures 2 through 4 show photomicrographs of a special-site nevus of the breast, which appropriately does not stain for PRAME; Figures 5 and 6 show an MIS at a special site that appropriately stains for PRAME.
Comment
The distinction between benign and malignant pigmented lesions at special sites presents a fair challenge for pathologists due to the larger degree of leniency for architectural distortion and cytologic atypia in benign lesions at these sites. The presence of architectural distortion or cytologic atypia at the lesion’s edge makes rendering a benign diagnosis especially difficult, and the need for a validated immunohistochemical stain is apparent. In our cohort, strong clonal PRAME expression provided a reliable immunohistochemical marker, allowing for the distinction of malignant lesions from benign nevi at special sites. Diffuse faint PRAME expression was present in several benign nevi within our cohort, and these lesions were considered negative (0) in our analysis.
Given the described test characteristics, we support the implementation of PRAME immunohistochemistry with a positivity threshold of 4+ expression as an ancillary test supporting the diagnosis of IM or MIS in special sites, which would allow clinicians to leverage the high specificity of 4+ PRAME expression to distinguish an IM or MIS from a benign nevus occurring at a special site. We do not recommend the use of 4+ PRAME expression as a screening test for melanoma or MIS among special-site nevi due to its comparatively low sensitivity; however, no one marker is always reliable, and we recommend continued clinicopathologic correlation for all cases. Although PRAME can assist in the delineation of malignant lesions from benign ones, microscopic examination of hematoxylin and eosin–stained section remains the gold standard for diagnosing malignant melanoma and MIS.
Although our case series included nevi and MIS/IM from all special sites, we were limited in the number of acrogenital and ear nevi included due to a relative paucity of biopsied benign nevi from these locations at the University of Virginia. Additionally, although the magnitude of the difference in PRAME expression between the study and control groups is sufficient to demonstrate statistical significance, the overall strength of our argument would be increased with a larger study group. We were limited by the number of cases available at our institution, which did not utilize PRAME during the initial diagnosis of the case; including these cases in the study group would have undermined the integrity of our argument because the differentiation of benign vs malignant initially was made using PRAME immunohistochemistry.
Conclusion
Due to their atypical features, special-site nevi can be challenging to assess. In this study, we showed that PRAME expression can be a reliable marker to distinguish benign from malignant lesions. Our results showed that 100% of benign special-site nevi demonstrated 3+ expression or less, with 56% (20/36) demonstrating no expression at all. The presence of diffuse PRAME expression (4+ PRAME staining) appears to be a specific indicator of a malignant lesion, but results should always be interpreted with respect to the patient’s clinical history and the lesion’s histomorphologic features. Further study of a larger sample size would allow refinement of the sensitivity and specificity of diffuse PRAME expression in the determination of malignancy for special-site lesions.
Acknowledgment—The authors thank the pathologistsat the University of Virginia Biorepository and Tissue Research Facility (Charlottesville, Virginia) for their skill and expertise in performing immunohistochemical staining for this study.