The anxiety of caring for a child in imminent peril may cause even an experienced clinician to forget to ask important questions about ingestions and exposures that can be critical to the patient’s management. Though emergency physicians (EPs) routinely ask about household medications when obtaining a history from family members, they occasionally gloss over a detail of utmost importance: topical medications.
The use of topical medications is extremely prevalent in the United States, in turn resulting in accidental ingestion—particularly in the pediatric population. In 2015, there were 56,455 calls to US Poison Control Centers for pediatric (children ≤5 years) exposures to topical preparations.1 Topical drug-delivery-system formulations include drops, ointments, gels, and patches. Intentional and unintentional misuse or overdose of any of these formulations can cause toxicity. Unintentional overdose of these drugs can occur secondary to exploratory ingestions, therapeutic errors, or medication overuse due to the perception of safety associated with topical preparations.
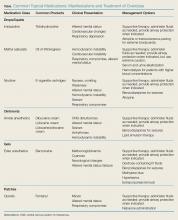
Through the example of commonly used prescription and nonprescription topical medications, this article reviews the clinical manifestations of toxicity in each of these topical delivery systems, along with respective treatment strategies (Table).Drops
Topical liquid medications such as ophthalmic and otologic drops can be fatal when ingested or used inappropriately. The following sections review commonly used prescription and nonprescription formulations, associated toxicological manifestations, and appropriate management.
Ophthalmic Drops
A common class of ophthalmic drops includes imidazoline-derived agents such as tetrahydrozoline (eg, Opti-Clear, Visine). Tetrahydrozoline hydrochloride is an agonist of alpha-1, alpha-2, and imidazoline receptors.2-4 Stimulation of alpha-1and imidazoline receptors impede sympathetic output, leading to bradycardia and hypotension. Imidazoline derivatives can mimic clonidine toxicity, with miosis, lethargy, diminished bowel sounds, respiratory depression, and apnea.2
Treatment. Management of overdose of imidazoline agents depends greatly on the patient’s presentation and is largely supportive. Overdoses of these agents and clonidine are similar: Patients can be extremely somnolent, but may transiently improve when a painful stimulus is applied. Activated charcoal may be useful for recent ingestions,3 but it should only be considered in patients whose airway is patent or protected. Intravenous fluids are indicated if the patient is hypotensive. Atropine may be considered for symptomatic bradycardia,3 and transcutaneous pacing should be considered if the patient is hemodynamically unstable. Intubation may be required if there is concern for airway compromise, though such compromise is a rare occurrence in ophthalmic ingestion of imidazoline-derived agents.
Although not well studied due to a lack of data, some sources recommend naloxone administration, given the similarities of imidazoline agents to clonidine in the overdose scenario.3,4 Although the optimal dose is unknown, high doses of naloxone (ie, pediatric patients, 0.4 mg, followed by 2 mg, then 10 mg, if no response) are typically required and should be considered in symptomatic patients after an ingestion. After successful supportive management, most patients continue to do well during their hospital course and have a full recovery.
Methyl Salicylate
Methyl salicylate (oil of wintergreen) is a common ingredient in muscular pain-relieving creams and ointments that can have devastating consequences in overdose. Significant toxicity from these compounds is rare, as large exposures are needed to reach a toxic threshold. However, oil of wintergreen is also available as a liquid preparation with 98% methyl salicylate.5 At this concentration, 1 teaspoon (5 mL) is roughly equivalent to 7 g of acetylsalicylate,5 and this amount of oil of wintergreen is severely toxic and may be lethal to a child. Because it is a liquid, oil of wintergreen is more rapidly absorbed than creams and ointments and can cause rapid toxicity in small quantities.
Methyl salicylate overdose initially causes stimulation of the brain’s respiratory center, which leads to a respiratory alkalosis. Uncoupling of oxidative phosphorylation later causes an anion gap metabolic acidosis. The combination of these two processes leads to a mixed acid-base disturbance. Common signs and symptoms of toxicity include tinnitus, hyperpnea, tachypnea, hyperthermia, nausea, vomiting, multisystem organ dysfunction, altered mental status, and death.
Treatment. Supportive care is critically important. Clinicians must be sure the patient’s airway is patent, particularly in those with altered sensorium or in patients who are becoming fatigued secondary to work of breathing. Extreme caution should be used when intubating these patients, as the patient’s respiratory rate (RR) must be matched if placed on a ventilator. If the RR is too low, the patient will become increasingly acidotic and may become hemodynamically unstable. Activated charcoal should be considered if the patient is mentating well or if the airway is protected.5,6 Adequate fluid resuscitation is essential.
Serum alkalinization is critical in helping to prevent central nervous system (CNS) toxicity. Urinary alkalinization with sodium bicarbonate will augment the salicylate excretion rate and may also help correct the patient’s acidemia.
Current guidelines recommend hemodialysis in asymptomatic patients whose serum salicylate concentration is greater than 100 mg/dL, or in patients with consequential findings, such as altered mental status.7
In infants with severe salicylate toxicity, exchange transfusion can be considered, given the limitations of hemodialysis at this age.8 Clinical outcomes are generally good if managed appropriately, though oil of wintergreen ingestion can be fatal.
Liquids
Liquid nicotine also poses a major threat to the pediatric population. Since the early 2000s, electronic cigarettes (e-cigarettes) have gained popularity. E-cigarette cartridges contain highly concentrated liquid nicotine, and, until May 2016, were not regulated by the US Food and Drug Administration (FDA).9 Since then, the FDA’s updated rule now extends to all tobacco products, including e-cigarettes.10
Some of the recent literature suggest oral lethal doses of nicotine occur at levels as low as 0.8 mg/kg,11 though this is likely an overly conservative level. At this dose, even relatively diluted products with a 1.8% nicotine solution could be fatal.12
Liquid nicotine comes in thousands of flavors,13 and while this may make its use more enjoyable for adults, it poses a significant risk to small children. Children may be enticed to ingest liquid nicotine products due to their flavor-enhanced scents.12
At relatively low serum levels, nicotine acts as a nicotinic acetylcholine receptor agonist. Symptoms such as nausea, vomiting, diarrhea, abdominal discomfort, increased salivation, and weakness can occur early on in toxicity.13 Once nicotine concentrations reach higher levels, patients develop altered mental status, hemodynamic instability, seizure, muscle weakness, and respiratory compromise.
Treatment. Supportive therapy should be initiated when caring for patients with nicotine ingestion. Airway management is paramount, particularly if the patient has altered mental status. In some cases, intubation may be necessary, especially in patients with altered mental status and excessive salivation/bronchorrhea. Intravenous fluid administration is pivotal in patients with hypotension, particularly for those at risk for dehydration secondary to vomiting and diarrhea. Although there is no definitive antidote, atropine can be used to treat patients who are symptomatic from excessive muscarinic cholinergic stimulation.13,14 If seizures occur, they can be treated with benzodiazepines as needed.
The use of activated charcoal has little mention in the current literature. Because of its liquid formulation, nicotine will likely be absorbed quickly. If ingestion occurred shortly prior to presentation and the patient’s airway is patent or secured, a dose of activated charcoal may be cautiously administered.15 The prognosis is poor if large amounts of liquid nicotine have been consumed.
Topical Ointments
Ointments are semisolid preparations, typically for topical application. Topical anesthetics are available in a variety of prescription and nonprescription ointments. Of the local prescription and nonprescription anesthetics currently available, amide-type local anesthetics have become especially popular for their rapid and reliable onset of local anesthesia and low occurrence of hypersensitive reactions. Increased popularity raises the likelihood of accidental ingestion—especially in pediatric patients.
Dibucaine, an amide anesthetic, is available as a nonprescription medication. Its uses include treating pain associated with external hemorrhoids and pain after episiotomy. Compared with lidocaine, dibucaine is significantly more potent, and toxicity can occur at much lower levels.16
Therapeutically, local anesthetics act by binding to sodium channels, which are necessary for propagation of action potentials17; this blocks signal transduction in local sensory nerves. Toxicity occurs when these agents exert systemic effects, especially on the CNS and heart. Patients with toxic ingestion typically exhibit CNS effects, such as gait disturbances, visual changes, agitation, altered mental status, and seizure; mortality can occur in severe cases. At higher doses, cardiovascular effects may manifest and lead to vasodilation, hemodynamic instability, and dysrhythmias. QRS prolongation, which likely results from sodium channel blockade, can precipitate dysrhythmias; wide-complex bradycardia, ventricular tachycardia, ventricular fibrillation, and asystole have all been reported.16,17Treatment. Supportive care, including airway management and fluid resuscitation, should be initiated as early as possible. Although not well documented in the literature, activated charcoal may be administered if there is no concern for the patency of the patient’s airway or if the airway has been secured.16,17
Patients with clinically significant dibucaine ingestions typically exhibit the CNS findings previously described. Seizures require aggressive management because they can cause a metabolic acidosis that potentiates the toxicity of dibucaine. Benzodiazepines are good first-line agents, though pentobarbital, phenobarbital, or propofol can be used if the patient continues to seize.17
Fluid resuscitation should be maximized in hemodynamically unstable patients prior to administering vasopressors, which are often warranted if blood pressure does not respond to fluids. Evidence supports the use of lipid emulsion therapy in hemodynamically unstable patients18; several authors have reported successful resuscitation after administrating lipid emulsion to treat amide anesthetic toxicity (generally bupivacaine toxicity). Fatalities associated with dibucaine ingestion have been reported16; therefore, ingestion of any topical anesthetic must be recognized and treated promptly.
Gels
Gels are a common topical drug-delivery system. In pediatric patients, these medications are typically used to help decrease teething pain.19
Benzocaine
Benzocaine (eg, Anbesol, Oragel), an ester anesthetic, is one of the most common medications used to alleviate teething pain in infants. Though benzocaine gels possess analgesic properties at therapeutic dosing, severe toxicity can develop in cases of overdose.
Benzocaine is metabolized into oxidizing compounds that lead to methemoglobin formation. Humans normally reduce methemoglobin to hemoglobin through the cytochrome b5 reductase pathway20; however, when an oxidizing agent overwhelms the reducing system, concentrations of methemoglobin begin to rise. Methemoglobin has a decreased oxygen-carrying capacity, and also has a higher subunit binding affinity that leads to a leftward shift of the oxygen dissociation curve.
Findings of benzocaine toxicity range greatly and depend on the amount of methemoglobin formed. Patients can develop asymptomatic cyanosis with low-methemoglobin concentrations (around 15%). At levels of 30% to 40%, neurological complaints may manifest, including weakness, disturbances in coordination, and headaches. High concentrations of methemoglobin (55% to 70%) can cause altered mental status, unresponsiveness, and seizures. When levels are extremely high (>70%), patients are at risk for life-threatening hemodynamic instability and death.21Treatment. For patients with methemoglobinemia, treatment depends upon the serum concentration of methemoglobin. Supportive care, including airway and circulatory management, is critical. If methemoglobin concentrations are low (<15%), close observation can be considered, as healthy individuals can reduce methemoglobin quickly.20 In patients with severe methemoglobinemia (a level above 25%, or clinical findings such as shortness of breath or altered mental status), treatment with methylene blue should be initiated. Methylene blue, an oxidizing agent, initiates a series of events that culminates with the reduction of methemoglobin into hemoglobin.22 Methylene blue is typically dosed 1 to 2 mg/kg17,21,22; dosing can be repeated to a maximum of 4 mg/kg in infants and 7 mg/kg in children.20-22 One should use caution when dosing methylene blue: As an oxidizing agent, when given in excess, methylene blue can worsen methemoglobinemia. Furthermore, methylene blue should not be given to patients with glucose-6-phosphate dehydrogenase deficiency, as this combination can cause massive hemolysis.17,20-22
Though rare, if patients are hemodynamically unstable or have life-threatening methemoglobinemia, hyperbaric oxygen therapy, exchange transfusion, or hemodialysis can be attempted—if these are readily available.17,20-22
Recognizing methemoglobinemia early is essential, and when a patient receives prompt treatment, mortality from methemoglobinemia secondary to benzocaine overdose is extremely low.
Transdermal Patches
Transdermal drug delivery is a relatively new route of administration—one that has gained increasingly in popularity. Patches are being used more frequently because they are easy to administer, have improved compliance due to decreased dosing frequency, allow concealment, and avoid first-pass metabolism, which increases the concentration of the parent compound.23
Although patches have several clinical advantages, they can pose a significant threat, particularly to pediatric patients, for several reasons. Patches, which work by delivering medication transdermally through a concentration gradient, are often impregnated with high concentrations of medication. If the patch is heated or damaged, this can significantly increase the amount of medication released onto the skin, leading to an overdose. Patches also normally contain high concentrations of medication even after they are worn for the prescribed time, though retained quantities vary depending on the drug and device.23,24 One study using fentanyl patches found 28% to 84.4% of the original drug remained in the patch after its clinical use.25 Toxicity from patches normally occurs from transdermal exposure as well as oral exposure/ingestion.
Fentanyl Patch
Fentanyl, a powerful synthetic opioid, has been available via transdermal delivery route since the early 1990s. Use of fentanyl patches has proven to be popular and efficacious in pain management. Unintentional exposure in pediatric patients is especially dangerous because children are often opioid-naive, and even small doses of fentanyl can be toxic.
Several cases of pediatric fentanyl toxicity secondary to transdermal exposure have been described in the literature. Though fewer in number, cases involving toxicity from patch ingestion have also been reported in adult patients26; to the best of our knowledge, no cases have been published on pediatric fentanyl-patch ingestions, though this should be considered when evaluating a patient with an opioid toxidrome.
Fentanyl, a mu-opioid agonist, can lead to significant morbidity and mortality. Findings from fentanyl toxicity are dose-dependent but include miosis, altered mental status, bradypnea, respiratory arrest, coma, and death, if left untreated.
Treatment. Airway protection is essential, and once opioid toxicity is suspected, patients who lack spontaneous respiration should receive immediate noninvasive respiratory support followed by naloxone administration; mechanical ventilation is sometimes required in patients with severe overdose. A thorough physical examination is crucial, and transdermal patches must be immediately identified and removed to prevent further drug absorption.
If a patch is found, the area should be thoroughly cleansed to remove any residual drug from the affected area. Removal of the patch does not result in an immediate reversal of toxicity. Due to the reservoir in the skin, spontaneous reversal may take up to 1 day. Oral ingestion can lead to a fatal outcome, so if ingestion is suspected, providers must examine the oral cavity to ensure that no piece of the patch is present.27Naloxone, a competitive opioid receptor antagonist, is used to reverse opioid overdose. It is typically dosed at 0.001 mg/kg28 and can be increased incrementally up to 0.01 mg/kg, or even higher if findings do not improve. Many patients require sequential doses of naloxone due to its relatively short half-life compared to the prolonged elimination of transdermal or ingested fentanyl.28,29
Naloxone infusions are commonly needed for these patients, and are typically dosed at about two-thirds of the dose required for initial opioid reversal.28 Given the prolonged duration of possible toxicity, any patient who presents to the ED with signs of opioid overdose from transdermal exposure or oral ingestion of a patch should be admitted to the hospital30 and monitored for 24 hours28,31 to ensure that symptoms do not rebound, especially once the naloxone drip is weaned. Patients should be monitored for 4 to 6 hours after cessation of a naloxone infusion. Fortunately, timely and adequate management can result in positive clinical outcomes in most of these situations.
Conclusion
Ingestions of topical products are relatively common occurrences, particularly in pediatric patients. During the history taking, clinicians should be vigilant and always inquire about any topical medications within the home any time a pediatric patient presents with signs and symptoms indicative of a toxic ingestion. Family members should also be counseled on the dangers of accidental topical medication ingestion or misuse. Providers should give recommendations for proper storage and disposal of all prescription and nonprescription medications, which may help not only save a repeat visit to the ED, but may in fact save a life.