STOCKHOLM – Adding liraglutide to multiple daily insulin injections helped stabilize blood glucose in patients with type 2 diabetes, while also resulting in weight loss and reduction of daily insulin doses.
All this was accomplished without an increase in the risk of hypoglycemia, Dr. Marcus Lind said at the annual meeting of the European Association for the Study of Diabetes.
A randomized, placebo-controlled study showed that the approach was successful in patients with long-standing disease, said Dr. Lind of the University of Gothenburg, Sweden. “We used to think that incretin-based therapies were most helpful in early type 2 diabetes. This seems to confirm that they are effective during the entire disease process.”
The soon-to-be-published 24-week study examined the addition of liraglutide in 124 patients with type 2 diabetes of long duration (a mean of 17 years). They were overweight, with a mean body mass index of 33.5 (about 218 pounds). At baseline, their mean hemoglobin A1c was 9%; they were taking a mean of 105 units of insulin each day in about four injections.
The study’s primary endpoint was change in HbA1c at 24 weeks. This declined significantly more among those taking liraglutide than those taking a placebo (–1.6% vs. –0.4%). “This is a difference of 1.1%, which is very clinically significant,” Dr. Lind said.
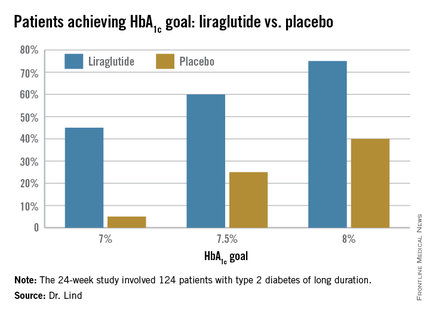
Dr. Lind assigned individualized HbA1c goals to patients, with the cutpoints of 7%, 7.5%, and 8%. Significantly more patients taking liraglutide were able to achieve those goals (see chart).
They also lost about 8 pounds more weight than did the placebo patients, and experienced a greater decrease in systolic blood pressure (–5.5 mm Hg more than placebo). There was no effect on diastolic blood pressure or lipids.
There were no severe hypoglycemic events in either group, nor any between-group difference in nonsevere events.
There were three serious adverse events among three patients taking liraglutide, and eight among four patients taking placebo; none of these were pancreatitis or pancreatic cancer.
Patients taking the study drug experienced significantly more nausea, especially at the beginning of the study, compared to the end of the study (22% vs. 5%).
The study was investigator initiated but received financial support from Novo Nordisk, which provided the drug. Dr. Lind disclosed financial relationships with numerous pharmaceutical companies, including honoraria from Novo Nordisk.