Pediatric asthma is the most commonly encountered childhood chronic disease, occurring in approximately 13.5% of children.1 Due to the interplay between patient, family physician (FP), and the environment, asthma often proves challenging to control. Although national guidelines for the treatment of asthma have not changed since 2007, significant research continues to examine the optimal means of preventing, controlling, and treating asthma in children. This review summarizes the evidence to date so that you can maximize your care for these young patients.
A stepwise approach to asthma control
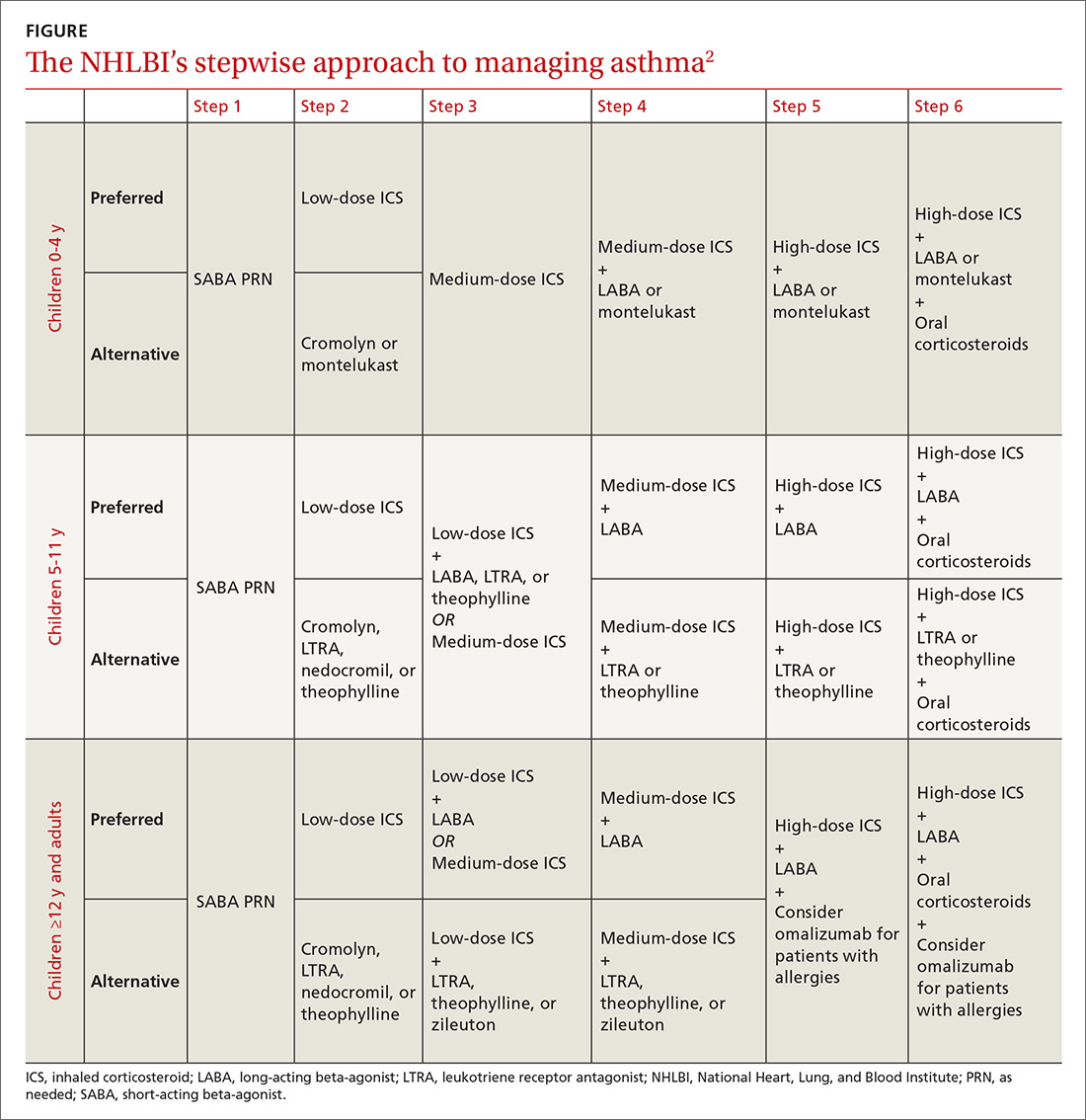
The 2007 National Heart, Lung, and Blood Institute (NHLBI) guidelines provide a common pathway for the management of asthma (FIGURE).2 These guidelines emphasize stepwise treatment, based on symptom severity, which maximizes quality of life while minimizing morbidity. Treatment is escalated after careful assessment of frequency of daytime symptoms, frequency of nighttime symptoms, forced expiratory volume in one second (FEV1), and the number of exacerbations requiring systemic steroids over the past year. It’s also appropriate to de-escalate care when symptoms are controlled to minimize adverse effects.
The 2017 Global Initiative for Asthma (GINA) guidelines recommend a similar stepwise approach, with generally the same progression of control medications as the NHLBI guidelines. One variation is that GINA guidelines recommend considering inhaled corticosteroids (ICS) for all asthma patients—even those with intermittent symptoms.3
The Asthma Control Test and Asthma Control Questionnaire are also available for assessment of efficacy of asthma control and can help guide FPs with the decision of when to escalate control medications. (They are available at: http://bit.ly/2o3CzGX and http://bit.ly/2p1LvAc, respectively.)
A systematic review found that both tools were effective in determining if a patient was well controlled vs not well controlled.4 Similarly, a 2012 study found that using the Asthma Control Test score was useful for assessing control and directing changes to treatment.5 In addition, using these tools consistently in a primary care setting increased the frequency of assessment without negatively impacting patient flow through a clinic.6
Short-acting beta-agonists: A mainstay for intermittent asthma
Inhaled short-acting beta-agonists (SABAs) are the mainstay of treatment for intermittent asthma as well as asthma exacerbations. Short-acting beta-agonists are effective for symptomatic relief and preventing exacerbations prior to known exposures because they dilate smooth muscle in bronchioles and relieve bronchospasm.
Albuterol, the most commonly used SABA, is a mixture of the active R-enantiomer and inactive L-enantiomer. Levalbuterol, which consists of only the active R-enantiomer, is also available and is theoretically more effective with fewer adverse effects. Studies examining the difference in efficacy between albuterol and levalbuterol, however, have been mixed, and current guidelines do not recommend one over the other.7
Metered-dose inhalers vs nebulizers
SABAs are typically prescribed in metered-dose inhalers (MDIs), dry powder inhalers, or nebulizers. A meta-analysis comparing the efficacy of nebulizers to MDIs with spacers in both the outpatient and emergency department (ED) settings indicated that MDIs work at least as well as nebulizers and may also reduce length of ED stay.8 This trend appears consistent even with children younger than 24 months.9
If you are prescribing an MDI, be sure to routinely prescribe spacers to help ensure the medication is properly administered. Various types of spacers are available; some consist of an extension of the mouthpiece, while others serve as a chamber with a one-way valve to help improve the ability of the child to inhale the medication. Spacers that do not have antistatic coating should be gently washed with water and detergent.
Masks are also available for younger children and should be properly sized. The same spacer can be used for multiple medications, although they should be administered one at a time. Generally, albuterol should be administered prior to other medications to maximize distribution of subsequent inhaled medications.
Start low with inhaled corticosteroids
Treatment of persistent asthma consists of regular use of inhaled corticosteroids (ICSs; first line) with SABAs, as needed, for exacerbations. Guidelines recommend starting at a low dose and increasing the dose based on symptom control. Patients must consistently use ICSs for one to 2 weeks prior to obtaining full effect of the medication, and parents should be counseled to set appropriate expectations.2,3 ICSs are dispensed as MDIs, dry powder inhalers, and nebulizers; spacers should be considered.
Studies have shown a slight decrease in height for children on ICSs, with long-term studies indicating a persistent 1- to 2-cm decrease in adult height.10,11
Patient isn’t well controlled? Time for a long-acting beta-agonist
For patients not well controlled on low-dose ICSs, the dose can be increased or a long-acting beta-agonist (LABA) or leukotriene receptor antagonist (LTRA) can be added. A recent meta-analysis examining children not well controlled with ICSs, found that the addition of LABAs resulted in improved FEV1 and nighttime symptoms, and reduced the requirement for rescue inhalers when compared to increasing the ICS dose alone.12 In addition, there was no difference in adverse events between the 2 agents, although patients taking ICSs and LABAs had greater growth in height than those with an increased dose of ICS.12 Adding a LABA, however, did not decrease the need for systemic steroids and also did not reduce the number of exacerbations requiring hospitalizations.12