The Advisory Committee on Immunization Practices (ACIP) made relatively few new vaccine recommendations in 2017. One pertained to prevention of hepatitis B virus (HBV) infection in infants born to HBV-infected mothers. Another recommended a new vaccine to prevent shingles. A third advised considering an additional dose of mumps vaccine during an outbreak. This year’s recommendations pertaining to influenza vaccines were covered in a previous Practice Alert.1
Perinatal HBV prevention: New strategy if revaccination is required
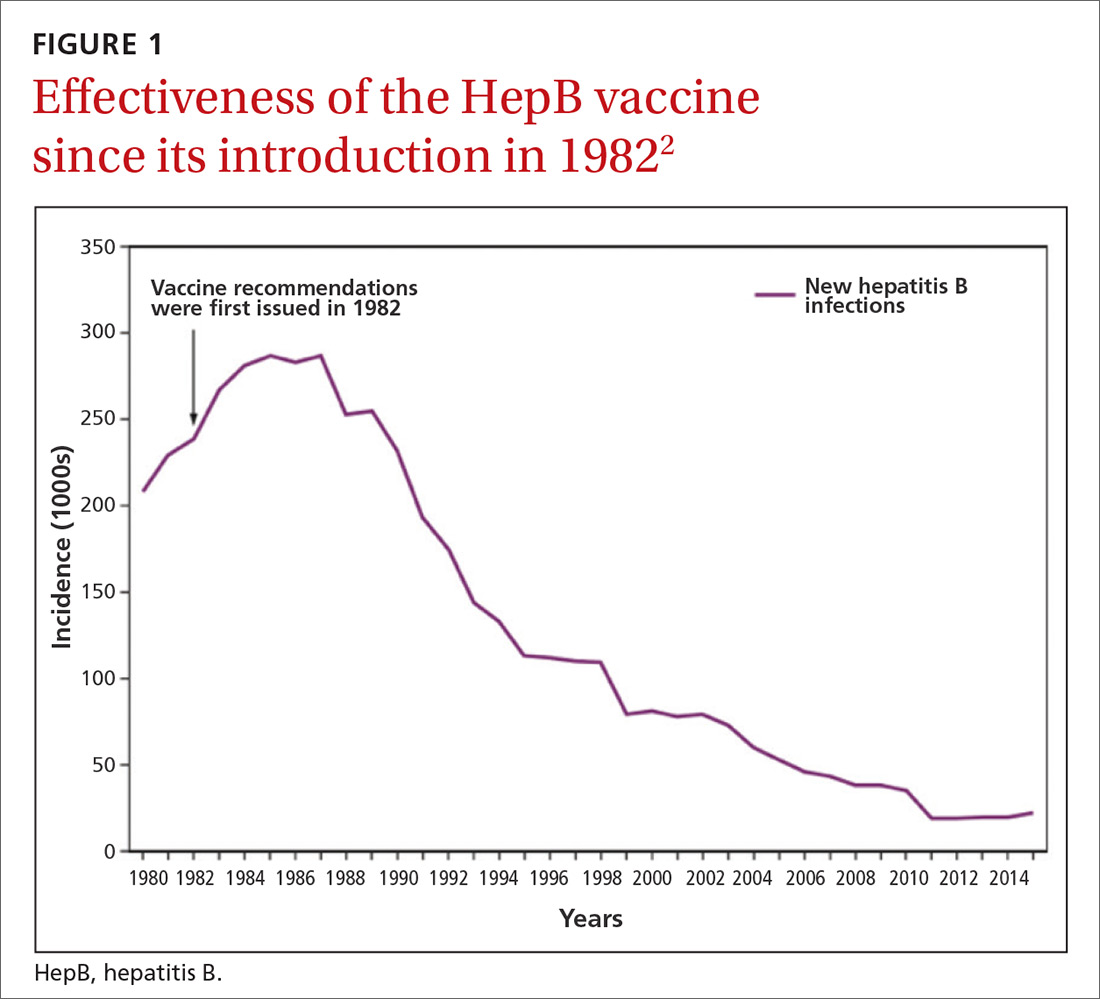
Hepatitis B prevention programs in the United States have decreased the incidence of HBV infections from 9.6 cases per 100,000 population in 1982 (the year the hepatitis B [HepB] vaccine was first available) to 1.1 cases per 100,000 population in 2015 (FIGURE 1).2 One major route of HBV dissemination worldwide is perinatal transmission to infants by HBV-infected mothers. However, this route of infection has been greatly diminished in the United States because of widespread screening of pregnant women and because newborns of mothers with known active HBV infection receive prophylaxis with hepatitis B immune globulin and HBV vaccine.
Each year in the United States an estimated 25,000 infants are born to mothers who are positive for hepatitis B surface antigen (HBsAg).3 Without post-exposure prophylaxis, 85% of these infants would develop HBV infection if the mother is also hepatitis B e antigen (HBeAg) positive; 30% would develop HBV infection if the mother is HBeAg negative.2 Eighty percent to 90% of infected infants develop chronic HBV infection and are at increased risk of chronic liver disease.2 Of all infants receiving the recommended post-exposure prophylaxis, only about 1% develop infection.2
Available HepB vaccines. HepB vaccine consists of HBsAg derived from yeast using recombinant DNA technology, which is then purified by biochemical separation techniques. Three vaccine products are available for newborns and infants in the United States. Two are single-antigen vaccines—Engerix-B (GlaxoSmithKline Biologicals) and Recombivax HB (Merck & Co.)—and both can be used starting at birth. One combination vaccine, Pediarix (GlaxoSmithKline Biologicals) is used for children ages 6 weeks to 6 years. It contains HBsAg as do the other 2 vaccines, as well as diphtheria and tetanus toxoids, acellular pertussis adsorbed, and inactivated poliovirus (DTaP-HepB-IPV).
Until December 31, 2014, a vaccine combining HBsAg and haemophilus-B antigen, Comvax (Merck and Co.), was available for infants 6 weeks or older. Comvax is no longer produced.
Factors affecting the dosing schedule. For infants born to HBsAg-positive mothers, the final dose of the HepB series should be completed at age 6 months with either one of the monovalent HepB vaccines or the DTaP-HepB-IPV vaccine. When the now-discontinued Comvax was used to complete the series, the final dose was administered at 12 to 15 months. The timing of HepB vaccine at birth and at subsequent intervals, and a decision on whether to give hepatitis B immune globulin, depend on the baby’s birth weight, the mother’s HBsAg status, and type of vaccine used.2
Post-vaccination assessment. ACIP recommends that babies born to HBsAg-positive mothers and having received the final dose of the vaccine series be serologically tested for immunity to HBV at age 9 to 12 months; or if the series is delayed, at one to 2 months after the final dose.4 Infants without evidence of active infection (ie, HBsAg negative) and with levels of antibody to HBsAg ≥10 mIU/mL are considered protected and need no further vaccinations.4 Revaccination is advised for those with antibody levels <10 mIU/mL—who account for only about 2% of infants having received the recommended schedule.4
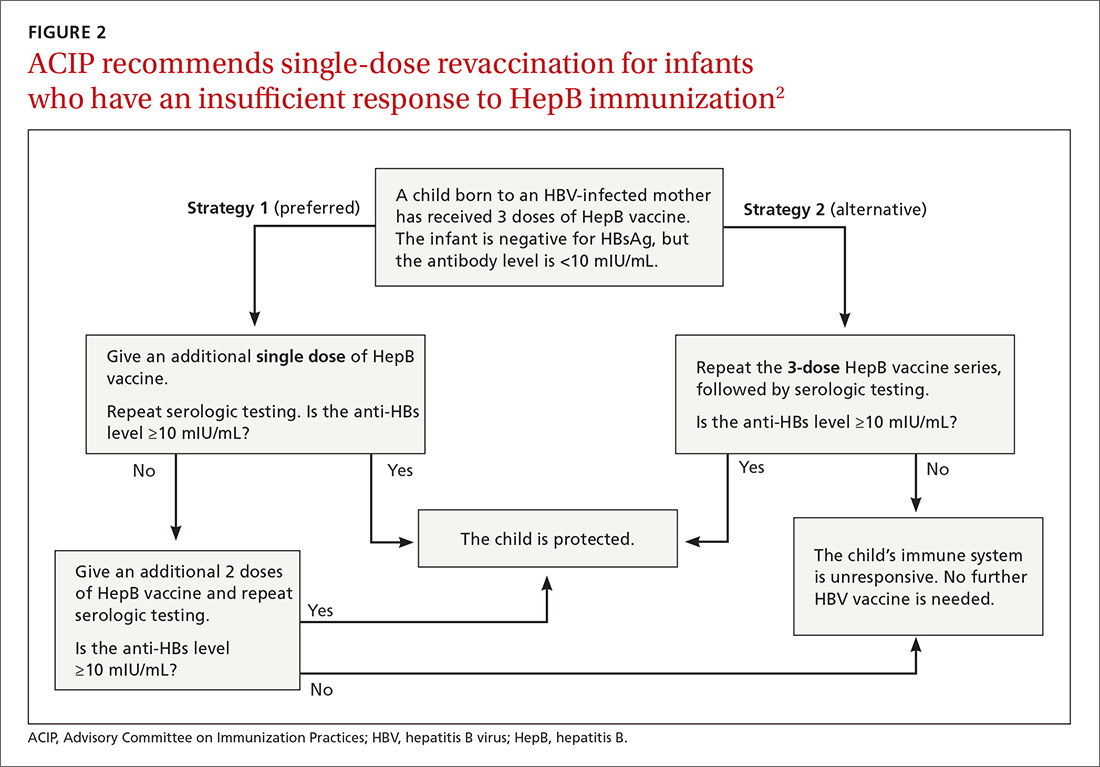
New revaccination strategy. The previous recommendation on revaccination advised a second 3-dose series with repeat serologic testing one to 2 months after the final dose of vaccine. Although this strategy is still acceptable, the new recommendation for infants with antibody levels <10 mIU/mL favors (for cost savings and convenience) administration of a single dose of HepB vaccine with retesting one to 2 months later.2
Several studies presented at the ACIP meeting in February 2017 showed that more than 90% of infants revaccinated with the single dose will develop a protective antibody level.4 Infants whose anti-HBs remain <10 mIU/mL following the single-dose re-vaccination should receive 2 additional doses of HepB vaccine, followed by testing one to 2 months after the last dose4 (FIGURE 22).
(A new HepB vaccine, HEPLISAV-B [Dynavax Technologies Corp]), has been approved for use in adults. More on this in a bit.)