The US Preventive Services Task Force (USPSTF) had a productive year in 2022. In total, the USPSTF
- reviewed and made recommendations on 4 new topics
- re-assessed 19 previous recommendations on 11 topics
- made 24 separate recommendations, including 1 “A,” 3 “B,” 3 “C,” and 5 “D” recommendations and 12 “I” statements (see TABLE 11).
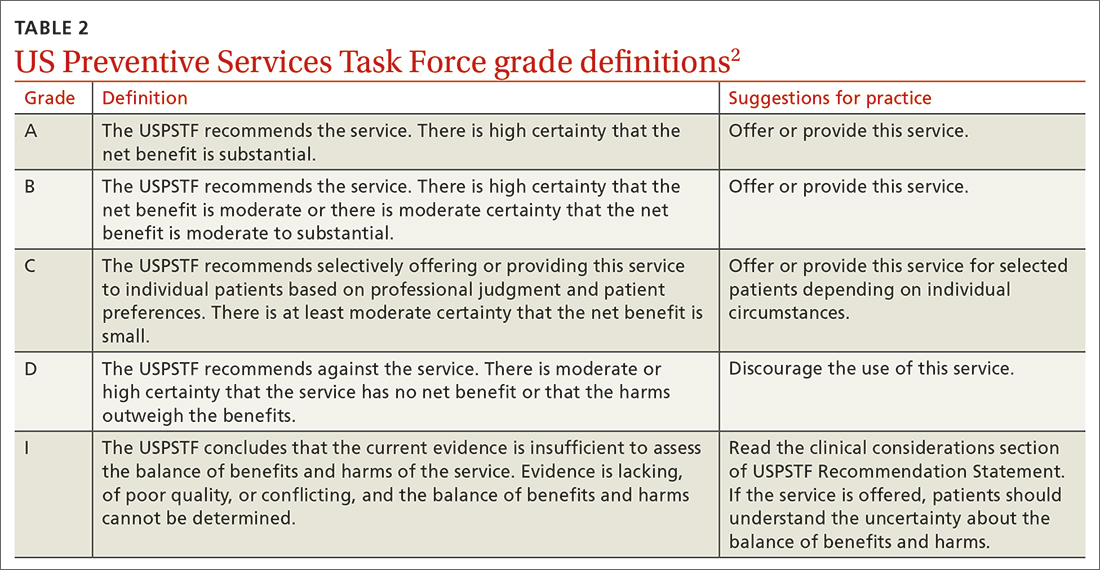
A note about grading. TABLE 22 outlines the USPSTF’s grade definitions and suggestions for practice. The importance of an “A” or “B” recommendation rests historically with the requirement in the Affordable Care Act (ACA) that all USPSTF-recommended services with either of these grades have to be provided by commercial health insurance plans with no co-pay or deductible applied. (The legal challenge in Texas to the ACA’s preventive care provision may change that.)
What’s new?
The USPSTF’s review of 4 new topics exceeds the entity’s output in each of the prior 4 years, when the Task Force was able to add only 1 or 2 topics annually. However, 3 of the 4 new topics in 2022 resulted in an insufficient evidence or “I” statement, which means there was not enough evidence to judge the relative benefits and harms of the intervention.
These 3 included screening for type 2 diabetes in children and adolescents younger than 18 years; screening for obstructive sleep apnea in the general adult population (ages ≥ 18 years); and screening for eating disorders in adolescents and adults. The fourth new topic, screening for anxiety in children and adolescents, resulted in a “B” recommendation and was described in a recent Practice Alert.3
Major revision to 1 prior recommendation
Only 1 of the 19 revisited recommendations resulted in a major revision: the use of daily aspirin for primary prevention of cardiovascular disease (CVD). Note that it does not apply to those who have established CVD, in whom the use of aspirin would be considered tertiary prevention or harm reduction.
In 2016, the USPSTF recommended (with a “B” grade) the use of daily low-dose aspirin for those ages 50 to 59 years who had a 10-year risk for a CVD event > 10%; no increased risk for bleeding; at least a 10-year life expectancy; and a willingness to take aspirin for 10 years. For those ages 60 to 69 years with a 10-year risk for a CVD event > 10%, the recommendation was a “C.” For those younger than 50 and older than 70, an “I” statement was issued.
In 2022, the USPSTF was much less enthusiastic about daily aspirin as a primary preventative.4 The recommendation is now a “C” for those ages 40 to 59 years who have a 10-year CVD risk ≥ 10%. Those most likely to benefit have a 10-year CVD risk > 15%.
Continue to: The recommendation pertains...