Evidence summary
Adding ezetimibe reduces nonfatal events but does not improve mortality
A 2018 Cochrane meta-analysis included 10 RCTs (N = 21,919 patients) that evaluated the efficacy and safety of ezetimibe plus a statin (dual therapy) vs a statin alone or plus placebo (monotherapy) for the secondary prevention of CVD. Mean age of patients ranged from 55 to 84 years. Almost all of the patients (> 99%) included in the analyses had existing ASCVD. The dose of ezetimibe was 10 mg; statins used included atorvastatin 10 to 80 mg, pitavastatin 2 to 4 mg, rosuvastatin 10 mg, and simvastatin 20 to 80 mg.1
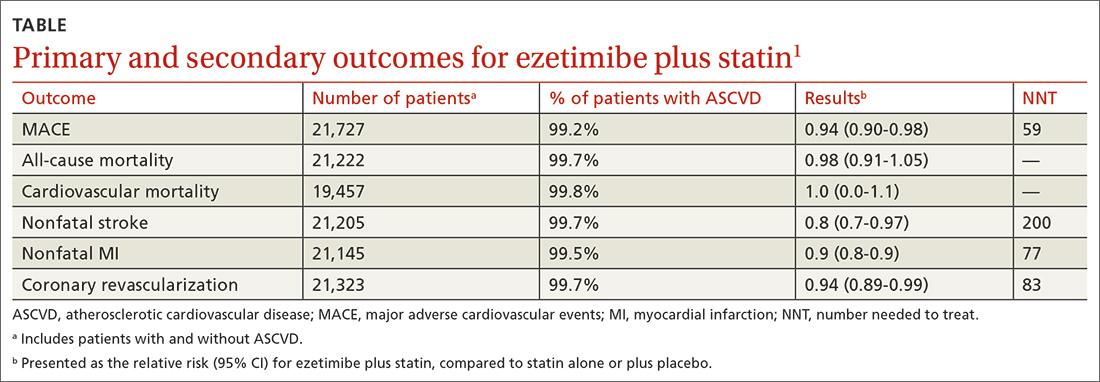
The primary outcomes were MACE and all-cause mortality. MACE is defined as a composite of CVD, nonfatal myocardial infarction (MI), nonfatal stroke, hospitalization for unstable angina, or coronary revascularization procedures. The TABLE1 provides a detailed breakdown of each of the outcomes.
The dual-therapy group compared to the monotherapy group had a lower risk for MACE (26.6% vs 28.3%; 1.7% absolute risk reduction; 6% relative risk reduction; NNT = 59) and little or no difference in the reduction of all-cause mortality. For secondary outcomes, the dual-therapy group had a lower risk for nonfatal MI, nonfatal stroke, and coronary revascularization. There was no difference in cardiovascular mortality or adverse events between the 2 groups. The quality of evidence was high for all-cause mortality and moderate for cardiovascular mortality, MACE, MI, and stroke.1
The 2015 IMPROVE-IT study, the largest included in the Cochrane review, was a double-blind RCT (N = 18,144) conducted at 1147 sites in 39 countries comparing simvastatin 40 mg/d plus ezetimibe 10 mg/d (dual therapy) vs simvastatin 40 mg/d plus placebo (monotherapy). Patients were at least 50 years old (average age, 64 years) and had been hospitalized for acute coronary syndrome (ACS) within the previous 10 days; 76% were male and 84% were White. The average low-density lipoprotein (LDL) concentration at baseline was 94 mg/dL in both groups.2
The primary endpoint was a composite of cardiovascular death, a major coronary event (nonfatal MI, unstable angina requiring hospitalization, coronary revascularization at least 30 days after randomization), or nonfatal stroke, with a median follow-up of 6 years. The simvastatin plus ezetimibe group compared to the simvastatin-only group had a lower risk for the primary end point (HR = 0.94; 95% CI, 0.89-0.99; NNT = 50), but no differences in cardiovascular or all-cause mortality. Since the study only recruited patients with recent ACS, results are only applicable to that specific population.2
The 2022 RACING study was a multicenter, open-label, randomized, noninferiority trial that evaluated the combination of ezetimibe 10 mg and a moderate-intensity statin (rosuvastatin 10 mg) compared to a high-intensity statin alone (rosuvastatin 20 mg) in adults (N = 3780) with ASCVD. Included patients were ages 19 to 80 years (mean, 64 years) and had a baseline LDL concentration of 80 mg/dL (standard deviation, 64-100 mg/dL) with known ASCVD (defined by prior MI, ACS, history of coronary or other arterial revascularization, ischemic stroke, or peripheral artery disease); 75% were male.3
The primary outcome was a composite of cardiovascular death, major cardiovascular events, or nonfatal stroke. At 3 years, an intention-to-treat analysis found no significant difference between the combination and monotherapy groups (9% vs 9.9%; absolute difference, –0.78%; 95% CI, –2.39% to 0.83%). Dose reduction or discontinuation of the study drug(s) due to intolerance was lower in the combination group than in the monotherapy group (4.8% vs 8.2%; P < 0.0001). The study may be limited by the fact that it was nonblinded and all participants were South Korean, which limits generalizability.3
Recommendations from others
A 2022 evidence-based clinical practice guideline published in BMJ recommends adding ezetimibe to a statin to decrease all-cause mortality, cardiovascular mortality, nonfatal stroke, and nonfatal MI in patients with known CVD, regardless of their LDL concentration (weak recommendation based on a systematic review and network meta-analysis).4
In 2019, the American Heart Association and the American College of Cardiology recommended ezetimibe for patients with clinical ASCVD who are on maximally tolerated statin therapy and have an LDL concentration of 70 mg/dL or higher (Class 2b recommendation [meaning it can be considered] based on a meta-analysis of moderate-quality RCTs).5
Editor’s takeaway
The data on this important and well-studied question have inched closer to firm and clear answers. First, adding ezetimibe to a lower-intensity statin when a higher-intensity statin is not tolerated is an effective treatment. Second, adding ezetimibe to a statin improves nonfatal ASCVD outcomes but not fatal ones. What has not yet been made clear, because a noninferiority trial does not answer this question, is whether the highest intensity statin plus ezetimibe is superior to that high-intensity statin alone, regardless of LDL concentration.