The US Preventive Services Task Force (USPSTF) recently released their final recommendation update on the use of antiretroviral therapy to prevent HIV infection in adolescents and adults who are at increased risk.1 The Task Force last addressed this topic in 2019; since then, 2 additional antiretroviral regimens have been approved for preexposure prophylaxis (PrEP). The update also includes revised wording on who should consider receiving PrEP.
HIV remains a significant public health problem in the United States. The Centers for Disease Control and Prevention (CDC) estimates that 1.2 million people in the United States are living with HIV, and approximately 30,000 new infections occur each year.2 Men who have sex with men account for 68% of new infections, and there are marked racial disparities in both incidence and prevalence of infection, with Black/African Americans accounting for 42% of new infections.2
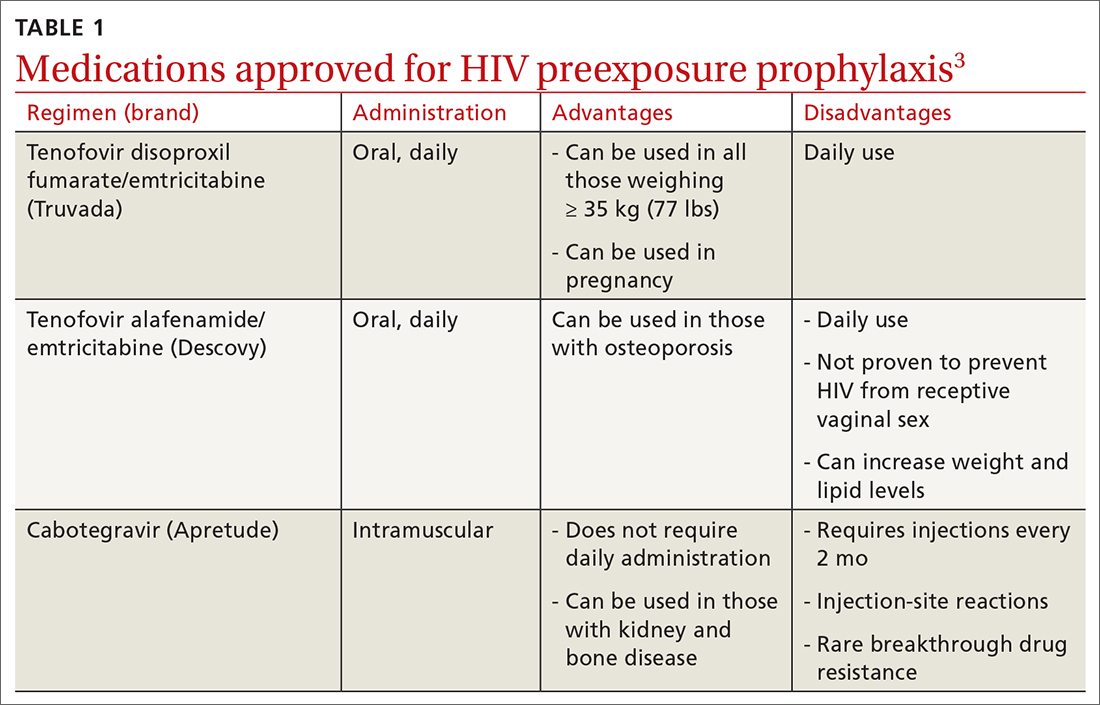
PrEP decreases the risk for HIV by about 50% overall, with higher rates of protection correlated to higher adherence (close to 100% protection with daily adherence to oral regimens).3 The 3 approved regimens for PrEP are outlined in TABLE 13.
Who’s at increased risk? The USPSTF did not find any risk assessment tools with proven accuracy in identifying those at increased risk for HIV infection but did document risk factors and behaviors that can be used to predict risk. They encourage discussion about HIV prevention with all adults and adolescents who are sexually active or who inject drugs.
Those people for whom the Task Force recommends considering PrEP are listed in TABLE 21. However, the USPSTF recommends providing PrEP to anyone who requests it, as they may not want to disclose their risk factors.
What to keep in mind. Family physicians are encouraged to read the full USPSTF report and refer to CDC guidelines on prescribing PrEP, which provide details on each regimen and the routine laboratory testing that should be performed.4 The most important clinical considerations described in the USPSTF report are:
- Before starting PrEP, document a negative HIV antigen/antibody test result and continue to test for HIV every 3 months. PrEP regimens should not be used to treat HIV.
- Document a negative HIV RNA assay if the patient has taken oral PrEP in the past 3 months or injectable PrEP in the past 12 months.
- At PrEP initiation, consider ordering other recommended tests, such as those for kidney function, chronic hepatitis B infection (if using tenofovir disoproxil fumarate/emtricitabine), lipid levels (if using tenofovir alafenamide/emtricitabine), and other sexually transmitted infection (STIs).
- Encourage the use of condoms, as PrEP does not protect from other STIs.
- Follow up regularly, and at each patient visit stress the need for medication adherence to achieve maximum protection.