In the past year, there has been significant progress in the availability of interventions to prevent respiratory syncytial virus (RSV) and its complications. Four products have been approved by the US Food and Drug Administration (FDA) and recommended by the Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP). They include 2 vaccines for adults ages 60 years and older, a monoclonal antibody for infants and high-risk children, and a maternal vaccine to prevent RSV infection in newborns.
RSV in adults
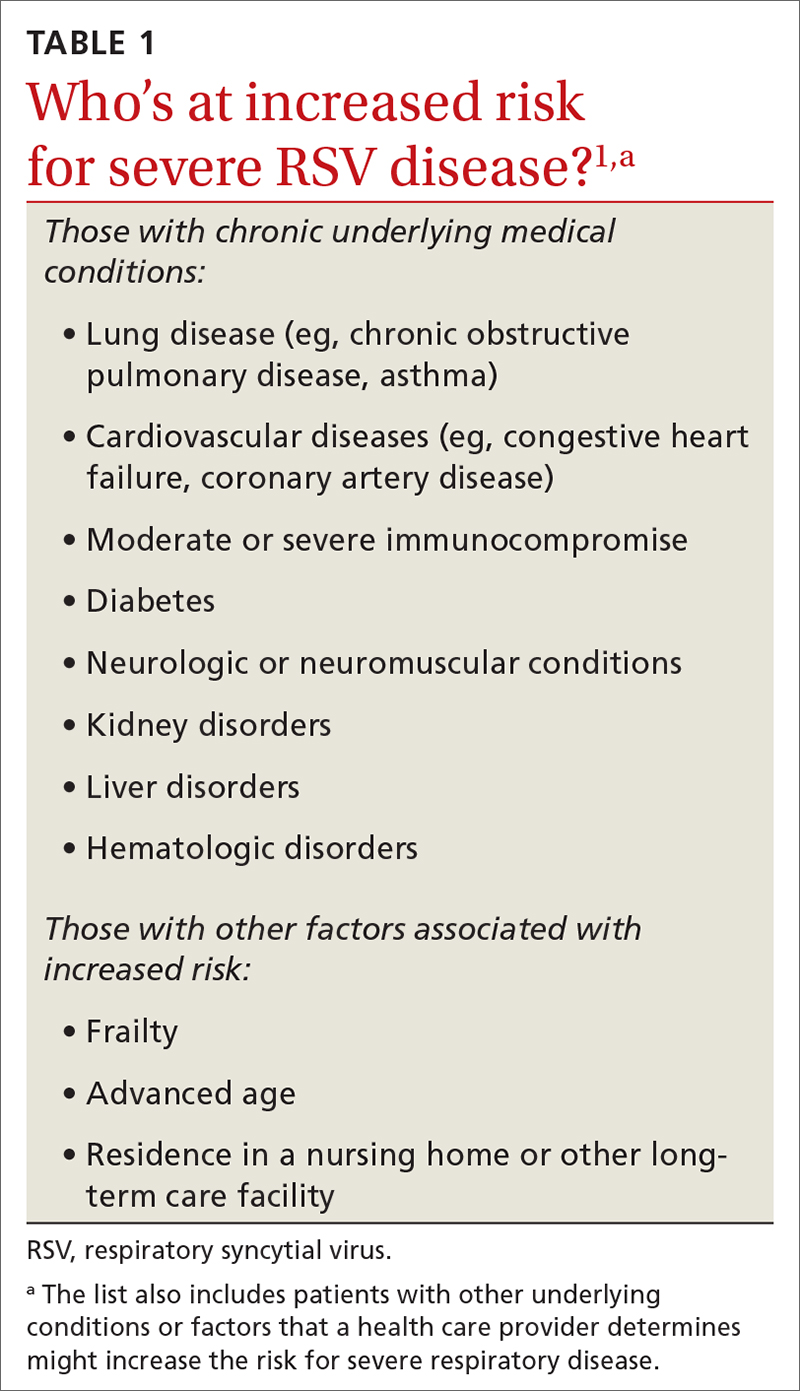
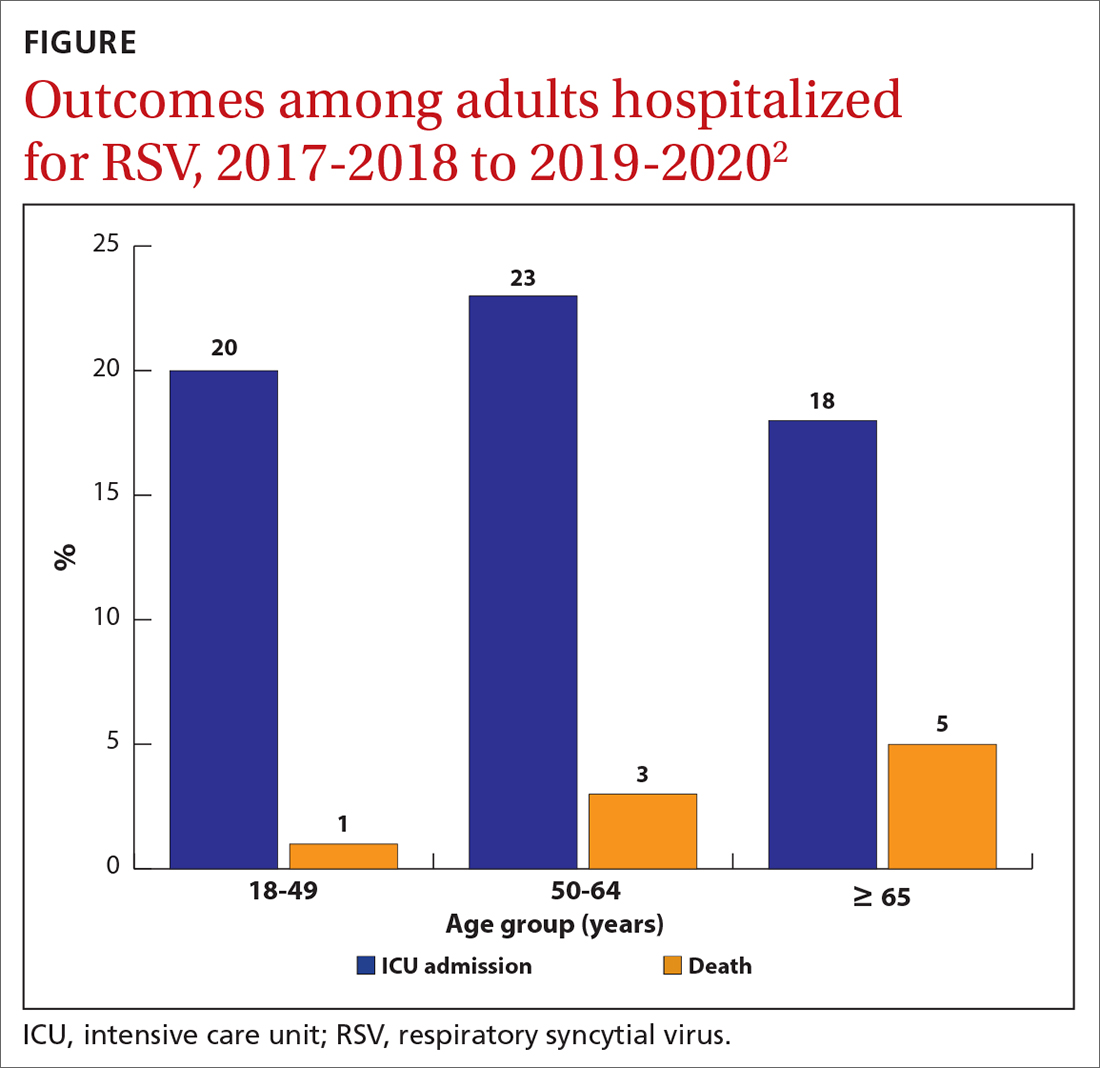
While there is some uncertainty about the total burden of RSV in adults in the United States, the CDC estimates that each year it causes 0.9 to 1.4 million medical encounters, 60,000 to 160,000 hospitalizations, and 6000 to 10,000 deaths.1 The rate of RSV-caused hospitalization increases with age,2 and the infection is more severe in those with certain chronic medical conditions (TABLE 11). The FIGURE2 demonstrates the outcomes of adults who are hospitalized for RSV. Adults older than 65 years have a 5% mortality rate if hospitalized for RSV infection.2
Vaccine options for adults
Two vaccines were recently approved for the prevention of RSV-associated lower respiratory tract disease (LRTD) in those ages 60 years and older: RSVPreF3 (Arexvy, GSK), which is an adjuvanted recombinant F protein vaccine, and RSVpreF (Abrysvo, Pfizer), which is a recombinant stabilized vaccine. Both require only a single dose (0.5 mL IM), which provides protection for 2 years.
The efficacy of the GSK vaccine in preventing laboratory-confirmed, RSV-associated LRTD was 82.6% during the first RSV season and 56.1% during the second season. The efficacy of the Pfizer vaccine in preventing symptomatic, laboratory-confirmed LRTD was 88.9% during the first RSV season and 78.6% during the second season.1 However, the trials leading to licensure of both vaccines were underpowered to show efficacy in the oldest adults and those who are frail or to show efficacy against RSV-caused hospitalization.
Safety of the adult RSV vaccines. The safety trials for both vaccines had a total of 38,177 participants. There were a total of 6 neurologic inflammatory conditions that developed within 42 days of vaccination, including 2 cases of suspected Guillain-Barré syndrome (GBS), 2 cases of possible acute disseminated encephalomyelitis, and 1 case each of chronic inflammatory demyelinating polyneuropathy and undifferentiated motor-sensory axonal polyneuropathy.1 That is a rate of 1 case of a neurologic inflammatory condition for every 6363 people vaccinated. Since the trials were not powered to determine whether the small number of cases were due to chance, postmarketing surveillance will be needed to clarify the true risk for GBS or other neurologic inflammatory events from RSV vaccination.
The lack of efficacy data for the most vulnerable older adults and the lingering questions about safety prompted the ACIP to recommend that adults ages 60 years and older may receive a single dose of RSV vaccine, using shared clinical decision-making—which is different from a routine or risk-based vaccine recommendation. For RSV vaccination, the decision to vaccinate should be based on a risk/benefit discussion between the clinician and the patient. Those most likely to benefit from the vaccine are listed in TABLE 1.1
While data on coadministration of RSV vaccines with other adult vaccines are sparse, the ACIP states that co-administration with other vaccines is acceptable.1 It is not known yet whether boosters will be needed after 2 years.
Continue to: RSV in infants and children