The Long Route of Research
The 1995 Nelson-Grether study compared VLBW (< 1500 g) infants who survived and developed moderate/severe cerebral palsy within 3 years to randomly selected VLBW controls with respect to whether their mothers had received MgSO4 to prevent seizures in preeclampsia or as a tocolytic agent.5 In a population of more than 155,000 children born between 1983 and 1985, in utero exposure to MgSO4 was reported in 7.1% of 42 VLBW infants with cerebral palsy and 36% of 75 VLBW controls (odds ratio [OR], 0.14; 95% CI, 0.05-0.51). In women without preeclampsia the OR increased to 0.25.
This motivating study had been preceded by several observational studies showing that infants born to women with preeclampsia who received MgSO4 had significantly lower risks of developing intraventricular hemorrhage (IVH) and germinal matrix hemorrhage (GMH). In one of these studies, published in 1992, Karl C. Kuban, MD, and coauthors reported that “maternal receipt of magnesium sulfate was associated with diminished risk of GMH-IVH even in those babies born to mothers who apparently did not have preeclampsia.”9
In the several years following the 1995 Nelson-Grether study, several other case-control/observational studies were reported, with conflicting conclusions, and investigators around the world began designing and conducting needed randomized controlled trials.
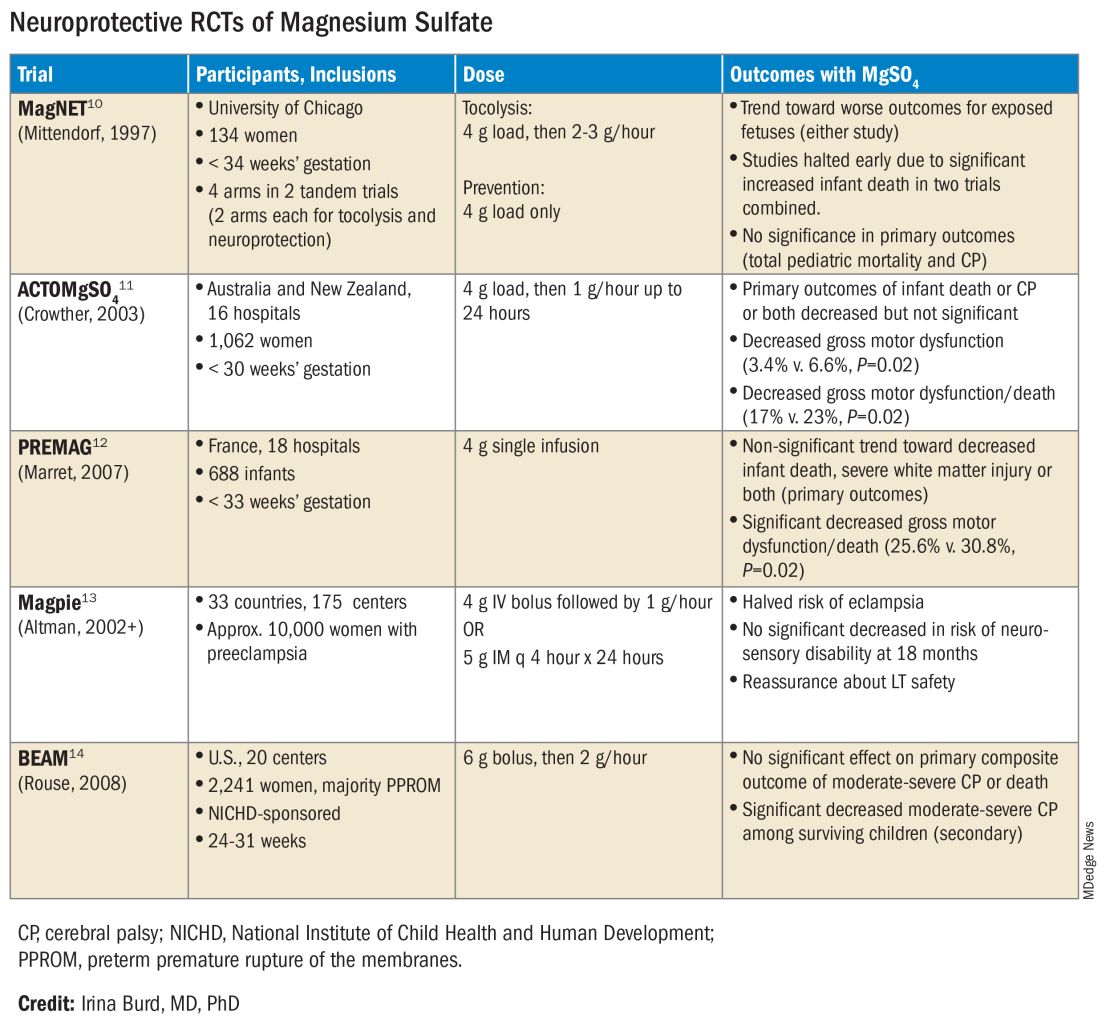
The six published randomized controlled trials looking at MgSO4 and neuroprotection varied in their inclusion and exclusion criteria, their recruitment and enrollment style, the gestational ages for MgSO4 administration, loading and maintenance doses, how cerebral palsy or neuroprotection was assessed, and other factors (See Table for RCT characteristics and main outcomes).10-14 One of the trials aimed primarily at evaluating the efficacy of MgSO4 for preventing preeclampsia.
Again, none of the randomized controlled trials demonstrated statistical significance for their primary outcomes or concluded that there was a significant neuroprotective effect for cerebral palsy. Rather, most suggested benefit through secondary analyses. Moreover, as mentioned earlier, research that proceeded after the first published randomized controlled trial — the Magnesium and Neurologic Endpoints (MAGnet) trial — was suspended early when an interim analysis showed a significantly increased risk of mortality in MgSO4-exposed fetuses. All told, it wasn’t until researchers obtained unpublished data and conducted meta-analyses and systematic reviews that a significant effect of MgSO4 on cerebral palsy could be seen.
The three systematic reviews and the Cochrane review, each of which used slightly different methodologies, were published in rapid succession in 2009. One review calculated a relative risk of cerebral palsy of 0.71 (95% CI, 0.55-0.91) — and a relative risk for the combined outcome of death and cerebral palsy at 0.85 (95% CI, 0.74-0.98) — when women at risk of preterm birth were given MgSO4.15 The number needed to treat (NNT) to prevent one case of cerebral palsy was 63, investigators determined, and the NNT to prevent one case of cerebral palsy or infant death was 44.
Another review estimated the NNT for prevention of one case of cerebral palsy at 52 when MgSO4 is given at less than 34 weeks’ gestation, and similarly concluded that MgSO4 is associated with a significantly “reduced risk of moderate/severe CP and substantial gross motor dysfunction without any statistically significant effect on the risk of total pediatric mortality.”16
A third review, from the National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU), estimated an NNT of 46 to prevent one case of cerebral palsy in infants exposed to MgSO4 before 30 weeks, and an NNT of 56 when exposure occurs before 32-34 weeks.17
The Cochrane Review, meanwhile, reported a relative reduction in the risk of cerebral palsy of 0.68 (95% CI, 0.54-0.87) when antenatal MgSO4 is given at less than 37 weeks’ gestation, as well as a significant reduction in the rate of substantial gross motor dysfunction (RR, 0.61; 95% CI, 0.44-0.85).18 The NNT to avoid one case of cerebral palsy, researchers reported, was 63.