THE TAKEAWAY: Wait at least 2 weeks to conduct monospot testing in patients with routine tonsillitis. If strong clinical suspicion exists, proceed with specific IgM and IgG serologic testing.24,25,27,28
5. Evaluating prescription drug levels: Which factors interfere?
Correct interpretation of lab tests conducted to measure prescription drug levels has major implications with regard to patient safety, particularly for medications with a narrow therapeutic index.
Most drug level tests measure the total concentration, which includes both bound and unbound (free) forms. The unbound forms are the active components of the drug; thus, for an accurate evaluation, it is important to be aware of factors that increase free drug concentration. Chief among them is low protein levels, or hypoalbuminemia.30
Risk factors for hypoalbuminemia include significant burns, advanced age, pregnancy, malnutrition, and human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS).30 HIV/AIDS is a particularly high risk because certain protease inhibitors are highly protein bound.
Drug protein binding is classified as low, moderate, or high. The main proteins involved in the process are albumin, alpha-1-acid glycoprotein, and lipoprotein. Medications that are highly protein bound (>80%) are the most affected by low protein levels: Problems can arise when drugs completely bind to all the available proteins and excess drug availability increases free drug levels.
Medications that are most likely to be affected by a high degree of protein binding include carbamazepine, cyclosporine, mycophenolic acid, phenytoin, protease inhibitors (with the exception of indinavir), tacrolimus, and valproic acid. It is important to consider free levels when you order medication assays for these drugs to avoid misinterpreting the serum levels as being too low-a scenario that raises the risk of drug toxicity and adverse outcomes.30,31
A study of 119 phenytoin samples from 70 patients found significantly higher free phenytoin levels in patients with lower albumin levels.32 Higher free phenytoin levels were also seen in older patients and in those with diminished renal function (creatinine clearance <25 mL/min).32 The degree of protein binding is affected by both the serum drug concentration and the albumin level, with saturable protein binding occurring at higher drug levels.33
Calculate phenytoin levels with this equation. To calculate corrected phenytoin levels in patients with low albumin levels, use the following formula, known as the Sheiner-Tozer equation:34
Concentration adjusted=concentration reported/([adjustment x serum albumin] + 0.1); adjustment=0.2 for creatinine clearance ≥20 or 0.1 for creatinine clearance <20.
Additional causes of misinterpreted drug levels. While hypoalbuminemia plays a major role in the misinterpretation of drug levels, other factors affect serum drug concentration, as well. These include drug-drug interactions, which can significantly increase the concentration of the medications involved, and the timing of the test with regard to medication administration. Digoxin levels, in particular, need to be drawn at least 6 to 8 hours after the last dose is taken to allow for appropriate drug distribution.35
THE TAKEAWAY: It is essential to consider free drug level monitoring in patients who either have hypoalbuminemia or have one or more risk factors for hypoalbuminemia to avoid falsely low estimation of drug levels.36,37
6 Liver function tests: Necessary for patients on statin therapy?
Since statins gained US Food and Drug Administration (FDA) approval, the drugs have been associated with increased liver function tests (LFTs). Indeed, there had been a long-standing belief, based on clinical trials, that by monitoring alanine aminotransferase (ALT) and maintaining it at <3 times the upper limit normal (ULN), hepatotoxicity could be avoided.38 In clinical practice, however, further ALT elevation is frequently allowed based on patient tolerability.
In February 2012, the FDA revised its safety data to reflect this practice.39 The FDA update confirmed that routine LFT monitoring is unnecessary for patients on statins—and that it is not very effective in identifying or preventing liver damage.
Overall, serious hepatotoxicity is very rare, with an incidence ≤2 per 1 million patient-years.39 The National Lipid Association Statin Safety Assessment Task Force recommends repeating LFTs that are 3 to 5 times the ULN within 6 months and continuing with the statin dose if the patient is asymptomatic.38
THE TAKEAWAY: Routine liver function monitoring is not necessary for patients on statins. A better approach: Obtain baseline ALT levels, and repeat the testing only as clinically indicated thereafter.38,39
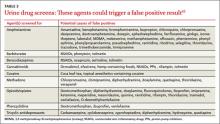
7. Urine drug screens: Which factors affect their accuracy?
The gold standard for testing for drugs of abuse, urine drug screens (UDS) have good sensitivity and specificity, easy administration, and reasonable cost.40 UDS can detect various narcotics, such as morphine, oxycodone, ,and methadone, and identify other illicit drugs, although which drugs and metabolites are tested for is laboratory- and test-specific.