Advances in genomics and technology perpetually change and improve therapies in oncology. Enhanced comprehension of cellular signaling, division, and replication has created a platform to selectively restrict neoplastic growth while preserving the integrity of benign cells.
This article reviews therapies that were newly approved in 2018, as well as those previously approved whose indications were expanded this past year. The list highlights the most clinically important approvals, as well as adverse events that are unique or especially severe.
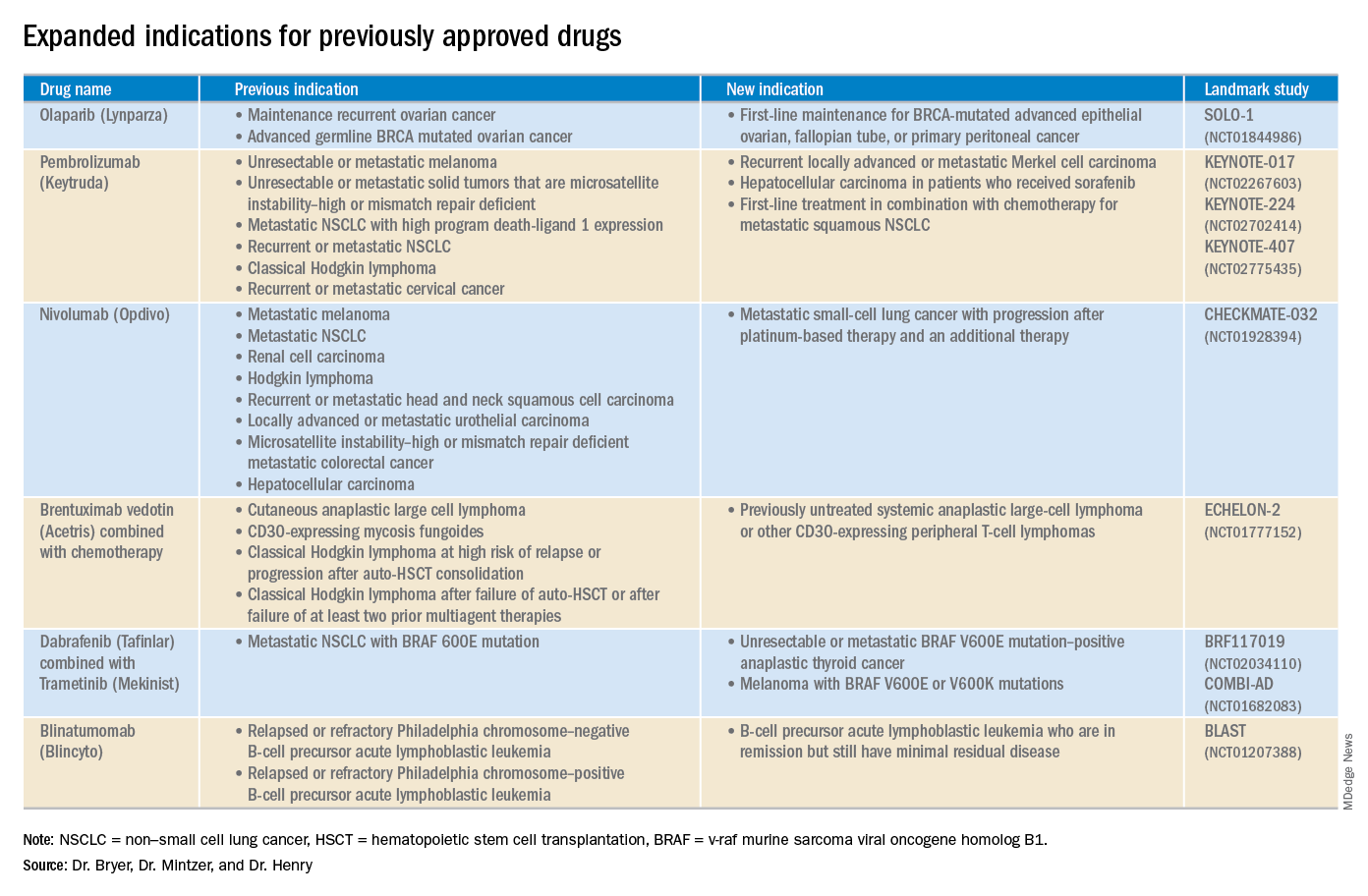
Click on the PDF above to download the full article and charts in an easy-to-print format.
Apalutamide (Erleada)
Class: Androgen receptor inhibitor.
Disease: Nonmetastatic castration-resistant prostate cancer.
Dose: 240 mg orally, once daily.
Adverse Events (AEs): Hyperkalemia and increased risks of seizures, falls, and fractures.
Phase 3 SPARTAN trial (NCT01946204): 40.5-month metastasis-free survival rate, compared with 16.2 months in the placebo group.
Cemiplimab (Libtayo)
Class: Antibody against programmed cell death protein-1 (PD-1).
Disease: Metastatic cutaneous squamous cell carcinoma (CSCC) or locally advanced CSCC that is ineligible for curative surgery/radiation.
Dose: 350 mg intravenous infusion every 3 weeks.
AEs: Pneumonitis, autoimmune myocarditis, hepatitis, and aseptic meningitis.
1423 and 1540 trials (NCT02383212 and NCT02760498): 47.2% of patients who received cemiplimab had complete disappearance of the tumor or a decrease in tumor size.
Dacomitinib (Vizimpro)
Class: Second-generation tyrosine kinase inhibitor.
Disease: Metastatic non–small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 19 deletion or exon 21 L858R substitution mutation.
Dose: 45 mg orally once daily.
AEs: Dermatotoxicity and diarrhea.
ARCHER1050 trial (NCT01774721): Patients who received dacomitinib demonstrated an improved overall survival, with a median of 34.1 months, compared with 26.8 months with gefitinib.
Duvelisib (Copiktra)
Class: Dual inhibitor of phosphatidylinositol 3-kinase delta and gamma.
Disease: Relapsed or refractory chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma, or relapsed or refractory follicular lymphoma after at least two prior systemic therapies.
Dose: 25 mg orally twice daily.
AEs: Infection, diarrhea or colitis, and pneumonia.
Phase 3 DUO trial (NCT02004522): Progression-free survival in the duvelisib arm was 7.3 months longer than that in the ofatumumab arm. The overall response rate for patients receiving duvelisib was 78%, compared with 39% for those receiving ofatumumab.