ESTES PARK, COLO. – The investigational HZ/su vaccine looks like it could be the herpes zoster vaccine of the future for older adults, Dr. Laura P. Hurley said at a conference on internal medicine sponsored by the University of Colorado.
The vaccine demonstrated unprecedented efficacy in the recently reported international ZOE-50 study, which featured 15,411 participants aged 50 years and older who were randomized to either vaccine or placebo.
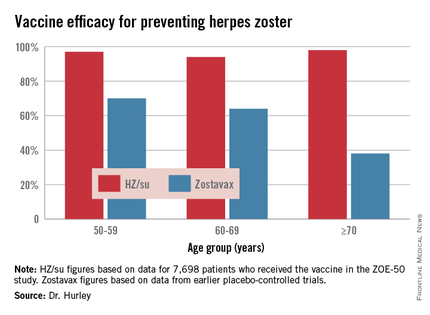
During a mean 3.2 years of follow-up, the vaccine efficacy was 97.2% for prevention of herpes zoster in adults aged 50 and older. Herpes zoster occurred in 6 subjects in the vaccine group and 210 in the placebo arm. That’s an incidence rate of 0.3 versus 9.1 cases per 1,000 person-years (N Engl J Med. 2015 May 28;372[22]:2087-96).
The HZ/su vaccine, a subunit vaccine containing varicella-zoster virus glycoprotein E and a proprietary adjuvant system, crushed the current Zostavax vaccine in terms of vaccine efficacy in all older-age subgroups based upon comparative data from the earlier large placebo-controlled trials of Zostavax. Efficacy of the HZ/su vaccine was independent of age and was as good in persons over age 70 – the age group where the incidence of herpes zoster and its complications is highest – as in the 50- to 59-year-olds, noted Dr. Hurley, a general internist and vaccination researcher at the university.
Follow-up in ZOE-50 is ongoing and planned for at least 10 years in order to determine the duration of protection. Thus far, however, there is no signal of waning efficacy. In contrast, a recent study of nearly 7,000 Zostavax recipients showed that its efficacy declined over time and remained statistically significant only through year 8 post vaccination (Clin Infect Dis. 2015 Mar 15;60[6]:900-9), she observed.
Dr. Hurley described another big advantage of the investigational vaccine, in addition to its almost-too-good-to-be-true efficacy: the fact that, unlike Zostavax, it’s not a live-virus vaccine. That means it doesn’t require freezer storage, thereby removing a major impediment to convenient vaccination. It also means it can potentially be safe for use in immunocompromised patients. Such patients were excluded from ZOE-50 but are the focus of ongoing studies conducted in patients with hematologic and solid organ malignancies, as well as renal transplant recipients.
The HZ/su vaccine has two major drawbacks. One is that it’s delivered in a series of two intramuscular doses with the injections given 2 months apart. “A two-shot series always creates problems with implementation,” according to Dr. Hurley.
The other shortcoming is the high rate of local and systemic reactions during the first week post vaccination. Most of the reactions were mild to moderate in severity and lasted only 1 or 2 days.
“Patients who had a large local reaction to the first shot and were asked to come back 2 months later for the second might be less inclined to do so,” the physician said.
The HZ/su vaccine is being developed by GlaxoSmithKline. Dr. Hurley reported having no financial conflicts of interest.