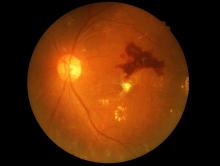
The Food and Drug Administration has permitted the marketing of IDx-DR, a retinal imaging device that uses artificial intelligence (AI) to detect greater than a mild level of diabetic retinopathy in adult patients with diabetes. It also is the first authorized device that provides a screening tool without the need of an eye care specialist, making it ideal for health care providers who do not specialize in eye care.
“Early detection of retinopathy is an important part of managing care for the millions of people with diabetes, yet many patients with diabetes are not adequately screened for diabetic retinopathy.” About 50% of people with diabetes do not have the recommended annual retinopathy screening exam, Malvina Eydelman, MD, director of the division of ophthalmic, and ear, nose, and throat devices at the FDA’s Center for Devices and Radiological Health noted in a press release.
IDx-DR is the first device authorized for marketing that provides a screening decision without the need for a clinician to interpret the image or results, which makes it usable by health care providers who may not normally be involved in eye care, according to the FDA.The software behind IDx-DR utilizes an artificial intelligence program to analyze retinal images captured by a retinal camera, the Topcon NW400. After one or more images are taken, they are uploaded to a Cloud server, on which IDx-DR software is installed, where they can be analyzed using an algorithm. In cases in which the images are of sufficient quality, the software provides the doctor with one of two results: “More than mild diabetic retinopathy detected: refer to an eye care professional” or “Negative for more than mild diabetic retinopathy; rescreen in 12 months.”
If a positive result is detected, patients should see an eye care specialist for further diagnostic evaluation and treatment as soon as possible.
The FDA reviewed data obtained from a clinical study prior to approving IDx-DR for marketing. The study looked at retinal images from 900 patients at 10 primary care sites. The aim of the study was to determine how often IDx-DR was correct in identifying mild diabetic retinopathy. The device was accurate nearly 90% of the time, correctly identifying mild diabetic retinopathy 87.4% of the time. It was also able to identify correctly patients who did not have mild diabetic retinopathy 89.5% of the time.
IDx-DR was approved via the FDA’s De Novo premarket review pathway, which offers a way to approve novel, low to moderate risk devices for which there are no similar devices previously approved. It also was granted a Breakthrough Device designation, because of its effectiveness in treating or diagnosing a irreversibly debilitating disease or condition.