To the Editor:
COVID-19 infection has resulted in 6.9 million deaths worldwide. India has the third highest mortality from COVID-19 infection after the United States and Brazil.1 Vaccination plays a crucial role in containing COVID-19 infection and reducing its severity. At present, 11 vaccines have been approved by the World Health Organization. India started its vaccination program on January 16, 2021, with approval for use of Covaxin (Bharat Biotech) and Covishield (Oxford/AstraZeneca formulation)(Serum Institute of India). More than 2 billion doses have been administered since then.2,3
Patients with psoriasis are prone to develop a severe form of COVID-19 due to comorbidities and the intake of immunosuppressive drugs.4 These patients often are hesitant to receive the vaccine without an expert opinion. COVID-19 vaccines are considered to increase tumor necrosis factor α (TNF-α) and IFN-γ production by CD4+ T cells. Tumor necrosis factor α is a key proinflammatory cytokine implicated in the pathogenesis of psoriasis. COVID-19 messenger RNA vaccines induce elevation of IL-6 and helper T cells (TH17), which can induce a flare of psoriasis in a subset of patients.5The International Psoriasis Council recommends that patients with psoriasis receive one of the vaccines approved to prevent COVID-19 infection as soon as possible.6 Reports of new-onset psoriasis and flare of psoriasis after the use of COVID-19 vaccines, such as those manufactured by Pfizer-BioNTech, Moderna, and AstraZeneca, have been published from different parts of the world.7 India used locally developed whole virion inactivated BBV152 (Covaxin) and nonreplicating viral vaccine ChAdOx1 nCoV-19 (Covishield) in its vaccination program and exported them to other developing countries. There is a dearth of data on the safety of these vaccines in patients with psoriasis, which needs to be assessed. Later, Covaxin, ZyCoV-D (DNA plasmid vaccine; Cadila Healthcare), and CorbeVax (protein subunit vaccine; Biological E) were approved for usage in children.8 We conducted a cross-sectional study using the direct interview method.
Patients with psoriasis who attended the outpatient department of the Postgraduate Institute of Medical Education and Research (Chandigarh, India) from April 2022 to June 2022 were invited to participate in the study after written informed consent was received. Patients 18 years and older with chronic plaque psoriasis who had received a COVID-19 vaccine dose in the last 90 days were enrolled. Data on demographics, comorbidities, treatment received for psoriasis, vaccination concerns, history of COVID-19 infection, type of vaccine received with doses, adverse effects, and psoriasis flare after receiving the vaccine (considered up to 2 weeks from the date of vaccination) were collected. Ordinal logistic regression was used to identify factors associated with a psoriasis flare following vaccination. P<.05 was considered statistically significant.
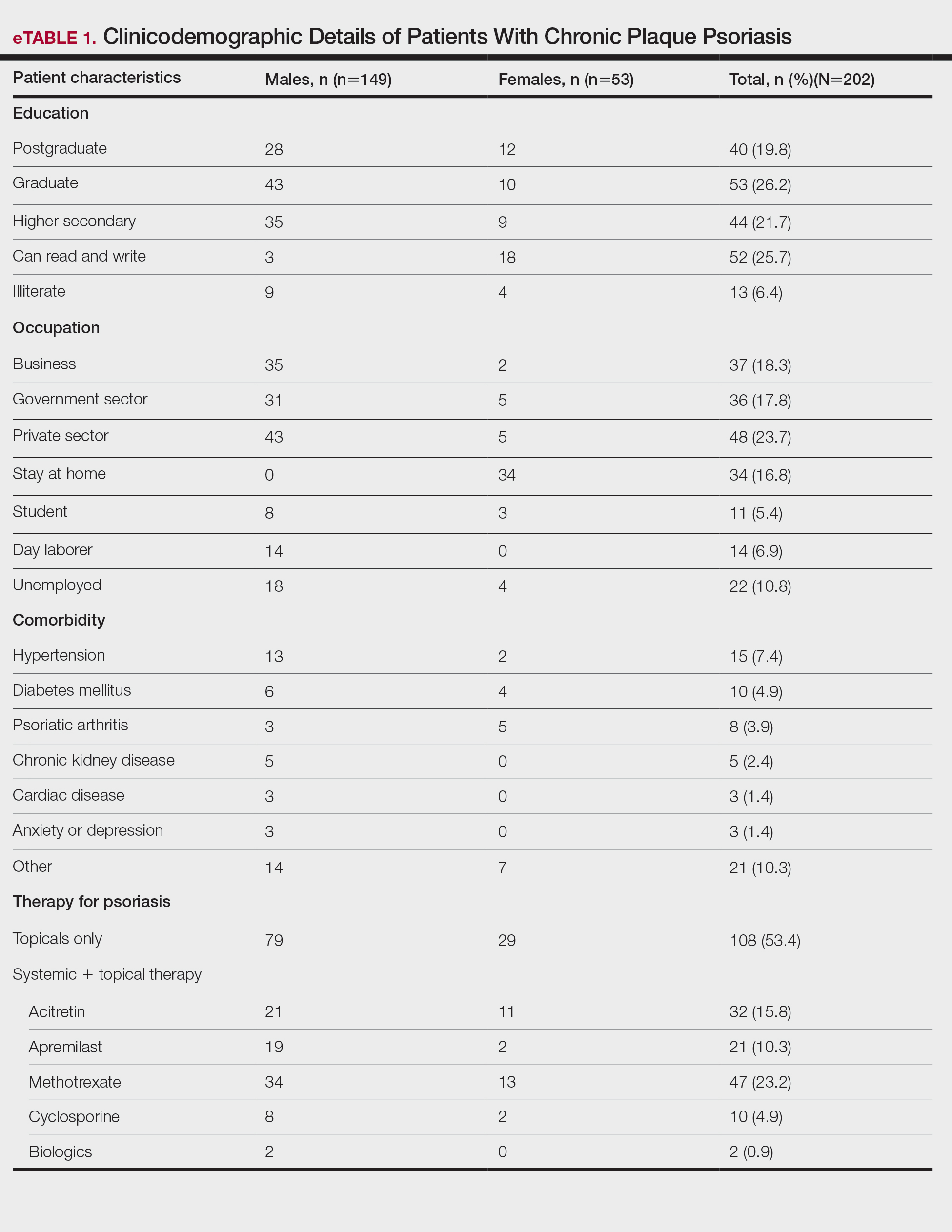
A total of 202 patients with chronic plaque psoriasis who received either Covaxin or Covishield were enrolled during the study period. The mean age (SD) was 40.3 (13.1) years, and 149 (73.8%) patients were male. One hundred thirty-five (66.8%) patients completed 2 doses of the vaccine. eTable 1 provides the clinicodemographic details of the patients. Eighty-three (41.1%) patients had a fear of psoriasis flare after vaccination. Seventy-two (35.6%) patients received the vaccine after clearance from their treating physician/dermatologist. One hundred sixty-four (81.2%) patients received the Covishield vaccine, and 38 (18.8%) patients received Covaxin. Eighty-three (41.1%) patients reported flulike symptoms, such as fever, myalgia, or body pain, within the first week of vaccination. Sixty-one (30.2%) patients reported a psoriasis flare after vaccination in the form of new lesions or worsening of pre-existing lesions. Of these patients, 51 reported a flare after receiving the first dose of vaccine, 8 patients reported a flare after receiving the second dose of vaccine, and 2 patients reported a flare after receiving both doses of vaccine. The mean (SD) flare onset was 8.1 (3.4) days after the vaccination. Eighteen patients considered the flare to be severe. Seventeen (8.4%) patients reported a positive history of COVID-19 infection before vaccination. None of the patients reported breakthrough COVID-19 infection or pustular aggravation of psoriasis following the vaccination.
The self-reported psoriasis flare after receiving the COVID-19 vaccine was significantly higher in patients who experienced immediate adverse effects (P=.005), which included fever, myalgia, joint pain, and injection-site reaction. The reported postvaccination psoriasis flare was not significantly associated with patient sex, history of COVID-19 infection, type of vaccine received, comorbidities, or therapy for psoriasis (eTable 2).
Nearly 30% of our patients reported a postvaccination psoriasis flare, which was more common after the first vaccine dose. Sotiriou et al7 reported 14 cases of psoriasis flare in patients after receiving Pfizer-BioNTech, Moderna, and AstraZeneca COVID-19 vaccines. These patients experienced an exacerbation of disease soon after the second dose of vaccine (mean [SD], 10.36 [7.71] days), and 21% of the 713 enrolled patients wanted to forego the immunization due to concern of a postvaccination psoriasis flare.7 In another report, 14 (27%) patients developed a psoriasis flare after COVID-19 vaccination; the mean (SD) flare onset was 9.3 (4.3) days after vaccination.9
Data on the safety of the COVID-19 vaccine in patients using immunosuppressive drugs are limited. We did not find a significant association between the psoriasis flare and use of immunosuppressive drugs or type of vaccine received. Huang and Tsai9 observed similar results, with no association between psoriasis flare and use of immunosuppressive drugs or biologics, while Damiani et al10 demonstrated a protective role of biologics in preventing vaccine-induced psoriasis flare.