Four functional domains
Historically, HF exercise or rehab trials have excluded patients hospitalized with acute decompensation, and third-party reimbursement often has not covered such programs because of a lack of supporting evidence and a supposed potential for harm, Dr. Kitzman said.
Entry to REHAB-HF required the patients to be fit enough to walk 4 meters, with or without a walker or other assistant device, and to have been in the hospital for at least 24 hours with a primary diagnosis of acute decompensated HF.
The intervention relied on exercises aimed at improving the four functional domains of strength, balance, mobility, and – when those three were sufficiently developed – endurance, Dr. Kitzman and associates wrote in their published report.
“The intervention was initiated in the hospital when feasible and was subsequently transitioned to an outpatient facility as soon as possible after discharge,” they wrote. Afterward, “a key goal of the intervention during the first 3 months [the outpatient phase] was to prepare the patient to transition to the independent maintenance phase (months 4-6).”
The study’s control patients “received frequent calls from study staff to try to approximate the increased attention received by the intervention group,” Dr. Kitzman said in an interview. “They were allowed to receive all usual care as ordered by their treating physicians. This included, if ordered, standard physical therapy or cardiac rehabilitation” in 43% of the control cohort. Of the trial’s 349 patients, those assigned to the intervention scored significantly higher on the three-component Short Physical Performance Battery (SPPB) at 12 weeks than those assigned to a usual care approach that included, for some, more conventional cardiac rehabilitation (8.3 vs. 6.9; P < .001).
The SPPB, validated in trials as a proxy for clinical outcomes includes tests of balance while standing, gait speed during a 4-minute walk, and strength. The latter is the test that measures time needed to rise from a chair five times.
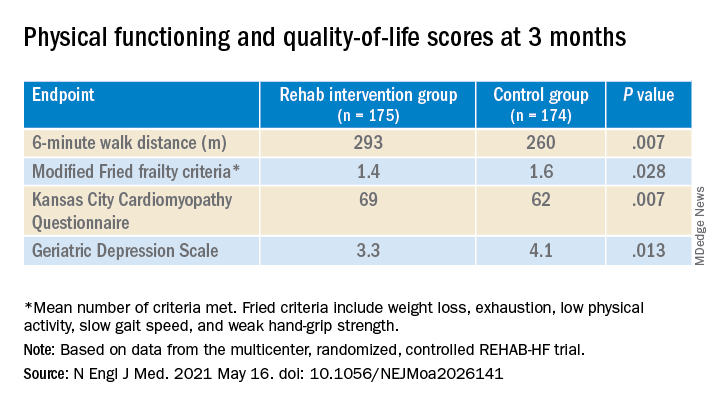
They also showed consistent gains in other measures of physical functioning and quality of life by 12 weeks months.
The observed SPPB treatment effect is “impressive” and “compares very favorably with previously reported estimates,” observed an accompanying editorial from Stefan D. Anker, MD, PhD, of the German Center for Cardiovascular Research and Charité Universitätsmedizin, Berlin, and Andrew J.S. Coats, DM, of the University of Warwick, Coventry, England.
“Similarly, the between-group differences seen in 6-minute walk distance (34 m) and gait speed (0.12 m/s) are clinically meaningful and sizable.”
They propose that some of the substantial quality-of-life benefit in the intervention group “may be due to better physical performance, and that part may be due to improvements in psychosocial factors and mood. It appears that exercise also resulted in patients becoming happier, or at least less depressed, as evidenced by the positive results on the Geriatric Depression Scale.”
Similar results across most subgroups
In subgroup analyses, the intervention was successful against the standard-care approach in both men and women at all ages and regardless of ejection fraction; symptom status; and whether the patient had diabetes, ischemic heart disease, or atrial fibrillation, or was obese.
Clinical outcomes were not significantly different at 6 months. The rate of death from any cause was 13% for the intervention group and 10% for the control group. There were 194 and 213 hospitalizations from any cause, respectively.
Not included in the trial’s current publication but soon to be published, Dr. Kitzman said when interviewed, is a comparison of outcomes in patients with HFpEF and HFrEF. “We found at baseline that those with HFpEF had worse impairment in physical function, quality of life, and frailty. After the intervention, there appeared to be consistently larger improvements in all outcomes, including SPPB, 6-minute walk, qualify of life, and frailty, in HFpEF versus HFrEF.”
The signals of potential benefit in HFpEF extended to clinical endpoints, he said. In contrast to similar rates of all-cause rehospitalization in HFrEF, “in patients with HFpEF, rehospitalizations were 17% lower in the intervention group, compared to the control group.” Still, he noted, the interaction P value wasn’t significant.
However, Dr. Kitzman added, mortality in the intervention group, compared with the control group, was reduced by 35% among patients with HFpEF, “but was 250% higher in HFrEF,” with a significant interaction P value.
He was careful to note that, as a phase 2 trial, REHAB-HF was underpowered for clinical events, “and even the results in the HFpEF group should not be seen as adequate evidence to change clinical care.” They were from an exploratory analysis that included relatively few events.
“Because definitive demonstration of improvement in clinical events is critical for altering clinical care guidelines and for third-party payer reimbursement decisions, we believe that a subsequent phase 3 trial is needed and are currently planning toward that,” Dr. Kitzman said.
The study was supported by research grants from the National Institutes of Health, the Kermit Glenn Phillips II Chair in Cardiovascular Medicine, and the Oristano Family Fund at Wake Forest. Dr. Kitzman disclosed receiving consulting fees or honoraria from AbbVie, AstraZeneca, Bayer Healthcare, Boehringer Ingelheim, CinRx, Corviamedical, GlaxoSmithKline, and Merck; and having an unspecified relationship with Gilead. Dr. Handberg disclosed receiving grants from Aastom Biosciences, Abbott Laboratories, Amgen, Amorcyte, AstraZeneca, Biocardia, Boehringer Ingelheim, Capricor, Cytori Therapeutics, Department of Defense, Direct Flow Medical, Everyfit, Gilead, Ionis, Medtronic, Merck, Mesoblast, Relypsa, and Sanofi-Aventis. Dr. Bittner discloses receiving consulting fees or honoraria from Pfizer and Sanofi; receiving research grants from Amgen and The Medicines Company; and having unspecified relationships with AstraZeneca, DalCor, Esperion, and Sanofi-Aventis. Dr. Anker reported receiving grants and personal fees from Abbott Vascular and Vifor; personal fees from Bayer, Boehringer Ingelheim, Novartis, Servier, Cardiac Dimensions, Thermo Fisher Scientific, AstraZeneca, Occlutech, Actimed, and Respicardia. Dr. Coats disclosed receiving personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Menarini, Novartis, Nutricia, Servier, Vifor, Abbott, Actimed, Arena, Cardiac Dimensions, Corvia, CVRx, Enopace, ESN Cleer, Faraday, WL Gore, Impulse Dynamics, and Respicardia.
A version of this article first appeared on Medscape.com.