User login
Increased lymphoma risk in patients with PIDD

© ASCO/Zach Boyden-Holmes
CHICAGO—Investigators have found an increased risk in cancer incidence for patients with primary immunodeficiency diseases (PIDD), and in particular, a significant increase in lymphoma cases.
Investigators reviewed records of patients registered in the United States Immune Deficiency Network (USIDNET) and found they had a 42% increase in cancer incidence overall compared to the general population in the Surveillance, Epidemiology and End Results (SEER) database.
And lymphoma incidence was 10 times higher among men and 8 times higher among women in the USIDNET registry.
The USIDNET registry collects information, including clinical, laboratory, and outcome data, on patients affected by PIDD. Site-specific cancer incidence rates are included in the registry.
Investigators compared data from the 2 registries based on age and gender. They abstracted data on 3665 patients from the USIDNET Registry from 2003 to 2015 and generated site-specific incidence rates for them. They also generated age adjusted incidence rates using the SEER database for comparison.
The investigators observed a 1.34-fold excess relative risk of cancer (P<0.001) in patients with PIDD compared to the age-adjusted SEER population.
They also discovered that in men, the relative risk increased to 1.8-fold (P<0.001), while in women, the excess relative risk of cancer was not significant.
Men also had a statistically significant increase in skin cancer (4.45-fold excess relative risk, P<0.001) and thyroid cancer (4-fold excess relative risk, P=0.002).
Women had a statistically significant increase in skin (3.19-fold excess relative risk, P<0.001) and stomach cancer (3-fold excess relative risk, P=0.015).
And both men and women had a statistically significant increase in lymphoma, at a significance of P<0.001 for each gender.
“This study found that patients with primary immunodeficiency disorders have a modest increase in overall cancer incidence,” senior author, Brahm Segal, MD, of Roswell Park Cancer Institute in Buffalo, New York, said, “driven by specific primary immunodeficiency disorders predisposing to specific cancers, particularly lymphoma.”
The investigators did not observe an increased risk for the most common solid tumor cancers, including breast, lung, prostate, and colon.
The investigators believe the findings point to a “restricted role of the immune system in protecting from specific cancers and question the role of immunosurveillance in incident risk of common solid tumor cancers.”
They reported their findings at the 2016 ASCO Annual Meeting as abstract 1520. ![]()

© ASCO/Zach Boyden-Holmes
CHICAGO—Investigators have found an increased risk in cancer incidence for patients with primary immunodeficiency diseases (PIDD), and in particular, a significant increase in lymphoma cases.
Investigators reviewed records of patients registered in the United States Immune Deficiency Network (USIDNET) and found they had a 42% increase in cancer incidence overall compared to the general population in the Surveillance, Epidemiology and End Results (SEER) database.
And lymphoma incidence was 10 times higher among men and 8 times higher among women in the USIDNET registry.
The USIDNET registry collects information, including clinical, laboratory, and outcome data, on patients affected by PIDD. Site-specific cancer incidence rates are included in the registry.
Investigators compared data from the 2 registries based on age and gender. They abstracted data on 3665 patients from the USIDNET Registry from 2003 to 2015 and generated site-specific incidence rates for them. They also generated age adjusted incidence rates using the SEER database for comparison.
The investigators observed a 1.34-fold excess relative risk of cancer (P<0.001) in patients with PIDD compared to the age-adjusted SEER population.
They also discovered that in men, the relative risk increased to 1.8-fold (P<0.001), while in women, the excess relative risk of cancer was not significant.
Men also had a statistically significant increase in skin cancer (4.45-fold excess relative risk, P<0.001) and thyroid cancer (4-fold excess relative risk, P=0.002).
Women had a statistically significant increase in skin (3.19-fold excess relative risk, P<0.001) and stomach cancer (3-fold excess relative risk, P=0.015).
And both men and women had a statistically significant increase in lymphoma, at a significance of P<0.001 for each gender.
“This study found that patients with primary immunodeficiency disorders have a modest increase in overall cancer incidence,” senior author, Brahm Segal, MD, of Roswell Park Cancer Institute in Buffalo, New York, said, “driven by specific primary immunodeficiency disorders predisposing to specific cancers, particularly lymphoma.”
The investigators did not observe an increased risk for the most common solid tumor cancers, including breast, lung, prostate, and colon.
The investigators believe the findings point to a “restricted role of the immune system in protecting from specific cancers and question the role of immunosurveillance in incident risk of common solid tumor cancers.”
They reported their findings at the 2016 ASCO Annual Meeting as abstract 1520. ![]()

© ASCO/Zach Boyden-Holmes
CHICAGO—Investigators have found an increased risk in cancer incidence for patients with primary immunodeficiency diseases (PIDD), and in particular, a significant increase in lymphoma cases.
Investigators reviewed records of patients registered in the United States Immune Deficiency Network (USIDNET) and found they had a 42% increase in cancer incidence overall compared to the general population in the Surveillance, Epidemiology and End Results (SEER) database.
And lymphoma incidence was 10 times higher among men and 8 times higher among women in the USIDNET registry.
The USIDNET registry collects information, including clinical, laboratory, and outcome data, on patients affected by PIDD. Site-specific cancer incidence rates are included in the registry.
Investigators compared data from the 2 registries based on age and gender. They abstracted data on 3665 patients from the USIDNET Registry from 2003 to 2015 and generated site-specific incidence rates for them. They also generated age adjusted incidence rates using the SEER database for comparison.
The investigators observed a 1.34-fold excess relative risk of cancer (P<0.001) in patients with PIDD compared to the age-adjusted SEER population.
They also discovered that in men, the relative risk increased to 1.8-fold (P<0.001), while in women, the excess relative risk of cancer was not significant.
Men also had a statistically significant increase in skin cancer (4.45-fold excess relative risk, P<0.001) and thyroid cancer (4-fold excess relative risk, P=0.002).
Women had a statistically significant increase in skin (3.19-fold excess relative risk, P<0.001) and stomach cancer (3-fold excess relative risk, P=0.015).
And both men and women had a statistically significant increase in lymphoma, at a significance of P<0.001 for each gender.
“This study found that patients with primary immunodeficiency disorders have a modest increase in overall cancer incidence,” senior author, Brahm Segal, MD, of Roswell Park Cancer Institute in Buffalo, New York, said, “driven by specific primary immunodeficiency disorders predisposing to specific cancers, particularly lymphoma.”
The investigators did not observe an increased risk for the most common solid tumor cancers, including breast, lung, prostate, and colon.
The investigators believe the findings point to a “restricted role of the immune system in protecting from specific cancers and question the role of immunosurveillance in incident risk of common solid tumor cancers.”
They reported their findings at the 2016 ASCO Annual Meeting as abstract 1520. ![]()
Adjuvant AI therapy for breast cancer: 10 years is superior to 5 years
CHICAGO – Extending adjuvant aromatase inhibitor (AI) therapy from 5 years to 10 years further reduces the risk of recurrence and new breast cancer in postmenopausal women treated for early disease, according to findings of the MA17.R trial.
In the phase III trial conducted by the Canadian Cancer Trials Group and the North American Breast Cancer Group, 1,918 postmenopausal women who had completed about 5 years of AI therapy (preceded by tamoxifen in the majority of cases) were randomized to take the AI letrozole or to stop therapy by taking placebo for an additional 5 years.
Compared with peers who stopped, women who continued AI therapy for 5 more years had a 34% lower risk of recurrence or contralateral breast cancer, investigators reported in sessions and a press briefing at the annual meeting of the American Society of Clinical Oncology. The trade-off was a small increase in the rates of skeletal adverse events such as fractures; quality of life was essentially unaffected.
“MA17.R is the first study to show the benefit of extending an adjuvant aromatase inhibitor beyond 5 years,” commented lead investigator Paul E. Goss, M.D., director of the Breast Cancer Research Program at the Massachusetts General Hospital and a professor of medicine at Harvard Medical School, both in Boston. “Unlike many anticancer therapies, aromatase inhibitors are readily accessible around the world, and therefore, our results will further improve the outcome of many women with breast cancer.”
The disease-free survival curves will likely separate further, given a “legacy effect” of endocrine therapy that persists after it ends, he predicted. “And I think overall survival will eventually become positive in MA.17R. … The Food and Drug Administration has taken the opinion that overall survival follows disease-free survival like night follows day for endocrine therapies, and I think they are correct, I think that’s what we see in all the trials.”
Implications
It remains unclear how patients should be managed after 10 years of an AI, according to Dr. Goss. “That’s uncharted waters,” he elaborated. “We know from the curve of the natural history of this disease that it chronically relapses, and we continue to see patients 25 years after their primary diagnosis with a recurrence.” Some data in mice suggest there may be benefit from continuing the AI until recurrence. “I don’t think that would be entered into in clinical practice as a rule, because there is no clinical trial of that,” he said. “But this data will now take the aromatase inhibitors out to 10 years.”
The MA.17R trial’s findings show that extended duration of AI therapy is “clinically valuable,” according to ASCO expert Harold J. Burstein, M.D., Ph.D., a senior physician at the Dana-Farber Cancer Institute and an associate professor of medicine at the Harvard Medical School in Boston. He predicted the findings will have at least two consequences.
“One is tremendous interest in longer durations of therapy with the AI, but also some tailoring of treatment based on how the patient has fared with their therapy and on their baseline risk of recurrence. So women who have less risky cancer will probably be less inclined to pursue these longer durations, and women who have higher-risk cancers will be more inclined,” he explained.
Also, “women who have finished 5 years of an AI without any prior tamoxifen I think are going to be very compelled by these data, whereas women who have had 5 years of tamoxifen, 5 years of an AI, and are already 10 years out probably get less benefit numerically from yet longer duration of therapy,” he added. “And we are certainly not at the point of saying that women should be on these drugs for the rest of their lives.”
MA.17R design
Postmenopausal women were eligible for MA.17R if they had undergone resection of hormone receptor–positive early breast cancer and had already completed about 5 years of therapy with any of the three AIs currently on the market. In the large majority of cases, they also had previously received tamoxifen.
Many of the women came from the parent MA.17 trial, which tested an initial 5 years of letrozole (brand name Femara) against placebo after tamoxifen therapy. Longer-term results of that trial, previously reported (J Clin Oncol. 2012;30:718-21), showed that this duration of letrozole had a significant disease-free survival benefit (hazard ratio, .52) and overall survival benefit (HR, .61).
In the MA.17R trial, the women were randomized to letrozole or a placebo for an additional 5 years, with a primary endpoint of disease-free survival and secondary endpoints including safety and quality of life.
Efficacy and safety
After a median follow-up of 6.3 years, the 5-year rate of disease-free survival was 95% with letrozole and 91% with placebo, according to results reported by Dr. Goss in a plenary session at the meeting and simultaneously published (N Engl J Med. 2016. June 5 doi: 10.1056/NEJMoa1604700).
The difference translated to a more than one-third reduction in the risk of disease recurrence or the occurrence of contralateral breast cancer (HR, .66; P = .01). Roughly three-fourths of recurrences were distant.
The groups did not differ significantly with respect to the rate of overall survival, which was 93% with letrozole and 94% with placebo.
The annual incidence of contralateral breast cancer was sharply lower in the letrozole group, at 0.21%, than in the placebo group, at 0.49%, translating to a more than one-half reduction in this risk of this outcome (HR, .42; P = .007).
No new toxicities or emergent symptoms were noted from extending AI therapy, according to Dr. Goss. However, the letrozole group significantly more commonly experienced bone pain (18% vs. 14%), bone fractures (14% vs. 9%), and new-onset osteoporosis (11% vs. 6%). Therefore, “bone health remains important for risk-benefit consideration,” he said.
Patient-reported outcomes
In a separate session and the press briefing, Dr. Julie Lemieux, a researcher at the Centre Hospitalier affilié Universitaire de Québec, reported the trial’s patient-reported outcomes, ascertained from questionnaires completed at baseline and annually out to 5 years.
In general, both the letrozole and placebo groups had small deteriorations over time in global quality of life as assessed from summary scores on the mental and physical scales of the 36-item Short Form Health Survey (SF-36). But both also had small improvements over time in scores on the Menopause-Specific Quality of Life (MENQOL) scale.
When compared, the two groups were statistically indistinguishable on most of these measures. The only significant difference was greater worsening in the letrozole group on the role function-physical subscale of the SF-36, which pertains to difficulty performing work or physical activity due to physical health. However, the difference averaged just 3.2 points, which fell short of the 5 points that the investigators considered clinically important.
“The limitation of this analysis was that it was a highly selected population. All of these women had already tolerated 5 years of an aromatase inhibitor, and about 70% had received 5 years of tamoxifen before,” Dr. Lemieux commented. “Also, they were clinical trial participants.”
Nonetheless, these findings “are very reassuring for those women who want a longer duration of adjuvant endocrine therapy that they can expect a preserved quality of life,” she concluded.
Dr. Goss disclosed that he had no relevant conflicts of interest. Dr. Lemieux disclosed that she had no relevant conflicts of interest. The trial received support from Novartis.
CHICAGO – Extending adjuvant aromatase inhibitor (AI) therapy from 5 years to 10 years further reduces the risk of recurrence and new breast cancer in postmenopausal women treated for early disease, according to findings of the MA17.R trial.
In the phase III trial conducted by the Canadian Cancer Trials Group and the North American Breast Cancer Group, 1,918 postmenopausal women who had completed about 5 years of AI therapy (preceded by tamoxifen in the majority of cases) were randomized to take the AI letrozole or to stop therapy by taking placebo for an additional 5 years.
Compared with peers who stopped, women who continued AI therapy for 5 more years had a 34% lower risk of recurrence or contralateral breast cancer, investigators reported in sessions and a press briefing at the annual meeting of the American Society of Clinical Oncology. The trade-off was a small increase in the rates of skeletal adverse events such as fractures; quality of life was essentially unaffected.
“MA17.R is the first study to show the benefit of extending an adjuvant aromatase inhibitor beyond 5 years,” commented lead investigator Paul E. Goss, M.D., director of the Breast Cancer Research Program at the Massachusetts General Hospital and a professor of medicine at Harvard Medical School, both in Boston. “Unlike many anticancer therapies, aromatase inhibitors are readily accessible around the world, and therefore, our results will further improve the outcome of many women with breast cancer.”
The disease-free survival curves will likely separate further, given a “legacy effect” of endocrine therapy that persists after it ends, he predicted. “And I think overall survival will eventually become positive in MA.17R. … The Food and Drug Administration has taken the opinion that overall survival follows disease-free survival like night follows day for endocrine therapies, and I think they are correct, I think that’s what we see in all the trials.”
Implications
It remains unclear how patients should be managed after 10 years of an AI, according to Dr. Goss. “That’s uncharted waters,” he elaborated. “We know from the curve of the natural history of this disease that it chronically relapses, and we continue to see patients 25 years after their primary diagnosis with a recurrence.” Some data in mice suggest there may be benefit from continuing the AI until recurrence. “I don’t think that would be entered into in clinical practice as a rule, because there is no clinical trial of that,” he said. “But this data will now take the aromatase inhibitors out to 10 years.”
The MA.17R trial’s findings show that extended duration of AI therapy is “clinically valuable,” according to ASCO expert Harold J. Burstein, M.D., Ph.D., a senior physician at the Dana-Farber Cancer Institute and an associate professor of medicine at the Harvard Medical School in Boston. He predicted the findings will have at least two consequences.
“One is tremendous interest in longer durations of therapy with the AI, but also some tailoring of treatment based on how the patient has fared with their therapy and on their baseline risk of recurrence. So women who have less risky cancer will probably be less inclined to pursue these longer durations, and women who have higher-risk cancers will be more inclined,” he explained.
Also, “women who have finished 5 years of an AI without any prior tamoxifen I think are going to be very compelled by these data, whereas women who have had 5 years of tamoxifen, 5 years of an AI, and are already 10 years out probably get less benefit numerically from yet longer duration of therapy,” he added. “And we are certainly not at the point of saying that women should be on these drugs for the rest of their lives.”
MA.17R design
Postmenopausal women were eligible for MA.17R if they had undergone resection of hormone receptor–positive early breast cancer and had already completed about 5 years of therapy with any of the three AIs currently on the market. In the large majority of cases, they also had previously received tamoxifen.
Many of the women came from the parent MA.17 trial, which tested an initial 5 years of letrozole (brand name Femara) against placebo after tamoxifen therapy. Longer-term results of that trial, previously reported (J Clin Oncol. 2012;30:718-21), showed that this duration of letrozole had a significant disease-free survival benefit (hazard ratio, .52) and overall survival benefit (HR, .61).
In the MA.17R trial, the women were randomized to letrozole or a placebo for an additional 5 years, with a primary endpoint of disease-free survival and secondary endpoints including safety and quality of life.
Efficacy and safety
After a median follow-up of 6.3 years, the 5-year rate of disease-free survival was 95% with letrozole and 91% with placebo, according to results reported by Dr. Goss in a plenary session at the meeting and simultaneously published (N Engl J Med. 2016. June 5 doi: 10.1056/NEJMoa1604700).
The difference translated to a more than one-third reduction in the risk of disease recurrence or the occurrence of contralateral breast cancer (HR, .66; P = .01). Roughly three-fourths of recurrences were distant.
The groups did not differ significantly with respect to the rate of overall survival, which was 93% with letrozole and 94% with placebo.
The annual incidence of contralateral breast cancer was sharply lower in the letrozole group, at 0.21%, than in the placebo group, at 0.49%, translating to a more than one-half reduction in this risk of this outcome (HR, .42; P = .007).
No new toxicities or emergent symptoms were noted from extending AI therapy, according to Dr. Goss. However, the letrozole group significantly more commonly experienced bone pain (18% vs. 14%), bone fractures (14% vs. 9%), and new-onset osteoporosis (11% vs. 6%). Therefore, “bone health remains important for risk-benefit consideration,” he said.
Patient-reported outcomes
In a separate session and the press briefing, Dr. Julie Lemieux, a researcher at the Centre Hospitalier affilié Universitaire de Québec, reported the trial’s patient-reported outcomes, ascertained from questionnaires completed at baseline and annually out to 5 years.
In general, both the letrozole and placebo groups had small deteriorations over time in global quality of life as assessed from summary scores on the mental and physical scales of the 36-item Short Form Health Survey (SF-36). But both also had small improvements over time in scores on the Menopause-Specific Quality of Life (MENQOL) scale.
When compared, the two groups were statistically indistinguishable on most of these measures. The only significant difference was greater worsening in the letrozole group on the role function-physical subscale of the SF-36, which pertains to difficulty performing work or physical activity due to physical health. However, the difference averaged just 3.2 points, which fell short of the 5 points that the investigators considered clinically important.
“The limitation of this analysis was that it was a highly selected population. All of these women had already tolerated 5 years of an aromatase inhibitor, and about 70% had received 5 years of tamoxifen before,” Dr. Lemieux commented. “Also, they were clinical trial participants.”
Nonetheless, these findings “are very reassuring for those women who want a longer duration of adjuvant endocrine therapy that they can expect a preserved quality of life,” she concluded.
Dr. Goss disclosed that he had no relevant conflicts of interest. Dr. Lemieux disclosed that she had no relevant conflicts of interest. The trial received support from Novartis.
CHICAGO – Extending adjuvant aromatase inhibitor (AI) therapy from 5 years to 10 years further reduces the risk of recurrence and new breast cancer in postmenopausal women treated for early disease, according to findings of the MA17.R trial.
In the phase III trial conducted by the Canadian Cancer Trials Group and the North American Breast Cancer Group, 1,918 postmenopausal women who had completed about 5 years of AI therapy (preceded by tamoxifen in the majority of cases) were randomized to take the AI letrozole or to stop therapy by taking placebo for an additional 5 years.
Compared with peers who stopped, women who continued AI therapy for 5 more years had a 34% lower risk of recurrence or contralateral breast cancer, investigators reported in sessions and a press briefing at the annual meeting of the American Society of Clinical Oncology. The trade-off was a small increase in the rates of skeletal adverse events such as fractures; quality of life was essentially unaffected.
“MA17.R is the first study to show the benefit of extending an adjuvant aromatase inhibitor beyond 5 years,” commented lead investigator Paul E. Goss, M.D., director of the Breast Cancer Research Program at the Massachusetts General Hospital and a professor of medicine at Harvard Medical School, both in Boston. “Unlike many anticancer therapies, aromatase inhibitors are readily accessible around the world, and therefore, our results will further improve the outcome of many women with breast cancer.”
The disease-free survival curves will likely separate further, given a “legacy effect” of endocrine therapy that persists after it ends, he predicted. “And I think overall survival will eventually become positive in MA.17R. … The Food and Drug Administration has taken the opinion that overall survival follows disease-free survival like night follows day for endocrine therapies, and I think they are correct, I think that’s what we see in all the trials.”
Implications
It remains unclear how patients should be managed after 10 years of an AI, according to Dr. Goss. “That’s uncharted waters,” he elaborated. “We know from the curve of the natural history of this disease that it chronically relapses, and we continue to see patients 25 years after their primary diagnosis with a recurrence.” Some data in mice suggest there may be benefit from continuing the AI until recurrence. “I don’t think that would be entered into in clinical practice as a rule, because there is no clinical trial of that,” he said. “But this data will now take the aromatase inhibitors out to 10 years.”
The MA.17R trial’s findings show that extended duration of AI therapy is “clinically valuable,” according to ASCO expert Harold J. Burstein, M.D., Ph.D., a senior physician at the Dana-Farber Cancer Institute and an associate professor of medicine at the Harvard Medical School in Boston. He predicted the findings will have at least two consequences.
“One is tremendous interest in longer durations of therapy with the AI, but also some tailoring of treatment based on how the patient has fared with their therapy and on their baseline risk of recurrence. So women who have less risky cancer will probably be less inclined to pursue these longer durations, and women who have higher-risk cancers will be more inclined,” he explained.
Also, “women who have finished 5 years of an AI without any prior tamoxifen I think are going to be very compelled by these data, whereas women who have had 5 years of tamoxifen, 5 years of an AI, and are already 10 years out probably get less benefit numerically from yet longer duration of therapy,” he added. “And we are certainly not at the point of saying that women should be on these drugs for the rest of their lives.”
MA.17R design
Postmenopausal women were eligible for MA.17R if they had undergone resection of hormone receptor–positive early breast cancer and had already completed about 5 years of therapy with any of the three AIs currently on the market. In the large majority of cases, they also had previously received tamoxifen.
Many of the women came from the parent MA.17 trial, which tested an initial 5 years of letrozole (brand name Femara) against placebo after tamoxifen therapy. Longer-term results of that trial, previously reported (J Clin Oncol. 2012;30:718-21), showed that this duration of letrozole had a significant disease-free survival benefit (hazard ratio, .52) and overall survival benefit (HR, .61).
In the MA.17R trial, the women were randomized to letrozole or a placebo for an additional 5 years, with a primary endpoint of disease-free survival and secondary endpoints including safety and quality of life.
Efficacy and safety
After a median follow-up of 6.3 years, the 5-year rate of disease-free survival was 95% with letrozole and 91% with placebo, according to results reported by Dr. Goss in a plenary session at the meeting and simultaneously published (N Engl J Med. 2016. June 5 doi: 10.1056/NEJMoa1604700).
The difference translated to a more than one-third reduction in the risk of disease recurrence or the occurrence of contralateral breast cancer (HR, .66; P = .01). Roughly three-fourths of recurrences were distant.
The groups did not differ significantly with respect to the rate of overall survival, which was 93% with letrozole and 94% with placebo.
The annual incidence of contralateral breast cancer was sharply lower in the letrozole group, at 0.21%, than in the placebo group, at 0.49%, translating to a more than one-half reduction in this risk of this outcome (HR, .42; P = .007).
No new toxicities or emergent symptoms were noted from extending AI therapy, according to Dr. Goss. However, the letrozole group significantly more commonly experienced bone pain (18% vs. 14%), bone fractures (14% vs. 9%), and new-onset osteoporosis (11% vs. 6%). Therefore, “bone health remains important for risk-benefit consideration,” he said.
Patient-reported outcomes
In a separate session and the press briefing, Dr. Julie Lemieux, a researcher at the Centre Hospitalier affilié Universitaire de Québec, reported the trial’s patient-reported outcomes, ascertained from questionnaires completed at baseline and annually out to 5 years.
In general, both the letrozole and placebo groups had small deteriorations over time in global quality of life as assessed from summary scores on the mental and physical scales of the 36-item Short Form Health Survey (SF-36). But both also had small improvements over time in scores on the Menopause-Specific Quality of Life (MENQOL) scale.
When compared, the two groups were statistically indistinguishable on most of these measures. The only significant difference was greater worsening in the letrozole group on the role function-physical subscale of the SF-36, which pertains to difficulty performing work or physical activity due to physical health. However, the difference averaged just 3.2 points, which fell short of the 5 points that the investigators considered clinically important.
“The limitation of this analysis was that it was a highly selected population. All of these women had already tolerated 5 years of an aromatase inhibitor, and about 70% had received 5 years of tamoxifen before,” Dr. Lemieux commented. “Also, they were clinical trial participants.”
Nonetheless, these findings “are very reassuring for those women who want a longer duration of adjuvant endocrine therapy that they can expect a preserved quality of life,” she concluded.
Dr. Goss disclosed that he had no relevant conflicts of interest. Dr. Lemieux disclosed that she had no relevant conflicts of interest. The trial received support from Novartis.
AT ASCO 2016
Key clinical point: Extending adjuvant AI therapy out to 10 years improves disease-free survival and is generally safe and well tolerated.
Major finding: Patients who continued taking an AI out to 10 years had a 34% lower risk of recurrence or contralateral breast cancer than peers who stopped after 5 years.
Data source: A randomized placebo-controlled phase III trial among 1,918 postmenopausal women who had already completed 5 years of AI therapy (MA17.R trial).
Disclosures: Dr. Goss disclosed that he had no relevant conflicts of interest. Dr. Lemieux disclosed that she had no relevant conflicts of interest. The trial received support from Novartis.
Web app boosts lung cancer survival
CHICAGO – A simple Web-based mobile application (web-app) improved survival time and quality of life of patients with advanced lung cancer, according to a randomized study presented at the annual meeting of the American Society of Clinical Oncology.
The study was stopped at the planned interim survival analysis that occurred after 121 evaluable patients because of survival benefit favoring the web-app arm. The application, called Moovcare, allowed patients to report symptoms over time and stay in close touch with their care providers after their initial surgery, chemotherapy, or radiation therapy.
“The 1-year survival was 75% in the Moovcare vs. 49% in the control arm,” said lead author Dr. Fabrice Denis of the Institut Inter-régional de Cancérologie Jean Bernard in LeMans, France, in a press conference.
Dr. Denis identified several reasons why a web-app could be useful in treating patients with lung cancer. Even with more than 1 million lung cancer deaths a year worldwide, there is no standard follow-up, and relapses do not occur on a 3 or 6-month schedule of planned visits. So patients often wait several weeks until their next visit to report symptoms indicative of a relapse. They may also be reluctant to report symptoms because of shame over how they contracted the disease, for example, from smoking. And patients are often hesitant to “bother” the doctor with symptoms between visits. All these reasons can contribute to suboptimal therapy and worse outcomes.
Investigators designed Moovcare to allow patients to report symptoms weekly, facilitating early detection of relapse or dangerous medical conditions and triggering early supportive care. They compared the web-app to a control of usual, nonpersonalized follow-up in a French multicenter prospective, randomized trial.
Patients (n = 121) with stage II/node-positive to stage IV (90% stage III/IV) nonprogressive small cell or non–small cell lung cancer were randomly assigned 1:1 to the two arms of the trial. They had to have Internet access, prior experience with email, performance status of 0-2, and an initial symptom score less than 7. Patients could be taking tyrosine kinase inhibitors or on maintenance therapy. Monitoring visits were the same for both groups every 3 months or more frequently. Patients in the control arm received more frequent computed tomographic (CT) imaging than did ones in the web-app arm, and CT scans could be performed at any time in either group based on the investigator’s clinical judgment, or in the case of the web-app, as suggested by patient report in the algorithm.
The median follow up was 9 months. Relapse rates were close to 50% for both groups. The 1-year survival of 75% in the Moovcare to 49% in the control arm gave a 1-year absolute survival increase of 26%. Median survival was 19 months vs. 12 months, a 7-month improvement in median survival for the Moovcare arm. The hazard ratio for death in the web-app arm, compared with the control arm was 0.325 (95% confidence interval, 0.157-0.672; P = .0025).
When they relapsed, 77% of patients in the web-app arm had a good performance status, compared with 33% in the control arm. “This led to 74% of patients receiving optimal therapy in the Moovcare arm vs. 33% in the control arm,” Dr. Denis said. “And the number of imaging [procedures] was reduced by 50% per patient per year.”
Overall quality of life was better in the web-app arm, as assessed using standard quality of life questionnaires.
Moovcare works by having patients or their relatives report 12 symptoms weekly (for example, asthenia, cough, dyspnea, anorexia, etc.) using a smartphone, tablet, or computer. An algorithm analyzes an association of symptoms and triggers email alerts to health care providers if relapse or dangerous medical conditions may be occurring. Providers follow up alerts by phone and schedule visits and imaging. “The sensitivity of the algorithm was high and was validated in two prospective studies,” Dr. Denis said. Sensitivity was 86%-100%.
Moovcare allowed earlier detection of relapse and improved overall survival for three reasons. “It allowed higher performance status at relapse, leading to more optimal therapy for relapsing patients. Dangerous medical conditions were detected earlier and treated earlier. It favored earlier supportive care, which improved quality of life. Less imaging was needed and performed at the right time,” Dr. Denis said.
Patients were monitored on a weekly basis, allowing more personalized care. The Moovcare web-app has been evaluated prospectively in about 300 patients, providing a high level of evidence of its utility in improving outcomes for patients with advanced lung cancer.
Press conference moderator Dr. Patricia Ganz commented that Moovcare is an example of a new way to improve the delivery of high-quality care to patients. “If we had a drug or some new intervention that caused this level of survival benefit, wouldn’t we want to go out and use it?” she asked. “This is a tremendous advance. This is personalized medicine. This is really tailoring it to the patient, and you can see how simple it is to collect this kind of data from the patient and then bring them in in between what would have been a scheduled visit.” She said the app overcomes the barrier of patients putting off reporting symptoms until their next visit or their reluctance to “bother the doctor.”
She said the app alerts the health care team to potential problems and prompts them to “use tests when appropriate, not on a schedule, [which] leads to avoidance of waste in the follow-up of care of our patients.”
CHICAGO – A simple Web-based mobile application (web-app) improved survival time and quality of life of patients with advanced lung cancer, according to a randomized study presented at the annual meeting of the American Society of Clinical Oncology.
The study was stopped at the planned interim survival analysis that occurred after 121 evaluable patients because of survival benefit favoring the web-app arm. The application, called Moovcare, allowed patients to report symptoms over time and stay in close touch with their care providers after their initial surgery, chemotherapy, or radiation therapy.
“The 1-year survival was 75% in the Moovcare vs. 49% in the control arm,” said lead author Dr. Fabrice Denis of the Institut Inter-régional de Cancérologie Jean Bernard in LeMans, France, in a press conference.
Dr. Denis identified several reasons why a web-app could be useful in treating patients with lung cancer. Even with more than 1 million lung cancer deaths a year worldwide, there is no standard follow-up, and relapses do not occur on a 3 or 6-month schedule of planned visits. So patients often wait several weeks until their next visit to report symptoms indicative of a relapse. They may also be reluctant to report symptoms because of shame over how they contracted the disease, for example, from smoking. And patients are often hesitant to “bother” the doctor with symptoms between visits. All these reasons can contribute to suboptimal therapy and worse outcomes.
Investigators designed Moovcare to allow patients to report symptoms weekly, facilitating early detection of relapse or dangerous medical conditions and triggering early supportive care. They compared the web-app to a control of usual, nonpersonalized follow-up in a French multicenter prospective, randomized trial.
Patients (n = 121) with stage II/node-positive to stage IV (90% stage III/IV) nonprogressive small cell or non–small cell lung cancer were randomly assigned 1:1 to the two arms of the trial. They had to have Internet access, prior experience with email, performance status of 0-2, and an initial symptom score less than 7. Patients could be taking tyrosine kinase inhibitors or on maintenance therapy. Monitoring visits were the same for both groups every 3 months or more frequently. Patients in the control arm received more frequent computed tomographic (CT) imaging than did ones in the web-app arm, and CT scans could be performed at any time in either group based on the investigator’s clinical judgment, or in the case of the web-app, as suggested by patient report in the algorithm.
The median follow up was 9 months. Relapse rates were close to 50% for both groups. The 1-year survival of 75% in the Moovcare to 49% in the control arm gave a 1-year absolute survival increase of 26%. Median survival was 19 months vs. 12 months, a 7-month improvement in median survival for the Moovcare arm. The hazard ratio for death in the web-app arm, compared with the control arm was 0.325 (95% confidence interval, 0.157-0.672; P = .0025).
When they relapsed, 77% of patients in the web-app arm had a good performance status, compared with 33% in the control arm. “This led to 74% of patients receiving optimal therapy in the Moovcare arm vs. 33% in the control arm,” Dr. Denis said. “And the number of imaging [procedures] was reduced by 50% per patient per year.”
Overall quality of life was better in the web-app arm, as assessed using standard quality of life questionnaires.
Moovcare works by having patients or their relatives report 12 symptoms weekly (for example, asthenia, cough, dyspnea, anorexia, etc.) using a smartphone, tablet, or computer. An algorithm analyzes an association of symptoms and triggers email alerts to health care providers if relapse or dangerous medical conditions may be occurring. Providers follow up alerts by phone and schedule visits and imaging. “The sensitivity of the algorithm was high and was validated in two prospective studies,” Dr. Denis said. Sensitivity was 86%-100%.
Moovcare allowed earlier detection of relapse and improved overall survival for three reasons. “It allowed higher performance status at relapse, leading to more optimal therapy for relapsing patients. Dangerous medical conditions were detected earlier and treated earlier. It favored earlier supportive care, which improved quality of life. Less imaging was needed and performed at the right time,” Dr. Denis said.
Patients were monitored on a weekly basis, allowing more personalized care. The Moovcare web-app has been evaluated prospectively in about 300 patients, providing a high level of evidence of its utility in improving outcomes for patients with advanced lung cancer.
Press conference moderator Dr. Patricia Ganz commented that Moovcare is an example of a new way to improve the delivery of high-quality care to patients. “If we had a drug or some new intervention that caused this level of survival benefit, wouldn’t we want to go out and use it?” she asked. “This is a tremendous advance. This is personalized medicine. This is really tailoring it to the patient, and you can see how simple it is to collect this kind of data from the patient and then bring them in in between what would have been a scheduled visit.” She said the app overcomes the barrier of patients putting off reporting symptoms until their next visit or their reluctance to “bother the doctor.”
She said the app alerts the health care team to potential problems and prompts them to “use tests when appropriate, not on a schedule, [which] leads to avoidance of waste in the follow-up of care of our patients.”
CHICAGO – A simple Web-based mobile application (web-app) improved survival time and quality of life of patients with advanced lung cancer, according to a randomized study presented at the annual meeting of the American Society of Clinical Oncology.
The study was stopped at the planned interim survival analysis that occurred after 121 evaluable patients because of survival benefit favoring the web-app arm. The application, called Moovcare, allowed patients to report symptoms over time and stay in close touch with their care providers after their initial surgery, chemotherapy, or radiation therapy.
“The 1-year survival was 75% in the Moovcare vs. 49% in the control arm,” said lead author Dr. Fabrice Denis of the Institut Inter-régional de Cancérologie Jean Bernard in LeMans, France, in a press conference.
Dr. Denis identified several reasons why a web-app could be useful in treating patients with lung cancer. Even with more than 1 million lung cancer deaths a year worldwide, there is no standard follow-up, and relapses do not occur on a 3 or 6-month schedule of planned visits. So patients often wait several weeks until their next visit to report symptoms indicative of a relapse. They may also be reluctant to report symptoms because of shame over how they contracted the disease, for example, from smoking. And patients are often hesitant to “bother” the doctor with symptoms between visits. All these reasons can contribute to suboptimal therapy and worse outcomes.
Investigators designed Moovcare to allow patients to report symptoms weekly, facilitating early detection of relapse or dangerous medical conditions and triggering early supportive care. They compared the web-app to a control of usual, nonpersonalized follow-up in a French multicenter prospective, randomized trial.
Patients (n = 121) with stage II/node-positive to stage IV (90% stage III/IV) nonprogressive small cell or non–small cell lung cancer were randomly assigned 1:1 to the two arms of the trial. They had to have Internet access, prior experience with email, performance status of 0-2, and an initial symptom score less than 7. Patients could be taking tyrosine kinase inhibitors or on maintenance therapy. Monitoring visits were the same for both groups every 3 months or more frequently. Patients in the control arm received more frequent computed tomographic (CT) imaging than did ones in the web-app arm, and CT scans could be performed at any time in either group based on the investigator’s clinical judgment, or in the case of the web-app, as suggested by patient report in the algorithm.
The median follow up was 9 months. Relapse rates were close to 50% for both groups. The 1-year survival of 75% in the Moovcare to 49% in the control arm gave a 1-year absolute survival increase of 26%. Median survival was 19 months vs. 12 months, a 7-month improvement in median survival for the Moovcare arm. The hazard ratio for death in the web-app arm, compared with the control arm was 0.325 (95% confidence interval, 0.157-0.672; P = .0025).
When they relapsed, 77% of patients in the web-app arm had a good performance status, compared with 33% in the control arm. “This led to 74% of patients receiving optimal therapy in the Moovcare arm vs. 33% in the control arm,” Dr. Denis said. “And the number of imaging [procedures] was reduced by 50% per patient per year.”
Overall quality of life was better in the web-app arm, as assessed using standard quality of life questionnaires.
Moovcare works by having patients or their relatives report 12 symptoms weekly (for example, asthenia, cough, dyspnea, anorexia, etc.) using a smartphone, tablet, or computer. An algorithm analyzes an association of symptoms and triggers email alerts to health care providers if relapse or dangerous medical conditions may be occurring. Providers follow up alerts by phone and schedule visits and imaging. “The sensitivity of the algorithm was high and was validated in two prospective studies,” Dr. Denis said. Sensitivity was 86%-100%.
Moovcare allowed earlier detection of relapse and improved overall survival for three reasons. “It allowed higher performance status at relapse, leading to more optimal therapy for relapsing patients. Dangerous medical conditions were detected earlier and treated earlier. It favored earlier supportive care, which improved quality of life. Less imaging was needed and performed at the right time,” Dr. Denis said.
Patients were monitored on a weekly basis, allowing more personalized care. The Moovcare web-app has been evaluated prospectively in about 300 patients, providing a high level of evidence of its utility in improving outcomes for patients with advanced lung cancer.
Press conference moderator Dr. Patricia Ganz commented that Moovcare is an example of a new way to improve the delivery of high-quality care to patients. “If we had a drug or some new intervention that caused this level of survival benefit, wouldn’t we want to go out and use it?” she asked. “This is a tremendous advance. This is personalized medicine. This is really tailoring it to the patient, and you can see how simple it is to collect this kind of data from the patient and then bring them in in between what would have been a scheduled visit.” She said the app overcomes the barrier of patients putting off reporting symptoms until their next visit or their reluctance to “bother the doctor.”
She said the app alerts the health care team to potential problems and prompts them to “use tests when appropriate, not on a schedule, [which] leads to avoidance of waste in the follow-up of care of our patients.”
AT THE 2016 ASCO ANNUAL MEETING
Key clinical point: A Web-based app improves survival for advanced lung cancer patients.
Major finding: Survival improved by 26% for web-app patients vs. controls.
Data source: Multicenter, prospective, phase III, randomized trial of 121 patients.
Disclosures: Dr. Denis has received honoraria and expenses from several pharmaceutical companies and has received institutional research funding from Sivan. Dr. Patricia Ganz reported stock and other ownership interest in Abbott Laboratories, GlaxoSmithKline, Johnson & Johnson, Merck, Novartis, Pfizer, and Teva.
End-of-life aggressive cancer care continues despite recommendations
CHICAGO – Aggressive care for most patients with incurable solid tumors continued in the final 30 days of life, and one-third died in the hospital, a recent, large retrospective study of health claims data on more than 28,000 patients shows, and there was no decrease in this practice over a recent 7-year period, despite recommendations to the contrary.
“Aggressive medical care for patients with incurable cancers at the end of life is widely recognized to be harmful to patients and their families,” Dr. Ronald Chen of the University of North Carolina at Chapel Hill said at the annual meeting of the American Society of Clinical Oncology.
As part of the Choosing Wisely campaign in 2012, the American Society of Clinical Oncology’s first recommendation was not to use cancer-directed therapy for patients with solid tumors when there is no strong evidence supporting the clinical value of further anticancer treatment. It also recommended more use of palliative and supportive care. However, the impact of these recommendations on clinical care in younger cancer populations was unknown.
In a study of patients younger than 65 years with solid tumors who died in 2007-2014, Dr. Chen and his associates studied the use of several items that could be considered to be aggressive therapy. They included chemotherapy, radiotherapy, invasive procedures, emergency room visits, hospitalization, intensive care use, and in-hospital death. Patients had any of five common metastatic diseases: breast, lung, prostate, colorectal, or pancreatic cancers (n = 5,855; 12,764; 1,508; 5,207; 3,397, respectively).
The source material for the study was large commercial insurance claims data on patients across 14 states. Investigators evaluated the proportion of patients who received forms of aggressive care in the final 30 days of life.
“Overall, the findings are remarkably consistent across the five diseases. And overall, about three-quarters of patients received at least one form of aggressive care in the last 30 days of life,” Dr. Chen said (range, 71.2%-75.9% of patients). Almost two-thirds of patients (61.6%-65.1%) were admitted to the hospital or went to the emergency department, about 20% of patients (15.9%-20.6%) received intensive care, and one-third of patients (30.3%-35.4%) died in the hospital instead of at home. About 25%-30% of patients received chemotherapy or an invasive procedure, such as a biopsy or a form of surgery. Radiation therapy was used the least and was administered to about 5%-20% of patients.
Looking at the overall use of aggressive care for each of the cancers studied, the researchers found virtually no trend over time, that is, from the second quarter of 2012, when ASCO issued its Choosing Wisely guidelines, through the fourth quarter of 2014. For each of the cancers, aggressive care was delivered to just about 75% of patients across all quarters. Looking further back, the investigators found the same proportions of patients receiving aggressive care in the last 30 days of life during the years 2007-2011.
They also looked specifically at the use of chemotherapy and did not find a change after the Choosing Wisely recommendations, “nor did we find a significant increase in the use of hospice from before 2012 to afterward,” Dr. Chen said. “Additional efforts are critically needed to improve end-of-life care for patients with terminal cancers to ensure that the care provided meets the goals and preferences of patients and their families.” Fewer than one-fifth of patients used hospice care.
Press conference moderator Dr. Patricia Ganz, director of cancer prevention and control research at the Jonsson Comprehensive Cancer Center of the University of California, Los Angeles, called the study “interesting and important for several reasons.” First, there have been very few studies on the topic on the younger (up to age 65) cancer population although the SEER-Medicare database has been used as a source of claims data for older cancer patients. One may like to know if the younger population is being treated more aggressively than the older population is, as well as other patterns of care.
“Giving chemotherapy in the last 30 days of life has been a coping measure for a very long time,” she said. “It’s been nationally looked at as one of our failures in giving good end-of-life care, and so the fact that there wasn’t any dramatic change at 2012 doesn’t bother me in the sense that we’ve been talking about this for a very long time, and we haven’t seen any movement.” She said there is a lot left to do in delivering high quality end-of-life care.
Dr. Chen said more education of both patients and physicians is needed to improve conversations about goals and expectations, as well as palliative care and hospice. These types of care need to be made more accessible, he said.
Limitations of the study include a lack of information on the cause of death (whether related to the cancer, the treatment received, or other), and researchers did not review the medical records to investigate the medical reasons for the use of aggressive care near the end of life.
CHICAGO – Aggressive care for most patients with incurable solid tumors continued in the final 30 days of life, and one-third died in the hospital, a recent, large retrospective study of health claims data on more than 28,000 patients shows, and there was no decrease in this practice over a recent 7-year period, despite recommendations to the contrary.
“Aggressive medical care for patients with incurable cancers at the end of life is widely recognized to be harmful to patients and their families,” Dr. Ronald Chen of the University of North Carolina at Chapel Hill said at the annual meeting of the American Society of Clinical Oncology.
As part of the Choosing Wisely campaign in 2012, the American Society of Clinical Oncology’s first recommendation was not to use cancer-directed therapy for patients with solid tumors when there is no strong evidence supporting the clinical value of further anticancer treatment. It also recommended more use of palliative and supportive care. However, the impact of these recommendations on clinical care in younger cancer populations was unknown.
In a study of patients younger than 65 years with solid tumors who died in 2007-2014, Dr. Chen and his associates studied the use of several items that could be considered to be aggressive therapy. They included chemotherapy, radiotherapy, invasive procedures, emergency room visits, hospitalization, intensive care use, and in-hospital death. Patients had any of five common metastatic diseases: breast, lung, prostate, colorectal, or pancreatic cancers (n = 5,855; 12,764; 1,508; 5,207; 3,397, respectively).
The source material for the study was large commercial insurance claims data on patients across 14 states. Investigators evaluated the proportion of patients who received forms of aggressive care in the final 30 days of life.
“Overall, the findings are remarkably consistent across the five diseases. And overall, about three-quarters of patients received at least one form of aggressive care in the last 30 days of life,” Dr. Chen said (range, 71.2%-75.9% of patients). Almost two-thirds of patients (61.6%-65.1%) were admitted to the hospital or went to the emergency department, about 20% of patients (15.9%-20.6%) received intensive care, and one-third of patients (30.3%-35.4%) died in the hospital instead of at home. About 25%-30% of patients received chemotherapy or an invasive procedure, such as a biopsy or a form of surgery. Radiation therapy was used the least and was administered to about 5%-20% of patients.
Looking at the overall use of aggressive care for each of the cancers studied, the researchers found virtually no trend over time, that is, from the second quarter of 2012, when ASCO issued its Choosing Wisely guidelines, through the fourth quarter of 2014. For each of the cancers, aggressive care was delivered to just about 75% of patients across all quarters. Looking further back, the investigators found the same proportions of patients receiving aggressive care in the last 30 days of life during the years 2007-2011.
They also looked specifically at the use of chemotherapy and did not find a change after the Choosing Wisely recommendations, “nor did we find a significant increase in the use of hospice from before 2012 to afterward,” Dr. Chen said. “Additional efforts are critically needed to improve end-of-life care for patients with terminal cancers to ensure that the care provided meets the goals and preferences of patients and their families.” Fewer than one-fifth of patients used hospice care.
Press conference moderator Dr. Patricia Ganz, director of cancer prevention and control research at the Jonsson Comprehensive Cancer Center of the University of California, Los Angeles, called the study “interesting and important for several reasons.” First, there have been very few studies on the topic on the younger (up to age 65) cancer population although the SEER-Medicare database has been used as a source of claims data for older cancer patients. One may like to know if the younger population is being treated more aggressively than the older population is, as well as other patterns of care.
“Giving chemotherapy in the last 30 days of life has been a coping measure for a very long time,” she said. “It’s been nationally looked at as one of our failures in giving good end-of-life care, and so the fact that there wasn’t any dramatic change at 2012 doesn’t bother me in the sense that we’ve been talking about this for a very long time, and we haven’t seen any movement.” She said there is a lot left to do in delivering high quality end-of-life care.
Dr. Chen said more education of both patients and physicians is needed to improve conversations about goals and expectations, as well as palliative care and hospice. These types of care need to be made more accessible, he said.
Limitations of the study include a lack of information on the cause of death (whether related to the cancer, the treatment received, or other), and researchers did not review the medical records to investigate the medical reasons for the use of aggressive care near the end of life.
CHICAGO – Aggressive care for most patients with incurable solid tumors continued in the final 30 days of life, and one-third died in the hospital, a recent, large retrospective study of health claims data on more than 28,000 patients shows, and there was no decrease in this practice over a recent 7-year period, despite recommendations to the contrary.
“Aggressive medical care for patients with incurable cancers at the end of life is widely recognized to be harmful to patients and their families,” Dr. Ronald Chen of the University of North Carolina at Chapel Hill said at the annual meeting of the American Society of Clinical Oncology.
As part of the Choosing Wisely campaign in 2012, the American Society of Clinical Oncology’s first recommendation was not to use cancer-directed therapy for patients with solid tumors when there is no strong evidence supporting the clinical value of further anticancer treatment. It also recommended more use of palliative and supportive care. However, the impact of these recommendations on clinical care in younger cancer populations was unknown.
In a study of patients younger than 65 years with solid tumors who died in 2007-2014, Dr. Chen and his associates studied the use of several items that could be considered to be aggressive therapy. They included chemotherapy, radiotherapy, invasive procedures, emergency room visits, hospitalization, intensive care use, and in-hospital death. Patients had any of five common metastatic diseases: breast, lung, prostate, colorectal, or pancreatic cancers (n = 5,855; 12,764; 1,508; 5,207; 3,397, respectively).
The source material for the study was large commercial insurance claims data on patients across 14 states. Investigators evaluated the proportion of patients who received forms of aggressive care in the final 30 days of life.
“Overall, the findings are remarkably consistent across the five diseases. And overall, about three-quarters of patients received at least one form of aggressive care in the last 30 days of life,” Dr. Chen said (range, 71.2%-75.9% of patients). Almost two-thirds of patients (61.6%-65.1%) were admitted to the hospital or went to the emergency department, about 20% of patients (15.9%-20.6%) received intensive care, and one-third of patients (30.3%-35.4%) died in the hospital instead of at home. About 25%-30% of patients received chemotherapy or an invasive procedure, such as a biopsy or a form of surgery. Radiation therapy was used the least and was administered to about 5%-20% of patients.
Looking at the overall use of aggressive care for each of the cancers studied, the researchers found virtually no trend over time, that is, from the second quarter of 2012, when ASCO issued its Choosing Wisely guidelines, through the fourth quarter of 2014. For each of the cancers, aggressive care was delivered to just about 75% of patients across all quarters. Looking further back, the investigators found the same proportions of patients receiving aggressive care in the last 30 days of life during the years 2007-2011.
They also looked specifically at the use of chemotherapy and did not find a change after the Choosing Wisely recommendations, “nor did we find a significant increase in the use of hospice from before 2012 to afterward,” Dr. Chen said. “Additional efforts are critically needed to improve end-of-life care for patients with terminal cancers to ensure that the care provided meets the goals and preferences of patients and their families.” Fewer than one-fifth of patients used hospice care.
Press conference moderator Dr. Patricia Ganz, director of cancer prevention and control research at the Jonsson Comprehensive Cancer Center of the University of California, Los Angeles, called the study “interesting and important for several reasons.” First, there have been very few studies on the topic on the younger (up to age 65) cancer population although the SEER-Medicare database has been used as a source of claims data for older cancer patients. One may like to know if the younger population is being treated more aggressively than the older population is, as well as other patterns of care.
“Giving chemotherapy in the last 30 days of life has been a coping measure for a very long time,” she said. “It’s been nationally looked at as one of our failures in giving good end-of-life care, and so the fact that there wasn’t any dramatic change at 2012 doesn’t bother me in the sense that we’ve been talking about this for a very long time, and we haven’t seen any movement.” She said there is a lot left to do in delivering high quality end-of-life care.
Dr. Chen said more education of both patients and physicians is needed to improve conversations about goals and expectations, as well as palliative care and hospice. These types of care need to be made more accessible, he said.
Limitations of the study include a lack of information on the cause of death (whether related to the cancer, the treatment received, or other), and researchers did not review the medical records to investigate the medical reasons for the use of aggressive care near the end of life.
AT THE 2016 ASCO ANNUAL MEETING
Key clinical point: End-of-life aggressive cancer care continues unabated despite Choosing Wisely recommendations.
Major finding: Three-quarters of cancer patients received aggressive therapies at end of life.
Data source: Retrospective study of health claims data on 28,731 patients younger than 65 years with incurable cancers.
Disclosures: The study received funding from the North Carolina Translational and Clinical Sciences Institute. Dr. Chen reported consulting or advisory roles with Medivation/Astellas and research funding from Accuray. Dr. Patricia Ganz reported stock and other ownership interest in Abbott Laboratories, GlaxoSmithKline, Johnson & Johnson, Merck, Novartis, Pfizer, and Teva.
Vandetanib shows variable response, toxicity in RET-positive NSCLC
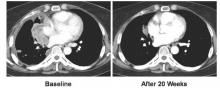
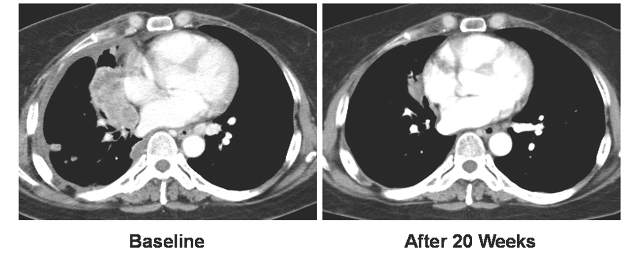
CHICAGO – The RET tyrosine kinase inhibitor vandetanib shows marked yet variable antitumor activity and toxicity in patients whose RET-positive non–small cell lung cancer (NSCLC) was unsuccessfully treated with chemotherapy, according to results of two small phase II trials presented at the annual meeting of the American Society of Clinical Oncology.
The overall response rates were 53% and 61% in two independent trials conducted by Dr. Takashi Seto of the National Kyushu Cancer Center, Japan, and by Dr. Se-Hoon Lee of Sungkyunkwan University, South Korea, respectively.
Compared to previous studies with other RET inhibitors, though all had small cohorts, there were many similarities in response rates. Progression-free survival was the most variable, but was much higher in the cabozantinib data reported at the 2015 ASCO annual meeting, according to moderator Dr. Karen Reckamp of the City of Hope Comprehensive Cancer Center, Duarte, Calif. “Vandetanib may have lower response rates than some of the others,” she said.
RET is a tyrosine kinase domain that fuses to and undergoes rearrangements with KIF5B and CCDC6 genes. This fusion and subsequent rearrangement results in ligand-dependent dimerization, which causes tumor growth. RET rearrangements were first identified in thyroid cancers.
“RET fusions were identified as new driver oncogenes of NSCLC in 2012 and observed in 1%-2% of all NSCLC,” Dr. Seto said. “Non–small cell lung cancer with RET rearrangement is regarded as a unique entity in terms of pathogenesis,” said Dr. Lee. There are currently multiple RET inhibitors in various stages of development.
In the trial headed by Dr. Seto, the Japanese genetic screening network was utilized to identify 34 NSCLC patients with RET rearrangements. Among the 34 patients, 17 met the eligibility requirements of having failed at least one prior chemotherapy treatment. Of those 17 patients, the median age was 59 years, 74% were female, all had adenocarcinomas, and 68% were nonsmokers.
All 17 patients received vandetanib at a dose of 300 mg once daily. The overall response rate was 53% (90% confidence interval, 31-74), and the disease control rate was 88%. The median progression-free survival was 4.7 months (90% CI, 2.8-8.3).
There was a marked difference in overall response rate and progression-free survival among RET fusion subtypes. For CCDC6-RET, the overall response was 83% and the progression-free survival was 8.3 months. For KIF5B-RET, the overall response was 20% with a median progression-free survival of 2.9 months.
Dr. Seto noted that there was no known biological explanation for the observed discrepancy in response rate or survival.
Dr. Seto reported that the safety profile was similar to previous reports. Four patients ended treatment due to adverse events while 16 experienced dose interruptions due to treatment-related toxicities. The most common grade three and four toxicities were hypertension (58%), rash (16%), and diarrhea (11%).
In the trial headed by Dr. Lee, 18 patients with RET rearrangements (confirmed by fluorescent in situ hybridization) met the eligibility requirement of having failed platinum-based chemotherapy. The median age of the cohort was 55 years, and 33% were female.
Similar to Dr. Seto’s study, all 18 patients received vandetanib at 300 mg once daily. Of the 18 patient cohort, 17% achieved partial remission and 44% achieved stable disease. Seven patients had no remission or stabilization. There were no treatment-related mortalities or grade 4 adverse events. Two grade 3 adverse events were reported.
“Looking at these two studies together, I think the important thing about the characteristics you see [is] that the age range is very similar [with a] median in the 50’s,” commented Dr. Reckamp. “The male to female ratio is actually opposite in both so [this] can occur in both men and women. The smoking status, interestingly is similar in both, where about a third of patients were former smokers. Most of the patients had adenocarcinoma. Many of these patients were highly previously treated. Only the Seto group looked at RET fusion partner, which may be important in looking at efficacy for these agents.” Vandetanib is a “challenging drug to tolerate,” Dr. Reckamp also noted.
“Is there a preferred RET inhibitor in small cell lung cancer?” Dr. Reckamp asked. “There are many RET inhibitors approved for other cancer types at this point, and they are multitargeted tyrosine kinases. In small studies they have similar efficacy. Toxicities vary because of the off-target effects, and most of the [treatment] decisions were made based on potential toxicities rather than differing efficacy. So none is really differentiated as the best choice, and it is unlikely that we are going to have the trials to evaluate them head to head.”
Dr. Reckamp suggested that “most patients with adenocarcinoma” should be tested for RET rearrangements. “Both men and women, both smokers and nonsmokers. And if you don’t test, you won’t find it. And if you don’t find it, you won’t be able to treat.” Dr. Reckamp also noted that next generation sequencing (NGS) should be the preferred method of identifying RET status because NGS uses less tissue, provides genetic sequencing, and allows for the identification of binding partners.
“Does targeting RET improve patient outcomes?” she continued. “Because that is really the question we need to answer if we need to move forward with RET inhibition for lung cancer. So there are variable response rates, less than other inhibitors of other oncogenic-driven tumors, that’s for sure... But if you look at the data, and these are [for] heavily pretreated patients for the most part, the response rates are better than second-line cytotoxic chemotherapy that we have had in the past and similar to unselected checkpoint inhibition. So there is potential for improving outcomes, and again if we don’t know someone is RET, we potentially are not going to offer a treatment that could help them live longer or better.”
When asked how she would treat a newly-diagnosed patient with RET-positive NSCLC, Dr. Reckamp said she would treat with first-line chemotherapy rather than a tyrosine kinase inhibitor (TKI) but would enroll the patient in whatever TKI trial was ongoing at that point in time.
“There are multiple trials that are ongoing,” Dr. Reckamp said. “It is unlikely that a comparison trial will be completed and so we are going to have to look at these trials next to each other and differentiate based on toxicity [and] perceived efficacy.”
Specifically, Dr. Reckamp believes the medical community needs to move toward “universal testing” for RET status in lung cancer patients. Resistance and combination therapies will also need to be assessed in future studies.
“RET is important in lung cancer, and should be targeted. We now need to find the best way to do that,” Dr. Reckamp concluded.
The trial headed by Dr. Seto was funded by the Japan Agency for Medical Research and Development, AMED, and AstraZeneca. Dr. Seto reported receiving honoraria and research funding from multiple companies including AstraZeneca. The trial headed by Dr. Lee was funded by AstraZeneca Korea. Dr. Lee reported having a consulting or advisory role and receiving honoraria and research funding from AstraZeneca, Pfizer, and Roche/Genentech.
CHICAGO – The RET tyrosine kinase inhibitor vandetanib shows marked yet variable antitumor activity and toxicity in patients whose RET-positive non–small cell lung cancer (NSCLC) was unsuccessfully treated with chemotherapy, according to results of two small phase II trials presented at the annual meeting of the American Society of Clinical Oncology.
The overall response rates were 53% and 61% in two independent trials conducted by Dr. Takashi Seto of the National Kyushu Cancer Center, Japan, and by Dr. Se-Hoon Lee of Sungkyunkwan University, South Korea, respectively.
Compared to previous studies with other RET inhibitors, though all had small cohorts, there were many similarities in response rates. Progression-free survival was the most variable, but was much higher in the cabozantinib data reported at the 2015 ASCO annual meeting, according to moderator Dr. Karen Reckamp of the City of Hope Comprehensive Cancer Center, Duarte, Calif. “Vandetanib may have lower response rates than some of the others,” she said.
RET is a tyrosine kinase domain that fuses to and undergoes rearrangements with KIF5B and CCDC6 genes. This fusion and subsequent rearrangement results in ligand-dependent dimerization, which causes tumor growth. RET rearrangements were first identified in thyroid cancers.
“RET fusions were identified as new driver oncogenes of NSCLC in 2012 and observed in 1%-2% of all NSCLC,” Dr. Seto said. “Non–small cell lung cancer with RET rearrangement is regarded as a unique entity in terms of pathogenesis,” said Dr. Lee. There are currently multiple RET inhibitors in various stages of development.
In the trial headed by Dr. Seto, the Japanese genetic screening network was utilized to identify 34 NSCLC patients with RET rearrangements. Among the 34 patients, 17 met the eligibility requirements of having failed at least one prior chemotherapy treatment. Of those 17 patients, the median age was 59 years, 74% were female, all had adenocarcinomas, and 68% were nonsmokers.
All 17 patients received vandetanib at a dose of 300 mg once daily. The overall response rate was 53% (90% confidence interval, 31-74), and the disease control rate was 88%. The median progression-free survival was 4.7 months (90% CI, 2.8-8.3).
There was a marked difference in overall response rate and progression-free survival among RET fusion subtypes. For CCDC6-RET, the overall response was 83% and the progression-free survival was 8.3 months. For KIF5B-RET, the overall response was 20% with a median progression-free survival of 2.9 months.
Dr. Seto noted that there was no known biological explanation for the observed discrepancy in response rate or survival.
Dr. Seto reported that the safety profile was similar to previous reports. Four patients ended treatment due to adverse events while 16 experienced dose interruptions due to treatment-related toxicities. The most common grade three and four toxicities were hypertension (58%), rash (16%), and diarrhea (11%).
In the trial headed by Dr. Lee, 18 patients with RET rearrangements (confirmed by fluorescent in situ hybridization) met the eligibility requirement of having failed platinum-based chemotherapy. The median age of the cohort was 55 years, and 33% were female.
Similar to Dr. Seto’s study, all 18 patients received vandetanib at 300 mg once daily. Of the 18 patient cohort, 17% achieved partial remission and 44% achieved stable disease. Seven patients had no remission or stabilization. There were no treatment-related mortalities or grade 4 adverse events. Two grade 3 adverse events were reported.
“Looking at these two studies together, I think the important thing about the characteristics you see [is] that the age range is very similar [with a] median in the 50’s,” commented Dr. Reckamp. “The male to female ratio is actually opposite in both so [this] can occur in both men and women. The smoking status, interestingly is similar in both, where about a third of patients were former smokers. Most of the patients had adenocarcinoma. Many of these patients were highly previously treated. Only the Seto group looked at RET fusion partner, which may be important in looking at efficacy for these agents.” Vandetanib is a “challenging drug to tolerate,” Dr. Reckamp also noted.
“Is there a preferred RET inhibitor in small cell lung cancer?” Dr. Reckamp asked. “There are many RET inhibitors approved for other cancer types at this point, and they are multitargeted tyrosine kinases. In small studies they have similar efficacy. Toxicities vary because of the off-target effects, and most of the [treatment] decisions were made based on potential toxicities rather than differing efficacy. So none is really differentiated as the best choice, and it is unlikely that we are going to have the trials to evaluate them head to head.”
Dr. Reckamp suggested that “most patients with adenocarcinoma” should be tested for RET rearrangements. “Both men and women, both smokers and nonsmokers. And if you don’t test, you won’t find it. And if you don’t find it, you won’t be able to treat.” Dr. Reckamp also noted that next generation sequencing (NGS) should be the preferred method of identifying RET status because NGS uses less tissue, provides genetic sequencing, and allows for the identification of binding partners.
“Does targeting RET improve patient outcomes?” she continued. “Because that is really the question we need to answer if we need to move forward with RET inhibition for lung cancer. So there are variable response rates, less than other inhibitors of other oncogenic-driven tumors, that’s for sure... But if you look at the data, and these are [for] heavily pretreated patients for the most part, the response rates are better than second-line cytotoxic chemotherapy that we have had in the past and similar to unselected checkpoint inhibition. So there is potential for improving outcomes, and again if we don’t know someone is RET, we potentially are not going to offer a treatment that could help them live longer or better.”
When asked how she would treat a newly-diagnosed patient with RET-positive NSCLC, Dr. Reckamp said she would treat with first-line chemotherapy rather than a tyrosine kinase inhibitor (TKI) but would enroll the patient in whatever TKI trial was ongoing at that point in time.
“There are multiple trials that are ongoing,” Dr. Reckamp said. “It is unlikely that a comparison trial will be completed and so we are going to have to look at these trials next to each other and differentiate based on toxicity [and] perceived efficacy.”
Specifically, Dr. Reckamp believes the medical community needs to move toward “universal testing” for RET status in lung cancer patients. Resistance and combination therapies will also need to be assessed in future studies.
“RET is important in lung cancer, and should be targeted. We now need to find the best way to do that,” Dr. Reckamp concluded.
The trial headed by Dr. Seto was funded by the Japan Agency for Medical Research and Development, AMED, and AstraZeneca. Dr. Seto reported receiving honoraria and research funding from multiple companies including AstraZeneca. The trial headed by Dr. Lee was funded by AstraZeneca Korea. Dr. Lee reported having a consulting or advisory role and receiving honoraria and research funding from AstraZeneca, Pfizer, and Roche/Genentech.
CHICAGO – The RET tyrosine kinase inhibitor vandetanib shows marked yet variable antitumor activity and toxicity in patients whose RET-positive non–small cell lung cancer (NSCLC) was unsuccessfully treated with chemotherapy, according to results of two small phase II trials presented at the annual meeting of the American Society of Clinical Oncology.
The overall response rates were 53% and 61% in two independent trials conducted by Dr. Takashi Seto of the National Kyushu Cancer Center, Japan, and by Dr. Se-Hoon Lee of Sungkyunkwan University, South Korea, respectively.
Compared to previous studies with other RET inhibitors, though all had small cohorts, there were many similarities in response rates. Progression-free survival was the most variable, but was much higher in the cabozantinib data reported at the 2015 ASCO annual meeting, according to moderator Dr. Karen Reckamp of the City of Hope Comprehensive Cancer Center, Duarte, Calif. “Vandetanib may have lower response rates than some of the others,” she said.
RET is a tyrosine kinase domain that fuses to and undergoes rearrangements with KIF5B and CCDC6 genes. This fusion and subsequent rearrangement results in ligand-dependent dimerization, which causes tumor growth. RET rearrangements were first identified in thyroid cancers.
“RET fusions were identified as new driver oncogenes of NSCLC in 2012 and observed in 1%-2% of all NSCLC,” Dr. Seto said. “Non–small cell lung cancer with RET rearrangement is regarded as a unique entity in terms of pathogenesis,” said Dr. Lee. There are currently multiple RET inhibitors in various stages of development.
In the trial headed by Dr. Seto, the Japanese genetic screening network was utilized to identify 34 NSCLC patients with RET rearrangements. Among the 34 patients, 17 met the eligibility requirements of having failed at least one prior chemotherapy treatment. Of those 17 patients, the median age was 59 years, 74% were female, all had adenocarcinomas, and 68% were nonsmokers.
All 17 patients received vandetanib at a dose of 300 mg once daily. The overall response rate was 53% (90% confidence interval, 31-74), and the disease control rate was 88%. The median progression-free survival was 4.7 months (90% CI, 2.8-8.3).
There was a marked difference in overall response rate and progression-free survival among RET fusion subtypes. For CCDC6-RET, the overall response was 83% and the progression-free survival was 8.3 months. For KIF5B-RET, the overall response was 20% with a median progression-free survival of 2.9 months.
Dr. Seto noted that there was no known biological explanation for the observed discrepancy in response rate or survival.
Dr. Seto reported that the safety profile was similar to previous reports. Four patients ended treatment due to adverse events while 16 experienced dose interruptions due to treatment-related toxicities. The most common grade three and four toxicities were hypertension (58%), rash (16%), and diarrhea (11%).
In the trial headed by Dr. Lee, 18 patients with RET rearrangements (confirmed by fluorescent in situ hybridization) met the eligibility requirement of having failed platinum-based chemotherapy. The median age of the cohort was 55 years, and 33% were female.
Similar to Dr. Seto’s study, all 18 patients received vandetanib at 300 mg once daily. Of the 18 patient cohort, 17% achieved partial remission and 44% achieved stable disease. Seven patients had no remission or stabilization. There were no treatment-related mortalities or grade 4 adverse events. Two grade 3 adverse events were reported.
“Looking at these two studies together, I think the important thing about the characteristics you see [is] that the age range is very similar [with a] median in the 50’s,” commented Dr. Reckamp. “The male to female ratio is actually opposite in both so [this] can occur in both men and women. The smoking status, interestingly is similar in both, where about a third of patients were former smokers. Most of the patients had adenocarcinoma. Many of these patients were highly previously treated. Only the Seto group looked at RET fusion partner, which may be important in looking at efficacy for these agents.” Vandetanib is a “challenging drug to tolerate,” Dr. Reckamp also noted.
“Is there a preferred RET inhibitor in small cell lung cancer?” Dr. Reckamp asked. “There are many RET inhibitors approved for other cancer types at this point, and they are multitargeted tyrosine kinases. In small studies they have similar efficacy. Toxicities vary because of the off-target effects, and most of the [treatment] decisions were made based on potential toxicities rather than differing efficacy. So none is really differentiated as the best choice, and it is unlikely that we are going to have the trials to evaluate them head to head.”
Dr. Reckamp suggested that “most patients with adenocarcinoma” should be tested for RET rearrangements. “Both men and women, both smokers and nonsmokers. And if you don’t test, you won’t find it. And if you don’t find it, you won’t be able to treat.” Dr. Reckamp also noted that next generation sequencing (NGS) should be the preferred method of identifying RET status because NGS uses less tissue, provides genetic sequencing, and allows for the identification of binding partners.
“Does targeting RET improve patient outcomes?” she continued. “Because that is really the question we need to answer if we need to move forward with RET inhibition for lung cancer. So there are variable response rates, less than other inhibitors of other oncogenic-driven tumors, that’s for sure... But if you look at the data, and these are [for] heavily pretreated patients for the most part, the response rates are better than second-line cytotoxic chemotherapy that we have had in the past and similar to unselected checkpoint inhibition. So there is potential for improving outcomes, and again if we don’t know someone is RET, we potentially are not going to offer a treatment that could help them live longer or better.”
When asked how she would treat a newly-diagnosed patient with RET-positive NSCLC, Dr. Reckamp said she would treat with first-line chemotherapy rather than a tyrosine kinase inhibitor (TKI) but would enroll the patient in whatever TKI trial was ongoing at that point in time.
“There are multiple trials that are ongoing,” Dr. Reckamp said. “It is unlikely that a comparison trial will be completed and so we are going to have to look at these trials next to each other and differentiate based on toxicity [and] perceived efficacy.”
Specifically, Dr. Reckamp believes the medical community needs to move toward “universal testing” for RET status in lung cancer patients. Resistance and combination therapies will also need to be assessed in future studies.
“RET is important in lung cancer, and should be targeted. We now need to find the best way to do that,” Dr. Reckamp concluded.
The trial headed by Dr. Seto was funded by the Japan Agency for Medical Research and Development, AMED, and AstraZeneca. Dr. Seto reported receiving honoraria and research funding from multiple companies including AstraZeneca. The trial headed by Dr. Lee was funded by AstraZeneca Korea. Dr. Lee reported having a consulting or advisory role and receiving honoraria and research funding from AstraZeneca, Pfizer, and Roche/Genentech.
AT THE 2016 ASCO ANNUAL MEETING
Key clinical point: Vandetanib shows marked yet variable anti-tumor activity and toxicity in patients with RET-rearranged NSCLC.
Major finding: The overall response rates were 53% and 61% in two independent trials.
Data source: Two independently-conducted multicenter phase II trials of patients with RET-rearranged non-small cell lung cancer.
Disclosures: The trial headed by Dr. Seto was funded by the Japan Agency for Medical Research and Development, AMED, and AstraZeneca. Dr. Seto reported receiving honoraria and research funding from multiple companies including AstraZeneca. The trial headed by Dr. Lee was funded by AstraZeneca Korea Ltd. Dr. Lee reported having a consulting or advisory role and receiving honoraria and research funding from AstraZeneca, Pfizer, and Roche/Genentech.
Mogamulizumab achieves objective responses in relapsed/refractory adult T-cell leukemia-lymphoma
CHICAGO – The anti-CCR4 monoclonal antibody mogamulizumab was superior to other investigator-selected therapies for the treatment of patients with relapsed/refractory adult T-cell leukemia-lymphoma (ATL), based on results from 71 patients in a prospective, multicenter, randomized study reported at the annual meeting of the American Society of Clinical Oncology.
Commonly used cytotoxic regimens provided limited therapeutic benefit for these patients, but mogamulizumab resulted in an objective response rate that supports its therapeutic potential in this setting, reported Dr. Adrienne Alise Phillips of New York Presbyterian/Weill Cornell Medical College, New York.
A malignancy of T-cells infected with HTLV-1, ATL has a poor prognosis with a median overall survival of less than 3 months in patients with relapsed/refractory disease. CCR4 is expressed in over 90% of ATL patients, and mogamulizumab is approved in Japan for ATL as well as for peripheral T-cell lymphoma and cutaneous T-cell lymphoma.
The 71 patients in the study were from the United States, the European Union and Latin America. The study is the largest randomized clinical trial of relapsed/refractory adult T-cell leukemia-lymphoma thus far conducted. The patients were randomized in 2:1 fashion 47:24 patients) to mogamulizumab, 1.0 mg/kg, given weekly for the first 4-week cycle and then biweekly, or to one of three investigator choice regimens [gemcitabine and oxaliplatin, DHAP (dexamethasone, high-dose cytarabine, and cisplatin), or pralatrexate]. Patients who were in the investigator-choice arm and whose disease progressed were permitted to cross over to mogamulizumab.
The primary endpoint was objective response rate based on modified Tsukasaki criteria and assessed by the treating investigator and in blinded fashion by independent review.
The objective response rate in the mogamulizumab-treated group was 23.4% (11 of 47) by independent review and 34% (16 of 47) by the treating investigator. In the investigator choice group, the overall response rate was 2 of 24 by independent review and 0 of 24 by the treating investigator.
The confirmed objective response rate after 1 month in the mogamulizumab-treated group was 10.6% by independent review and 14.9% by the treating investigator; there were no confirmed responses in the investigator-choice arm. Of 18 patients who crossed over to mogamulizumab, 3 responded. The median duration of response for mogamulizumab was 5 months; one patient had a complete response that lasted over 9 months and the survival data are not yet mature.
Mogamulizumab had few drug-related adverse events, primarily infusion reactions (46.8%), rash/drug eruption (25.5%) and infections (14.9%).
Dr. Phillips disclosed ties to Celgene, Genentech, and Takeda, as well as research funding from Kyowa Hakko Kirin, the sponsor of the study.
On Twitter @maryjodales
 |
| Mary Jo Dales/Frontline Medical News Dr. Sonali M. Smith |
Mogamulizumab was superior to investigator’s choice therapy in the largest prospective randomized trial of this very rare disease. Approximately one-third of patients responded, while the response to investigator’s choice therapies was zero. The potential impact of mogamulizumab on T-cell regulation is intriguing. Could it have applications in other T-cell non-Hodgkin’s lymphomas and cutaneous T-cell lymphomas?
Dr. Sonali M. Smith is with the University of Chicago and was the invited discussant of the study.
 |
| Mary Jo Dales/Frontline Medical News Dr. Sonali M. Smith |
Mogamulizumab was superior to investigator’s choice therapy in the largest prospective randomized trial of this very rare disease. Approximately one-third of patients responded, while the response to investigator’s choice therapies was zero. The potential impact of mogamulizumab on T-cell regulation is intriguing. Could it have applications in other T-cell non-Hodgkin’s lymphomas and cutaneous T-cell lymphomas?
Dr. Sonali M. Smith is with the University of Chicago and was the invited discussant of the study.
 |
| Mary Jo Dales/Frontline Medical News Dr. Sonali M. Smith |
Mogamulizumab was superior to investigator’s choice therapy in the largest prospective randomized trial of this very rare disease. Approximately one-third of patients responded, while the response to investigator’s choice therapies was zero. The potential impact of mogamulizumab on T-cell regulation is intriguing. Could it have applications in other T-cell non-Hodgkin’s lymphomas and cutaneous T-cell lymphomas?
Dr. Sonali M. Smith is with the University of Chicago and was the invited discussant of the study.
CHICAGO – The anti-CCR4 monoclonal antibody mogamulizumab was superior to other investigator-selected therapies for the treatment of patients with relapsed/refractory adult T-cell leukemia-lymphoma (ATL), based on results from 71 patients in a prospective, multicenter, randomized study reported at the annual meeting of the American Society of Clinical Oncology.
Commonly used cytotoxic regimens provided limited therapeutic benefit for these patients, but mogamulizumab resulted in an objective response rate that supports its therapeutic potential in this setting, reported Dr. Adrienne Alise Phillips of New York Presbyterian/Weill Cornell Medical College, New York.
A malignancy of T-cells infected with HTLV-1, ATL has a poor prognosis with a median overall survival of less than 3 months in patients with relapsed/refractory disease. CCR4 is expressed in over 90% of ATL patients, and mogamulizumab is approved in Japan for ATL as well as for peripheral T-cell lymphoma and cutaneous T-cell lymphoma.
The 71 patients in the study were from the United States, the European Union and Latin America. The study is the largest randomized clinical trial of relapsed/refractory adult T-cell leukemia-lymphoma thus far conducted. The patients were randomized in 2:1 fashion 47:24 patients) to mogamulizumab, 1.0 mg/kg, given weekly for the first 4-week cycle and then biweekly, or to one of three investigator choice regimens [gemcitabine and oxaliplatin, DHAP (dexamethasone, high-dose cytarabine, and cisplatin), or pralatrexate]. Patients who were in the investigator-choice arm and whose disease progressed were permitted to cross over to mogamulizumab.
The primary endpoint was objective response rate based on modified Tsukasaki criteria and assessed by the treating investigator and in blinded fashion by independent review.
The objective response rate in the mogamulizumab-treated group was 23.4% (11 of 47) by independent review and 34% (16 of 47) by the treating investigator. In the investigator choice group, the overall response rate was 2 of 24 by independent review and 0 of 24 by the treating investigator.
The confirmed objective response rate after 1 month in the mogamulizumab-treated group was 10.6% by independent review and 14.9% by the treating investigator; there were no confirmed responses in the investigator-choice arm. Of 18 patients who crossed over to mogamulizumab, 3 responded. The median duration of response for mogamulizumab was 5 months; one patient had a complete response that lasted over 9 months and the survival data are not yet mature.
Mogamulizumab had few drug-related adverse events, primarily infusion reactions (46.8%), rash/drug eruption (25.5%) and infections (14.9%).
Dr. Phillips disclosed ties to Celgene, Genentech, and Takeda, as well as research funding from Kyowa Hakko Kirin, the sponsor of the study.
On Twitter @maryjodales
CHICAGO – The anti-CCR4 monoclonal antibody mogamulizumab was superior to other investigator-selected therapies for the treatment of patients with relapsed/refractory adult T-cell leukemia-lymphoma (ATL), based on results from 71 patients in a prospective, multicenter, randomized study reported at the annual meeting of the American Society of Clinical Oncology.
Commonly used cytotoxic regimens provided limited therapeutic benefit for these patients, but mogamulizumab resulted in an objective response rate that supports its therapeutic potential in this setting, reported Dr. Adrienne Alise Phillips of New York Presbyterian/Weill Cornell Medical College, New York.
A malignancy of T-cells infected with HTLV-1, ATL has a poor prognosis with a median overall survival of less than 3 months in patients with relapsed/refractory disease. CCR4 is expressed in over 90% of ATL patients, and mogamulizumab is approved in Japan for ATL as well as for peripheral T-cell lymphoma and cutaneous T-cell lymphoma.
The 71 patients in the study were from the United States, the European Union and Latin America. The study is the largest randomized clinical trial of relapsed/refractory adult T-cell leukemia-lymphoma thus far conducted. The patients were randomized in 2:1 fashion 47:24 patients) to mogamulizumab, 1.0 mg/kg, given weekly for the first 4-week cycle and then biweekly, or to one of three investigator choice regimens [gemcitabine and oxaliplatin, DHAP (dexamethasone, high-dose cytarabine, and cisplatin), or pralatrexate]. Patients who were in the investigator-choice arm and whose disease progressed were permitted to cross over to mogamulizumab.
The primary endpoint was objective response rate based on modified Tsukasaki criteria and assessed by the treating investigator and in blinded fashion by independent review.
The objective response rate in the mogamulizumab-treated group was 23.4% (11 of 47) by independent review and 34% (16 of 47) by the treating investigator. In the investigator choice group, the overall response rate was 2 of 24 by independent review and 0 of 24 by the treating investigator.
The confirmed objective response rate after 1 month in the mogamulizumab-treated group was 10.6% by independent review and 14.9% by the treating investigator; there were no confirmed responses in the investigator-choice arm. Of 18 patients who crossed over to mogamulizumab, 3 responded. The median duration of response for mogamulizumab was 5 months; one patient had a complete response that lasted over 9 months and the survival data are not yet mature.
Mogamulizumab had few drug-related adverse events, primarily infusion reactions (46.8%), rash/drug eruption (25.5%) and infections (14.9%).
Dr. Phillips disclosed ties to Celgene, Genentech, and Takeda, as well as research funding from Kyowa Hakko Kirin, the sponsor of the study.
On Twitter @maryjodales
AT THE 2016 ASCO ANNUAL MEETING
Key clinical point: The anti-CCR4 monoclonal antibody mogamulizumab was superior to other investigator-selected therapies for the treatment of patients with relapsed/refractory adult T-cell leukemia-lymphoma.
Major finding: The confirmed objective response rate after 1 month in the mogamulizumab-treated group was 10.6% by independent review and 14.9% by the treating investigator; there were no confirmed responses in the investigator-choice arm.
Data source: Prospective, multicenter, randomized study of 71 patients from the United States, the European Union, and Latin America.
Disclosures: Dr. Phillips disclosed ties to Celgene, Genentech, and Takeda, as well as research funding from Kyowa Hakko Kirin, the sponsor of the study.
Alemtuzumab plus CHOP didn’t boost survival in elderly patients with peripheral T-cell lymphomas
CHICAGO – Adding the monoclonal anti-CD52 antibody alemtuzumab to CHOP (A-CHOP) increased response rates in elderly patients with peripheral T-cell lymphomas but did not improve their survival, based on the final results from 116 patients treated in the international ACT-2 phase III trial.
Complete responses were seen in 43% of 58 patients given CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone) and in 60% of 58 patients given A-CHOP in the trial. However, trial participants did not significantly differ in event-free survival and progression-free survival at 3 years.
Further, overall survival at 3 years was 38% for the patients given A-CHOP and 56% for the patients given CHOP. The poorer overall survival was mainly the result of treatment-related toxicity, Dr. Lorenz H. Trümper reported at the annual meeting of the American Society of Clinical Oncology.
The estimated 3-year disease-free survival is 25% for elderly patients with peripheral T-cell lymphomas. Previous phase II trials had indicated that alemtuzumab was active in primary and relapsed T-cell lymphoma, prompting the study of adjuvant alemtuzumab in combination with dose-dense CHOP-14 in patients with previously untreated peripheral T-cell lymphoma, he said.
Although the treatment protocol demanded stringent monitoring for cytomegalovirus and Epstein-Barr virus and anti-infective prophylaxis, there were more grade 3 or higher infections in the A-CHOP group (40%) than the CHOP group (21%). The higher infection rates were attributed to higher rates of grade 3/4 hematotoxicity in patients given A-CHOP. Grade 4 leukocytopenia was seen in 70% with A-CHOP and 54% with CHOP; grade 3/4 thrombocytopenia was seen in 19% given A-CHOP and 13% given CHOP, according to Dr. Trümper of the University of Göttingen, Germany.
For the study, 116 patients from 52 centers were randomized to receive either six cycles of CHOP or A-CHOP given at 14-day intervals with granulocyte-colony stimulating factor (G-CSF) support. Initially, patients received a total of 360 mg of alemtuzumab (90 mg given at each of the first four cycles of CHOP). After patient 39 was enrolled, the dose was reduced to 120 mg (30 mg given at each of the first four cycles of CHOP). Median patient age was 69 years, and 58% of the trial participants were men.
Treatment was completed as planned in 79% of the CHOP patients and in 57% of the A-CHOP patients.
The study was sponsored by the University of Göttingen. Dr. Trümper is a consultant or adviser to Hexal and Janssen-Ortho, and receives research funding from Genzyme, the maker of alemtuzumab (Lemtrada), as well as other drug companies.
On Twitter @maryjodales
CHICAGO – Adding the monoclonal anti-CD52 antibody alemtuzumab to CHOP (A-CHOP) increased response rates in elderly patients with peripheral T-cell lymphomas but did not improve their survival, based on the final results from 116 patients treated in the international ACT-2 phase III trial.
Complete responses were seen in 43% of 58 patients given CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone) and in 60% of 58 patients given A-CHOP in the trial. However, trial participants did not significantly differ in event-free survival and progression-free survival at 3 years.
Further, overall survival at 3 years was 38% for the patients given A-CHOP and 56% for the patients given CHOP. The poorer overall survival was mainly the result of treatment-related toxicity, Dr. Lorenz H. Trümper reported at the annual meeting of the American Society of Clinical Oncology.
The estimated 3-year disease-free survival is 25% for elderly patients with peripheral T-cell lymphomas. Previous phase II trials had indicated that alemtuzumab was active in primary and relapsed T-cell lymphoma, prompting the study of adjuvant alemtuzumab in combination with dose-dense CHOP-14 in patients with previously untreated peripheral T-cell lymphoma, he said.
Although the treatment protocol demanded stringent monitoring for cytomegalovirus and Epstein-Barr virus and anti-infective prophylaxis, there were more grade 3 or higher infections in the A-CHOP group (40%) than the CHOP group (21%). The higher infection rates were attributed to higher rates of grade 3/4 hematotoxicity in patients given A-CHOP. Grade 4 leukocytopenia was seen in 70% with A-CHOP and 54% with CHOP; grade 3/4 thrombocytopenia was seen in 19% given A-CHOP and 13% given CHOP, according to Dr. Trümper of the University of Göttingen, Germany.
For the study, 116 patients from 52 centers were randomized to receive either six cycles of CHOP or A-CHOP given at 14-day intervals with granulocyte-colony stimulating factor (G-CSF) support. Initially, patients received a total of 360 mg of alemtuzumab (90 mg given at each of the first four cycles of CHOP). After patient 39 was enrolled, the dose was reduced to 120 mg (30 mg given at each of the first four cycles of CHOP). Median patient age was 69 years, and 58% of the trial participants were men.
Treatment was completed as planned in 79% of the CHOP patients and in 57% of the A-CHOP patients.
The study was sponsored by the University of Göttingen. Dr. Trümper is a consultant or adviser to Hexal and Janssen-Ortho, and receives research funding from Genzyme, the maker of alemtuzumab (Lemtrada), as well as other drug companies.
On Twitter @maryjodales
CHICAGO – Adding the monoclonal anti-CD52 antibody alemtuzumab to CHOP (A-CHOP) increased response rates in elderly patients with peripheral T-cell lymphomas but did not improve their survival, based on the final results from 116 patients treated in the international ACT-2 phase III trial.
Complete responses were seen in 43% of 58 patients given CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone) and in 60% of 58 patients given A-CHOP in the trial. However, trial participants did not significantly differ in event-free survival and progression-free survival at 3 years.
Further, overall survival at 3 years was 38% for the patients given A-CHOP and 56% for the patients given CHOP. The poorer overall survival was mainly the result of treatment-related toxicity, Dr. Lorenz H. Trümper reported at the annual meeting of the American Society of Clinical Oncology.
The estimated 3-year disease-free survival is 25% for elderly patients with peripheral T-cell lymphomas. Previous phase II trials had indicated that alemtuzumab was active in primary and relapsed T-cell lymphoma, prompting the study of adjuvant alemtuzumab in combination with dose-dense CHOP-14 in patients with previously untreated peripheral T-cell lymphoma, he said.
Although the treatment protocol demanded stringent monitoring for cytomegalovirus and Epstein-Barr virus and anti-infective prophylaxis, there were more grade 3 or higher infections in the A-CHOP group (40%) than the CHOP group (21%). The higher infection rates were attributed to higher rates of grade 3/4 hematotoxicity in patients given A-CHOP. Grade 4 leukocytopenia was seen in 70% with A-CHOP and 54% with CHOP; grade 3/4 thrombocytopenia was seen in 19% given A-CHOP and 13% given CHOP, according to Dr. Trümper of the University of Göttingen, Germany.
For the study, 116 patients from 52 centers were randomized to receive either six cycles of CHOP or A-CHOP given at 14-day intervals with granulocyte-colony stimulating factor (G-CSF) support. Initially, patients received a total of 360 mg of alemtuzumab (90 mg given at each of the first four cycles of CHOP). After patient 39 was enrolled, the dose was reduced to 120 mg (30 mg given at each of the first four cycles of CHOP). Median patient age was 69 years, and 58% of the trial participants were men.
Treatment was completed as planned in 79% of the CHOP patients and in 57% of the A-CHOP patients.
The study was sponsored by the University of Göttingen. Dr. Trümper is a consultant or adviser to Hexal and Janssen-Ortho, and receives research funding from Genzyme, the maker of alemtuzumab (Lemtrada), as well as other drug companies.
On Twitter @maryjodales
AT ASCO 16
Key clinical point: Adding the monoclonal anti-CD52 antibody alemtuzumab to CHOP (A-CHOP) increased response rates in elderly patients with peripheral T-cell lymphomas but did not improve their survival.
Major finding: Overall survival at 3 years was 38% for the patients given A-CHOP and 56% for the patients given CHOP.
Data source: Results from 116 patients treated in the international ACT-2 phase III trial.
Disclosures: The study was sponsored by the University of Göttingen, Germany. Dr. Trümper is a consultant or adviser to Hexal and Janssen-Ortho, and receives research funding from Genzyme, the maker of alemtuzumab, as well as other drug companies.
Temozolomide + RT boosts survival in elderly with glioblastoma
CHICAGO – Adding concomitant and adjuvant temozolomide to a shorter course of radiotherapy (RT) for elderly patients with glioblastoma improves progression-free survival, according to a phase III study presented at the annual meeting of the American Society of Clinical Oncology.
Both patients with and without MGMT methylated promoters in their tumors benefited, with the greatest benefit accruing to patients with promoter methylation, reported Dr. James Perry, head of the division of neurology at Sunnybrook Health Sciences Centre, Toronto.
The age of peak incidence of glioblastoma is 64 years, and the incidence is increasing. Best practice has been surgical resection and 6 weeks of radiation combined with oral temozolomide. A pivotal trial just over 10 years ago was restricted to patients younger than age 70 years and included few patients older than 65 years. It showed decreasing benefit of temozolomide with increasing age. Furthermore, trials in the elderly have compared only head-to-head radiation schedules or radiation alone with temozolomide alone. There has been no evidence on which to base practice for combining radiotherapy with temozolomide, Dr. Perry said.
To address this deficiency, Dr. Perry and his colleagues randomly assigned patients 65 years or older with newly diagnosed glioblastoma either to a short course of RT, consisting of 40 Gy in 15 fractions over 3 weeks or to the same radiotherapy plus 3 weeks of concomitant temozolomide and then adjuvant temozolomide for 12 months or until progression.
Patients (n = 562; 281 in each arm) averaged 73 years (range, 65-90 years), 77% had Eastern Cooperative Oncology Group performance score (PS) 0/1 and the rest PS 2, and 58% had had tumor resection.
Median overall survival was 9.3 months with the combined therapy and 7.6 months with radiation alone. Median progression-free survival was 5.3 vs. 3.9 months, respectively, with a hazard ratio of 0.50 (P less than .0001).
MGMT promoter methylation is a predictive marker for benefit from chemotherapy and a prognostic factor for survival. Forty-six percent of the tumors had this promoter methylation.
Temozolomide use added relatively more benefit if promoters were methylated. Patients with methylated promoters had an overall survival of 7.7 months with radiation alone and 13.5 months with combined radiation and temozolomide (HR, 0.53; P = .0001). For unmethylated promoters, overall survival with radiation alone was 7.9 months and was 10.0 months when temozolomide was added (HR, 0.75; P = .055), suggesting some benefit from temozolomide, although not as great as with tumors with methylated promoters.
“The advantage of this combined treatment with chemoradiation was achieved with minimal side effects,” Dr. Perry reported. Mild nausea and vomiting occurred mostly in the first weeks of therapy, and a slight increase in grade 3/4 hematologic toxicity was seen but occurred in less than 5% of patients. No differences in quality of life were reported between the treatment arms.
Most patients could easily complete the treatment plan, with 97% adherence to the 3 weeks of chemoradiation. “This is quite important because the elderly often have difficulties with mobility, distance from treatment centers, and sometimes don’t have caregivers [who] are able to bring them back and forth for treatment,” Dr. Perry said. He suggested that the shorter course of radiotherapy may have been one factor in the high adherence rate.
Based on these results of higher efficacy using concomitant and adjuvant temozolomide with manageable toxicities and no sacrifice in quality of life, “oncologists now have evidence to consider radiation with chemotherapy in all newly diagnosed elderly patients with glioblastoma,” he said.
Dr. Julie Vose, president of the American Society of Clinical Oncology, said the study was important because it compared the treatments in the appropriate patient population, that is, in the elderly who have the highest incidence of glioblastoma.
CHICAGO – Adding concomitant and adjuvant temozolomide to a shorter course of radiotherapy (RT) for elderly patients with glioblastoma improves progression-free survival, according to a phase III study presented at the annual meeting of the American Society of Clinical Oncology.
Both patients with and without MGMT methylated promoters in their tumors benefited, with the greatest benefit accruing to patients with promoter methylation, reported Dr. James Perry, head of the division of neurology at Sunnybrook Health Sciences Centre, Toronto.
The age of peak incidence of glioblastoma is 64 years, and the incidence is increasing. Best practice has been surgical resection and 6 weeks of radiation combined with oral temozolomide. A pivotal trial just over 10 years ago was restricted to patients younger than age 70 years and included few patients older than 65 years. It showed decreasing benefit of temozolomide with increasing age. Furthermore, trials in the elderly have compared only head-to-head radiation schedules or radiation alone with temozolomide alone. There has been no evidence on which to base practice for combining radiotherapy with temozolomide, Dr. Perry said.
To address this deficiency, Dr. Perry and his colleagues randomly assigned patients 65 years or older with newly diagnosed glioblastoma either to a short course of RT, consisting of 40 Gy in 15 fractions over 3 weeks or to the same radiotherapy plus 3 weeks of concomitant temozolomide and then adjuvant temozolomide for 12 months or until progression.
Patients (n = 562; 281 in each arm) averaged 73 years (range, 65-90 years), 77% had Eastern Cooperative Oncology Group performance score (PS) 0/1 and the rest PS 2, and 58% had had tumor resection.
Median overall survival was 9.3 months with the combined therapy and 7.6 months with radiation alone. Median progression-free survival was 5.3 vs. 3.9 months, respectively, with a hazard ratio of 0.50 (P less than .0001).
MGMT promoter methylation is a predictive marker for benefit from chemotherapy and a prognostic factor for survival. Forty-six percent of the tumors had this promoter methylation.
Temozolomide use added relatively more benefit if promoters were methylated. Patients with methylated promoters had an overall survival of 7.7 months with radiation alone and 13.5 months with combined radiation and temozolomide (HR, 0.53; P = .0001). For unmethylated promoters, overall survival with radiation alone was 7.9 months and was 10.0 months when temozolomide was added (HR, 0.75; P = .055), suggesting some benefit from temozolomide, although not as great as with tumors with methylated promoters.
“The advantage of this combined treatment with chemoradiation was achieved with minimal side effects,” Dr. Perry reported. Mild nausea and vomiting occurred mostly in the first weeks of therapy, and a slight increase in grade 3/4 hematologic toxicity was seen but occurred in less than 5% of patients. No differences in quality of life were reported between the treatment arms.
Most patients could easily complete the treatment plan, with 97% adherence to the 3 weeks of chemoradiation. “This is quite important because the elderly often have difficulties with mobility, distance from treatment centers, and sometimes don’t have caregivers [who] are able to bring them back and forth for treatment,” Dr. Perry said. He suggested that the shorter course of radiotherapy may have been one factor in the high adherence rate.
Based on these results of higher efficacy using concomitant and adjuvant temozolomide with manageable toxicities and no sacrifice in quality of life, “oncologists now have evidence to consider radiation with chemotherapy in all newly diagnosed elderly patients with glioblastoma,” he said.
Dr. Julie Vose, president of the American Society of Clinical Oncology, said the study was important because it compared the treatments in the appropriate patient population, that is, in the elderly who have the highest incidence of glioblastoma.
CHICAGO – Adding concomitant and adjuvant temozolomide to a shorter course of radiotherapy (RT) for elderly patients with glioblastoma improves progression-free survival, according to a phase III study presented at the annual meeting of the American Society of Clinical Oncology.
Both patients with and without MGMT methylated promoters in their tumors benefited, with the greatest benefit accruing to patients with promoter methylation, reported Dr. James Perry, head of the division of neurology at Sunnybrook Health Sciences Centre, Toronto.
The age of peak incidence of glioblastoma is 64 years, and the incidence is increasing. Best practice has been surgical resection and 6 weeks of radiation combined with oral temozolomide. A pivotal trial just over 10 years ago was restricted to patients younger than age 70 years and included few patients older than 65 years. It showed decreasing benefit of temozolomide with increasing age. Furthermore, trials in the elderly have compared only head-to-head radiation schedules or radiation alone with temozolomide alone. There has been no evidence on which to base practice for combining radiotherapy with temozolomide, Dr. Perry said.
To address this deficiency, Dr. Perry and his colleagues randomly assigned patients 65 years or older with newly diagnosed glioblastoma either to a short course of RT, consisting of 40 Gy in 15 fractions over 3 weeks or to the same radiotherapy plus 3 weeks of concomitant temozolomide and then adjuvant temozolomide for 12 months or until progression.
Patients (n = 562; 281 in each arm) averaged 73 years (range, 65-90 years), 77% had Eastern Cooperative Oncology Group performance score (PS) 0/1 and the rest PS 2, and 58% had had tumor resection.
Median overall survival was 9.3 months with the combined therapy and 7.6 months with radiation alone. Median progression-free survival was 5.3 vs. 3.9 months, respectively, with a hazard ratio of 0.50 (P less than .0001).
MGMT promoter methylation is a predictive marker for benefit from chemotherapy and a prognostic factor for survival. Forty-six percent of the tumors had this promoter methylation.
Temozolomide use added relatively more benefit if promoters were methylated. Patients with methylated promoters had an overall survival of 7.7 months with radiation alone and 13.5 months with combined radiation and temozolomide (HR, 0.53; P = .0001). For unmethylated promoters, overall survival with radiation alone was 7.9 months and was 10.0 months when temozolomide was added (HR, 0.75; P = .055), suggesting some benefit from temozolomide, although not as great as with tumors with methylated promoters.
“The advantage of this combined treatment with chemoradiation was achieved with minimal side effects,” Dr. Perry reported. Mild nausea and vomiting occurred mostly in the first weeks of therapy, and a slight increase in grade 3/4 hematologic toxicity was seen but occurred in less than 5% of patients. No differences in quality of life were reported between the treatment arms.
Most patients could easily complete the treatment plan, with 97% adherence to the 3 weeks of chemoradiation. “This is quite important because the elderly often have difficulties with mobility, distance from treatment centers, and sometimes don’t have caregivers [who] are able to bring them back and forth for treatment,” Dr. Perry said. He suggested that the shorter course of radiotherapy may have been one factor in the high adherence rate.
Based on these results of higher efficacy using concomitant and adjuvant temozolomide with manageable toxicities and no sacrifice in quality of life, “oncologists now have evidence to consider radiation with chemotherapy in all newly diagnosed elderly patients with glioblastoma,” he said.
Dr. Julie Vose, president of the American Society of Clinical Oncology, said the study was important because it compared the treatments in the appropriate patient population, that is, in the elderly who have the highest incidence of glioblastoma.
AT THE 2016 ASCO ANNUAL MEETING
Key clinical point: Combining temozolomide with radiotherapy prolongs survival for elderly with glioblastoma.
Major finding: Combining temozolomide with radiotherapy increased survival by 33% vs. radiation alone.
Data source: Global phase III study of 562 elderly patients with newly diagnosed glioblastoma randomized to temozolomide plus radiation vs radiation alone.
Disclosures: Dr. Perry reported stock or other ownership interests in DelMar Pharmaceuticals and VBL Therapeutics. Dr. Vose reported receiving honoraria from Sanofi-Aventis and Seattle Genetics; consulting for Bio Connections; and receiving research funding to her institution from Acerta, Bristol-Myers Squibb, Celgene, Genentech, GlaxoSmithKline, Incyte, Janssen Biotech, Kite Pharma, Pharmacyclics, and Spectrum Pharmaceuticals.
Tandem ASCT for neuroblastoma comes with caveat

Photo by Chad McNeeley
CHICAGO—A phase 3 study of tandem autologous stem cell transplant (ASCT) for children with high-risk neuroblastoma has shown that tandem transplant as consolidation significantly improved event-free survival (EFS).
Nevertheless, the improvement comes “with an important caveat,” according to Julie R. Park, MD, “that this is in children who survive induction without disease progression after induction or severe induction-related toxicity.”
Of note, the tandem transplant did not increase toxicity or regimen-related mortality.
Dr Park, of the Seattle Children’s Hospital and University of Washington, presented the study data on behalf of the Children’s Oncology Group and the COG ANBL0532 Study Committee during the plenary session at the 2016 ASCO Annual Meeting (LBA3*).
Neuroblastoma, Dr Park explained, is a disease that occurs in young children and is the most common extracranial tumor of childhood. Patients with high-risk neuroblastoma account for 50% of all children diagnosed with the disease, and neuroblastoma accounts for more than 10% of all childhood cancer mortality.
The outcome for high-risk neuroblastoma patients is dismal: fewer than 50% survive following current multi-agent, aggressive therapy, she said.
Randomized clinical trials performed over the last 25 years “have taught us that dose intensification of therapy is important,” Dr Park said, “and the treatment of minimal residual disease with non-cross resistant therapies is equally important.”
Pilot studies of tandem ASCT demonstrated tolerable toxicity and suggested efficacy, and clinical trials demonstrated that collecting peripheral blood stem cells in small children with neuroblastoma was feasible.
So the investigators undertook the current study to improve 3-year EFS of high-risk neuroblastoma patients using a strategy of tandem transplant consolidation.
Eligibility
Patients with newly diagnosed high-risk neuroblastoma were eligible. They had to have metastatic disease (INSS stage 4) and be older than 18 months to be eligible for the trial.
They could be any age if they had INSS stage 2, 3, or 4 with MYCN amplification.
“Our prior studies had identified that the group with regional disease and toddlers with MYCN non-amplified metastatic disease had a better outcome compared to other children with high-risk neuroblastoma,” Dr Park commented.
“Therefore, these children were non-randomly assigned to single transplant and are not included in the randomization results provided today,” she explained.
All children had to have normal cardiac, liver, and renal function.
Trial design: Induction
Induction therapy consisted of 6 cycles of chemotherapy—2 cycles of cyclophosphamide/topotecan, followed by 4 alternating cycles of cisplatin/etoposide and cyclophosphamide/vincristine/doxorubicin.
Peripheral blood stem cells (PBSCs) were harvested after the first 2 cycles of dose intensive cyclophosphamide and topotecan.
Patients had surgery on the primary tumor after 5 cycles of induction.
Trial design: Consolidation
Following completion of induction therapy, patients were assessed for eligibility for consolidation. These criteria included sufficient PBSC harvest, adequate organ function, no evidence of disease progression, and consent for post induction therapy.
Children eligible for consolidation were randomized to either the standard transplant with carboplatin, etoposide, melphalan or tandem transplant with cyclophosphamide and thiotepa followed 6 – 8 weeks later by the second transplant with carboplatin, etoposide, and melphalan.
Patients in both arms received radiotherapy to their primary tumor site.
Trial Design: Post-consolidation
Patients then went on for post consolidation chemotherapy with isotretinoin.
“During the conduct of ANBL0532,” Dr Park pointed out, “there was an additional trial running within the Children’s Oncology Group for which patients were eligible to enroll.”
So from 2007 – 2009, the additional trial (ANBL0032) randomized patients to their then standard of isotretinoin or isotretinoin plus the anti-GD2 antibody dinutuximab and cytokines.
“In 2009, results of that study were released and the dinutuximab arm was superior,” she noted, so patients were no longer randomized to isotretinoin only.
Prior studies indicated that 40% of patients went off trial at the end of induction therapy, so the investigators enrolled 665 patients to ensure a randomization of at least 332 patients.
Data cut-off for the presentation was March 31, 2016.
Patient demographics
Of the 665 patients accrued, 13 were ineligible. Twenty-seven patients with favorable prognosis were non-randomly assigned to single ASCT.
Forty percent (229 patients) went off protocol therapy prior to randomization. The main reasons for this were death (8 patients), progressive disease (48 patients), and physician and patient discretion (206 patients).
Investigators randomized 355 patients, 179 to single ASCT and 176 to tandem ASCT.
Patients were a median age of 37.2 months, 38.2% had MYCN amplification, and 88% were INSS stage 4.
The randomized cohorts of patients were similar to the overall patient cohort in terms of age, MYCN amplification, and stage 4 disease.
The randomizations were also well balanced in terms of MYCN amplifications, stage 4 disease, and response to induction chemotherapy.
The investigators retrospectively reviewed the patient characteristics, including age at diagnosis, early response to induction, and assignment to receive immunotherapy, “and there was no statistically significant difference between the randomized cohorts for these characteristics,” Dr Park said.
Safety
The most commonly observed grade 3 or greater non-hematologic toxicities in the single and tandem arms included infection (18.3% and 17.9%, respectively), mucosal toxicities (17.2% and 13.6%, respectively), and hepatic toxicities, including sinusoidal obstructive disorder (6.5% and 6.2%, respectively).
The investigators observed no significant differences between the arms in regard to the rate of these toxicities. In addition, they observed no difference between the arms in the rate of regimen-related mortality, 4.1% in the single arm, and 1.2% in the tandem arm.
Efficacy
For all patients (n=652) from time of enrollment, the 3-year EFS was 51.0% and the 3-year OS was 68.3%.
In randomized patients (n=355), from the time of randomization, the 3-year EFS was 54.8% and the 3-year OS, 71.5%, with a median survival time of 4.6 years.
There was a statistically significant improvement in EFS for those patients assigned to tandem transplant, with a 3-year EFS of 61.4% as compared to those assigned to single transplant, who had a 3-year EFS of 48.4% (P=0.0081).
There was not a statistically significant difference in OS. However, Dr Park pointed out that the trial was not powered to detect a difference in overall survival.
When looking at the cohort of stage 4 patients who were older than 18 months, investigators again saw a statistically significant improvement in EFS. The 3-year EFS was 59%.1 for tandem transplants vs 45.5% for single transplants, P=0.0083.
Again, there was no difference in OS in the INSS stage 4 patients older than 18 months.
“And finally, when we analyzed the outcome for children who were assigned to receive immunotherapy,” Dr Park pointed out, “from the time of start of immunotherapy, there was a statistically significant improvement in both event-free survival and overall survival for those children who received tandem transplants.”
The 3-year EFS for patients who received a tandem transplant and immunotherapy was 73.7% compared to the 3-year EFS of 56.0% for patients who received a single transplant and immunotherapy (P=0.0033).
The 3-year overall survival was also significantly improved for patients who received tandem transplants and immunotherapy (83.7%) compared to those who received a single transplant and immunotherapy (74.4%) (P=0.0322).
Conclusions
“Tandem transplant consolidation improves outcome in patients with high-risk neuroblastoma,” Dr Park said, with the important caveat mentioned earlier.
The tandem transplant does not increase toxicity or regimen-related mortality, and the benefit of tandem ASCT remains following anti-GD2-directed immunotherapy. ![]()
*Data in the presentation differ slightly from the abstract

Photo by Chad McNeeley
CHICAGO—A phase 3 study of tandem autologous stem cell transplant (ASCT) for children with high-risk neuroblastoma has shown that tandem transplant as consolidation significantly improved event-free survival (EFS).
Nevertheless, the improvement comes “with an important caveat,” according to Julie R. Park, MD, “that this is in children who survive induction without disease progression after induction or severe induction-related toxicity.”
Of note, the tandem transplant did not increase toxicity or regimen-related mortality.
Dr Park, of the Seattle Children’s Hospital and University of Washington, presented the study data on behalf of the Children’s Oncology Group and the COG ANBL0532 Study Committee during the plenary session at the 2016 ASCO Annual Meeting (LBA3*).
Neuroblastoma, Dr Park explained, is a disease that occurs in young children and is the most common extracranial tumor of childhood. Patients with high-risk neuroblastoma account for 50% of all children diagnosed with the disease, and neuroblastoma accounts for more than 10% of all childhood cancer mortality.
The outcome for high-risk neuroblastoma patients is dismal: fewer than 50% survive following current multi-agent, aggressive therapy, she said.
Randomized clinical trials performed over the last 25 years “have taught us that dose intensification of therapy is important,” Dr Park said, “and the treatment of minimal residual disease with non-cross resistant therapies is equally important.”
Pilot studies of tandem ASCT demonstrated tolerable toxicity and suggested efficacy, and clinical trials demonstrated that collecting peripheral blood stem cells in small children with neuroblastoma was feasible.
So the investigators undertook the current study to improve 3-year EFS of high-risk neuroblastoma patients using a strategy of tandem transplant consolidation.
Eligibility
Patients with newly diagnosed high-risk neuroblastoma were eligible. They had to have metastatic disease (INSS stage 4) and be older than 18 months to be eligible for the trial.
They could be any age if they had INSS stage 2, 3, or 4 with MYCN amplification.
“Our prior studies had identified that the group with regional disease and toddlers with MYCN non-amplified metastatic disease had a better outcome compared to other children with high-risk neuroblastoma,” Dr Park commented.
“Therefore, these children were non-randomly assigned to single transplant and are not included in the randomization results provided today,” she explained.
All children had to have normal cardiac, liver, and renal function.
Trial design: Induction
Induction therapy consisted of 6 cycles of chemotherapy—2 cycles of cyclophosphamide/topotecan, followed by 4 alternating cycles of cisplatin/etoposide and cyclophosphamide/vincristine/doxorubicin.
Peripheral blood stem cells (PBSCs) were harvested after the first 2 cycles of dose intensive cyclophosphamide and topotecan.
Patients had surgery on the primary tumor after 5 cycles of induction.
Trial design: Consolidation
Following completion of induction therapy, patients were assessed for eligibility for consolidation. These criteria included sufficient PBSC harvest, adequate organ function, no evidence of disease progression, and consent for post induction therapy.
Children eligible for consolidation were randomized to either the standard transplant with carboplatin, etoposide, melphalan or tandem transplant with cyclophosphamide and thiotepa followed 6 – 8 weeks later by the second transplant with carboplatin, etoposide, and melphalan.
Patients in both arms received radiotherapy to their primary tumor site.
Trial Design: Post-consolidation
Patients then went on for post consolidation chemotherapy with isotretinoin.
“During the conduct of ANBL0532,” Dr Park pointed out, “there was an additional trial running within the Children’s Oncology Group for which patients were eligible to enroll.”
So from 2007 – 2009, the additional trial (ANBL0032) randomized patients to their then standard of isotretinoin or isotretinoin plus the anti-GD2 antibody dinutuximab and cytokines.
“In 2009, results of that study were released and the dinutuximab arm was superior,” she noted, so patients were no longer randomized to isotretinoin only.
Prior studies indicated that 40% of patients went off trial at the end of induction therapy, so the investigators enrolled 665 patients to ensure a randomization of at least 332 patients.
Data cut-off for the presentation was March 31, 2016.
Patient demographics
Of the 665 patients accrued, 13 were ineligible. Twenty-seven patients with favorable prognosis were non-randomly assigned to single ASCT.
Forty percent (229 patients) went off protocol therapy prior to randomization. The main reasons for this were death (8 patients), progressive disease (48 patients), and physician and patient discretion (206 patients).
Investigators randomized 355 patients, 179 to single ASCT and 176 to tandem ASCT.
Patients were a median age of 37.2 months, 38.2% had MYCN amplification, and 88% were INSS stage 4.
The randomized cohorts of patients were similar to the overall patient cohort in terms of age, MYCN amplification, and stage 4 disease.
The randomizations were also well balanced in terms of MYCN amplifications, stage 4 disease, and response to induction chemotherapy.
The investigators retrospectively reviewed the patient characteristics, including age at diagnosis, early response to induction, and assignment to receive immunotherapy, “and there was no statistically significant difference between the randomized cohorts for these characteristics,” Dr Park said.
Safety
The most commonly observed grade 3 or greater non-hematologic toxicities in the single and tandem arms included infection (18.3% and 17.9%, respectively), mucosal toxicities (17.2% and 13.6%, respectively), and hepatic toxicities, including sinusoidal obstructive disorder (6.5% and 6.2%, respectively).
The investigators observed no significant differences between the arms in regard to the rate of these toxicities. In addition, they observed no difference between the arms in the rate of regimen-related mortality, 4.1% in the single arm, and 1.2% in the tandem arm.
Efficacy
For all patients (n=652) from time of enrollment, the 3-year EFS was 51.0% and the 3-year OS was 68.3%.
In randomized patients (n=355), from the time of randomization, the 3-year EFS was 54.8% and the 3-year OS, 71.5%, with a median survival time of 4.6 years.
There was a statistically significant improvement in EFS for those patients assigned to tandem transplant, with a 3-year EFS of 61.4% as compared to those assigned to single transplant, who had a 3-year EFS of 48.4% (P=0.0081).
There was not a statistically significant difference in OS. However, Dr Park pointed out that the trial was not powered to detect a difference in overall survival.
When looking at the cohort of stage 4 patients who were older than 18 months, investigators again saw a statistically significant improvement in EFS. The 3-year EFS was 59%.1 for tandem transplants vs 45.5% for single transplants, P=0.0083.
Again, there was no difference in OS in the INSS stage 4 patients older than 18 months.
“And finally, when we analyzed the outcome for children who were assigned to receive immunotherapy,” Dr Park pointed out, “from the time of start of immunotherapy, there was a statistically significant improvement in both event-free survival and overall survival for those children who received tandem transplants.”
The 3-year EFS for patients who received a tandem transplant and immunotherapy was 73.7% compared to the 3-year EFS of 56.0% for patients who received a single transplant and immunotherapy (P=0.0033).
The 3-year overall survival was also significantly improved for patients who received tandem transplants and immunotherapy (83.7%) compared to those who received a single transplant and immunotherapy (74.4%) (P=0.0322).
Conclusions
“Tandem transplant consolidation improves outcome in patients with high-risk neuroblastoma,” Dr Park said, with the important caveat mentioned earlier.
The tandem transplant does not increase toxicity or regimen-related mortality, and the benefit of tandem ASCT remains following anti-GD2-directed immunotherapy. ![]()
*Data in the presentation differ slightly from the abstract

Photo by Chad McNeeley
CHICAGO—A phase 3 study of tandem autologous stem cell transplant (ASCT) for children with high-risk neuroblastoma has shown that tandem transplant as consolidation significantly improved event-free survival (EFS).
Nevertheless, the improvement comes “with an important caveat,” according to Julie R. Park, MD, “that this is in children who survive induction without disease progression after induction or severe induction-related toxicity.”
Of note, the tandem transplant did not increase toxicity or regimen-related mortality.
Dr Park, of the Seattle Children’s Hospital and University of Washington, presented the study data on behalf of the Children’s Oncology Group and the COG ANBL0532 Study Committee during the plenary session at the 2016 ASCO Annual Meeting (LBA3*).
Neuroblastoma, Dr Park explained, is a disease that occurs in young children and is the most common extracranial tumor of childhood. Patients with high-risk neuroblastoma account for 50% of all children diagnosed with the disease, and neuroblastoma accounts for more than 10% of all childhood cancer mortality.
The outcome for high-risk neuroblastoma patients is dismal: fewer than 50% survive following current multi-agent, aggressive therapy, she said.
Randomized clinical trials performed over the last 25 years “have taught us that dose intensification of therapy is important,” Dr Park said, “and the treatment of minimal residual disease with non-cross resistant therapies is equally important.”
Pilot studies of tandem ASCT demonstrated tolerable toxicity and suggested efficacy, and clinical trials demonstrated that collecting peripheral blood stem cells in small children with neuroblastoma was feasible.
So the investigators undertook the current study to improve 3-year EFS of high-risk neuroblastoma patients using a strategy of tandem transplant consolidation.
Eligibility
Patients with newly diagnosed high-risk neuroblastoma were eligible. They had to have metastatic disease (INSS stage 4) and be older than 18 months to be eligible for the trial.
They could be any age if they had INSS stage 2, 3, or 4 with MYCN amplification.
“Our prior studies had identified that the group with regional disease and toddlers with MYCN non-amplified metastatic disease had a better outcome compared to other children with high-risk neuroblastoma,” Dr Park commented.
“Therefore, these children were non-randomly assigned to single transplant and are not included in the randomization results provided today,” she explained.
All children had to have normal cardiac, liver, and renal function.
Trial design: Induction
Induction therapy consisted of 6 cycles of chemotherapy—2 cycles of cyclophosphamide/topotecan, followed by 4 alternating cycles of cisplatin/etoposide and cyclophosphamide/vincristine/doxorubicin.
Peripheral blood stem cells (PBSCs) were harvested after the first 2 cycles of dose intensive cyclophosphamide and topotecan.
Patients had surgery on the primary tumor after 5 cycles of induction.
Trial design: Consolidation
Following completion of induction therapy, patients were assessed for eligibility for consolidation. These criteria included sufficient PBSC harvest, adequate organ function, no evidence of disease progression, and consent for post induction therapy.
Children eligible for consolidation were randomized to either the standard transplant with carboplatin, etoposide, melphalan or tandem transplant with cyclophosphamide and thiotepa followed 6 – 8 weeks later by the second transplant with carboplatin, etoposide, and melphalan.
Patients in both arms received radiotherapy to their primary tumor site.
Trial Design: Post-consolidation
Patients then went on for post consolidation chemotherapy with isotretinoin.
“During the conduct of ANBL0532,” Dr Park pointed out, “there was an additional trial running within the Children’s Oncology Group for which patients were eligible to enroll.”
So from 2007 – 2009, the additional trial (ANBL0032) randomized patients to their then standard of isotretinoin or isotretinoin plus the anti-GD2 antibody dinutuximab and cytokines.
“In 2009, results of that study were released and the dinutuximab arm was superior,” she noted, so patients were no longer randomized to isotretinoin only.
Prior studies indicated that 40% of patients went off trial at the end of induction therapy, so the investigators enrolled 665 patients to ensure a randomization of at least 332 patients.
Data cut-off for the presentation was March 31, 2016.
Patient demographics
Of the 665 patients accrued, 13 were ineligible. Twenty-seven patients with favorable prognosis were non-randomly assigned to single ASCT.
Forty percent (229 patients) went off protocol therapy prior to randomization. The main reasons for this were death (8 patients), progressive disease (48 patients), and physician and patient discretion (206 patients).
Investigators randomized 355 patients, 179 to single ASCT and 176 to tandem ASCT.
Patients were a median age of 37.2 months, 38.2% had MYCN amplification, and 88% were INSS stage 4.
The randomized cohorts of patients were similar to the overall patient cohort in terms of age, MYCN amplification, and stage 4 disease.
The randomizations were also well balanced in terms of MYCN amplifications, stage 4 disease, and response to induction chemotherapy.
The investigators retrospectively reviewed the patient characteristics, including age at diagnosis, early response to induction, and assignment to receive immunotherapy, “and there was no statistically significant difference between the randomized cohorts for these characteristics,” Dr Park said.
Safety
The most commonly observed grade 3 or greater non-hematologic toxicities in the single and tandem arms included infection (18.3% and 17.9%, respectively), mucosal toxicities (17.2% and 13.6%, respectively), and hepatic toxicities, including sinusoidal obstructive disorder (6.5% and 6.2%, respectively).
The investigators observed no significant differences between the arms in regard to the rate of these toxicities. In addition, they observed no difference between the arms in the rate of regimen-related mortality, 4.1% in the single arm, and 1.2% in the tandem arm.
Efficacy
For all patients (n=652) from time of enrollment, the 3-year EFS was 51.0% and the 3-year OS was 68.3%.
In randomized patients (n=355), from the time of randomization, the 3-year EFS was 54.8% and the 3-year OS, 71.5%, with a median survival time of 4.6 years.
There was a statistically significant improvement in EFS for those patients assigned to tandem transplant, with a 3-year EFS of 61.4% as compared to those assigned to single transplant, who had a 3-year EFS of 48.4% (P=0.0081).
There was not a statistically significant difference in OS. However, Dr Park pointed out that the trial was not powered to detect a difference in overall survival.
When looking at the cohort of stage 4 patients who were older than 18 months, investigators again saw a statistically significant improvement in EFS. The 3-year EFS was 59%.1 for tandem transplants vs 45.5% for single transplants, P=0.0083.
Again, there was no difference in OS in the INSS stage 4 patients older than 18 months.
“And finally, when we analyzed the outcome for children who were assigned to receive immunotherapy,” Dr Park pointed out, “from the time of start of immunotherapy, there was a statistically significant improvement in both event-free survival and overall survival for those children who received tandem transplants.”
The 3-year EFS for patients who received a tandem transplant and immunotherapy was 73.7% compared to the 3-year EFS of 56.0% for patients who received a single transplant and immunotherapy (P=0.0033).
The 3-year overall survival was also significantly improved for patients who received tandem transplants and immunotherapy (83.7%) compared to those who received a single transplant and immunotherapy (74.4%) (P=0.0322).
Conclusions
“Tandem transplant consolidation improves outcome in patients with high-risk neuroblastoma,” Dr Park said, with the important caveat mentioned earlier.
The tandem transplant does not increase toxicity or regimen-related mortality, and the benefit of tandem ASCT remains following anti-GD2-directed immunotherapy. ![]()
*Data in the presentation differ slightly from the abstract
Cancer drugs most affordable in Australia, least in India and China
CHICAGO – Cancer drugs are most expensive in the United States and least expensive in India and South Africa, but when taking into account a nation’s wealth and cost of living, the treatments are most affordable in Australia, and least affordable in India and China. The United States is somewhat in between, according to an analysis presented at the annual meeting of the American Society of Clinical Oncology.
“Value has become a major buzzword in cancer care. In addition to existing methods of cost effectiveness, new frameworks have been developed by ASCO, [the National Comprehensive Cancer Network], [the European Society for Medical Oncology], and [the American Board of Internal Medicine]. However, ultimately value boils down to a simple equation,” said Dr. Daniel A. Goldstein of Rabin Medical Center, Petah Tikva, Israel.
“Value is benefit divided by cost. In this study, we interrogated the denominator in this equation. Cost. And we did this on a global perspective.”
Dr. Goldstein and his associates collected drug prices of 15 generic and 8 patented drugs and calculated retail prices in seven countries (Australia, India, China, South Africa, Israel, the United Kingdom, and the United States) for 1 month of therapy using a standardized body surface area to determine dosage. The United States had the highest median monthly retail prices at just under $9,000 for patented drugs. China, Israel, the U.K., and Australia had similar median monthly retail prices that were just under $3,000. India and South Africa had the lowest median monthly retail prices for patented drugs.
Median monthly generic retail prices were fairly similar across the seven countries.
“It is currently unclear how the difference in prices relates to ability to pay for payers in different countries,” Dr. Goldstein said. To assess affordability, the investigators calculated the monthly drug price as a percentage of gross domestic product per capita at purchasing power parity (GDPcap), a measurement that takes into account a nation’s wealth and cost of living.
Cancer drugs were the most affordable in Australia. Monthly drug prices were 3% of GDPcap for generics and 71% for patented drugs. In the United States, the cost of generics was 14% of GDPcap while patented drugs were 192% of GDPcap. Cancer drugs were least affordable in India and China. Monthly drug prices were 48% of GDPcap for generic drugs in China, 288% for patented drugs, 33% for generic drugs in India, and 313% for patented drugs.
“Despite lower drug prices in India and China, these drugs appear to be less affordable than in other countries. In the USA despite having the highest GDP per capita, given the higher drug prices, the drugs appear to be less affordable than in other developed countries. And we see a similar pattern for generic drugs but with a lower magnitude. And this is mostly driven by differences in GDP per capita rather than differences in drug prices,” Dr. Rabin said.
“This is a very interesting international comparative study, and I think the concept of affordability is a novel one because we tend to just look at the cost and say it is so much less expensive in other parts of the world but looking at affordability adds another dimension to the many nuanced issues [of cost],” commented moderator Dr. Patricia Ganz of the University of California, Los Angeles.
A major limitation of this study was the inability to implicate the role of health insurance, drug discounts, rebates, and medical assistance programs into affordability. In response to a question from a reporter, Dr. Goldstein said that wealth disparities within countries would also be an important factor to more thoroughly understand the ability of patients to pay for cancer treatments.
Dr. Goldstein added that a better understanding of a drug’s benefit (improvements on life expectancy, toxicity, duration of therapy, and so on) is also important to truly understand the value of drugs on a global level.
“There are several questions this study does not answer,” Dr. Goldstein said. “Should drugs be priced the same globally? Is there an ethical duty to price drugs relative to an individual or population’s level of wealth? And is there a difference between drugs used in curative and metastatic setting[s]? Should governments or insurance companies provide all drugs with proven efficacy?”
This study was unfunded. Dr. Goldstein had no relevant disclosures to report. One coauthor disclosed a consulting or advisory role with Novartis, and travel, accommodations, or expenses from Genomic Health.
CHICAGO – Cancer drugs are most expensive in the United States and least expensive in India and South Africa, but when taking into account a nation’s wealth and cost of living, the treatments are most affordable in Australia, and least affordable in India and China. The United States is somewhat in between, according to an analysis presented at the annual meeting of the American Society of Clinical Oncology.
“Value has become a major buzzword in cancer care. In addition to existing methods of cost effectiveness, new frameworks have been developed by ASCO, [the National Comprehensive Cancer Network], [the European Society for Medical Oncology], and [the American Board of Internal Medicine]. However, ultimately value boils down to a simple equation,” said Dr. Daniel A. Goldstein of Rabin Medical Center, Petah Tikva, Israel.
“Value is benefit divided by cost. In this study, we interrogated the denominator in this equation. Cost. And we did this on a global perspective.”
Dr. Goldstein and his associates collected drug prices of 15 generic and 8 patented drugs and calculated retail prices in seven countries (Australia, India, China, South Africa, Israel, the United Kingdom, and the United States) for 1 month of therapy using a standardized body surface area to determine dosage. The United States had the highest median monthly retail prices at just under $9,000 for patented drugs. China, Israel, the U.K., and Australia had similar median monthly retail prices that were just under $3,000. India and South Africa had the lowest median monthly retail prices for patented drugs.
Median monthly generic retail prices were fairly similar across the seven countries.
“It is currently unclear how the difference in prices relates to ability to pay for payers in different countries,” Dr. Goldstein said. To assess affordability, the investigators calculated the monthly drug price as a percentage of gross domestic product per capita at purchasing power parity (GDPcap), a measurement that takes into account a nation’s wealth and cost of living.
Cancer drugs were the most affordable in Australia. Monthly drug prices were 3% of GDPcap for generics and 71% for patented drugs. In the United States, the cost of generics was 14% of GDPcap while patented drugs were 192% of GDPcap. Cancer drugs were least affordable in India and China. Monthly drug prices were 48% of GDPcap for generic drugs in China, 288% for patented drugs, 33% for generic drugs in India, and 313% for patented drugs.
“Despite lower drug prices in India and China, these drugs appear to be less affordable than in other countries. In the USA despite having the highest GDP per capita, given the higher drug prices, the drugs appear to be less affordable than in other developed countries. And we see a similar pattern for generic drugs but with a lower magnitude. And this is mostly driven by differences in GDP per capita rather than differences in drug prices,” Dr. Rabin said.
“This is a very interesting international comparative study, and I think the concept of affordability is a novel one because we tend to just look at the cost and say it is so much less expensive in other parts of the world but looking at affordability adds another dimension to the many nuanced issues [of cost],” commented moderator Dr. Patricia Ganz of the University of California, Los Angeles.
A major limitation of this study was the inability to implicate the role of health insurance, drug discounts, rebates, and medical assistance programs into affordability. In response to a question from a reporter, Dr. Goldstein said that wealth disparities within countries would also be an important factor to more thoroughly understand the ability of patients to pay for cancer treatments.
Dr. Goldstein added that a better understanding of a drug’s benefit (improvements on life expectancy, toxicity, duration of therapy, and so on) is also important to truly understand the value of drugs on a global level.
“There are several questions this study does not answer,” Dr. Goldstein said. “Should drugs be priced the same globally? Is there an ethical duty to price drugs relative to an individual or population’s level of wealth? And is there a difference between drugs used in curative and metastatic setting[s]? Should governments or insurance companies provide all drugs with proven efficacy?”
This study was unfunded. Dr. Goldstein had no relevant disclosures to report. One coauthor disclosed a consulting or advisory role with Novartis, and travel, accommodations, or expenses from Genomic Health.
CHICAGO – Cancer drugs are most expensive in the United States and least expensive in India and South Africa, but when taking into account a nation’s wealth and cost of living, the treatments are most affordable in Australia, and least affordable in India and China. The United States is somewhat in between, according to an analysis presented at the annual meeting of the American Society of Clinical Oncology.
“Value has become a major buzzword in cancer care. In addition to existing methods of cost effectiveness, new frameworks have been developed by ASCO, [the National Comprehensive Cancer Network], [the European Society for Medical Oncology], and [the American Board of Internal Medicine]. However, ultimately value boils down to a simple equation,” said Dr. Daniel A. Goldstein of Rabin Medical Center, Petah Tikva, Israel.
“Value is benefit divided by cost. In this study, we interrogated the denominator in this equation. Cost. And we did this on a global perspective.”
Dr. Goldstein and his associates collected drug prices of 15 generic and 8 patented drugs and calculated retail prices in seven countries (Australia, India, China, South Africa, Israel, the United Kingdom, and the United States) for 1 month of therapy using a standardized body surface area to determine dosage. The United States had the highest median monthly retail prices at just under $9,000 for patented drugs. China, Israel, the U.K., and Australia had similar median monthly retail prices that were just under $3,000. India and South Africa had the lowest median monthly retail prices for patented drugs.
Median monthly generic retail prices were fairly similar across the seven countries.
“It is currently unclear how the difference in prices relates to ability to pay for payers in different countries,” Dr. Goldstein said. To assess affordability, the investigators calculated the monthly drug price as a percentage of gross domestic product per capita at purchasing power parity (GDPcap), a measurement that takes into account a nation’s wealth and cost of living.
Cancer drugs were the most affordable in Australia. Monthly drug prices were 3% of GDPcap for generics and 71% for patented drugs. In the United States, the cost of generics was 14% of GDPcap while patented drugs were 192% of GDPcap. Cancer drugs were least affordable in India and China. Monthly drug prices were 48% of GDPcap for generic drugs in China, 288% for patented drugs, 33% for generic drugs in India, and 313% for patented drugs.
“Despite lower drug prices in India and China, these drugs appear to be less affordable than in other countries. In the USA despite having the highest GDP per capita, given the higher drug prices, the drugs appear to be less affordable than in other developed countries. And we see a similar pattern for generic drugs but with a lower magnitude. And this is mostly driven by differences in GDP per capita rather than differences in drug prices,” Dr. Rabin said.
“This is a very interesting international comparative study, and I think the concept of affordability is a novel one because we tend to just look at the cost and say it is so much less expensive in other parts of the world but looking at affordability adds another dimension to the many nuanced issues [of cost],” commented moderator Dr. Patricia Ganz of the University of California, Los Angeles.
A major limitation of this study was the inability to implicate the role of health insurance, drug discounts, rebates, and medical assistance programs into affordability. In response to a question from a reporter, Dr. Goldstein said that wealth disparities within countries would also be an important factor to more thoroughly understand the ability of patients to pay for cancer treatments.
Dr. Goldstein added that a better understanding of a drug’s benefit (improvements on life expectancy, toxicity, duration of therapy, and so on) is also important to truly understand the value of drugs on a global level.
“There are several questions this study does not answer,” Dr. Goldstein said. “Should drugs be priced the same globally? Is there an ethical duty to price drugs relative to an individual or population’s level of wealth? And is there a difference between drugs used in curative and metastatic setting[s]? Should governments or insurance companies provide all drugs with proven efficacy?”
This study was unfunded. Dr. Goldstein had no relevant disclosures to report. One coauthor disclosed a consulting or advisory role with Novartis, and travel, accommodations, or expenses from Genomic Health.
AT THE 2016 ASCO ANNUAL MEETING
Key clinical point: Taking into account a country’s wealth and cost of living, cancer drugs are most affordable in Australia, least affordable in India and China.
Major finding: In Australia, monthly drug prices were 3% of gross domestic product per capita at purchasing power parity (GDPcap) for generics and 71% for patented drugs. Monthly drug prices in China were 48% of GDPcap for generic drugs and 288% for patented drugs, while in India prices were 33% of GDPcap for generic drugs and 313% for patented drugs.
Data source: A multinational study of cancer drug prices, wealth, and cost of living.
Disclosures: This study was unfunded. Dr. Goldstein had no relevant disclosures to report. One coauthor disclosed a consulting or advisory role with Novartis, and travel, accommodations, or expenses from Genomic Health.