User login
Topical Combo Shows Early Promise in Moderate Psoriasis
SEOUL, SOUTH KOREA – The combination of calcipotriene and nicotinamide may be an attractive corticosteroid-sparing option for topical psoriasis therapy, according to results from a pilot trial.
"Both agents are somewhat effective for psoriasis; the combination of the two is markedly superior," Dr. Mark Lebwohl said in summarizing the findings of a pilot bilateral comparative study presented at the World Congress of Dermatology.
The investigational topical combination therapy is being developed by Dermipsor, an Israeli pharmaceutical company.
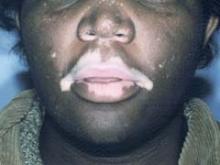
Dr. Lebwohl was a coinvestigator in the multicenter, double-blind, 12-week pilot study involving 168 patients with symmetric moderate psoriasis (defined as not more than 15% body surface area involvement).
The patients were randomized to two of seven treatments: calcipotriene 0.005% monotherapy, nicotinamide 1.4% alone, placebo, or calcipotriene 0.005% plus nicotinamide in a concentration of either 0.05%, 0.1%, 0.7%, or 1.4%. Patients treated the lesions on one side of the body with one of their two assigned treatments, and lesions on the opposite side with the other.
The primary end point was efficacy (defined as a clear to almost-clear outcome at week 12), which was achieved in 19% of patients who were treated with placebo, 25% of those on nicotinamide 1.4% alone, 31.5% of those treated with calcipotriene alone, and 50% of patients in the calcipotriene plus nicotinamide 1.4% group, reported Dr. Lebwohl, professor and chairman of the department of dermatology at Mount Sinai School of Medicine, New York.
The 50% clear or almost-clear rate with calcipotriene plus nicotinamide 1.4% is similar to the 48% rate of absent to very mild disease that was previously reported with a calcipotriol/betamethasone combination in a 52-week study (Dermatology 2006;213:319-26).
Thus, the calcipotriene/nicotinamide combination is an agent that will be investigated further in larger, lengthier studies as a possible steroid-sparing agent in comparative trials vs. calcipotriene-steroid combination therapy, Dr. Lebwohl said.
Topical combination therapy continues to be an active area in psoriasis research, he observed.
"It’s really a no-brainer: The combination of two ingredients is going to be superior to either one alone. It’s a very simple concept: Two is better than one," he said.
Dr. Lebwohl disclosed that he is a consultant to Dermipsor and has advisory board relationships with other pharmaceutical companies that are developing dermatologic drugs.
SEOUL, SOUTH KOREA – The combination of calcipotriene and nicotinamide may be an attractive corticosteroid-sparing option for topical psoriasis therapy, according to results from a pilot trial.
"Both agents are somewhat effective for psoriasis; the combination of the two is markedly superior," Dr. Mark Lebwohl said in summarizing the findings of a pilot bilateral comparative study presented at the World Congress of Dermatology.
The investigational topical combination therapy is being developed by Dermipsor, an Israeli pharmaceutical company.
Dr. Lebwohl was a coinvestigator in the multicenter, double-blind, 12-week pilot study involving 168 patients with symmetric moderate psoriasis (defined as not more than 15% body surface area involvement).
The patients were randomized to two of seven treatments: calcipotriene 0.005% monotherapy, nicotinamide 1.4% alone, placebo, or calcipotriene 0.005% plus nicotinamide in a concentration of either 0.05%, 0.1%, 0.7%, or 1.4%. Patients treated the lesions on one side of the body with one of their two assigned treatments, and lesions on the opposite side with the other.
The primary end point was efficacy (defined as a clear to almost-clear outcome at week 12), which was achieved in 19% of patients who were treated with placebo, 25% of those on nicotinamide 1.4% alone, 31.5% of those treated with calcipotriene alone, and 50% of patients in the calcipotriene plus nicotinamide 1.4% group, reported Dr. Lebwohl, professor and chairman of the department of dermatology at Mount Sinai School of Medicine, New York.
The 50% clear or almost-clear rate with calcipotriene plus nicotinamide 1.4% is similar to the 48% rate of absent to very mild disease that was previously reported with a calcipotriol/betamethasone combination in a 52-week study (Dermatology 2006;213:319-26).
Thus, the calcipotriene/nicotinamide combination is an agent that will be investigated further in larger, lengthier studies as a possible steroid-sparing agent in comparative trials vs. calcipotriene-steroid combination therapy, Dr. Lebwohl said.
Topical combination therapy continues to be an active area in psoriasis research, he observed.
"It’s really a no-brainer: The combination of two ingredients is going to be superior to either one alone. It’s a very simple concept: Two is better than one," he said.
Dr. Lebwohl disclosed that he is a consultant to Dermipsor and has advisory board relationships with other pharmaceutical companies that are developing dermatologic drugs.
SEOUL, SOUTH KOREA – The combination of calcipotriene and nicotinamide may be an attractive corticosteroid-sparing option for topical psoriasis therapy, according to results from a pilot trial.
"Both agents are somewhat effective for psoriasis; the combination of the two is markedly superior," Dr. Mark Lebwohl said in summarizing the findings of a pilot bilateral comparative study presented at the World Congress of Dermatology.
The investigational topical combination therapy is being developed by Dermipsor, an Israeli pharmaceutical company.
Dr. Lebwohl was a coinvestigator in the multicenter, double-blind, 12-week pilot study involving 168 patients with symmetric moderate psoriasis (defined as not more than 15% body surface area involvement).
The patients were randomized to two of seven treatments: calcipotriene 0.005% monotherapy, nicotinamide 1.4% alone, placebo, or calcipotriene 0.005% plus nicotinamide in a concentration of either 0.05%, 0.1%, 0.7%, or 1.4%. Patients treated the lesions on one side of the body with one of their two assigned treatments, and lesions on the opposite side with the other.
The primary end point was efficacy (defined as a clear to almost-clear outcome at week 12), which was achieved in 19% of patients who were treated with placebo, 25% of those on nicotinamide 1.4% alone, 31.5% of those treated with calcipotriene alone, and 50% of patients in the calcipotriene plus nicotinamide 1.4% group, reported Dr. Lebwohl, professor and chairman of the department of dermatology at Mount Sinai School of Medicine, New York.
The 50% clear or almost-clear rate with calcipotriene plus nicotinamide 1.4% is similar to the 48% rate of absent to very mild disease that was previously reported with a calcipotriol/betamethasone combination in a 52-week study (Dermatology 2006;213:319-26).
Thus, the calcipotriene/nicotinamide combination is an agent that will be investigated further in larger, lengthier studies as a possible steroid-sparing agent in comparative trials vs. calcipotriene-steroid combination therapy, Dr. Lebwohl said.
Topical combination therapy continues to be an active area in psoriasis research, he observed.
"It’s really a no-brainer: The combination of two ingredients is going to be superior to either one alone. It’s a very simple concept: Two is better than one," he said.
Dr. Lebwohl disclosed that he is a consultant to Dermipsor and has advisory board relationships with other pharmaceutical companies that are developing dermatologic drugs.
FROM THE WORLD CONGRESS OF DERMATOLOGY
Major Finding: In a study of 168 patients, 50% of patients with moderate psoriasis who used a combination of calcipotriene 0.005% plus nicotinamide 1.4% achieved a clear- to-almost-clear outcome at week 12, compared with 19% of patients using placebo.
Data Source: A double-blind, bilateral, comparative 12-week study of 168 patients.
Disclosures: Dr. Lebwohl disclosed that he is a consultant to Dermipsor and has advisory board relationships with other pharmaceutical companies that are developing dermatologic drugs.
Topical Combo Shows Early Promise in Moderate Psoriasis
SEOUL, SOUTH KOREA – The combination of calcipotriene and nicotinamide may be an attractive corticosteroid-sparing option for topical psoriasis therapy, according to results from a pilot trial.
"Both agents are somewhat effective for psoriasis; the combination of the two is markedly superior," Dr. Mark Lebwohl said in summarizing the findings of a pilot bilateral comparative study presented at the World Congress of Dermatology.
The investigational topical combination therapy is being developed by Dermipsor, an Israeli pharmaceutical company.
Dr. Lebwohl was a coinvestigator in the multicenter, double-blind, 12-week pilot study involving 168 patients with symmetric moderate psoriasis (defined as not more than 15% body surface area involvement).
The patients were randomized to two of seven treatments: calcipotriene 0.005% monotherapy, nicotinamide 1.4% alone, placebo, or calcipotriene 0.005% plus nicotinamide in a concentration of either 0.05%, 0.1%, 0.7%, or 1.4%. Patients treated the lesions on one side of the body with one of their two assigned treatments, and lesions on the opposite side with the other.
The primary end point was efficacy (defined as a clear to almost-clear outcome at week 12), which was achieved in 19% of patients who were treated with placebo, 25% of those on nicotinamide 1.4% alone, 31.5% of those treated with calcipotriene alone, and 50% of patients in the calcipotriene plus nicotinamide 1.4% group, reported Dr. Lebwohl, professor and chairman of the department of dermatology at Mount Sinai School of Medicine, New York.
The 50% clear or almost-clear rate with calcipotriene plus nicotinamide 1.4% is similar to the 48% rate of absent to very mild disease that was previously reported with a calcipotriol/betamethasone combination in a 52-week study (Dermatology 2006;213:319-26).
Thus, the calcipotriene/nicotinamide combination is an agent that will be investigated further in larger, lengthier studies as a possible steroid-sparing agent in comparative trials vs. calcipotriene-steroid combination therapy, Dr. Lebwohl said.
Topical combination therapy continues to be an active area in psoriasis research, he observed.
"It's really a no-brainer: The combination of two ingredients is going to be superior to either one alone. It's a very simple concept: Two is better than one," he said.
Dr. Lebwohl disclosed that he is a consultant to Dermipsor and has advisory board relationships with other pharmaceutical companies that are developing dermatologic drugs.
SEOUL, SOUTH KOREA – The combination of calcipotriene and nicotinamide may be an attractive corticosteroid-sparing option for topical psoriasis therapy, according to results from a pilot trial.
"Both agents are somewhat effective for psoriasis; the combination of the two is markedly superior," Dr. Mark Lebwohl said in summarizing the findings of a pilot bilateral comparative study presented at the World Congress of Dermatology.
The investigational topical combination therapy is being developed by Dermipsor, an Israeli pharmaceutical company.
Dr. Lebwohl was a coinvestigator in the multicenter, double-blind, 12-week pilot study involving 168 patients with symmetric moderate psoriasis (defined as not more than 15% body surface area involvement).
The patients were randomized to two of seven treatments: calcipotriene 0.005% monotherapy, nicotinamide 1.4% alone, placebo, or calcipotriene 0.005% plus nicotinamide in a concentration of either 0.05%, 0.1%, 0.7%, or 1.4%. Patients treated the lesions on one side of the body with one of their two assigned treatments, and lesions on the opposite side with the other.
The primary end point was efficacy (defined as a clear to almost-clear outcome at week 12), which was achieved in 19% of patients who were treated with placebo, 25% of those on nicotinamide 1.4% alone, 31.5% of those treated with calcipotriene alone, and 50% of patients in the calcipotriene plus nicotinamide 1.4% group, reported Dr. Lebwohl, professor and chairman of the department of dermatology at Mount Sinai School of Medicine, New York.
The 50% clear or almost-clear rate with calcipotriene plus nicotinamide 1.4% is similar to the 48% rate of absent to very mild disease that was previously reported with a calcipotriol/betamethasone combination in a 52-week study (Dermatology 2006;213:319-26).
Thus, the calcipotriene/nicotinamide combination is an agent that will be investigated further in larger, lengthier studies as a possible steroid-sparing agent in comparative trials vs. calcipotriene-steroid combination therapy, Dr. Lebwohl said.
Topical combination therapy continues to be an active area in psoriasis research, he observed.
"It's really a no-brainer: The combination of two ingredients is going to be superior to either one alone. It's a very simple concept: Two is better than one," he said.
Dr. Lebwohl disclosed that he is a consultant to Dermipsor and has advisory board relationships with other pharmaceutical companies that are developing dermatologic drugs.
SEOUL, SOUTH KOREA – The combination of calcipotriene and nicotinamide may be an attractive corticosteroid-sparing option for topical psoriasis therapy, according to results from a pilot trial.
"Both agents are somewhat effective for psoriasis; the combination of the two is markedly superior," Dr. Mark Lebwohl said in summarizing the findings of a pilot bilateral comparative study presented at the World Congress of Dermatology.
The investigational topical combination therapy is being developed by Dermipsor, an Israeli pharmaceutical company.
Dr. Lebwohl was a coinvestigator in the multicenter, double-blind, 12-week pilot study involving 168 patients with symmetric moderate psoriasis (defined as not more than 15% body surface area involvement).
The patients were randomized to two of seven treatments: calcipotriene 0.005% monotherapy, nicotinamide 1.4% alone, placebo, or calcipotriene 0.005% plus nicotinamide in a concentration of either 0.05%, 0.1%, 0.7%, or 1.4%. Patients treated the lesions on one side of the body with one of their two assigned treatments, and lesions on the opposite side with the other.
The primary end point was efficacy (defined as a clear to almost-clear outcome at week 12), which was achieved in 19% of patients who were treated with placebo, 25% of those on nicotinamide 1.4% alone, 31.5% of those treated with calcipotriene alone, and 50% of patients in the calcipotriene plus nicotinamide 1.4% group, reported Dr. Lebwohl, professor and chairman of the department of dermatology at Mount Sinai School of Medicine, New York.
The 50% clear or almost-clear rate with calcipotriene plus nicotinamide 1.4% is similar to the 48% rate of absent to very mild disease that was previously reported with a calcipotriol/betamethasone combination in a 52-week study (Dermatology 2006;213:319-26).
Thus, the calcipotriene/nicotinamide combination is an agent that will be investigated further in larger, lengthier studies as a possible steroid-sparing agent in comparative trials vs. calcipotriene-steroid combination therapy, Dr. Lebwohl said.
Topical combination therapy continues to be an active area in psoriasis research, he observed.
"It's really a no-brainer: The combination of two ingredients is going to be superior to either one alone. It's a very simple concept: Two is better than one," he said.
Dr. Lebwohl disclosed that he is a consultant to Dermipsor and has advisory board relationships with other pharmaceutical companies that are developing dermatologic drugs.
FROM THE WORLD CONGRESS OF DERMATOLOGY
Major Finding: In a study of 168 patients, 50% of patients with moderate psoriasis who used a combination of calcipotriene 0.005% plus nicotinamide 1.4% achieved a clear- to-almost-clear outcome at week 12, compared with 19% of patients using placebo.
Data Source: A double-blind, bilateral, comparative 12-week study of 168 patients.
Disclosures: Dr. Lebwohl disclosed that he is a consultant to Dermipsor and has advisory board relationships with other pharmaceutical companies that are developing dermatologic drugs.
Bimatoprost Repigments Vitiligo Patient Skin
SEOUL, SOUTH KOREA – Topical bimatoprost ophthalmic solution shows promise as a novel treatment for localized, stable vitiligo, a small pilot study suggests.
Patients with recalcitrant sequential or focal vitiligo, especially on the face, responded particularly well to the topical prostaglandin F2-alpha analogue in this small prospective trial with blinded outcome assessment, Dr. Tarun Narang said at the World Congress of Dermatology.
Advantages of this off-label treatment include its low cost, the fact that patients can self-apply it with no requirement for photoexposure, and the minimal side effects, added Dr. Narang of Gian Sagar Medical College in Banur, India.
He reported on 10 patients with localized vitiligo who applied bimatoprost 0.03% ophthalmic solution at a dose of 1 drop per 2 cm2 of affected skin twice daily for 4 months.
Seven of 10 patients showed pronounced repigmentation beginning on average after 2 months of treatment: 3 patients had 100% repigmentation, 3 others had 75%-99% repigmentation, and 1 patient showed 50%-75% repigmentation.
Patients with a disease duration of 6 months or less had the best results. Lesions on the face and scalp regimented fastest, after just 4-6 weeks of treatment. Facial lesions responded best, as all three patients who had 100% clearing had vitiligo on the face.
During 6 months of follow-up, three patients with lesions on the trunk or extremities that initially responded to treatment relapsed, typically 2-3 months after conclusion of the 4-month treatment period. Interestingly, all five patients with focal or segmental vitiligo had either 100% repigmentation or 75%-99% improvement, and none relapsed off therapy, Dr. Narang noted.
The only treatment side effect was a transient burning sensation, mainly on the lips, reported by two patients.
Dr. Narang said that although vitiligo involves the disappearance of dermal melanocytes, the mechanisms involved are not completely understood. However, it is known that prostaglandins in the skin help regulate melanocytes.
The topical prostaglandin analogues prescribed for treatment of glaucoma cause increased melanogenesis as evidenced by their common side effects: periocular skin hyperpigmentation, hypertrichosis, and iris hyperpigmentation.
Ophthalmologic colleagues say these side effects are more pronounced with bimatoprost than other topical prostaglandin analogues, which is why Dr. Narang said he decided to conduct this first-ever study of the drug as a treatment for vitiligo. The long-term effects of daily bimatoprost for vitiligo will require further study, he stressed.
Dr. Narang declared having no relevant financial disclosures.
SEOUL, SOUTH KOREA – Topical bimatoprost ophthalmic solution shows promise as a novel treatment for localized, stable vitiligo, a small pilot study suggests.
Patients with recalcitrant sequential or focal vitiligo, especially on the face, responded particularly well to the topical prostaglandin F2-alpha analogue in this small prospective trial with blinded outcome assessment, Dr. Tarun Narang said at the World Congress of Dermatology.
Advantages of this off-label treatment include its low cost, the fact that patients can self-apply it with no requirement for photoexposure, and the minimal side effects, added Dr. Narang of Gian Sagar Medical College in Banur, India.
He reported on 10 patients with localized vitiligo who applied bimatoprost 0.03% ophthalmic solution at a dose of 1 drop per 2 cm2 of affected skin twice daily for 4 months.
Seven of 10 patients showed pronounced repigmentation beginning on average after 2 months of treatment: 3 patients had 100% repigmentation, 3 others had 75%-99% repigmentation, and 1 patient showed 50%-75% repigmentation.
Patients with a disease duration of 6 months or less had the best results. Lesions on the face and scalp regimented fastest, after just 4-6 weeks of treatment. Facial lesions responded best, as all three patients who had 100% clearing had vitiligo on the face.
During 6 months of follow-up, three patients with lesions on the trunk or extremities that initially responded to treatment relapsed, typically 2-3 months after conclusion of the 4-month treatment period. Interestingly, all five patients with focal or segmental vitiligo had either 100% repigmentation or 75%-99% improvement, and none relapsed off therapy, Dr. Narang noted.
The only treatment side effect was a transient burning sensation, mainly on the lips, reported by two patients.
Dr. Narang said that although vitiligo involves the disappearance of dermal melanocytes, the mechanisms involved are not completely understood. However, it is known that prostaglandins in the skin help regulate melanocytes.
The topical prostaglandin analogues prescribed for treatment of glaucoma cause increased melanogenesis as evidenced by their common side effects: periocular skin hyperpigmentation, hypertrichosis, and iris hyperpigmentation.
Ophthalmologic colleagues say these side effects are more pronounced with bimatoprost than other topical prostaglandin analogues, which is why Dr. Narang said he decided to conduct this first-ever study of the drug as a treatment for vitiligo. The long-term effects of daily bimatoprost for vitiligo will require further study, he stressed.
Dr. Narang declared having no relevant financial disclosures.
SEOUL, SOUTH KOREA – Topical bimatoprost ophthalmic solution shows promise as a novel treatment for localized, stable vitiligo, a small pilot study suggests.
Patients with recalcitrant sequential or focal vitiligo, especially on the face, responded particularly well to the topical prostaglandin F2-alpha analogue in this small prospective trial with blinded outcome assessment, Dr. Tarun Narang said at the World Congress of Dermatology.
Advantages of this off-label treatment include its low cost, the fact that patients can self-apply it with no requirement for photoexposure, and the minimal side effects, added Dr. Narang of Gian Sagar Medical College in Banur, India.
He reported on 10 patients with localized vitiligo who applied bimatoprost 0.03% ophthalmic solution at a dose of 1 drop per 2 cm2 of affected skin twice daily for 4 months.
Seven of 10 patients showed pronounced repigmentation beginning on average after 2 months of treatment: 3 patients had 100% repigmentation, 3 others had 75%-99% repigmentation, and 1 patient showed 50%-75% repigmentation.
Patients with a disease duration of 6 months or less had the best results. Lesions on the face and scalp regimented fastest, after just 4-6 weeks of treatment. Facial lesions responded best, as all three patients who had 100% clearing had vitiligo on the face.
During 6 months of follow-up, three patients with lesions on the trunk or extremities that initially responded to treatment relapsed, typically 2-3 months after conclusion of the 4-month treatment period. Interestingly, all five patients with focal or segmental vitiligo had either 100% repigmentation or 75%-99% improvement, and none relapsed off therapy, Dr. Narang noted.
The only treatment side effect was a transient burning sensation, mainly on the lips, reported by two patients.
Dr. Narang said that although vitiligo involves the disappearance of dermal melanocytes, the mechanisms involved are not completely understood. However, it is known that prostaglandins in the skin help regulate melanocytes.
The topical prostaglandin analogues prescribed for treatment of glaucoma cause increased melanogenesis as evidenced by their common side effects: periocular skin hyperpigmentation, hypertrichosis, and iris hyperpigmentation.
Ophthalmologic colleagues say these side effects are more pronounced with bimatoprost than other topical prostaglandin analogues, which is why Dr. Narang said he decided to conduct this first-ever study of the drug as a treatment for vitiligo. The long-term effects of daily bimatoprost for vitiligo will require further study, he stressed.
Dr. Narang declared having no relevant financial disclosures.
FROM THE WORLD CONGRESS OF DERMATOLOGY
Major Finding: Seven of 10 vitiligo patients showed pronounced repigmentation beginning on average after 2 months of treatment: 3 patients had 100% repigmentation, 3 patients had 75%-99% repigmentation, and 1 patient showed 50%-75% repigmentation.
Data Source: A small pilot study to evaluate an off-label use of topical bimatoprost ophthalmic solution.
Disclosures: Dr. Narang declared having no relevant financial disclosures.
Only One in Four Women Equate Beauty With Youth
SEOUL, SOUTH KOREA – European women are more interested in aesthetic procedures to improve their stomach or abdomen than their face, according to a large survey.
A majority of the women surveyed indicated they were most satisfied with how they looked about 10 years ago, when most were in their late 20s. Three-quarters of the women said they didn’t mind looking their age – they just didn’t want to look older. Eighty percent believed beauty is under an individual’s control and can be enhanced or shaped.
The Face Value Beauty Survey included 2,939 women from Italy, Spain, France, the United Kingdom, and Russia with an interest in minimally invasive aesthetic procedures. The survey was conducted online by Harris Interactive and released by Merz, its sponsor, at the World Congress of Dermatology.
Only one-quarter of women surveyed equated beauty with youth. Instead, the top five attributes cited as making women beautiful were healthy skin, an overall well-groomed appearance, beautiful hair, a charming personality, and confidence, according to Steven Basta, who presented the findings.
Fifty-six percent of European women indicated they would like to change their abdomen or stomach. Forty-four percent reported they would like to target their overall weight, 41% their breasts, 38% their face, and 38% their buttocks.
The most popular aesthetic procedure that the surveyed women had undergone was laser hair removal, at 18%. Rounding out the top five most popular procedures were chemical peels, cellulite treatments, facial dermal fillers, and facial botulinum toxin injections, each tried by 12%-13% of respondents, said Mr. Basta, CEO of Merz.
One in five European women described their personal style as "glamorous" or "extravagant." Fifty-seven percent said beauty arises from a natural appearance; half as many women said beauty stems from a made-up or glamorous look.
Three in five facial filler and/or botulinum toxin users said they had experienced a positive life change due to their treatments.
The majority of facial injectable users reported never admitting to their significant other, close friends, or family that they had undergone such treatments, or they did so only selectively. Instead, they typically claimed their new look was the result of facials, makeup, diet, exercise, or being in love.
SEOUL, SOUTH KOREA – European women are more interested in aesthetic procedures to improve their stomach or abdomen than their face, according to a large survey.
A majority of the women surveyed indicated they were most satisfied with how they looked about 10 years ago, when most were in their late 20s. Three-quarters of the women said they didn’t mind looking their age – they just didn’t want to look older. Eighty percent believed beauty is under an individual’s control and can be enhanced or shaped.
The Face Value Beauty Survey included 2,939 women from Italy, Spain, France, the United Kingdom, and Russia with an interest in minimally invasive aesthetic procedures. The survey was conducted online by Harris Interactive and released by Merz, its sponsor, at the World Congress of Dermatology.
Only one-quarter of women surveyed equated beauty with youth. Instead, the top five attributes cited as making women beautiful were healthy skin, an overall well-groomed appearance, beautiful hair, a charming personality, and confidence, according to Steven Basta, who presented the findings.
Fifty-six percent of European women indicated they would like to change their abdomen or stomach. Forty-four percent reported they would like to target their overall weight, 41% their breasts, 38% their face, and 38% their buttocks.
The most popular aesthetic procedure that the surveyed women had undergone was laser hair removal, at 18%. Rounding out the top five most popular procedures were chemical peels, cellulite treatments, facial dermal fillers, and facial botulinum toxin injections, each tried by 12%-13% of respondents, said Mr. Basta, CEO of Merz.
One in five European women described their personal style as "glamorous" or "extravagant." Fifty-seven percent said beauty arises from a natural appearance; half as many women said beauty stems from a made-up or glamorous look.
Three in five facial filler and/or botulinum toxin users said they had experienced a positive life change due to their treatments.
The majority of facial injectable users reported never admitting to their significant other, close friends, or family that they had undergone such treatments, or they did so only selectively. Instead, they typically claimed their new look was the result of facials, makeup, diet, exercise, or being in love.
SEOUL, SOUTH KOREA – European women are more interested in aesthetic procedures to improve their stomach or abdomen than their face, according to a large survey.
A majority of the women surveyed indicated they were most satisfied with how they looked about 10 years ago, when most were in their late 20s. Three-quarters of the women said they didn’t mind looking their age – they just didn’t want to look older. Eighty percent believed beauty is under an individual’s control and can be enhanced or shaped.
The Face Value Beauty Survey included 2,939 women from Italy, Spain, France, the United Kingdom, and Russia with an interest in minimally invasive aesthetic procedures. The survey was conducted online by Harris Interactive and released by Merz, its sponsor, at the World Congress of Dermatology.
Only one-quarter of women surveyed equated beauty with youth. Instead, the top five attributes cited as making women beautiful were healthy skin, an overall well-groomed appearance, beautiful hair, a charming personality, and confidence, according to Steven Basta, who presented the findings.
Fifty-six percent of European women indicated they would like to change their abdomen or stomach. Forty-four percent reported they would like to target their overall weight, 41% their breasts, 38% their face, and 38% their buttocks.
The most popular aesthetic procedure that the surveyed women had undergone was laser hair removal, at 18%. Rounding out the top five most popular procedures were chemical peels, cellulite treatments, facial dermal fillers, and facial botulinum toxin injections, each tried by 12%-13% of respondents, said Mr. Basta, CEO of Merz.
One in five European women described their personal style as "glamorous" or "extravagant." Fifty-seven percent said beauty arises from a natural appearance; half as many women said beauty stems from a made-up or glamorous look.
Three in five facial filler and/or botulinum toxin users said they had experienced a positive life change due to their treatments.
The majority of facial injectable users reported never admitting to their significant other, close friends, or family that they had undergone such treatments, or they did so only selectively. Instead, they typically claimed their new look was the result of facials, makeup, diet, exercise, or being in love.
FROM THE WORLD CONGRESS OF DERMATOLOGY
Major Finding: Fifty-six percent of European women indicated they would like to change their abdomen or stomach. Forty-four percent reported they would like to target their overall weight, 41% their breasts, 38% their face, and 38% their buttocks.
Data Source: The Face Value Beauty Survey included 2,939 women from Italy, Spain, France, the United Kingdom, and Russia with an interest in minimally invasive aesthetic procedures..
Disclosures: The study was sponsored and released by Merz.
Only One in Four Women Equate Beauty With Youth
SEOUL, SOUTH KOREA – European women are more interested in aesthetic procedures to improve their stomach or abdomen than their face, according to a large survey.
A majority of the women surveyed indicated they were most satisfied with how they looked about 10 years ago, when most were in their late 20s. Three-quarters of the women said they didn’t mind looking their age – they just didn’t want to look older. Eighty percent believed beauty is under an individual’s control and can be enhanced or shaped.
The Face Value Beauty Survey included 2,939 women from Italy, Spain, France, the United Kingdom, and Russia with an interest in minimally invasive aesthetic procedures. The survey was conducted online by Harris Interactive and released by Merz, its sponsor, at the World Congress of Dermatology.
Only one-quarter of women surveyed equated beauty with youth. Instead, the top five attributes cited as making women beautiful were healthy skin, an overall well-groomed appearance, beautiful hair, a charming personality, and confidence, according to Steven Basta, who presented the findings.
Fifty-six percent of European women indicated they would like to change their abdomen or stomach. Forty-four percent reported they would like to target their overall weight, 41% their breasts, 38% their face, and 38% their buttocks.
The most popular aesthetic procedure that the surveyed women had undergone was laser hair removal, at 18%. Rounding out the top five most popular procedures were chemical peels, cellulite treatments, facial dermal fillers, and facial botulinum toxin injections, each tried by 12%-13% of respondents, said Mr. Basta, CEO of Merz.
One in five European women described their personal style as "glamorous" or "extravagant." Fifty-seven percent said beauty arises from a natural appearance; half as many women said beauty stems from a made-up or glamorous look.
Three in five facial filler and/or botulinum toxin users said they had experienced a positive life change due to their treatments.
The majority of facial injectable users reported never admitting to their significant other, close friends, or family that they had undergone such treatments, or they did so only selectively. Instead, they typically claimed their new look was the result of facials, makeup, diet, exercise, or being in love.
SEOUL, SOUTH KOREA – European women are more interested in aesthetic procedures to improve their stomach or abdomen than their face, according to a large survey.
A majority of the women surveyed indicated they were most satisfied with how they looked about 10 years ago, when most were in their late 20s. Three-quarters of the women said they didn’t mind looking their age – they just didn’t want to look older. Eighty percent believed beauty is under an individual’s control and can be enhanced or shaped.
The Face Value Beauty Survey included 2,939 women from Italy, Spain, France, the United Kingdom, and Russia with an interest in minimally invasive aesthetic procedures. The survey was conducted online by Harris Interactive and released by Merz, its sponsor, at the World Congress of Dermatology.
Only one-quarter of women surveyed equated beauty with youth. Instead, the top five attributes cited as making women beautiful were healthy skin, an overall well-groomed appearance, beautiful hair, a charming personality, and confidence, according to Steven Basta, who presented the findings.
Fifty-six percent of European women indicated they would like to change their abdomen or stomach. Forty-four percent reported they would like to target their overall weight, 41% their breasts, 38% their face, and 38% their buttocks.
The most popular aesthetic procedure that the surveyed women had undergone was laser hair removal, at 18%. Rounding out the top five most popular procedures were chemical peels, cellulite treatments, facial dermal fillers, and facial botulinum toxin injections, each tried by 12%-13% of respondents, said Mr. Basta, CEO of Merz.
One in five European women described their personal style as "glamorous" or "extravagant." Fifty-seven percent said beauty arises from a natural appearance; half as many women said beauty stems from a made-up or glamorous look.
Three in five facial filler and/or botulinum toxin users said they had experienced a positive life change due to their treatments.
The majority of facial injectable users reported never admitting to their significant other, close friends, or family that they had undergone such treatments, or they did so only selectively. Instead, they typically claimed their new look was the result of facials, makeup, diet, exercise, or being in love.
SEOUL, SOUTH KOREA – European women are more interested in aesthetic procedures to improve their stomach or abdomen than their face, according to a large survey.
A majority of the women surveyed indicated they were most satisfied with how they looked about 10 years ago, when most were in their late 20s. Three-quarters of the women said they didn’t mind looking their age – they just didn’t want to look older. Eighty percent believed beauty is under an individual’s control and can be enhanced or shaped.
The Face Value Beauty Survey included 2,939 women from Italy, Spain, France, the United Kingdom, and Russia with an interest in minimally invasive aesthetic procedures. The survey was conducted online by Harris Interactive and released by Merz, its sponsor, at the World Congress of Dermatology.
Only one-quarter of women surveyed equated beauty with youth. Instead, the top five attributes cited as making women beautiful were healthy skin, an overall well-groomed appearance, beautiful hair, a charming personality, and confidence, according to Steven Basta, who presented the findings.
Fifty-six percent of European women indicated they would like to change their abdomen or stomach. Forty-four percent reported they would like to target their overall weight, 41% their breasts, 38% their face, and 38% their buttocks.
The most popular aesthetic procedure that the surveyed women had undergone was laser hair removal, at 18%. Rounding out the top five most popular procedures were chemical peels, cellulite treatments, facial dermal fillers, and facial botulinum toxin injections, each tried by 12%-13% of respondents, said Mr. Basta, CEO of Merz.
One in five European women described their personal style as "glamorous" or "extravagant." Fifty-seven percent said beauty arises from a natural appearance; half as many women said beauty stems from a made-up or glamorous look.
Three in five facial filler and/or botulinum toxin users said they had experienced a positive life change due to their treatments.
The majority of facial injectable users reported never admitting to their significant other, close friends, or family that they had undergone such treatments, or they did so only selectively. Instead, they typically claimed their new look was the result of facials, makeup, diet, exercise, or being in love.
FROM THE WORLD CONGRESS OF DERMATOLOGY
Major Finding: Fifty-six percent of European women indicated they would like to change their abdomen or stomach. Forty-four percent reported they would like to target their overall weight, 41% their breasts, 38% their face, and 38% their buttocks.
Data Source: The Face Value Beauty Survey included 2,939 women from Italy, Spain, France, the United Kingdom, and Russia with an interest in minimally invasive aesthetic procedures..
Disclosures: The study was sponsored and released by Merz.
Only One in Four Women Equate Beauty With Youth
SEOUL, SOUTH KOREA – European women are more interested in aesthetic procedures to improve their stomach or abdomen than their face, according to a large survey.
A majority of the women surveyed indicated they were most satisfied with how they looked about 10 years ago, when most were in their late 20s. Three-quarters of the women said they didn’t mind looking their age – they just didn’t want to look older. Eighty percent believed beauty is under an individual’s control and can be enhanced or shaped.
The Face Value Beauty Survey included 2,939 women from Italy, Spain, France, the United Kingdom, and Russia with an interest in minimally invasive aesthetic procedures. The survey was conducted online by Harris Interactive and released by Merz, its sponsor, at the World Congress of Dermatology.
Only one-quarter of women surveyed equated beauty with youth. Instead, the top five attributes cited as making women beautiful were healthy skin, an overall well-groomed appearance, beautiful hair, a charming personality, and confidence, according to Steven Basta, who presented the findings.
Fifty-six percent of European women indicated they would like to change their abdomen or stomach. Forty-four percent reported they would like to target their overall weight, 41% their breasts, 38% their face, and 38% their buttocks.
The most popular aesthetic procedure that the surveyed women had undergone was laser hair removal, at 18%. Rounding out the top five most popular procedures were chemical peels, cellulite treatments, facial dermal fillers, and facial botulinum toxin injections, each tried by 12%-13% of respondents, said Mr. Basta, CEO of Merz.
One in five European women described their personal style as "glamorous" or "extravagant." Fifty-seven percent said beauty arises from a natural appearance; half as many women said beauty stems from a made-up or glamorous look.
Three in five facial filler and/or botulinum toxin users said they had experienced a positive life change due to their treatments.
The majority of facial injectable users reported never admitting to their significant other, close friends, or family that they had undergone such treatments, or they did so only selectively. Instead, they typically claimed their new look was the result of facials, makeup, diet, exercise, or being in love.
SEOUL, SOUTH KOREA – European women are more interested in aesthetic procedures to improve their stomach or abdomen than their face, according to a large survey.
A majority of the women surveyed indicated they were most satisfied with how they looked about 10 years ago, when most were in their late 20s. Three-quarters of the women said they didn’t mind looking their age – they just didn’t want to look older. Eighty percent believed beauty is under an individual’s control and can be enhanced or shaped.
The Face Value Beauty Survey included 2,939 women from Italy, Spain, France, the United Kingdom, and Russia with an interest in minimally invasive aesthetic procedures. The survey was conducted online by Harris Interactive and released by Merz, its sponsor, at the World Congress of Dermatology.
Only one-quarter of women surveyed equated beauty with youth. Instead, the top five attributes cited as making women beautiful were healthy skin, an overall well-groomed appearance, beautiful hair, a charming personality, and confidence, according to Steven Basta, who presented the findings.
Fifty-six percent of European women indicated they would like to change their abdomen or stomach. Forty-four percent reported they would like to target their overall weight, 41% their breasts, 38% their face, and 38% their buttocks.
The most popular aesthetic procedure that the surveyed women had undergone was laser hair removal, at 18%. Rounding out the top five most popular procedures were chemical peels, cellulite treatments, facial dermal fillers, and facial botulinum toxin injections, each tried by 12%-13% of respondents, said Mr. Basta, CEO of Merz.
One in five European women described their personal style as "glamorous" or "extravagant." Fifty-seven percent said beauty arises from a natural appearance; half as many women said beauty stems from a made-up or glamorous look.
Three in five facial filler and/or botulinum toxin users said they had experienced a positive life change due to their treatments.
The majority of facial injectable users reported never admitting to their significant other, close friends, or family that they had undergone such treatments, or they did so only selectively. Instead, they typically claimed their new look was the result of facials, makeup, diet, exercise, or being in love.
SEOUL, SOUTH KOREA – European women are more interested in aesthetic procedures to improve their stomach or abdomen than their face, according to a large survey.
A majority of the women surveyed indicated they were most satisfied with how they looked about 10 years ago, when most were in their late 20s. Three-quarters of the women said they didn’t mind looking their age – they just didn’t want to look older. Eighty percent believed beauty is under an individual’s control and can be enhanced or shaped.
The Face Value Beauty Survey included 2,939 women from Italy, Spain, France, the United Kingdom, and Russia with an interest in minimally invasive aesthetic procedures. The survey was conducted online by Harris Interactive and released by Merz, its sponsor, at the World Congress of Dermatology.
Only one-quarter of women surveyed equated beauty with youth. Instead, the top five attributes cited as making women beautiful were healthy skin, an overall well-groomed appearance, beautiful hair, a charming personality, and confidence, according to Steven Basta, who presented the findings.
Fifty-six percent of European women indicated they would like to change their abdomen or stomach. Forty-four percent reported they would like to target their overall weight, 41% their breasts, 38% their face, and 38% their buttocks.
The most popular aesthetic procedure that the surveyed women had undergone was laser hair removal, at 18%. Rounding out the top five most popular procedures were chemical peels, cellulite treatments, facial dermal fillers, and facial botulinum toxin injections, each tried by 12%-13% of respondents, said Mr. Basta, CEO of Merz.
One in five European women described their personal style as "glamorous" or "extravagant." Fifty-seven percent said beauty arises from a natural appearance; half as many women said beauty stems from a made-up or glamorous look.
Three in five facial filler and/or botulinum toxin users said they had experienced a positive life change due to their treatments.
The majority of facial injectable users reported never admitting to their significant other, close friends, or family that they had undergone such treatments, or they did so only selectively. Instead, they typically claimed their new look was the result of facials, makeup, diet, exercise, or being in love.
FROM THE WORLD CONGRESS OF DERMATOLOGY
Major Finding: Fifty-six percent of European women indicated they would like to change their abdomen or stomach. Forty-four percent reported they would like to target their overall weight, 41% their breasts, 38% their face, and 38% their buttocks.
Data Source: The Face Value Beauty Survey included 2,939 women from Italy, Spain, France, the United Kingdom, and Russia with an interest in minimally invasive aesthetic procedures.
Disclosures: The study was sponsored and released by Merz.
Dapsone Gel More Effective for Acne in Women
SEOUL, SOUTH KOREA – Topical dapsone gel 5% is a particularly advantageous acne treatment in adult women, according to Dr. Valerie D. Callender.
A gender difference in outcomes with the therapy has become apparent, with women faring significantly better than men in terms of lesion count reductions and acne clearance rates, she said at the World Congress of Dermatology.
Dr. Callender cited a meta-analysis of clinical trial data presented last year at the Fall Clinical Dermatology Conference in Las Vegas by Dr. Julie Harper (of the University of Alabama, Birmingham). Among 2,898 acne patients aged older than 12 years, roughly equally divided into men and women, 12 weeks of daily treatment with topical dapsone gel 5% (Aczone) resulted in an overall mean 48% reduction in inflammatory lesions and a 32% decrease in noninflammatory lesions. Forty-one percent of dapsone-treated patients had no or minimal acne after 12 weeks, compared with 33% of subjects randomized to a vehicle.
At each of the biweekly patient assessments conducted during the 12-week study, dapsone-treated women had significantly greater reductions in both inflammatory and noninflammatory lesions than did men. At 12 weeks, women on dapsone gel had an absolute 7.6% greater reduction in inflammatory acne lesions and a 10.7% greater absolute decrease in noninflammatory lesions than did men. The total lesion count after 12 weeks of topical dapsone, compared with baseline, was an absolute 9.7% less in women than in men.
Side effects, mostly application site dryness or redness, didn't differ between men and women.
Topical dapsone gel is a sulfone antibiotic with anti-inflammatory properties. It is not clear exactly how it works in the treatment of acne. In vitro studies suggest dapsone may suppress neutrophil recruitment and inhibit generation of oxygen free radicals, according to Dr. Callender, a dermatologist at Howard University, Washington.
She disclosed that she serves as a clinical researcher, consultant, and/or speaker for Allergan, which markets topical dapsone, as well as for Coria, Galderma, Medicis, and Stiefel.
SEOUL, SOUTH KOREA – Topical dapsone gel 5% is a particularly advantageous acne treatment in adult women, according to Dr. Valerie D. Callender.
A gender difference in outcomes with the therapy has become apparent, with women faring significantly better than men in terms of lesion count reductions and acne clearance rates, she said at the World Congress of Dermatology.
Dr. Callender cited a meta-analysis of clinical trial data presented last year at the Fall Clinical Dermatology Conference in Las Vegas by Dr. Julie Harper (of the University of Alabama, Birmingham). Among 2,898 acne patients aged older than 12 years, roughly equally divided into men and women, 12 weeks of daily treatment with topical dapsone gel 5% (Aczone) resulted in an overall mean 48% reduction in inflammatory lesions and a 32% decrease in noninflammatory lesions. Forty-one percent of dapsone-treated patients had no or minimal acne after 12 weeks, compared with 33% of subjects randomized to a vehicle.
At each of the biweekly patient assessments conducted during the 12-week study, dapsone-treated women had significantly greater reductions in both inflammatory and noninflammatory lesions than did men. At 12 weeks, women on dapsone gel had an absolute 7.6% greater reduction in inflammatory acne lesions and a 10.7% greater absolute decrease in noninflammatory lesions than did men. The total lesion count after 12 weeks of topical dapsone, compared with baseline, was an absolute 9.7% less in women than in men.
Side effects, mostly application site dryness or redness, didn't differ between men and women.
Topical dapsone gel is a sulfone antibiotic with anti-inflammatory properties. It is not clear exactly how it works in the treatment of acne. In vitro studies suggest dapsone may suppress neutrophil recruitment and inhibit generation of oxygen free radicals, according to Dr. Callender, a dermatologist at Howard University, Washington.
She disclosed that she serves as a clinical researcher, consultant, and/or speaker for Allergan, which markets topical dapsone, as well as for Coria, Galderma, Medicis, and Stiefel.
SEOUL, SOUTH KOREA – Topical dapsone gel 5% is a particularly advantageous acne treatment in adult women, according to Dr. Valerie D. Callender.
A gender difference in outcomes with the therapy has become apparent, with women faring significantly better than men in terms of lesion count reductions and acne clearance rates, she said at the World Congress of Dermatology.
Dr. Callender cited a meta-analysis of clinical trial data presented last year at the Fall Clinical Dermatology Conference in Las Vegas by Dr. Julie Harper (of the University of Alabama, Birmingham). Among 2,898 acne patients aged older than 12 years, roughly equally divided into men and women, 12 weeks of daily treatment with topical dapsone gel 5% (Aczone) resulted in an overall mean 48% reduction in inflammatory lesions and a 32% decrease in noninflammatory lesions. Forty-one percent of dapsone-treated patients had no or minimal acne after 12 weeks, compared with 33% of subjects randomized to a vehicle.
At each of the biweekly patient assessments conducted during the 12-week study, dapsone-treated women had significantly greater reductions in both inflammatory and noninflammatory lesions than did men. At 12 weeks, women on dapsone gel had an absolute 7.6% greater reduction in inflammatory acne lesions and a 10.7% greater absolute decrease in noninflammatory lesions than did men. The total lesion count after 12 weeks of topical dapsone, compared with baseline, was an absolute 9.7% less in women than in men.
Side effects, mostly application site dryness or redness, didn't differ between men and women.
Topical dapsone gel is a sulfone antibiotic with anti-inflammatory properties. It is not clear exactly how it works in the treatment of acne. In vitro studies suggest dapsone may suppress neutrophil recruitment and inhibit generation of oxygen free radicals, according to Dr. Callender, a dermatologist at Howard University, Washington.
She disclosed that she serves as a clinical researcher, consultant, and/or speaker for Allergan, which markets topical dapsone, as well as for Coria, Galderma, Medicis, and Stiefel.
EXPERT ANALYSIS FROM THE WORLD CONGRESS OF DERMATOLOGY
Dapsone Gel More Effective for Acne in Women
SEOUL, SOUTH KOREA – Topical dapsone gel 5% is a particularly advantageous acne treatment in adult women, according to Dr. Valerie D. Callender.
A gender difference in outcomes with the therapy has become apparent, with women faring significantly better than men in terms of lesion count reductions and acne clearance rates, she said at the World Congress of Dermatology.
Dr. Callender cited a meta-analysis of clinical trial data presented last year at the Fall Clinical Dermatology Conference in Las Vegas by Dr. Julie Harper (of the University of Alabama, Birmingham). Among 2,898 acne patients aged older than 12 years, roughly equally divided into men and women, 12 weeks of daily treatment with topical dapsone gel 5% (Aczone) resulted in an overall mean 48% reduction in inflammatory lesions and a 32% decrease in noninflammatory lesions. Forty-one percent of dapsone-treated patients had no or minimal acne after 12 weeks, compared with 33% of subjects randomized to a vehicle.
At each of the biweekly patient assessments conducted during the 12-week study, dapsone-treated women had significantly greater reductions in both inflammatory and noninflammatory lesions than did men. At 12 weeks, women on dapsone gel had an absolute 7.6% greater reduction in inflammatory acne lesions and a 10.7% greater absolute decrease in noninflammatory lesions than did men. The total lesion count after 12 weeks of topical dapsone, compared with baseline, was an absolute 9.7% less in women than in men.
Side effects, mostly application site dryness or redness, didn't differ between men and women.
Topical dapsone gel is a sulfone antibiotic with anti-inflammatory properties. It is not clear exactly how it works in the treatment of acne. In vitro studies suggest dapsone may suppress neutrophil recruitment and inhibit generation of oxygen free radicals, according to Dr. Callender, a dermatologist at Howard University, Washington.
She disclosed that she serves as a clinical researcher, consultant, and/or speaker for Allergan, which markets topical dapsone, as well as for Coria, Galderma, Medicis, and Stiefel.
SEOUL, SOUTH KOREA – Topical dapsone gel 5% is a particularly advantageous acne treatment in adult women, according to Dr. Valerie D. Callender.
A gender difference in outcomes with the therapy has become apparent, with women faring significantly better than men in terms of lesion count reductions and acne clearance rates, she said at the World Congress of Dermatology.
Dr. Callender cited a meta-analysis of clinical trial data presented last year at the Fall Clinical Dermatology Conference in Las Vegas by Dr. Julie Harper (of the University of Alabama, Birmingham). Among 2,898 acne patients aged older than 12 years, roughly equally divided into men and women, 12 weeks of daily treatment with topical dapsone gel 5% (Aczone) resulted in an overall mean 48% reduction in inflammatory lesions and a 32% decrease in noninflammatory lesions. Forty-one percent of dapsone-treated patients had no or minimal acne after 12 weeks, compared with 33% of subjects randomized to a vehicle.
At each of the biweekly patient assessments conducted during the 12-week study, dapsone-treated women had significantly greater reductions in both inflammatory and noninflammatory lesions than did men. At 12 weeks, women on dapsone gel had an absolute 7.6% greater reduction in inflammatory acne lesions and a 10.7% greater absolute decrease in noninflammatory lesions than did men. The total lesion count after 12 weeks of topical dapsone, compared with baseline, was an absolute 9.7% less in women than in men.
Side effects, mostly application site dryness or redness, didn't differ between men and women.
Topical dapsone gel is a sulfone antibiotic with anti-inflammatory properties. It is not clear exactly how it works in the treatment of acne. In vitro studies suggest dapsone may suppress neutrophil recruitment and inhibit generation of oxygen free radicals, according to Dr. Callender, a dermatologist at Howard University, Washington.
She disclosed that she serves as a clinical researcher, consultant, and/or speaker for Allergan, which markets topical dapsone, as well as for Coria, Galderma, Medicis, and Stiefel.
SEOUL, SOUTH KOREA – Topical dapsone gel 5% is a particularly advantageous acne treatment in adult women, according to Dr. Valerie D. Callender.
A gender difference in outcomes with the therapy has become apparent, with women faring significantly better than men in terms of lesion count reductions and acne clearance rates, she said at the World Congress of Dermatology.
Dr. Callender cited a meta-analysis of clinical trial data presented last year at the Fall Clinical Dermatology Conference in Las Vegas by Dr. Julie Harper (of the University of Alabama, Birmingham). Among 2,898 acne patients aged older than 12 years, roughly equally divided into men and women, 12 weeks of daily treatment with topical dapsone gel 5% (Aczone) resulted in an overall mean 48% reduction in inflammatory lesions and a 32% decrease in noninflammatory lesions. Forty-one percent of dapsone-treated patients had no or minimal acne after 12 weeks, compared with 33% of subjects randomized to a vehicle.
At each of the biweekly patient assessments conducted during the 12-week study, dapsone-treated women had significantly greater reductions in both inflammatory and noninflammatory lesions than did men. At 12 weeks, women on dapsone gel had an absolute 7.6% greater reduction in inflammatory acne lesions and a 10.7% greater absolute decrease in noninflammatory lesions than did men. The total lesion count after 12 weeks of topical dapsone, compared with baseline, was an absolute 9.7% less in women than in men.
Side effects, mostly application site dryness or redness, didn't differ between men and women.
Topical dapsone gel is a sulfone antibiotic with anti-inflammatory properties. It is not clear exactly how it works in the treatment of acne. In vitro studies suggest dapsone may suppress neutrophil recruitment and inhibit generation of oxygen free radicals, according to Dr. Callender, a dermatologist at Howard University, Washington.
She disclosed that she serves as a clinical researcher, consultant, and/or speaker for Allergan, which markets topical dapsone, as well as for Coria, Galderma, Medicis, and Stiefel.
EXPERT ANALYSIS FROM THE WORLD CONGRESS OF DERMATOLOGY
Biologics Don't Increase Herpes Zoster Risk
SEOUL, SOUTH KOREA – Treatment of psoriasis patients with biologic agents isn't associated with increased risk of herpes zoster, according to a large population-based Israeli study.
In contrast, the use of methotrexate, cyclosporine, acitretin, and systemic corticosteroids is associated with an increased risk of herpes zoster in psoriasis patients, Dr. Arnon D. Cohen said at the World Congress of Dermatology.
He reported on 22,330 psoriasis patients followed over 10 years at Clalit Health Services, Israel’s largest HMO. This was a real-world patient population, 53% of whom were female, in contrast to the highly selected populations in randomized clinical trials, which are typically 65%-69% male.
During nearly 220,000 person-years of follow-up there were 1,321 cases of herpes zoster. The baseline rate during periods when patients weren’t on any form of systemic therapy was 4.6 cases/1,000 patient-years. In contrast, the rate during cyclosporine therapy was more than 10-fold higher at 48.4 cases/1,000 patient-years.
Eighteen percent of psoriasis patients were on systemic corticosteroids at some point during the study period; that medication was associated with a herpes zoster rate of 25.7/1,000 patient-years of exposure.
Other therapies that conferred significantly increased risk were methotrexate at 17 cases/1,000 patient-years and acitretin (Soriatane) at 5.4 cases/1,000 patient-years, reported Dr. Cohen, director of the department of quality measures at Clalit and a dermatologist at Ben-Gurion University of the Negev, Beer-Sheva, Israel.
In contrast, no cases of herpes zoster occurred in psoriasis patients on Amevive, Raptiva, or, Humira. Nor was the incidence increased beyond baseline in patients on PUVA, UVB, or Enbrel.
In a , systemic corticosteroid therapy was associated with a 2.4-fold increased risk of developing herpes zoster. Being female was associated with a significant 16% increase in risk. Remicade therapy was associated with a 1.8-fold increase in risk, a trend that did not achieve statistical significance (P =.09).
Dr. Cohen and his coinvestigators conducted this study because, even though the biologic agents are recognized as conferring increased risk of tuberculosis and serious bacterial infections, very little is known about their association, if any, with viral infections.
Dr. Cohen declared having no relevant financial interests.
SEOUL, SOUTH KOREA – Treatment of psoriasis patients with biologic agents isn't associated with increased risk of herpes zoster, according to a large population-based Israeli study.
In contrast, the use of methotrexate, cyclosporine, acitretin, and systemic corticosteroids is associated with an increased risk of herpes zoster in psoriasis patients, Dr. Arnon D. Cohen said at the World Congress of Dermatology.
He reported on 22,330 psoriasis patients followed over 10 years at Clalit Health Services, Israel’s largest HMO. This was a real-world patient population, 53% of whom were female, in contrast to the highly selected populations in randomized clinical trials, which are typically 65%-69% male.
During nearly 220,000 person-years of follow-up there were 1,321 cases of herpes zoster. The baseline rate during periods when patients weren’t on any form of systemic therapy was 4.6 cases/1,000 patient-years. In contrast, the rate during cyclosporine therapy was more than 10-fold higher at 48.4 cases/1,000 patient-years.
Eighteen percent of psoriasis patients were on systemic corticosteroids at some point during the study period; that medication was associated with a herpes zoster rate of 25.7/1,000 patient-years of exposure.
Other therapies that conferred significantly increased risk were methotrexate at 17 cases/1,000 patient-years and acitretin (Soriatane) at 5.4 cases/1,000 patient-years, reported Dr. Cohen, director of the department of quality measures at Clalit and a dermatologist at Ben-Gurion University of the Negev, Beer-Sheva, Israel.
In contrast, no cases of herpes zoster occurred in psoriasis patients on Amevive, Raptiva, or, Humira. Nor was the incidence increased beyond baseline in patients on PUVA, UVB, or Enbrel.
In a , systemic corticosteroid therapy was associated with a 2.4-fold increased risk of developing herpes zoster. Being female was associated with a significant 16% increase in risk. Remicade therapy was associated with a 1.8-fold increase in risk, a trend that did not achieve statistical significance (P =.09).
Dr. Cohen and his coinvestigators conducted this study because, even though the biologic agents are recognized as conferring increased risk of tuberculosis and serious bacterial infections, very little is known about their association, if any, with viral infections.
Dr. Cohen declared having no relevant financial interests.
SEOUL, SOUTH KOREA – Treatment of psoriasis patients with biologic agents isn't associated with increased risk of herpes zoster, according to a large population-based Israeli study.
In contrast, the use of methotrexate, cyclosporine, acitretin, and systemic corticosteroids is associated with an increased risk of herpes zoster in psoriasis patients, Dr. Arnon D. Cohen said at the World Congress of Dermatology.
He reported on 22,330 psoriasis patients followed over 10 years at Clalit Health Services, Israel’s largest HMO. This was a real-world patient population, 53% of whom were female, in contrast to the highly selected populations in randomized clinical trials, which are typically 65%-69% male.
During nearly 220,000 person-years of follow-up there were 1,321 cases of herpes zoster. The baseline rate during periods when patients weren’t on any form of systemic therapy was 4.6 cases/1,000 patient-years. In contrast, the rate during cyclosporine therapy was more than 10-fold higher at 48.4 cases/1,000 patient-years.
Eighteen percent of psoriasis patients were on systemic corticosteroids at some point during the study period; that medication was associated with a herpes zoster rate of 25.7/1,000 patient-years of exposure.
Other therapies that conferred significantly increased risk were methotrexate at 17 cases/1,000 patient-years and acitretin (Soriatane) at 5.4 cases/1,000 patient-years, reported Dr. Cohen, director of the department of quality measures at Clalit and a dermatologist at Ben-Gurion University of the Negev, Beer-Sheva, Israel.
In contrast, no cases of herpes zoster occurred in psoriasis patients on Amevive, Raptiva, or, Humira. Nor was the incidence increased beyond baseline in patients on PUVA, UVB, or Enbrel.
In a , systemic corticosteroid therapy was associated with a 2.4-fold increased risk of developing herpes zoster. Being female was associated with a significant 16% increase in risk. Remicade therapy was associated with a 1.8-fold increase in risk, a trend that did not achieve statistical significance (P =.09).
Dr. Cohen and his coinvestigators conducted this study because, even though the biologic agents are recognized as conferring increased risk of tuberculosis and serious bacterial infections, very little is known about their association, if any, with viral infections.
Dr. Cohen declared having no relevant financial interests.
FROM THE WORLD CONGRESS OF DERMATOLOGY
Major Finding: No cases of herpes zoster occurred in psoriasis patients on alefacept (Amevive), efalizumab (Raptiva) or adalimumab (Humira).
Data Source: A population-based study of 22,330 psoriasis patients followed over 10 years at Clalit Health Services, Israel’s largest HMO.
Disclosures: Dr. Cohen reported having no financial conflicts.
Associated Herpes Zoster Risk Varies With Psoriasis Therapies
SEOUL, SOUTH KOREA – Treatment of psoriasis patients with biologic agents isn’t associated with increased risk of herpes zoster, according to a large population-based Israeli study.
In contrast, the use of methotrexate, cyclosporine, acitretin, and systemic corticosteroids is associated with an increased risk of herpes zoster in psoriasis patients, Dr. Arnon D. Cohen said at the World Congress of Dermatology.
He reported on 22,330 psoriasis patients followed over 10 years at Clalit Health Services, Israel’s largest HMO. This was a real-world patient population, 53% of whom were female, in contrast to the highly selected populations in randomized clinical trials, which are typically 65%-69% male.
During nearly 220,000 person-years of follow-up there were 1,321 cases of herpes zoster. The baseline rate during periods when patients weren’t on any form of systemic therapy was 4.6 cases/1,000 patient-years. In contrast, the rate during cyclosporine therapy was more than 10-fold higher at 48.4 cases/1,000 patient-years.
Eighteen percent of psoriasis patients were on systemic corticosteroids at some point during the study period; that medication was associated with a herpes zoster rate of 25.7/1,000 patient-years of exposure. Other therapies that conferred significantly increased risk were methotrexate at 17 cases/1,000 patient-years and acitretin (Soriatane) at 5.4 cases/1,000 patient-years, reported Dr. Cohen, director of the department of quality measures at Clalit and a dermatologist at Ben-Gurion University of the Negev, Beer-Sheva, Israel.
In contrast, no cases of herpes zoster occurred in psoriasis patients on alefacept (Amevive), efalizumab (Raptiva) or adalimumab (Humira). Nor was the incidence increased beyond baseline in patients on PUVA, UVB, or etanercept (Enbrel).
In a Cox logistic regression analysis, systemic corticosteroid therapy was associated with a 2.4-fold increased risk of developing herpes zoster. Being female was associated with a significant 16% increase in risk. Infliximab (Remicade) therapy was associated with a 1.8-fold increase in risk, a trend that did not achieve statistical significance (P =.09).
Dr. Cohen and his coinvestigators conducted this study because, even though the biologic agents are recognized as conferring increased risk of tuberculosis and serious bacterial infections, very little is known about their association, if any, with viral infections.
Dr. Cohen declared having no relevant financial interests.
SEOUL, SOUTH KOREA – Treatment of psoriasis patients with biologic agents isn’t associated with increased risk of herpes zoster, according to a large population-based Israeli study.
In contrast, the use of methotrexate, cyclosporine, acitretin, and systemic corticosteroids is associated with an increased risk of herpes zoster in psoriasis patients, Dr. Arnon D. Cohen said at the World Congress of Dermatology.
He reported on 22,330 psoriasis patients followed over 10 years at Clalit Health Services, Israel’s largest HMO. This was a real-world patient population, 53% of whom were female, in contrast to the highly selected populations in randomized clinical trials, which are typically 65%-69% male.
During nearly 220,000 person-years of follow-up there were 1,321 cases of herpes zoster. The baseline rate during periods when patients weren’t on any form of systemic therapy was 4.6 cases/1,000 patient-years. In contrast, the rate during cyclosporine therapy was more than 10-fold higher at 48.4 cases/1,000 patient-years.
Eighteen percent of psoriasis patients were on systemic corticosteroids at some point during the study period; that medication was associated with a herpes zoster rate of 25.7/1,000 patient-years of exposure. Other therapies that conferred significantly increased risk were methotrexate at 17 cases/1,000 patient-years and acitretin (Soriatane) at 5.4 cases/1,000 patient-years, reported Dr. Cohen, director of the department of quality measures at Clalit and a dermatologist at Ben-Gurion University of the Negev, Beer-Sheva, Israel.
In contrast, no cases of herpes zoster occurred in psoriasis patients on alefacept (Amevive), efalizumab (Raptiva) or adalimumab (Humira). Nor was the incidence increased beyond baseline in patients on PUVA, UVB, or etanercept (Enbrel).
In a Cox logistic regression analysis, systemic corticosteroid therapy was associated with a 2.4-fold increased risk of developing herpes zoster. Being female was associated with a significant 16% increase in risk. Infliximab (Remicade) therapy was associated with a 1.8-fold increase in risk, a trend that did not achieve statistical significance (P =.09).
Dr. Cohen and his coinvestigators conducted this study because, even though the biologic agents are recognized as conferring increased risk of tuberculosis and serious bacterial infections, very little is known about their association, if any, with viral infections.
Dr. Cohen declared having no relevant financial interests.
SEOUL, SOUTH KOREA – Treatment of psoriasis patients with biologic agents isn’t associated with increased risk of herpes zoster, according to a large population-based Israeli study.
In contrast, the use of methotrexate, cyclosporine, acitretin, and systemic corticosteroids is associated with an increased risk of herpes zoster in psoriasis patients, Dr. Arnon D. Cohen said at the World Congress of Dermatology.
He reported on 22,330 psoriasis patients followed over 10 years at Clalit Health Services, Israel’s largest HMO. This was a real-world patient population, 53% of whom were female, in contrast to the highly selected populations in randomized clinical trials, which are typically 65%-69% male.
During nearly 220,000 person-years of follow-up there were 1,321 cases of herpes zoster. The baseline rate during periods when patients weren’t on any form of systemic therapy was 4.6 cases/1,000 patient-years. In contrast, the rate during cyclosporine therapy was more than 10-fold higher at 48.4 cases/1,000 patient-years.
Eighteen percent of psoriasis patients were on systemic corticosteroids at some point during the study period; that medication was associated with a herpes zoster rate of 25.7/1,000 patient-years of exposure. Other therapies that conferred significantly increased risk were methotrexate at 17 cases/1,000 patient-years and acitretin (Soriatane) at 5.4 cases/1,000 patient-years, reported Dr. Cohen, director of the department of quality measures at Clalit and a dermatologist at Ben-Gurion University of the Negev, Beer-Sheva, Israel.
In contrast, no cases of herpes zoster occurred in psoriasis patients on alefacept (Amevive), efalizumab (Raptiva) or adalimumab (Humira). Nor was the incidence increased beyond baseline in patients on PUVA, UVB, or etanercept (Enbrel).
In a Cox logistic regression analysis, systemic corticosteroid therapy was associated with a 2.4-fold increased risk of developing herpes zoster. Being female was associated with a significant 16% increase in risk. Infliximab (Remicade) therapy was associated with a 1.8-fold increase in risk, a trend that did not achieve statistical significance (P =.09).
Dr. Cohen and his coinvestigators conducted this study because, even though the biologic agents are recognized as conferring increased risk of tuberculosis and serious bacterial infections, very little is known about their association, if any, with viral infections.
Dr. Cohen declared having no relevant financial interests.
FROM THE WORLD CONGRESS OF DERMATOLOGY
Major Finding: No cases of herpes zoster occurred in psoriasis patients on alefacept (Amevive), efalizumab (Raptiva) or adalimumab (Humira).
Data Source: A population-based study of 22,330 psoriasis patients followed over 10 years at Clalit Health Services, Israel’s largest HMO.
Disclosures: Dr. Cohen reported having no financial conflicts.