User login
American Academy of Dermatology (AAD): Summer Academy 2013
Apps for your smart phone
The number of health apps continues to grow at a rapid pace, and if you’re in search of more apps to download and experiment with, Dr. Craig Burkhart has a list for you.
To give a sense of how fast health apps are arriving in the market, Dr. Burkhart of the University of North Carolina at Chapel Hill, broke down the number of health applications for Apple devices at the times of American Academy of Dermatology’s meetings: At the 2012 AAD annual meeting, there were 5,000 iOS health apps. That number went up to 13,000 during the 2012 Summer AAD, and 40,000 at the 2013 AAD annual meeting.
He listed some of his favorites during the 2013 AAD summer academy meeting:
1password – to remember passwords
Byword – a simple writing app
Drafts – to automate text actions, also good for transcriptions
Dropbox – to store and share documents, large or small
Epocrates – for drug reference
Evernote – for note-taking
Flipboard – popular news reader
Google Drive – for documents and spreadsheets
Launch Center Pro – to get quick shortcuts for specific features buried in apps
Mind Node – for mind mapping
Omnifocus – for task management, based on GDT system
PDF Pen and Good Reader – PDF readers with annotating capabilities
PubMed Mobile – to search PubMed for journal articles
Read by QXMD – to keep up with medical and scientific research
Scanner Pro – to capture documents and receipts as PDF
Text Expander Touch – for those who write
Tweetbot – if you use twitter for news
What health apps would you recommend to your colleagues? Write to sknews@frontlinemedcom.com and let us know, or post your favorites on the Skin & Allergy News Facebook page.
Dr. Burkhart had no disclosures relevant to mobile apps.
nmiller@frontlinemedcom.com On Twitter @NaseemSMiller
The number of health apps continues to grow at a rapid pace, and if you’re in search of more apps to download and experiment with, Dr. Craig Burkhart has a list for you.
To give a sense of how fast health apps are arriving in the market, Dr. Burkhart of the University of North Carolina at Chapel Hill, broke down the number of health applications for Apple devices at the times of American Academy of Dermatology’s meetings: At the 2012 AAD annual meeting, there were 5,000 iOS health apps. That number went up to 13,000 during the 2012 Summer AAD, and 40,000 at the 2013 AAD annual meeting.
He listed some of his favorites during the 2013 AAD summer academy meeting:
1password – to remember passwords
Byword – a simple writing app
Drafts – to automate text actions, also good for transcriptions
Dropbox – to store and share documents, large or small
Epocrates – for drug reference
Evernote – for note-taking
Flipboard – popular news reader
Google Drive – for documents and spreadsheets
Launch Center Pro – to get quick shortcuts for specific features buried in apps
Mind Node – for mind mapping
Omnifocus – for task management, based on GDT system
PDF Pen and Good Reader – PDF readers with annotating capabilities
PubMed Mobile – to search PubMed for journal articles
Read by QXMD – to keep up with medical and scientific research
Scanner Pro – to capture documents and receipts as PDF
Text Expander Touch – for those who write
Tweetbot – if you use twitter for news
What health apps would you recommend to your colleagues? Write to sknews@frontlinemedcom.com and let us know, or post your favorites on the Skin & Allergy News Facebook page.
Dr. Burkhart had no disclosures relevant to mobile apps.
nmiller@frontlinemedcom.com On Twitter @NaseemSMiller
The number of health apps continues to grow at a rapid pace, and if you’re in search of more apps to download and experiment with, Dr. Craig Burkhart has a list for you.
To give a sense of how fast health apps are arriving in the market, Dr. Burkhart of the University of North Carolina at Chapel Hill, broke down the number of health applications for Apple devices at the times of American Academy of Dermatology’s meetings: At the 2012 AAD annual meeting, there were 5,000 iOS health apps. That number went up to 13,000 during the 2012 Summer AAD, and 40,000 at the 2013 AAD annual meeting.
He listed some of his favorites during the 2013 AAD summer academy meeting:
1password – to remember passwords
Byword – a simple writing app
Drafts – to automate text actions, also good for transcriptions
Dropbox – to store and share documents, large or small
Epocrates – for drug reference
Evernote – for note-taking
Flipboard – popular news reader
Google Drive – for documents and spreadsheets
Launch Center Pro – to get quick shortcuts for specific features buried in apps
Mind Node – for mind mapping
Omnifocus – for task management, based on GDT system
PDF Pen and Good Reader – PDF readers with annotating capabilities
PubMed Mobile – to search PubMed for journal articles
Read by QXMD – to keep up with medical and scientific research
Scanner Pro – to capture documents and receipts as PDF
Text Expander Touch – for those who write
Tweetbot – if you use twitter for news
What health apps would you recommend to your colleagues? Write to sknews@frontlinemedcom.com and let us know, or post your favorites on the Skin & Allergy News Facebook page.
Dr. Burkhart had no disclosures relevant to mobile apps.
nmiller@frontlinemedcom.com On Twitter @NaseemSMiller
Melanoma screening initiatives reveal complex ramifications
NEW YORK – A national, population-based melanoma screening program underway in Germany may provide the data to drive similar initiatives elsewhere, including the United States, but complex issues surround cancer screening programs of any kind, Dr. Allan C. Halpern said in a key address at the American Academy of Dermatology summer meeting.
Even if reduced mortality from the melanoma screening program in Germany equals the numbers seen in the regional initiative that prompted the national program, Dr. Halpern, chief of the dermatology service and co-leader of Memorial Sloan-Kettering Cancer Center (New York) melanoma disease management team, cautioned that U.S. policy makers will face several issues before adopting a similar program.
"We have begun to understand that no matter what you are screening for, you end up finding a lot of indolent disease, or what we now call overdiagnosis," Dr. Halpern said. In these cases, clinicians diagnose cancer or precancerous conditions "in patients who were never going to be hurt by their disease" but may incur harm from treatments or from the psychosocial stress of the diagnosis.
Melanoma is the only cancer for which mortality is increasing, despite simple and effective screening strategies, Dr. Halpern said. One problem is that only a proportion of those patients known to be at high risk for melanoma, such as those with a personal or family history of this disease, undergo regular surveillance. Despite a clear need for rigorous screening in high risk individuals, he suggested that "[dermatology] as a profession has not figured out how to do this consistently."
However, the program in Germany is not restricted to high-risk individuals. It was initiated after a screening program in Schleswig-Holstein, one of 16 German states, was credited with reducing melanoma mortality by 47% in women and 49% in men, based on rates 5 years after screening, compared with rates 4 years before screening (Cancer 2012;118:5395-402). Mortality rates in adjacent states over this period were unchanged.
In the German program, approximately 1,700 general practitioners were trained to provide whole body assessments for melanoma. Individuals aged 20 years and older were eligible, and more than 360,000 residents of Schleswig-Holstein were screened. Dr. Halpern said that the development of the program was largely because of the initiative of Dr. Eckhard W. Breitbart, a Schleswig-Holstein dermatologist who convinced public health authorities to provide funding.
The evidence of benefit was sufficient to generate a national program. So far, 13 million Germans have already been screened, Dr. Halpern said. Although the mortality reduction from the national program may not reach the magnitude seen at the regional level, any large reduction would provide "a huge endorsement for melanoma screening," Dr. Halpern noted.
Melanoma screening data are needed, Dr. Halpern added. The U.S. Preventive Services Task Force "specifically does not recommend melanoma screening for the population at large" because of lack of randomized, controlled trial evidence that it would provide an overall benefit, he said. Such trials have been proposed, and a pilot study was completed in Australia, Dr. Halpern said, but he said he does not believe a large scale trial is forthcoming. Rather, he said he believes that a study similar to the German study may be the best opportunity to show a benefit from screening.
Similar policy changes occurred after a Scandinavian initiative to screen cervical cancer demonstrated a large mortality benefit, according to Dr. Halpern, who called that experience the "poster child" for cancer screening initiatives. The mortality benefit data from the population-based program was so compelling that screening programs for cervical cancer are now broadly accepted worldwide, although a randomized controlled trial was never conducted. If the German data provide similar evidence of the benefits of melanoma screening, "this may be how we get to melanoma screening in this country," Dr. Halpern said.
Melanoma screening is "intuitively attractive," Dr. Halpern added, but he acknowledged the rationale for caution. While he said he believes there is a need to increase screening in high-risk populations, the risk of harm, including psychosocial harm, from population-based screening is not trivial. The German experience may provide the data to help determine whether population-based screening makes sense.
Dr. Halpern disclosed financial relationships with multiple companies including Canfield Scientific, DermTech International, Quintiles, Roche, and SciBase.
NEW YORK – A national, population-based melanoma screening program underway in Germany may provide the data to drive similar initiatives elsewhere, including the United States, but complex issues surround cancer screening programs of any kind, Dr. Allan C. Halpern said in a key address at the American Academy of Dermatology summer meeting.
Even if reduced mortality from the melanoma screening program in Germany equals the numbers seen in the regional initiative that prompted the national program, Dr. Halpern, chief of the dermatology service and co-leader of Memorial Sloan-Kettering Cancer Center (New York) melanoma disease management team, cautioned that U.S. policy makers will face several issues before adopting a similar program.
"We have begun to understand that no matter what you are screening for, you end up finding a lot of indolent disease, or what we now call overdiagnosis," Dr. Halpern said. In these cases, clinicians diagnose cancer or precancerous conditions "in patients who were never going to be hurt by their disease" but may incur harm from treatments or from the psychosocial stress of the diagnosis.
Melanoma is the only cancer for which mortality is increasing, despite simple and effective screening strategies, Dr. Halpern said. One problem is that only a proportion of those patients known to be at high risk for melanoma, such as those with a personal or family history of this disease, undergo regular surveillance. Despite a clear need for rigorous screening in high risk individuals, he suggested that "[dermatology] as a profession has not figured out how to do this consistently."
However, the program in Germany is not restricted to high-risk individuals. It was initiated after a screening program in Schleswig-Holstein, one of 16 German states, was credited with reducing melanoma mortality by 47% in women and 49% in men, based on rates 5 years after screening, compared with rates 4 years before screening (Cancer 2012;118:5395-402). Mortality rates in adjacent states over this period were unchanged.
In the German program, approximately 1,700 general practitioners were trained to provide whole body assessments for melanoma. Individuals aged 20 years and older were eligible, and more than 360,000 residents of Schleswig-Holstein were screened. Dr. Halpern said that the development of the program was largely because of the initiative of Dr. Eckhard W. Breitbart, a Schleswig-Holstein dermatologist who convinced public health authorities to provide funding.
The evidence of benefit was sufficient to generate a national program. So far, 13 million Germans have already been screened, Dr. Halpern said. Although the mortality reduction from the national program may not reach the magnitude seen at the regional level, any large reduction would provide "a huge endorsement for melanoma screening," Dr. Halpern noted.
Melanoma screening data are needed, Dr. Halpern added. The U.S. Preventive Services Task Force "specifically does not recommend melanoma screening for the population at large" because of lack of randomized, controlled trial evidence that it would provide an overall benefit, he said. Such trials have been proposed, and a pilot study was completed in Australia, Dr. Halpern said, but he said he does not believe a large scale trial is forthcoming. Rather, he said he believes that a study similar to the German study may be the best opportunity to show a benefit from screening.
Similar policy changes occurred after a Scandinavian initiative to screen cervical cancer demonstrated a large mortality benefit, according to Dr. Halpern, who called that experience the "poster child" for cancer screening initiatives. The mortality benefit data from the population-based program was so compelling that screening programs for cervical cancer are now broadly accepted worldwide, although a randomized controlled trial was never conducted. If the German data provide similar evidence of the benefits of melanoma screening, "this may be how we get to melanoma screening in this country," Dr. Halpern said.
Melanoma screening is "intuitively attractive," Dr. Halpern added, but he acknowledged the rationale for caution. While he said he believes there is a need to increase screening in high-risk populations, the risk of harm, including psychosocial harm, from population-based screening is not trivial. The German experience may provide the data to help determine whether population-based screening makes sense.
Dr. Halpern disclosed financial relationships with multiple companies including Canfield Scientific, DermTech International, Quintiles, Roche, and SciBase.
NEW YORK – A national, population-based melanoma screening program underway in Germany may provide the data to drive similar initiatives elsewhere, including the United States, but complex issues surround cancer screening programs of any kind, Dr. Allan C. Halpern said in a key address at the American Academy of Dermatology summer meeting.
Even if reduced mortality from the melanoma screening program in Germany equals the numbers seen in the regional initiative that prompted the national program, Dr. Halpern, chief of the dermatology service and co-leader of Memorial Sloan-Kettering Cancer Center (New York) melanoma disease management team, cautioned that U.S. policy makers will face several issues before adopting a similar program.
"We have begun to understand that no matter what you are screening for, you end up finding a lot of indolent disease, or what we now call overdiagnosis," Dr. Halpern said. In these cases, clinicians diagnose cancer or precancerous conditions "in patients who were never going to be hurt by their disease" but may incur harm from treatments or from the psychosocial stress of the diagnosis.
Melanoma is the only cancer for which mortality is increasing, despite simple and effective screening strategies, Dr. Halpern said. One problem is that only a proportion of those patients known to be at high risk for melanoma, such as those with a personal or family history of this disease, undergo regular surveillance. Despite a clear need for rigorous screening in high risk individuals, he suggested that "[dermatology] as a profession has not figured out how to do this consistently."
However, the program in Germany is not restricted to high-risk individuals. It was initiated after a screening program in Schleswig-Holstein, one of 16 German states, was credited with reducing melanoma mortality by 47% in women and 49% in men, based on rates 5 years after screening, compared with rates 4 years before screening (Cancer 2012;118:5395-402). Mortality rates in adjacent states over this period were unchanged.
In the German program, approximately 1,700 general practitioners were trained to provide whole body assessments for melanoma. Individuals aged 20 years and older were eligible, and more than 360,000 residents of Schleswig-Holstein were screened. Dr. Halpern said that the development of the program was largely because of the initiative of Dr. Eckhard W. Breitbart, a Schleswig-Holstein dermatologist who convinced public health authorities to provide funding.
The evidence of benefit was sufficient to generate a national program. So far, 13 million Germans have already been screened, Dr. Halpern said. Although the mortality reduction from the national program may not reach the magnitude seen at the regional level, any large reduction would provide "a huge endorsement for melanoma screening," Dr. Halpern noted.
Melanoma screening data are needed, Dr. Halpern added. The U.S. Preventive Services Task Force "specifically does not recommend melanoma screening for the population at large" because of lack of randomized, controlled trial evidence that it would provide an overall benefit, he said. Such trials have been proposed, and a pilot study was completed in Australia, Dr. Halpern said, but he said he does not believe a large scale trial is forthcoming. Rather, he said he believes that a study similar to the German study may be the best opportunity to show a benefit from screening.
Similar policy changes occurred after a Scandinavian initiative to screen cervical cancer demonstrated a large mortality benefit, according to Dr. Halpern, who called that experience the "poster child" for cancer screening initiatives. The mortality benefit data from the population-based program was so compelling that screening programs for cervical cancer are now broadly accepted worldwide, although a randomized controlled trial was never conducted. If the German data provide similar evidence of the benefits of melanoma screening, "this may be how we get to melanoma screening in this country," Dr. Halpern said.
Melanoma screening is "intuitively attractive," Dr. Halpern added, but he acknowledged the rationale for caution. While he said he believes there is a need to increase screening in high-risk populations, the risk of harm, including psychosocial harm, from population-based screening is not trivial. The German experience may provide the data to help determine whether population-based screening makes sense.
Dr. Halpern disclosed financial relationships with multiple companies including Canfield Scientific, DermTech International, Quintiles, Roche, and SciBase.
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY 2013
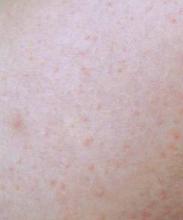
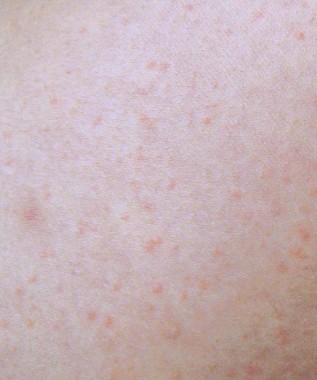
Tinea versicolor looks more like pasta primavera
NEW YORK – Clinical information evolves even for one of the most common dermatologic diseases, Malassezia infections; namely, progress in characterizing the species and the impact of a recent labeling change for oral ketoconazole, according to Dr. Nikki A. Levin of the University of Massachusetts, Worcester.
In a statement issued in July, "the [Food and Drug Administration] has limited the use or oral ketoconazole for invasive fungal infections because of concern about cases of fatal hepatotoxicity," Dr. Levin said.
Dr. Levin acknowledged ketoconazole as her go-to drug for tinea versicolor in patients not willing to use topical therapies, but she added that the new restrictions have changed her practice. She considers once-weekly fluconazole and itraconazole the best alternatives, noting only that the latter agent poses a higher risk of interactions in patients taking other drugs.
The relative efficacy of the FDA-approved options for topical therapy, including ketoconazole, econazole, clotrimazole, and miconazole, are difficult to judge because comparative studies are limited, Dr. Levin said. But the efficacy should be high for patients who apply any of these treatments twice a day for at least 3 weeks, she added.
A long list of agents not yet approved by the FDA have shown efficacy against tinea versicolor, but over-the-counter selenium sulfide lotion for skin infections or shampoo for scalp infections also are generally effective, Dr. Levin said.
Empirical use of these therapies is reasonable when patients show classic clinical signs of infection, said Dr. Levin. Malassezia prefers lipid-rich environments, and so it is most often found where sebaceous glands cluster, such as the face, scalp, and upper trunk. The creation of scales when a skin lesion is scraped (evoked scale sign), is generally sufficient to confirm the diagnosis when the KOH, or potassium hydroxide, test is positive, and cultures are rarely needed and can be problematic, Dr. Levin said. In fact, she cautioned that lipids are needed in the medium for the yeast to grow, so specimens sent to a lab should be accompanied by a specific request to demonstrate this infection.
In a presentation at the American Academy of Dermatology summer meeting, Dr. Levin also discussed evolving concepts that better characterize these yeast infections, including their appearance on light microscopy.
"In medical school, you are taught to recognize the spaghetti and meatball appearance of the short hyphae and the little spores, but since when are meatballs smaller than spaghetti? I am proposing that we change this to pasta primavera," said Dr. Levin, who likened the appearance of M. globus, which is one of the most common causes of tinea versicolor, to penne with peas.
The knowledge base on Malassezia overall and on species relevant to human infection in particular continues to grow, said Dr. Levin. She reported that the M. globosa genome was recently sequenced, and biochemistry studies have yielded new insights into how these organisms defeat immune defenses and alter melanin production to change skin pigmentation. However, she acknowledged that the immediate clinical relevance of this information is limited without further research.
The characterization of Malassezia continues to evolve, with an expansion of species to 14 from the previous 7, but differentiating among the species is not typically necessary in evaluating human infections, Dr. Levin said.
Malassezia infections, most commonly encountered as tinea versicolor, are extremely common, generally responsive to topical and oral therapies, and reasonably diagnosed in most cases on the basis of its classical clinical appearance without biopsy or culture.
"You may ask yourself, do I care about the taxonomy?" asked Dr. Levin. And the answer might be perhaps not. "In general, the treatment for these infections is the same," she said.
Dr. Levin disclosed a financial relationship with Amgen, but reported no disclosures relevant to her presentation.
NEW YORK – Clinical information evolves even for one of the most common dermatologic diseases, Malassezia infections; namely, progress in characterizing the species and the impact of a recent labeling change for oral ketoconazole, according to Dr. Nikki A. Levin of the University of Massachusetts, Worcester.
In a statement issued in July, "the [Food and Drug Administration] has limited the use or oral ketoconazole for invasive fungal infections because of concern about cases of fatal hepatotoxicity," Dr. Levin said.
Dr. Levin acknowledged ketoconazole as her go-to drug for tinea versicolor in patients not willing to use topical therapies, but she added that the new restrictions have changed her practice. She considers once-weekly fluconazole and itraconazole the best alternatives, noting only that the latter agent poses a higher risk of interactions in patients taking other drugs.
The relative efficacy of the FDA-approved options for topical therapy, including ketoconazole, econazole, clotrimazole, and miconazole, are difficult to judge because comparative studies are limited, Dr. Levin said. But the efficacy should be high for patients who apply any of these treatments twice a day for at least 3 weeks, she added.
A long list of agents not yet approved by the FDA have shown efficacy against tinea versicolor, but over-the-counter selenium sulfide lotion for skin infections or shampoo for scalp infections also are generally effective, Dr. Levin said.
Empirical use of these therapies is reasonable when patients show classic clinical signs of infection, said Dr. Levin. Malassezia prefers lipid-rich environments, and so it is most often found where sebaceous glands cluster, such as the face, scalp, and upper trunk. The creation of scales when a skin lesion is scraped (evoked scale sign), is generally sufficient to confirm the diagnosis when the KOH, or potassium hydroxide, test is positive, and cultures are rarely needed and can be problematic, Dr. Levin said. In fact, she cautioned that lipids are needed in the medium for the yeast to grow, so specimens sent to a lab should be accompanied by a specific request to demonstrate this infection.
In a presentation at the American Academy of Dermatology summer meeting, Dr. Levin also discussed evolving concepts that better characterize these yeast infections, including their appearance on light microscopy.
"In medical school, you are taught to recognize the spaghetti and meatball appearance of the short hyphae and the little spores, but since when are meatballs smaller than spaghetti? I am proposing that we change this to pasta primavera," said Dr. Levin, who likened the appearance of M. globus, which is one of the most common causes of tinea versicolor, to penne with peas.
The knowledge base on Malassezia overall and on species relevant to human infection in particular continues to grow, said Dr. Levin. She reported that the M. globosa genome was recently sequenced, and biochemistry studies have yielded new insights into how these organisms defeat immune defenses and alter melanin production to change skin pigmentation. However, she acknowledged that the immediate clinical relevance of this information is limited without further research.
The characterization of Malassezia continues to evolve, with an expansion of species to 14 from the previous 7, but differentiating among the species is not typically necessary in evaluating human infections, Dr. Levin said.
Malassezia infections, most commonly encountered as tinea versicolor, are extremely common, generally responsive to topical and oral therapies, and reasonably diagnosed in most cases on the basis of its classical clinical appearance without biopsy or culture.
"You may ask yourself, do I care about the taxonomy?" asked Dr. Levin. And the answer might be perhaps not. "In general, the treatment for these infections is the same," she said.
Dr. Levin disclosed a financial relationship with Amgen, but reported no disclosures relevant to her presentation.
NEW YORK – Clinical information evolves even for one of the most common dermatologic diseases, Malassezia infections; namely, progress in characterizing the species and the impact of a recent labeling change for oral ketoconazole, according to Dr. Nikki A. Levin of the University of Massachusetts, Worcester.
In a statement issued in July, "the [Food and Drug Administration] has limited the use or oral ketoconazole for invasive fungal infections because of concern about cases of fatal hepatotoxicity," Dr. Levin said.
Dr. Levin acknowledged ketoconazole as her go-to drug for tinea versicolor in patients not willing to use topical therapies, but she added that the new restrictions have changed her practice. She considers once-weekly fluconazole and itraconazole the best alternatives, noting only that the latter agent poses a higher risk of interactions in patients taking other drugs.
The relative efficacy of the FDA-approved options for topical therapy, including ketoconazole, econazole, clotrimazole, and miconazole, are difficult to judge because comparative studies are limited, Dr. Levin said. But the efficacy should be high for patients who apply any of these treatments twice a day for at least 3 weeks, she added.
A long list of agents not yet approved by the FDA have shown efficacy against tinea versicolor, but over-the-counter selenium sulfide lotion for skin infections or shampoo for scalp infections also are generally effective, Dr. Levin said.
Empirical use of these therapies is reasonable when patients show classic clinical signs of infection, said Dr. Levin. Malassezia prefers lipid-rich environments, and so it is most often found where sebaceous glands cluster, such as the face, scalp, and upper trunk. The creation of scales when a skin lesion is scraped (evoked scale sign), is generally sufficient to confirm the diagnosis when the KOH, or potassium hydroxide, test is positive, and cultures are rarely needed and can be problematic, Dr. Levin said. In fact, she cautioned that lipids are needed in the medium for the yeast to grow, so specimens sent to a lab should be accompanied by a specific request to demonstrate this infection.
In a presentation at the American Academy of Dermatology summer meeting, Dr. Levin also discussed evolving concepts that better characterize these yeast infections, including their appearance on light microscopy.
"In medical school, you are taught to recognize the spaghetti and meatball appearance of the short hyphae and the little spores, but since when are meatballs smaller than spaghetti? I am proposing that we change this to pasta primavera," said Dr. Levin, who likened the appearance of M. globus, which is one of the most common causes of tinea versicolor, to penne with peas.
The knowledge base on Malassezia overall and on species relevant to human infection in particular continues to grow, said Dr. Levin. She reported that the M. globosa genome was recently sequenced, and biochemistry studies have yielded new insights into how these organisms defeat immune defenses and alter melanin production to change skin pigmentation. However, she acknowledged that the immediate clinical relevance of this information is limited without further research.
The characterization of Malassezia continues to evolve, with an expansion of species to 14 from the previous 7, but differentiating among the species is not typically necessary in evaluating human infections, Dr. Levin said.
Malassezia infections, most commonly encountered as tinea versicolor, are extremely common, generally responsive to topical and oral therapies, and reasonably diagnosed in most cases on the basis of its classical clinical appearance without biopsy or culture.
"You may ask yourself, do I care about the taxonomy?" asked Dr. Levin. And the answer might be perhaps not. "In general, the treatment for these infections is the same," she said.
Dr. Levin disclosed a financial relationship with Amgen, but reported no disclosures relevant to her presentation.
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY 2013
New urticaria guidelines stress simplicity
NEW YORK – New guidelines for the diagnosis and treatment of urticaria have been endorsed by 15 professional organizations so far and are now being prepared for publication, according to a consensus meeting participant who summarized key points at the American Academy of Dermatology summer meeting.
The guidelines, developed at an earlier conference held in Berlin attended by experts from 39 countries, are straightforward, relatively simple, "and truly developed for global application," according to Dr. Kiran Godse of Patil Medical College and Hospital, Navi Mumbai, India. The guidelines represent a joint initiative of the Dermatology Section of the European Academy of Allergology and Clinical Immunology (EAACI), the Global Allergy and Asthma European Network (GA2LEN), the European Dermatology Forum (EDF), the American Academy of Allergy, Asthma and Immunology (AAAAI), and the World Allergy Organization (WAO).
The simplicity of the guidelines starts with the definition of urticaria. It consists of three characteristics: "wheals, angioedema, or both." While the definition goes on to specify that these conditions should be differentiated from autoinflammatory syndromes, hereditary angioedema, and other diseases that produce hives or swelling, the new guidelines abandon the term "idiopathic."
"Our understanding of the etiology and pathogenesis has advanced to the point that we can identify the causes in most cases," said Dr. Godse, indicating that classifying cases as "idiopathic" without further investigation is unhelpful when the goal is to find and avoid triggers.
A number of subclassifications, such as spontaneous urticaria, inducible urticaria, acute urticaria, and chronic urticaria, are defined and employed to guide clinical management. In patients with acute urticaria, diagnostic testing beyond a careful history is not recommended, except when avoidance strategies fail and recurrences are common.
Even in chronic urticaria, which is defined as symptoms persisting for at least 6 months, Dr. Godse said that the guidelines recommend "limited" initial diagnostic studies.
By relying on careful patient history rather than clinical tests to differentiate the major forms of this disease, such as cold urticaria, heat urticaria, delayed pressure urticaria, solar urticaria, and symptomatic dermographism, the guidelines in effect propose that underlying etiologies do not usually require an extensive workup. However, the guidelines do advise more extensive tests in individuals with persistent and significant disease, which can be measured with the Chronic Urticaria Quality of Life (CU-QoL) and the Angioedema Quality of Life (Ae-QoL) instruments. Both are strongly recommended for baseline assessment of symptom burden.
The treatment goal of the stepwise management is clear: complete absence of symptoms. "Treat the disease until it is gone," said Dr. Godse, summarizing this recommendation.
If symptoms cannot be eliminated simply by avoiding causes and aggravating factors, the guidelines identify second-generation, nonsedating H1 antihistamines as the first-line pharmacotherapy. Dr. Godse said that the guidelines specifically recommend continuous rather than on-demand regimens at the lowest effective dose. However, if symptoms persist after 1-4 weeks of therapy, the dosing frequency should be increased before moving to adjunctive use of additional therapies. Adjunctive therapies listed in the guidelines include omalizumab, cyclosporine A, and montelukast. The first two of these options received strong recommendations on the basis of a high level of evidence, but the third was given a weak recommendation on the basis of a low level of evidence.
In those who fail these therapies, the list of alternatives is lengthy and includes a short course of corticosteroids, immunomodulating therapies such as methotrexate, and intravenous immunoglobulins. While any one of these may be useful in an individual patient, the overall evidence of benefit was considered to be of relatively low quality.
Ultimately, the guidelines attempt to define an approach that is uniformly applicable across diverse populations, a full range of possible etiologies, and within different systems of medical care, according to Dr. Godse.
Asked for their opinion after hearing the guidelines explained at the meeting, Dr. Paul Schneiderman and Dr. Aaron Warshawsky said they were favorably impressed. Both thought the guidelines were clear, reasonable, and potentially helpful in clinical practice. Dr. Schneiderman, an associate clinical professor of dermatology at Yale University, New Haven, Conn., who maintains a private practice in Syosset, N.Y., reported that he will be able to better judge the clinical applicability of the new guidelines when he sees the full publication, but both he and Dr. Warshawsky, a dermatologist in private practice in Poughkeepsie, N.Y., agreed that advances in urticaria justify updated guidelines.
Dr. Godse reported no financial disclosures relevant to his presentation.
NEW YORK – New guidelines for the diagnosis and treatment of urticaria have been endorsed by 15 professional organizations so far and are now being prepared for publication, according to a consensus meeting participant who summarized key points at the American Academy of Dermatology summer meeting.
The guidelines, developed at an earlier conference held in Berlin attended by experts from 39 countries, are straightforward, relatively simple, "and truly developed for global application," according to Dr. Kiran Godse of Patil Medical College and Hospital, Navi Mumbai, India. The guidelines represent a joint initiative of the Dermatology Section of the European Academy of Allergology and Clinical Immunology (EAACI), the Global Allergy and Asthma European Network (GA2LEN), the European Dermatology Forum (EDF), the American Academy of Allergy, Asthma and Immunology (AAAAI), and the World Allergy Organization (WAO).
The simplicity of the guidelines starts with the definition of urticaria. It consists of three characteristics: "wheals, angioedema, or both." While the definition goes on to specify that these conditions should be differentiated from autoinflammatory syndromes, hereditary angioedema, and other diseases that produce hives or swelling, the new guidelines abandon the term "idiopathic."
"Our understanding of the etiology and pathogenesis has advanced to the point that we can identify the causes in most cases," said Dr. Godse, indicating that classifying cases as "idiopathic" without further investigation is unhelpful when the goal is to find and avoid triggers.
A number of subclassifications, such as spontaneous urticaria, inducible urticaria, acute urticaria, and chronic urticaria, are defined and employed to guide clinical management. In patients with acute urticaria, diagnostic testing beyond a careful history is not recommended, except when avoidance strategies fail and recurrences are common.
Even in chronic urticaria, which is defined as symptoms persisting for at least 6 months, Dr. Godse said that the guidelines recommend "limited" initial diagnostic studies.
By relying on careful patient history rather than clinical tests to differentiate the major forms of this disease, such as cold urticaria, heat urticaria, delayed pressure urticaria, solar urticaria, and symptomatic dermographism, the guidelines in effect propose that underlying etiologies do not usually require an extensive workup. However, the guidelines do advise more extensive tests in individuals with persistent and significant disease, which can be measured with the Chronic Urticaria Quality of Life (CU-QoL) and the Angioedema Quality of Life (Ae-QoL) instruments. Both are strongly recommended for baseline assessment of symptom burden.
The treatment goal of the stepwise management is clear: complete absence of symptoms. "Treat the disease until it is gone," said Dr. Godse, summarizing this recommendation.
If symptoms cannot be eliminated simply by avoiding causes and aggravating factors, the guidelines identify second-generation, nonsedating H1 antihistamines as the first-line pharmacotherapy. Dr. Godse said that the guidelines specifically recommend continuous rather than on-demand regimens at the lowest effective dose. However, if symptoms persist after 1-4 weeks of therapy, the dosing frequency should be increased before moving to adjunctive use of additional therapies. Adjunctive therapies listed in the guidelines include omalizumab, cyclosporine A, and montelukast. The first two of these options received strong recommendations on the basis of a high level of evidence, but the third was given a weak recommendation on the basis of a low level of evidence.
In those who fail these therapies, the list of alternatives is lengthy and includes a short course of corticosteroids, immunomodulating therapies such as methotrexate, and intravenous immunoglobulins. While any one of these may be useful in an individual patient, the overall evidence of benefit was considered to be of relatively low quality.
Ultimately, the guidelines attempt to define an approach that is uniformly applicable across diverse populations, a full range of possible etiologies, and within different systems of medical care, according to Dr. Godse.
Asked for their opinion after hearing the guidelines explained at the meeting, Dr. Paul Schneiderman and Dr. Aaron Warshawsky said they were favorably impressed. Both thought the guidelines were clear, reasonable, and potentially helpful in clinical practice. Dr. Schneiderman, an associate clinical professor of dermatology at Yale University, New Haven, Conn., who maintains a private practice in Syosset, N.Y., reported that he will be able to better judge the clinical applicability of the new guidelines when he sees the full publication, but both he and Dr. Warshawsky, a dermatologist in private practice in Poughkeepsie, N.Y., agreed that advances in urticaria justify updated guidelines.
Dr. Godse reported no financial disclosures relevant to his presentation.
NEW YORK – New guidelines for the diagnosis and treatment of urticaria have been endorsed by 15 professional organizations so far and are now being prepared for publication, according to a consensus meeting participant who summarized key points at the American Academy of Dermatology summer meeting.
The guidelines, developed at an earlier conference held in Berlin attended by experts from 39 countries, are straightforward, relatively simple, "and truly developed for global application," according to Dr. Kiran Godse of Patil Medical College and Hospital, Navi Mumbai, India. The guidelines represent a joint initiative of the Dermatology Section of the European Academy of Allergology and Clinical Immunology (EAACI), the Global Allergy and Asthma European Network (GA2LEN), the European Dermatology Forum (EDF), the American Academy of Allergy, Asthma and Immunology (AAAAI), and the World Allergy Organization (WAO).
The simplicity of the guidelines starts with the definition of urticaria. It consists of three characteristics: "wheals, angioedema, or both." While the definition goes on to specify that these conditions should be differentiated from autoinflammatory syndromes, hereditary angioedema, and other diseases that produce hives or swelling, the new guidelines abandon the term "idiopathic."
"Our understanding of the etiology and pathogenesis has advanced to the point that we can identify the causes in most cases," said Dr. Godse, indicating that classifying cases as "idiopathic" without further investigation is unhelpful when the goal is to find and avoid triggers.
A number of subclassifications, such as spontaneous urticaria, inducible urticaria, acute urticaria, and chronic urticaria, are defined and employed to guide clinical management. In patients with acute urticaria, diagnostic testing beyond a careful history is not recommended, except when avoidance strategies fail and recurrences are common.
Even in chronic urticaria, which is defined as symptoms persisting for at least 6 months, Dr. Godse said that the guidelines recommend "limited" initial diagnostic studies.
By relying on careful patient history rather than clinical tests to differentiate the major forms of this disease, such as cold urticaria, heat urticaria, delayed pressure urticaria, solar urticaria, and symptomatic dermographism, the guidelines in effect propose that underlying etiologies do not usually require an extensive workup. However, the guidelines do advise more extensive tests in individuals with persistent and significant disease, which can be measured with the Chronic Urticaria Quality of Life (CU-QoL) and the Angioedema Quality of Life (Ae-QoL) instruments. Both are strongly recommended for baseline assessment of symptom burden.
The treatment goal of the stepwise management is clear: complete absence of symptoms. "Treat the disease until it is gone," said Dr. Godse, summarizing this recommendation.
If symptoms cannot be eliminated simply by avoiding causes and aggravating factors, the guidelines identify second-generation, nonsedating H1 antihistamines as the first-line pharmacotherapy. Dr. Godse said that the guidelines specifically recommend continuous rather than on-demand regimens at the lowest effective dose. However, if symptoms persist after 1-4 weeks of therapy, the dosing frequency should be increased before moving to adjunctive use of additional therapies. Adjunctive therapies listed in the guidelines include omalizumab, cyclosporine A, and montelukast. The first two of these options received strong recommendations on the basis of a high level of evidence, but the third was given a weak recommendation on the basis of a low level of evidence.
In those who fail these therapies, the list of alternatives is lengthy and includes a short course of corticosteroids, immunomodulating therapies such as methotrexate, and intravenous immunoglobulins. While any one of these may be useful in an individual patient, the overall evidence of benefit was considered to be of relatively low quality.
Ultimately, the guidelines attempt to define an approach that is uniformly applicable across diverse populations, a full range of possible etiologies, and within different systems of medical care, according to Dr. Godse.
Asked for their opinion after hearing the guidelines explained at the meeting, Dr. Paul Schneiderman and Dr. Aaron Warshawsky said they were favorably impressed. Both thought the guidelines were clear, reasonable, and potentially helpful in clinical practice. Dr. Schneiderman, an associate clinical professor of dermatology at Yale University, New Haven, Conn., who maintains a private practice in Syosset, N.Y., reported that he will be able to better judge the clinical applicability of the new guidelines when he sees the full publication, but both he and Dr. Warshawsky, a dermatologist in private practice in Poughkeepsie, N.Y., agreed that advances in urticaria justify updated guidelines.
Dr. Godse reported no financial disclosures relevant to his presentation.
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY 2013
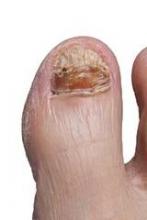
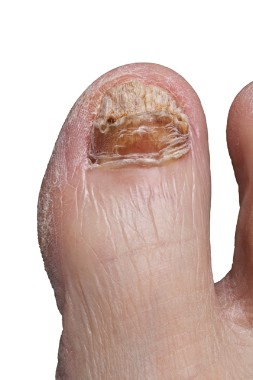
PCR reveals multiple pathogens in onychomyosis
NEW YORK – Diagnosis of fungal nail infection with polymerase chain reaction is demonstrating that the rates of mixed infection, including coinfection with dermatophyte and nondermatophyte molds, are substantially higher than that produced by cultures, according to a series of studies with implications for treatment selection.
The data may explain why some infections persist despite therapy and could lead to more frequent use of PCR as a diagnostic tool, Dr. Aditya K. Gupta reported at the American Academy of Dermatology summer meeting.
PCR is far more sensitive than culture for identification of dermatophytes and nondermatophytes, and, unlike culture, PCR can detect the presence of multiple fungi in the same sample, according to Dr. Gupta, a dermatologist at Sunnybrook and Women’s College Health Sciences Center, Toronto.
Based on studies at his institution, he reported that two or more pathogens are more common than previously appreciated "and this is really important," because it has immediate implications for selecting broader spectrum agents to increase the likelihood of mycologic cure.
"A lot of treatment failures that we attribute to lack of efficacy of the agent may in fact be a reflection of the fact that there is a nondermatophyte mold that is present, evading therapy," Dr. Gupta said.
In a series of 155 patients, PCR was positive for dermatophytes in 44% of patients when culture was positive in 20%. Positive findings were 14% and 7% for PCR and culture, respectively, for nondermatophytes.
PCR is now being used routinely at Dr. Gupta’s institution not only because of its ability to detect mixed infections but also because it is about twice as sensitive as culture for detecting nail fungi.
Dr. Gupta predicted wider use of PCR in onychomycosis because of its greater sensitivity, noting however, that cost may be an obstacle. The potential difficulty of obtaining third-party reimbursement for PCR, which is substantially more expensive than a culture is, was raised in the discussion period by several dermatologists who were impressed with these results. At Dr. Gupta’s center, cost has not been an issue because PCR is being performed as part of a research initiative, but the greater diagnostic accuracy may be relevant to a cost-benefit analysis that includes an opportunity to increase the proportion of patients initiated on an optimal therapy for the underlying pathogen, Dr. Gupta said.
Dr. Gupta reported that he has financial relationships with Valeant, NuvoLase, Bristol-Myers Squibb, Novartis, and Janssen. The study was not commercially sponsored.
NEW YORK – Diagnosis of fungal nail infection with polymerase chain reaction is demonstrating that the rates of mixed infection, including coinfection with dermatophyte and nondermatophyte molds, are substantially higher than that produced by cultures, according to a series of studies with implications for treatment selection.
The data may explain why some infections persist despite therapy and could lead to more frequent use of PCR as a diagnostic tool, Dr. Aditya K. Gupta reported at the American Academy of Dermatology summer meeting.
PCR is far more sensitive than culture for identification of dermatophytes and nondermatophytes, and, unlike culture, PCR can detect the presence of multiple fungi in the same sample, according to Dr. Gupta, a dermatologist at Sunnybrook and Women’s College Health Sciences Center, Toronto.
Based on studies at his institution, he reported that two or more pathogens are more common than previously appreciated "and this is really important," because it has immediate implications for selecting broader spectrum agents to increase the likelihood of mycologic cure.
"A lot of treatment failures that we attribute to lack of efficacy of the agent may in fact be a reflection of the fact that there is a nondermatophyte mold that is present, evading therapy," Dr. Gupta said.
In a series of 155 patients, PCR was positive for dermatophytes in 44% of patients when culture was positive in 20%. Positive findings were 14% and 7% for PCR and culture, respectively, for nondermatophytes.
PCR is now being used routinely at Dr. Gupta’s institution not only because of its ability to detect mixed infections but also because it is about twice as sensitive as culture for detecting nail fungi.
Dr. Gupta predicted wider use of PCR in onychomycosis because of its greater sensitivity, noting however, that cost may be an obstacle. The potential difficulty of obtaining third-party reimbursement for PCR, which is substantially more expensive than a culture is, was raised in the discussion period by several dermatologists who were impressed with these results. At Dr. Gupta’s center, cost has not been an issue because PCR is being performed as part of a research initiative, but the greater diagnostic accuracy may be relevant to a cost-benefit analysis that includes an opportunity to increase the proportion of patients initiated on an optimal therapy for the underlying pathogen, Dr. Gupta said.
Dr. Gupta reported that he has financial relationships with Valeant, NuvoLase, Bristol-Myers Squibb, Novartis, and Janssen. The study was not commercially sponsored.
NEW YORK – Diagnosis of fungal nail infection with polymerase chain reaction is demonstrating that the rates of mixed infection, including coinfection with dermatophyte and nondermatophyte molds, are substantially higher than that produced by cultures, according to a series of studies with implications for treatment selection.
The data may explain why some infections persist despite therapy and could lead to more frequent use of PCR as a diagnostic tool, Dr. Aditya K. Gupta reported at the American Academy of Dermatology summer meeting.
PCR is far more sensitive than culture for identification of dermatophytes and nondermatophytes, and, unlike culture, PCR can detect the presence of multiple fungi in the same sample, according to Dr. Gupta, a dermatologist at Sunnybrook and Women’s College Health Sciences Center, Toronto.
Based on studies at his institution, he reported that two or more pathogens are more common than previously appreciated "and this is really important," because it has immediate implications for selecting broader spectrum agents to increase the likelihood of mycologic cure.
"A lot of treatment failures that we attribute to lack of efficacy of the agent may in fact be a reflection of the fact that there is a nondermatophyte mold that is present, evading therapy," Dr. Gupta said.
In a series of 155 patients, PCR was positive for dermatophytes in 44% of patients when culture was positive in 20%. Positive findings were 14% and 7% for PCR and culture, respectively, for nondermatophytes.
PCR is now being used routinely at Dr. Gupta’s institution not only because of its ability to detect mixed infections but also because it is about twice as sensitive as culture for detecting nail fungi.
Dr. Gupta predicted wider use of PCR in onychomycosis because of its greater sensitivity, noting however, that cost may be an obstacle. The potential difficulty of obtaining third-party reimbursement for PCR, which is substantially more expensive than a culture is, was raised in the discussion period by several dermatologists who were impressed with these results. At Dr. Gupta’s center, cost has not been an issue because PCR is being performed as part of a research initiative, but the greater diagnostic accuracy may be relevant to a cost-benefit analysis that includes an opportunity to increase the proportion of patients initiated on an optimal therapy for the underlying pathogen, Dr. Gupta said.
Dr. Gupta reported that he has financial relationships with Valeant, NuvoLase, Bristol-Myers Squibb, Novartis, and Janssen. The study was not commercially sponsored.
AT THE AAD SUMMER ACADEMY 2013
Major finding: PCR is twice as effective as culture for the identification of pathogens causing onychomycosis.
Data source: Single-center study comparing diagnostic techniques in patient series
Disclosures: Dr. Gupta reported that he has financial relationships with Valeant, NuvoLase, Bristol-Myers Squibb, Novartis, and Janssen. The study was not commercially sponsored.