User login
ACC/AHA Cardiovascular Risk Equations Get a Thumbs-up
WASHINGTON – The controversial cardiovascular risk estimator introduced in the current American College of Cardiology/American Heart Association risk-assessment guidelines demonstrated "moderate to good" predictive performance when applied to a large U.S. cohort for whom consideration of statin therapy is clinically relevant, Paul Muntner, Ph.D., reported at the annual meeting of the American College of Cardiology.
"We believe that the current study supports the validity of the pooled cohort risk equations to inform clinical management decisions," said Dr. Muntner, professor of epidemiology and of medicine at the University of Alabama at Birmingham.
The risk estimator has come under strong criticism since the guidelines were released last November. When critics applied the risk estimator to participants in the Women's Health Study, the Physicians' Health Study, and the Women's Health Initiative, they found a big discrepancy between the observed atherosclerotic cardiovascular disease (ASCVD) event rates during follow-up and the predicted rates based on the risk calculator, with the ACC/AHA risk estimator tending to markedly overestimate risk. But those analyses involved studies lacking surveillance mechanisms to identify ASCVD events that weren’t reported by participants, according to Dr. Muntner.
"One of the challenges with those big studies is the underreporting of events. Let’s look at the Women’s Health Initiative. Roughly 25% of adjudicated events in that study were not detected because of the reliance on patient reporting. There were two reasons for this: Participants didn’t report a subsequently validated event, or hospital consent forms didn’t permit release of the chart to study investigators," he asserted.
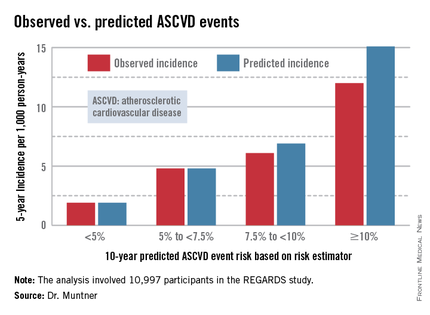
Dr. Muntner presented a new analysis in which the ASCVD risk estimator was applied to participants in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study, a prospective, observational, population-based study of more than 30,000 U.S. black and white patients. He and his coworkers compared the observed 5-year rates of the combined endpoint of death from coronary heart disease, nonfatal MI, or fatal or nonfatal stroke to rates projected by the risk equations.
The analysis was restricted to the 10,997 REGARDS participants who fell into the category of the population for whom the risk equations were designed as a guide in decision making regarding initiation of statin therapy: people aged 40-79 years without atherosclerotic cardiovascular disease or diabetes, not on a statin, and with an LDL cholesterol level of 70-189 mg/dL.
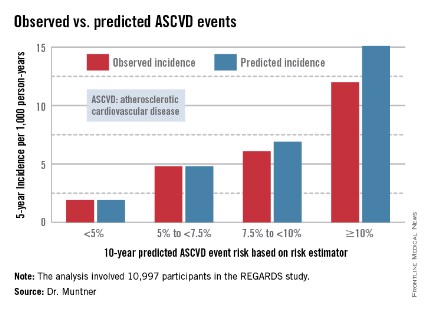
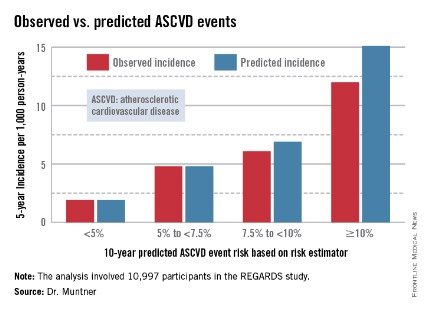
In participants in the lower 10-year ASCVD risk categories based on the equations, the predicted 5-year event rates were spot on with the observed rates. In patients at the higher end of the 10-year risk spectrum, the equations tended to overestimate the event risk (see chart). However, it should be noted that roughly 40% of the REGARDS cohort initiated statin therapy during the 5-year follow-up period, and that would have lowered their event rate, Dr. Muntner said.
The investigators also compared observed and predicted 5-year event rates in a separate REGARDS subgroup composed of 3,333 study participants with Medicare Part A insurance. In this older cohort, the risk equations tended to modestly underestimate the observed ASCVD event rate. "Overall, though, I would say this is pretty good calibration," the epidemiologist commented.
Simultaneous with Dr. Muntner’s presentation at ACC 14, the study results were published (JAMA 2014 April 9;311:1406-15).
The REGARDS study is funded by the National Institutes of Health, as was Dr. Muntner’s analysis. He reported having no relevant financial interests.
WASHINGTON – The controversial cardiovascular risk estimator introduced in the current American College of Cardiology/American Heart Association risk-assessment guidelines demonstrated "moderate to good" predictive performance when applied to a large U.S. cohort for whom consideration of statin therapy is clinically relevant, Paul Muntner, Ph.D., reported at the annual meeting of the American College of Cardiology.
"We believe that the current study supports the validity of the pooled cohort risk equations to inform clinical management decisions," said Dr. Muntner, professor of epidemiology and of medicine at the University of Alabama at Birmingham.

The risk estimator has come under strong criticism since the guidelines were released last November. When critics applied the risk estimator to participants in the Women's Health Study, the Physicians' Health Study, and the Women's Health Initiative, they found a big discrepancy between the observed atherosclerotic cardiovascular disease (ASCVD) event rates during follow-up and the predicted rates based on the risk calculator, with the ACC/AHA risk estimator tending to markedly overestimate risk. But those analyses involved studies lacking surveillance mechanisms to identify ASCVD events that weren’t reported by participants, according to Dr. Muntner.
"One of the challenges with those big studies is the underreporting of events. Let’s look at the Women’s Health Initiative. Roughly 25% of adjudicated events in that study were not detected because of the reliance on patient reporting. There were two reasons for this: Participants didn’t report a subsequently validated event, or hospital consent forms didn’t permit release of the chart to study investigators," he asserted.
Dr. Muntner presented a new analysis in which the ASCVD risk estimator was applied to participants in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study, a prospective, observational, population-based study of more than 30,000 U.S. black and white patients. He and his coworkers compared the observed 5-year rates of the combined endpoint of death from coronary heart disease, nonfatal MI, or fatal or nonfatal stroke to rates projected by the risk equations.
The analysis was restricted to the 10,997 REGARDS participants who fell into the category of the population for whom the risk equations were designed as a guide in decision making regarding initiation of statin therapy: people aged 40-79 years without atherosclerotic cardiovascular disease or diabetes, not on a statin, and with an LDL cholesterol level of 70-189 mg/dL.
In participants in the lower 10-year ASCVD risk categories based on the equations, the predicted 5-year event rates were spot on with the observed rates. In patients at the higher end of the 10-year risk spectrum, the equations tended to overestimate the event risk (see chart). However, it should be noted that roughly 40% of the REGARDS cohort initiated statin therapy during the 5-year follow-up period, and that would have lowered their event rate, Dr. Muntner said.
The investigators also compared observed and predicted 5-year event rates in a separate REGARDS subgroup composed of 3,333 study participants with Medicare Part A insurance. In this older cohort, the risk equations tended to modestly underestimate the observed ASCVD event rate. "Overall, though, I would say this is pretty good calibration," the epidemiologist commented.
Simultaneous with Dr. Muntner’s presentation at ACC 14, the study results were published (JAMA 2014 April 9;311:1406-15).
The REGARDS study is funded by the National Institutes of Health, as was Dr. Muntner’s analysis. He reported having no relevant financial interests.
WASHINGTON – The controversial cardiovascular risk estimator introduced in the current American College of Cardiology/American Heart Association risk-assessment guidelines demonstrated "moderate to good" predictive performance when applied to a large U.S. cohort for whom consideration of statin therapy is clinically relevant, Paul Muntner, Ph.D., reported at the annual meeting of the American College of Cardiology.
"We believe that the current study supports the validity of the pooled cohort risk equations to inform clinical management decisions," said Dr. Muntner, professor of epidemiology and of medicine at the University of Alabama at Birmingham.

The risk estimator has come under strong criticism since the guidelines were released last November. When critics applied the risk estimator to participants in the Women's Health Study, the Physicians' Health Study, and the Women's Health Initiative, they found a big discrepancy between the observed atherosclerotic cardiovascular disease (ASCVD) event rates during follow-up and the predicted rates based on the risk calculator, with the ACC/AHA risk estimator tending to markedly overestimate risk. But those analyses involved studies lacking surveillance mechanisms to identify ASCVD events that weren’t reported by participants, according to Dr. Muntner.
"One of the challenges with those big studies is the underreporting of events. Let’s look at the Women’s Health Initiative. Roughly 25% of adjudicated events in that study were not detected because of the reliance on patient reporting. There were two reasons for this: Participants didn’t report a subsequently validated event, or hospital consent forms didn’t permit release of the chart to study investigators," he asserted.
Dr. Muntner presented a new analysis in which the ASCVD risk estimator was applied to participants in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study, a prospective, observational, population-based study of more than 30,000 U.S. black and white patients. He and his coworkers compared the observed 5-year rates of the combined endpoint of death from coronary heart disease, nonfatal MI, or fatal or nonfatal stroke to rates projected by the risk equations.
The analysis was restricted to the 10,997 REGARDS participants who fell into the category of the population for whom the risk equations were designed as a guide in decision making regarding initiation of statin therapy: people aged 40-79 years without atherosclerotic cardiovascular disease or diabetes, not on a statin, and with an LDL cholesterol level of 70-189 mg/dL.
In participants in the lower 10-year ASCVD risk categories based on the equations, the predicted 5-year event rates were spot on with the observed rates. In patients at the higher end of the 10-year risk spectrum, the equations tended to overestimate the event risk (see chart). However, it should be noted that roughly 40% of the REGARDS cohort initiated statin therapy during the 5-year follow-up period, and that would have lowered their event rate, Dr. Muntner said.
The investigators also compared observed and predicted 5-year event rates in a separate REGARDS subgroup composed of 3,333 study participants with Medicare Part A insurance. In this older cohort, the risk equations tended to modestly underestimate the observed ASCVD event rate. "Overall, though, I would say this is pretty good calibration," the epidemiologist commented.
Simultaneous with Dr. Muntner’s presentation at ACC 14, the study results were published (JAMA 2014 April 9;311:1406-15).
The REGARDS study is funded by the National Institutes of Health, as was Dr. Muntner’s analysis. He reported having no relevant financial interests.
AT ACC 14
ACC/AHA cardiovascular risk equations get a thumbs-up
WASHINGTON – The controversial cardiovascular risk estimator introduced in the current American College of Cardiology/American Heart Association risk-assessment guidelines demonstrated "moderate to good" predictive performance when applied to a large U.S. cohort for whom consideration of statin therapy is clinically relevant, Paul Muntner, Ph.D., reported at the annual meeting of the American College of Cardiology.
"We believe that the current study supports the validity of the pooled cohort risk equations to inform clinical management decisions," said Dr. Muntner, professor of epidemiology and of medicine at the University of Alabama at Birmingham.
The risk estimator has come under strong criticism since the guidelines were released last November. When critics applied the risk estimator to participants in the Women's Health Study, the Physicians' Health Study, and the Women's Health Initiative, they found a big discrepancy between the observed atherosclerotic cardiovascular disease (ASCVD) event rates during follow-up and the predicted rates based on the risk calculator, with the ACC/AHA risk estimator tending to markedly overestimate risk. But those analyses involved studies lacking surveillance mechanisms to identify ASCVD events that weren’t reported by participants, according to Dr. Muntner.
"One of the challenges with those big studies is the underreporting of events. Let’s look at the Women’s Health Initiative. Roughly 25% of adjudicated events in that study were not detected because of the reliance on patient reporting. There were two reasons for this: Participants didn’t report a subsequently validated event, or hospital consent forms didn’t permit release of the chart to study investigators," he asserted.
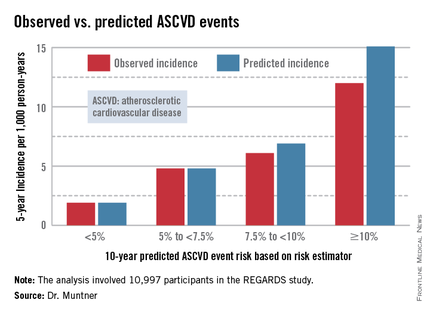
Dr. Muntner presented a new analysis in which the ASCVD risk estimator was applied to participants in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study, a prospective, observational, population-based study of more than 30,000 U.S. black and white patients. He and his coworkers compared the observed 5-year rates of the combined endpoint of death from coronary heart disease, nonfatal MI, or fatal or nonfatal stroke to rates projected by the risk equations.
The analysis was restricted to the 10,997 REGARDS participants who fell into the category of the population for whom the risk equations were designed as a guide in decision making regarding initiation of statin therapy: people aged 40-79 years without atherosclerotic cardiovascular disease or diabetes, not on a statin, and with an LDL cholesterol level of 70-189 mg/dL.
In participants in the lower 10-year ASCVD risk categories based on the equations, the predicted 5-year event rates were spot on with the observed rates. In patients at the higher end of the 10-year risk spectrum, the equations tended to overestimate the event risk (see chart). However, it should be noted that roughly 40% of the REGARDS cohort initiated statin therapy during the 5-year follow-up period, and that would have lowered their event rate, Dr. Muntner said.
The investigators also compared observed and predicted 5-year event rates in a separate REGARDS subgroup composed of 3,333 study participants with Medicare Part A insurance. In this older cohort, the risk equations tended to modestly underestimate the observed ASCVD event rate. "Overall, though, I would say this is pretty good calibration," the epidemiologist commented.
Simultaneous with Dr. Muntner’s presentation at ACC 14, the study results were published (JAMA 2014 April 9;311:1406-15).
The REGARDS study is funded by the National Institutes of Health, as was Dr. Muntner’s analysis. He reported having no relevant financial interests.
WASHINGTON – The controversial cardiovascular risk estimator introduced in the current American College of Cardiology/American Heart Association risk-assessment guidelines demonstrated "moderate to good" predictive performance when applied to a large U.S. cohort for whom consideration of statin therapy is clinically relevant, Paul Muntner, Ph.D., reported at the annual meeting of the American College of Cardiology.
"We believe that the current study supports the validity of the pooled cohort risk equations to inform clinical management decisions," said Dr. Muntner, professor of epidemiology and of medicine at the University of Alabama at Birmingham.

The risk estimator has come under strong criticism since the guidelines were released last November. When critics applied the risk estimator to participants in the Women's Health Study, the Physicians' Health Study, and the Women's Health Initiative, they found a big discrepancy between the observed atherosclerotic cardiovascular disease (ASCVD) event rates during follow-up and the predicted rates based on the risk calculator, with the ACC/AHA risk estimator tending to markedly overestimate risk. But those analyses involved studies lacking surveillance mechanisms to identify ASCVD events that weren’t reported by participants, according to Dr. Muntner.
"One of the challenges with those big studies is the underreporting of events. Let’s look at the Women’s Health Initiative. Roughly 25% of adjudicated events in that study were not detected because of the reliance on patient reporting. There were two reasons for this: Participants didn’t report a subsequently validated event, or hospital consent forms didn’t permit release of the chart to study investigators," he asserted.
Dr. Muntner presented a new analysis in which the ASCVD risk estimator was applied to participants in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study, a prospective, observational, population-based study of more than 30,000 U.S. black and white patients. He and his coworkers compared the observed 5-year rates of the combined endpoint of death from coronary heart disease, nonfatal MI, or fatal or nonfatal stroke to rates projected by the risk equations.
The analysis was restricted to the 10,997 REGARDS participants who fell into the category of the population for whom the risk equations were designed as a guide in decision making regarding initiation of statin therapy: people aged 40-79 years without atherosclerotic cardiovascular disease or diabetes, not on a statin, and with an LDL cholesterol level of 70-189 mg/dL.
In participants in the lower 10-year ASCVD risk categories based on the equations, the predicted 5-year event rates were spot on with the observed rates. In patients at the higher end of the 10-year risk spectrum, the equations tended to overestimate the event risk (see chart). However, it should be noted that roughly 40% of the REGARDS cohort initiated statin therapy during the 5-year follow-up period, and that would have lowered their event rate, Dr. Muntner said.
The investigators also compared observed and predicted 5-year event rates in a separate REGARDS subgroup composed of 3,333 study participants with Medicare Part A insurance. In this older cohort, the risk equations tended to modestly underestimate the observed ASCVD event rate. "Overall, though, I would say this is pretty good calibration," the epidemiologist commented.
Simultaneous with Dr. Muntner’s presentation at ACC 14, the study results were published (JAMA 2014 April 9;311:1406-15).
The REGARDS study is funded by the National Institutes of Health, as was Dr. Muntner’s analysis. He reported having no relevant financial interests.
WASHINGTON – The controversial cardiovascular risk estimator introduced in the current American College of Cardiology/American Heart Association risk-assessment guidelines demonstrated "moderate to good" predictive performance when applied to a large U.S. cohort for whom consideration of statin therapy is clinically relevant, Paul Muntner, Ph.D., reported at the annual meeting of the American College of Cardiology.
"We believe that the current study supports the validity of the pooled cohort risk equations to inform clinical management decisions," said Dr. Muntner, professor of epidemiology and of medicine at the University of Alabama at Birmingham.

The risk estimator has come under strong criticism since the guidelines were released last November. When critics applied the risk estimator to participants in the Women's Health Study, the Physicians' Health Study, and the Women's Health Initiative, they found a big discrepancy between the observed atherosclerotic cardiovascular disease (ASCVD) event rates during follow-up and the predicted rates based on the risk calculator, with the ACC/AHA risk estimator tending to markedly overestimate risk. But those analyses involved studies lacking surveillance mechanisms to identify ASCVD events that weren’t reported by participants, according to Dr. Muntner.
"One of the challenges with those big studies is the underreporting of events. Let’s look at the Women’s Health Initiative. Roughly 25% of adjudicated events in that study were not detected because of the reliance on patient reporting. There were two reasons for this: Participants didn’t report a subsequently validated event, or hospital consent forms didn’t permit release of the chart to study investigators," he asserted.
Dr. Muntner presented a new analysis in which the ASCVD risk estimator was applied to participants in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study, a prospective, observational, population-based study of more than 30,000 U.S. black and white patients. He and his coworkers compared the observed 5-year rates of the combined endpoint of death from coronary heart disease, nonfatal MI, or fatal or nonfatal stroke to rates projected by the risk equations.
The analysis was restricted to the 10,997 REGARDS participants who fell into the category of the population for whom the risk equations were designed as a guide in decision making regarding initiation of statin therapy: people aged 40-79 years without atherosclerotic cardiovascular disease or diabetes, not on a statin, and with an LDL cholesterol level of 70-189 mg/dL.
In participants in the lower 10-year ASCVD risk categories based on the equations, the predicted 5-year event rates were spot on with the observed rates. In patients at the higher end of the 10-year risk spectrum, the equations tended to overestimate the event risk (see chart). However, it should be noted that roughly 40% of the REGARDS cohort initiated statin therapy during the 5-year follow-up period, and that would have lowered their event rate, Dr. Muntner said.
The investigators also compared observed and predicted 5-year event rates in a separate REGARDS subgroup composed of 3,333 study participants with Medicare Part A insurance. In this older cohort, the risk equations tended to modestly underestimate the observed ASCVD event rate. "Overall, though, I would say this is pretty good calibration," the epidemiologist commented.
Simultaneous with Dr. Muntner’s presentation at ACC 14, the study results were published (JAMA 2014 April 9;311:1406-15).
The REGARDS study is funded by the National Institutes of Health, as was Dr. Muntner’s analysis. He reported having no relevant financial interests.
AT ACC 14
Major finding: The controversial cardiovascular risk equations at the heart of the latest ACC/AHA risk-assessment guidelines turned in a moderate to good performance in a validation study involving nearly 11,000 participants in a large, observational, prospective study.
Data source: The REGARDS study is a population-based study in which more than 30,000 U.S. black and white patients are being followed prospectively.
Disclosures: The analysis was funded by the National Institutes of Health. The presenter reported having no financial conflicts.
Crushing ticagrelor tablets sped platelet inhibition
WASHINGTON – Administration of crushed tablets of ticagrelor* may provide a way to dramatically accelerate the onset of platelet inhibition in patients with ST-elevation myocardial infarction.
In a proof-of-concept crossover study involving 36 healthy volunteers, crushing a whole 90-mg tablet of ticagrelor (Brilinta) and suspending it in 200 mL of water given orally resulted in a mean plasma ticagrelor concentration of 149 ng/mL 30 minutes post dose, compared with 34 ng/mL after a whole tablet was swallowed, Dr. Glenn F. Carlson reported at the annual meeting of the American College of Cardiology.
When the crushed tablet suspended in water was administered via nasogastric tube, the results were even more dramatic: a mean plasma ticagrelor concentration of 265 ng/mL 30 minutes post dose, or a ninefold greater mean plasma concentration compared with swallowing a whole tablet, said Dr. Carlson of AstraZeneca.
At 1 hour post dose, mean plasma concentrations of ticagrelor and its metabolite remained significantly greater with both of the crushed-tablet dosing strategies than with whole ticagrelor. However, 2 to 48 hours post dose, plasma concentrations were similar for all three drug administration strategies.
The clinical relevance of these findings lies in the well-established observation that in acute coronary syndromes – and especially in ST-elevation myocardial infarction – time is myocardium. That is, the more quickly that effective antiplatelet activity and reperfusion can be achieved, the less extensive the myocardial tissue damage, he noted.
The three modes of ticagrelor administration were found to be bioequivalent and equally safe.
The study was funded by AstraZeneca. Dr. Carlson is an employee of the company.
*CORRECTION, 4/29/2014: An earlier version of the article misstated the name of the drug ticagrelor.
WASHINGTON – Administration of crushed tablets of ticagrelor* may provide a way to dramatically accelerate the onset of platelet inhibition in patients with ST-elevation myocardial infarction.
In a proof-of-concept crossover study involving 36 healthy volunteers, crushing a whole 90-mg tablet of ticagrelor (Brilinta) and suspending it in 200 mL of water given orally resulted in a mean plasma ticagrelor concentration of 149 ng/mL 30 minutes post dose, compared with 34 ng/mL after a whole tablet was swallowed, Dr. Glenn F. Carlson reported at the annual meeting of the American College of Cardiology.
When the crushed tablet suspended in water was administered via nasogastric tube, the results were even more dramatic: a mean plasma ticagrelor concentration of 265 ng/mL 30 minutes post dose, or a ninefold greater mean plasma concentration compared with swallowing a whole tablet, said Dr. Carlson of AstraZeneca.
At 1 hour post dose, mean plasma concentrations of ticagrelor and its metabolite remained significantly greater with both of the crushed-tablet dosing strategies than with whole ticagrelor. However, 2 to 48 hours post dose, plasma concentrations were similar for all three drug administration strategies.
The clinical relevance of these findings lies in the well-established observation that in acute coronary syndromes – and especially in ST-elevation myocardial infarction – time is myocardium. That is, the more quickly that effective antiplatelet activity and reperfusion can be achieved, the less extensive the myocardial tissue damage, he noted.
The three modes of ticagrelor administration were found to be bioequivalent and equally safe.
The study was funded by AstraZeneca. Dr. Carlson is an employee of the company.
*CORRECTION, 4/29/2014: An earlier version of the article misstated the name of the drug ticagrelor.
WASHINGTON – Administration of crushed tablets of ticagrelor* may provide a way to dramatically accelerate the onset of platelet inhibition in patients with ST-elevation myocardial infarction.
In a proof-of-concept crossover study involving 36 healthy volunteers, crushing a whole 90-mg tablet of ticagrelor (Brilinta) and suspending it in 200 mL of water given orally resulted in a mean plasma ticagrelor concentration of 149 ng/mL 30 minutes post dose, compared with 34 ng/mL after a whole tablet was swallowed, Dr. Glenn F. Carlson reported at the annual meeting of the American College of Cardiology.
When the crushed tablet suspended in water was administered via nasogastric tube, the results were even more dramatic: a mean plasma ticagrelor concentration of 265 ng/mL 30 minutes post dose, or a ninefold greater mean plasma concentration compared with swallowing a whole tablet, said Dr. Carlson of AstraZeneca.
At 1 hour post dose, mean plasma concentrations of ticagrelor and its metabolite remained significantly greater with both of the crushed-tablet dosing strategies than with whole ticagrelor. However, 2 to 48 hours post dose, plasma concentrations were similar for all three drug administration strategies.
The clinical relevance of these findings lies in the well-established observation that in acute coronary syndromes – and especially in ST-elevation myocardial infarction – time is myocardium. That is, the more quickly that effective antiplatelet activity and reperfusion can be achieved, the less extensive the myocardial tissue damage, he noted.
The three modes of ticagrelor administration were found to be bioequivalent and equally safe.
The study was funded by AstraZeneca. Dr. Carlson is an employee of the company.
*CORRECTION, 4/29/2014: An earlier version of the article misstated the name of the drug ticagrelor.
AT ACC 14
Major finding: Mean plasma concentration of ticagralor was roughly fivefold greater 30 minutes post dose via a crushed tablet in water vs. a tablet swallowed whole, and ninefold greater when the medication was delivered in a suspension via nasogastric tube.
Data source: This was a single-center, randomized, crossover study involving 36 healthy volunteers who on separate occasions swallowed one 90-mg tablet of the antiplatelet agent ticagralor whole, drank a crushed tablet suspended in 200 mL of water, or received the suspension via nasogastric tube.
Disclosures: The study was funded by AstraZeneca. Dr. Carlson is an employee of the company.
Ultrasound-facilitated endovascular thrombolysis 'a game changer' in acute pulmonary embolism
WASHINGTON – Ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive or submassive pulmonary embolism significantly improves right ventricular function, reduces pulmonary hypertension and angiographic evidence of obstruction, and lessens the risk of fibrinolysis-associated intracranial hemorrhage, according to a prospective multicenter clinical trial.
"By minimizing the risk of intracranial bleeding, ultrasound-facilitated, catheter-directed, low-dose fibrinolysis represents a potential game changer in the treatment of high-risk pulmonary embolism patients," Dr. Gregory Piazza said in presenting the results of the SEATTLE II study at the annual meeting of the American College of Cardiology.
Full-dose systemic fibrinolysis has long been the go-to advanced therapy for high-risk pulmonary embolism (PE), but physicians are leery of the associated 2%-3% risk of catastrophic intracranial hemorrhage, noted Dr. Piazza, a cardiologist at Brigham and Women’s Hospital and Harvard University, Boston.
SEATTLE II was a single-arm, 21-site, prospective study in which 150 patients with high-risk PE underwent treatment using the commercially available EKOS EkoSonic Endovascular System.
Twenty-one percent of patients had massive PE, defined as presentation with syncope, cardiogenic shock, resuscitated cardiac arrest, or persistent hypotension. The remaining 79% had submassive PE, with normal blood pressure but evidence of right ventricular dysfunction. All participants had to have a right ventricular/left ventricular ratio (RV/LV) of 0.9 or greater on the same chest CT scan used in diagnosing the PE. This CT documentation of right ventricular dysfunction has been associated in a meta-analysis of patients with submassive PE with a 7.4-fold increased risk of death due to PE compared to normotensive PE patients with normal right ventricular function (J. Thromb. Haemost. 2013;11:1823-32).
The primary endpoint was change in RV/LV on chest CT from baseline to 48 hours after initiation of fibrinolysis. This ratio improved from 1.55 to 1.13, for a statistically and clinically significant 27% reduction. A similar-size improvement was seen in pulmonary artery systolic pressure – a secondary efficacy endpoint – which decreased from 51.4 mm Hg before treatment to 37.5 mm Hg post procedure and 36.9 mm Hg at 48 hours. Both efficacy endpoints improved to a similar extent regardless of whether patients had massive or submassive PE.
The mean Modified Miller Pulmonary Artery Angiographic Obstruction Score improved by 30%, from 22.5 pretreatment to 15.8 at 48 hours.
Three in-hospital deaths occurred. One was due to massive PE which occurred before the fibrinolysis procedure could be completed. The others were not directly attributable to PE: One involved overwhelming sepsis and the other was due to progressive respiratory failure. Major bleeding occurred in 11% of patients; however, 16 of the 17 events were classified as GUSTO moderate bleeds, with only a single GUSTO severe hemorrhage.
There were no intracranial hemorrhages.
The fibrinolytic agent used in SEATTLE II was tissue plasminogen activator, delivered at 1 mg/hr for a total dose of 24 mg. Patients with unilateral PE received a single device and 24 hours of infusion time. The 86% of patients who had bilateral disease got two devices and 12 hours of therapy.
The proprietary EKOS system consists of two catheters: an outer infusion catheter with side holes that elute the fibrinolytic agent, and an inner core catheter with ultrasound transducers placed at regular intervals. These transducers produce low-intensity ultrasound which serves two purposes. Through a process called acoustic streaming, the low-intensity ultrasound helps push the fibrinolytic agent closer to the thrombus. Plus, the ultrasound energy causes the clot fibrin to reconfigure from a tight lattice to a more porous structure that promotes deeper penetration of the fibrinolytic, Dr. Piazza explained.
Audience members greeted the study results enthusiastically.
"Most of us really do believe that for safety reasons this is the way to go, rather than systemic fibrinolysis," one said.
Dr. Piazza said the next step in defining the EKOS system’s role in clinical practice will be to study briefer infusion times as a means of achieving faster patient improvement with reduced use of hospital resources, including ICU and intermediate-care unit time.
The EKOS system has been approved in the United States since 2005 for treatment of blood clots in the arms and legs. In Europe it gained an additional indication for treatment of massive and submassive PE in 2011.
The SEATTLE II study was sponsored by EKOS Corp. Dr. Piazza reported receiving a research grant from the company.
WASHINGTON – Ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive or submassive pulmonary embolism significantly improves right ventricular function, reduces pulmonary hypertension and angiographic evidence of obstruction, and lessens the risk of fibrinolysis-associated intracranial hemorrhage, according to a prospective multicenter clinical trial.
"By minimizing the risk of intracranial bleeding, ultrasound-facilitated, catheter-directed, low-dose fibrinolysis represents a potential game changer in the treatment of high-risk pulmonary embolism patients," Dr. Gregory Piazza said in presenting the results of the SEATTLE II study at the annual meeting of the American College of Cardiology.
Full-dose systemic fibrinolysis has long been the go-to advanced therapy for high-risk pulmonary embolism (PE), but physicians are leery of the associated 2%-3% risk of catastrophic intracranial hemorrhage, noted Dr. Piazza, a cardiologist at Brigham and Women’s Hospital and Harvard University, Boston.
SEATTLE II was a single-arm, 21-site, prospective study in which 150 patients with high-risk PE underwent treatment using the commercially available EKOS EkoSonic Endovascular System.
Twenty-one percent of patients had massive PE, defined as presentation with syncope, cardiogenic shock, resuscitated cardiac arrest, or persistent hypotension. The remaining 79% had submassive PE, with normal blood pressure but evidence of right ventricular dysfunction. All participants had to have a right ventricular/left ventricular ratio (RV/LV) of 0.9 or greater on the same chest CT scan used in diagnosing the PE. This CT documentation of right ventricular dysfunction has been associated in a meta-analysis of patients with submassive PE with a 7.4-fold increased risk of death due to PE compared to normotensive PE patients with normal right ventricular function (J. Thromb. Haemost. 2013;11:1823-32).
The primary endpoint was change in RV/LV on chest CT from baseline to 48 hours after initiation of fibrinolysis. This ratio improved from 1.55 to 1.13, for a statistically and clinically significant 27% reduction. A similar-size improvement was seen in pulmonary artery systolic pressure – a secondary efficacy endpoint – which decreased from 51.4 mm Hg before treatment to 37.5 mm Hg post procedure and 36.9 mm Hg at 48 hours. Both efficacy endpoints improved to a similar extent regardless of whether patients had massive or submassive PE.
The mean Modified Miller Pulmonary Artery Angiographic Obstruction Score improved by 30%, from 22.5 pretreatment to 15.8 at 48 hours.
Three in-hospital deaths occurred. One was due to massive PE which occurred before the fibrinolysis procedure could be completed. The others were not directly attributable to PE: One involved overwhelming sepsis and the other was due to progressive respiratory failure. Major bleeding occurred in 11% of patients; however, 16 of the 17 events were classified as GUSTO moderate bleeds, with only a single GUSTO severe hemorrhage.
There were no intracranial hemorrhages.
The fibrinolytic agent used in SEATTLE II was tissue plasminogen activator, delivered at 1 mg/hr for a total dose of 24 mg. Patients with unilateral PE received a single device and 24 hours of infusion time. The 86% of patients who had bilateral disease got two devices and 12 hours of therapy.
The proprietary EKOS system consists of two catheters: an outer infusion catheter with side holes that elute the fibrinolytic agent, and an inner core catheter with ultrasound transducers placed at regular intervals. These transducers produce low-intensity ultrasound which serves two purposes. Through a process called acoustic streaming, the low-intensity ultrasound helps push the fibrinolytic agent closer to the thrombus. Plus, the ultrasound energy causes the clot fibrin to reconfigure from a tight lattice to a more porous structure that promotes deeper penetration of the fibrinolytic, Dr. Piazza explained.
Audience members greeted the study results enthusiastically.
"Most of us really do believe that for safety reasons this is the way to go, rather than systemic fibrinolysis," one said.
Dr. Piazza said the next step in defining the EKOS system’s role in clinical practice will be to study briefer infusion times as a means of achieving faster patient improvement with reduced use of hospital resources, including ICU and intermediate-care unit time.
The EKOS system has been approved in the United States since 2005 for treatment of blood clots in the arms and legs. In Europe it gained an additional indication for treatment of massive and submassive PE in 2011.
The SEATTLE II study was sponsored by EKOS Corp. Dr. Piazza reported receiving a research grant from the company.
WASHINGTON – Ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive or submassive pulmonary embolism significantly improves right ventricular function, reduces pulmonary hypertension and angiographic evidence of obstruction, and lessens the risk of fibrinolysis-associated intracranial hemorrhage, according to a prospective multicenter clinical trial.
"By minimizing the risk of intracranial bleeding, ultrasound-facilitated, catheter-directed, low-dose fibrinolysis represents a potential game changer in the treatment of high-risk pulmonary embolism patients," Dr. Gregory Piazza said in presenting the results of the SEATTLE II study at the annual meeting of the American College of Cardiology.
Full-dose systemic fibrinolysis has long been the go-to advanced therapy for high-risk pulmonary embolism (PE), but physicians are leery of the associated 2%-3% risk of catastrophic intracranial hemorrhage, noted Dr. Piazza, a cardiologist at Brigham and Women’s Hospital and Harvard University, Boston.
SEATTLE II was a single-arm, 21-site, prospective study in which 150 patients with high-risk PE underwent treatment using the commercially available EKOS EkoSonic Endovascular System.
Twenty-one percent of patients had massive PE, defined as presentation with syncope, cardiogenic shock, resuscitated cardiac arrest, or persistent hypotension. The remaining 79% had submassive PE, with normal blood pressure but evidence of right ventricular dysfunction. All participants had to have a right ventricular/left ventricular ratio (RV/LV) of 0.9 or greater on the same chest CT scan used in diagnosing the PE. This CT documentation of right ventricular dysfunction has been associated in a meta-analysis of patients with submassive PE with a 7.4-fold increased risk of death due to PE compared to normotensive PE patients with normal right ventricular function (J. Thromb. Haemost. 2013;11:1823-32).
The primary endpoint was change in RV/LV on chest CT from baseline to 48 hours after initiation of fibrinolysis. This ratio improved from 1.55 to 1.13, for a statistically and clinically significant 27% reduction. A similar-size improvement was seen in pulmonary artery systolic pressure – a secondary efficacy endpoint – which decreased from 51.4 mm Hg before treatment to 37.5 mm Hg post procedure and 36.9 mm Hg at 48 hours. Both efficacy endpoints improved to a similar extent regardless of whether patients had massive or submassive PE.
The mean Modified Miller Pulmonary Artery Angiographic Obstruction Score improved by 30%, from 22.5 pretreatment to 15.8 at 48 hours.
Three in-hospital deaths occurred. One was due to massive PE which occurred before the fibrinolysis procedure could be completed. The others were not directly attributable to PE: One involved overwhelming sepsis and the other was due to progressive respiratory failure. Major bleeding occurred in 11% of patients; however, 16 of the 17 events were classified as GUSTO moderate bleeds, with only a single GUSTO severe hemorrhage.
There were no intracranial hemorrhages.
The fibrinolytic agent used in SEATTLE II was tissue plasminogen activator, delivered at 1 mg/hr for a total dose of 24 mg. Patients with unilateral PE received a single device and 24 hours of infusion time. The 86% of patients who had bilateral disease got two devices and 12 hours of therapy.
The proprietary EKOS system consists of two catheters: an outer infusion catheter with side holes that elute the fibrinolytic agent, and an inner core catheter with ultrasound transducers placed at regular intervals. These transducers produce low-intensity ultrasound which serves two purposes. Through a process called acoustic streaming, the low-intensity ultrasound helps push the fibrinolytic agent closer to the thrombus. Plus, the ultrasound energy causes the clot fibrin to reconfigure from a tight lattice to a more porous structure that promotes deeper penetration of the fibrinolytic, Dr. Piazza explained.
Audience members greeted the study results enthusiastically.
"Most of us really do believe that for safety reasons this is the way to go, rather than systemic fibrinolysis," one said.
Dr. Piazza said the next step in defining the EKOS system’s role in clinical practice will be to study briefer infusion times as a means of achieving faster patient improvement with reduced use of hospital resources, including ICU and intermediate-care unit time.
The EKOS system has been approved in the United States since 2005 for treatment of blood clots in the arms and legs. In Europe it gained an additional indication for treatment of massive and submassive PE in 2011.
The SEATTLE II study was sponsored by EKOS Corp. Dr. Piazza reported receiving a research grant from the company.
AT ACC 14
Major finding: Ultrasound-facilitated, catheter-directed, low-dose fibrinolysis in patients with high-risk pulmonary embolism improved right ventricular function and reduced pulmonary hypertension with no intracranial hemorrhages or fatal bleeding events.
Data source: SEATTLE II, a multicenter, single-arm, prospective study of 150 patients with massive or submassive pulmonary embolism.
Disclosures: The study was sponsored by EKOS Corp. The presenter received a research grant from the company.
Off-label use of novel anticoagulants accelerates
WASHINGTON – The off-label use of novel oral anticoagulants for stroke prevention in patients with valvular atrial fibrillation has climbed steeply since the drugs reached the marketplace, mirroring the medications’ rapid adoption for the approved indication of preventing strokes in nonvalvular AF, according to Dr. Sandeep Mahrendra Jani*.
An analysis of 190,227 nonvalvular atrial fibrillation (NVAF) patients in 95 practices participating in the American College of Cardiology’s National Cardiovascular Data Registry – PINNACLE Registry – showed that during the first quarter of 2011, just 4.8% were on dabigatran, the sole novel oral anticoagulant then available. By the fourth quarter of 2012, however, 14.9% of NVAF patients were on a novel oral anticoagulant, either dabigatran or the subsequently approved rivaroxiban, he reported at the annual meeting of the ACC.
Similarly, among 2,142 registry participants with valvular atrial fibrillation (AF), the use of any novel oral anticoagulant shot up from 2.7% in the first quarter of 2011 to 13.8% in the fourth quarter of 2012, noted Dr. Jani of Medstar Washington (D.C.) Hospital Center.
During this time – prior to the arrival of apixiban on the market – the use of warfarin for stroke prevention in patients with NVAF declined from 47.9% to 44.3%. Among patients with valvular atrial fibrillation, the prevalence of warfarin therapy fell from 65.8% in the first quarter of 2011 to 60.1% in fourth quarter 2012.
During the first quarter of 2011, 51.2% of all patients with NVAF and 66.4% with valvular AF were on any oral anticoagulant. By fourth quarter 2012, these rates had increased to 56.9% and 66.8%, respectively.
The use of dabigatran in patients with valvular AF took a hit in late 2012 in response to the premature halt of the RE-ALIGN (Dabigatran Etexilate in Patients With Mechanical Heart Valves) trial, followed by the Food and Drug Administration’s warning against using dabigatran in patients with mechanical heart valves. Dabigatran was used by 2.7% of valvular AF patients in the first quarter of 2011, rising steadily to 12.1% by the third quarter of 2012, then plunging to just 1.4% in the year’s final quarter.
In light of the rapidly accelerating use of novel oral anticoagulants in patients with valvular AF, despite a lack of evidence of efficacy for stroke prevention in this setting, further studies are a priority, Dr. Jani said.
The PINNACLE registry is funded by the ACC, with founding sponsorship provided by Bristol-Myers Squibb and Pfizer. Dr. Jani reported having no relevant financial conflicts.
*Correction, 4/29/2014: An earlier version of this article misstated the name of study author Dr. Sandeep Mahrendra Jani.
WASHINGTON – The off-label use of novel oral anticoagulants for stroke prevention in patients with valvular atrial fibrillation has climbed steeply since the drugs reached the marketplace, mirroring the medications’ rapid adoption for the approved indication of preventing strokes in nonvalvular AF, according to Dr. Sandeep Mahrendra Jani*.
An analysis of 190,227 nonvalvular atrial fibrillation (NVAF) patients in 95 practices participating in the American College of Cardiology’s National Cardiovascular Data Registry – PINNACLE Registry – showed that during the first quarter of 2011, just 4.8% were on dabigatran, the sole novel oral anticoagulant then available. By the fourth quarter of 2012, however, 14.9% of NVAF patients were on a novel oral anticoagulant, either dabigatran or the subsequently approved rivaroxiban, he reported at the annual meeting of the ACC.
Similarly, among 2,142 registry participants with valvular atrial fibrillation (AF), the use of any novel oral anticoagulant shot up from 2.7% in the first quarter of 2011 to 13.8% in the fourth quarter of 2012, noted Dr. Jani of Medstar Washington (D.C.) Hospital Center.
During this time – prior to the arrival of apixiban on the market – the use of warfarin for stroke prevention in patients with NVAF declined from 47.9% to 44.3%. Among patients with valvular atrial fibrillation, the prevalence of warfarin therapy fell from 65.8% in the first quarter of 2011 to 60.1% in fourth quarter 2012.
During the first quarter of 2011, 51.2% of all patients with NVAF and 66.4% with valvular AF were on any oral anticoagulant. By fourth quarter 2012, these rates had increased to 56.9% and 66.8%, respectively.
The use of dabigatran in patients with valvular AF took a hit in late 2012 in response to the premature halt of the RE-ALIGN (Dabigatran Etexilate in Patients With Mechanical Heart Valves) trial, followed by the Food and Drug Administration’s warning against using dabigatran in patients with mechanical heart valves. Dabigatran was used by 2.7% of valvular AF patients in the first quarter of 2011, rising steadily to 12.1% by the third quarter of 2012, then plunging to just 1.4% in the year’s final quarter.
In light of the rapidly accelerating use of novel oral anticoagulants in patients with valvular AF, despite a lack of evidence of efficacy for stroke prevention in this setting, further studies are a priority, Dr. Jani said.
The PINNACLE registry is funded by the ACC, with founding sponsorship provided by Bristol-Myers Squibb and Pfizer. Dr. Jani reported having no relevant financial conflicts.
*Correction, 4/29/2014: An earlier version of this article misstated the name of study author Dr. Sandeep Mahrendra Jani.
WASHINGTON – The off-label use of novel oral anticoagulants for stroke prevention in patients with valvular atrial fibrillation has climbed steeply since the drugs reached the marketplace, mirroring the medications’ rapid adoption for the approved indication of preventing strokes in nonvalvular AF, according to Dr. Sandeep Mahrendra Jani*.
An analysis of 190,227 nonvalvular atrial fibrillation (NVAF) patients in 95 practices participating in the American College of Cardiology’s National Cardiovascular Data Registry – PINNACLE Registry – showed that during the first quarter of 2011, just 4.8% were on dabigatran, the sole novel oral anticoagulant then available. By the fourth quarter of 2012, however, 14.9% of NVAF patients were on a novel oral anticoagulant, either dabigatran or the subsequently approved rivaroxiban, he reported at the annual meeting of the ACC.
Similarly, among 2,142 registry participants with valvular atrial fibrillation (AF), the use of any novel oral anticoagulant shot up from 2.7% in the first quarter of 2011 to 13.8% in the fourth quarter of 2012, noted Dr. Jani of Medstar Washington (D.C.) Hospital Center.
During this time – prior to the arrival of apixiban on the market – the use of warfarin for stroke prevention in patients with NVAF declined from 47.9% to 44.3%. Among patients with valvular atrial fibrillation, the prevalence of warfarin therapy fell from 65.8% in the first quarter of 2011 to 60.1% in fourth quarter 2012.
During the first quarter of 2011, 51.2% of all patients with NVAF and 66.4% with valvular AF were on any oral anticoagulant. By fourth quarter 2012, these rates had increased to 56.9% and 66.8%, respectively.
The use of dabigatran in patients with valvular AF took a hit in late 2012 in response to the premature halt of the RE-ALIGN (Dabigatran Etexilate in Patients With Mechanical Heart Valves) trial, followed by the Food and Drug Administration’s warning against using dabigatran in patients with mechanical heart valves. Dabigatran was used by 2.7% of valvular AF patients in the first quarter of 2011, rising steadily to 12.1% by the third quarter of 2012, then plunging to just 1.4% in the year’s final quarter.
In light of the rapidly accelerating use of novel oral anticoagulants in patients with valvular AF, despite a lack of evidence of efficacy for stroke prevention in this setting, further studies are a priority, Dr. Jani said.
The PINNACLE registry is funded by the ACC, with founding sponsorship provided by Bristol-Myers Squibb and Pfizer. Dr. Jani reported having no relevant financial conflicts.
*Correction, 4/29/2014: An earlier version of this article misstated the name of study author Dr. Sandeep Mahrendra Jani.
AT ACC 14
Major finding: By the fourth quarter of 2012, 14.9% of patients with nonvalvular AF were on a novel anticoagulant. So were 13.8% of those with valvular AF, even though this is an off-label use of these drugs.
Data source: This study involved more than 190,000 patients with nonvalvular AF and 2,142 with valvular AF in 95 practices participating in the PINNACLE Registry.
Disclosures The PINNACLE Registry is funded by the ACC’s National Cardiovascular Data Registry. The presenter reported having no relevant financial conflicts.
'Favorable signal' for secondary coronary prevention in STABILITY
WASHINGTON – A novel pharmacologic approach to the prevention of ischemic events in patients with stable coronary heart disease failed to reduce the combined risk of cardiovascular death, acute myocardial infarction, or stroke in a nearly 16,000-patient megatrial known as STABILITY.
That being said, darapladib, the oral inhibitor of lipoprotein-associated phospholipase A2 (Lp-PLA2) under study, did significantly reduce the rate of the prespecified secondary endpoint of major coronary events, compared with placebo. And that’s an encouraging finding, Dr. Harvey D. White said at the annual meeting of the American College of Cardiology.
"We believe that there is a signal here on coronary events. This is the first large trial of a new treatment addressing a novel mechanism of inhibition of inflammation in the atherosclerotic plaque, and we believe this signal may be very important for patient care," said Dr. White, director of coronary care and cardiovascular research at Auckland (New Zealand) City Hospital.
Patients with stable coronary heart disease (CHD) constitute a population with an unmet need, as evidenced by the substantial clinical event rate in the placebo group, he added.
Lp-PLA2 is produced by white blood cells and macrophages and carried by low-density lipoprotein (LDL) cholesterol. Within an atheroma, Lp-PLA2 increases production of inflammatory products associated with necrotic core expansion. Stable atheromatous plaques contain little Lp-PLA2, but vulnerable or ruptured plaques contain it in high concentrations. A strong association between Lp-PLA2 activity and the risk of CHD has been shown in several dozen studies (Lancet 2010;375:1536-44). And darapladib reduces Lp-PLA2 levels by roughly 60%. In coronary imaging studies, the drug halts progression of coronary artery necrotic plaque core volume, the cardiologist noted.
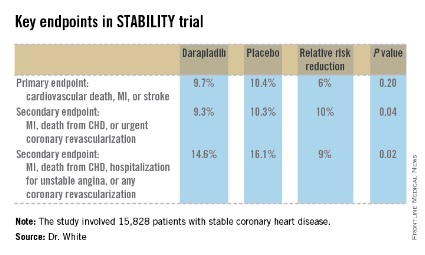
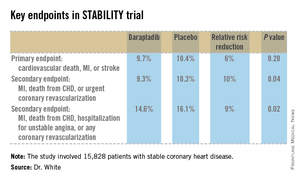
STABILITY (the Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) was a 39-nation clinical trial that included 15,828 patients with stable CHD who were randomized to receive once-daily darapladib at 160 mg or placebo and then followed prospectively for a median of 3.7 years. The combined rate of cardiovascular death, myocardial infarction, or stroke in patients randomized to darapladib was 9.7%, yielding a nonsignificant 6% relative risk reduction, compared with placebo. There was a consistent benefit favoring darapladib across all coronary-specific endpoints (see chart).
Diarrhea was significantly more common in the darapladib group. Three percent of patients randomized to darapladib discontinued treatment due to diarrhea, another 2% because of abnormal feces, 2% due to abnormal skin odor, and 1% for abnormal-smelling urine. This is believed to be a consequence of the sulfhydryl group in the drug molecule.
The STABILITY trial was hampered in that the study was designed before epidemiologic evidence showed Lp-PLA2 levels are unrelated to stroke risk. Yet stroke was a component of the primary endpoint. Also, the rate of background guideline-directed medical therapy in STABILITY participants was greater than in any previous secondary prevention trial: 93% of subjects were on aspirin and 97% on a statin – and statin therapy in and of itself has been shown to reduce Lp-PLA2 by up to 35%. One-third of STABILITY patients had a baseline LDL below 70 mg/dL, and only one-quarter had an LDL above 100 mg/dL.
In addition, three-quarters of subjects had previously undergone coronary revascularization, with one-third of participants having had coronary artery bypass surgery.
"This high rate of optimized background therapy may have made it hard to modulate events with darapladib," Dr. White observed.
Baseline levels of Lp-PLA2 are still being collected and will be reported later. Planned secondary analyses will assess whether darapladib has a more pronounced benefit in patients with high baseline Lp-PLA2.
A sister study known as SOLID, being led by the Harvard University–based TIMI group, will report results later this year. SOLID is evaluating darapladib for the prevention of ischemic events in patients started on the drug within 1 month after an acute coronary syndrome rather than in patients with stable coronary disease as in STABILITY.
"We can’t guess the results, but you could hypothesize that since there’s more inflammation in the cardiac core when the plaque is disrupted, SOLID will provide very interesting data," the cardiologist said.
Simultaneous with Dr. White’s presentation at ACC 14, the STABILITY results were published online at NEJM.org (doi: 10.1056/NEJMoa1315878).
STABILITY was sponsored by GlaxoSmithKline. Dr. White reported receiving research grant and travel support from the company.
WASHINGTON – A novel pharmacologic approach to the prevention of ischemic events in patients with stable coronary heart disease failed to reduce the combined risk of cardiovascular death, acute myocardial infarction, or stroke in a nearly 16,000-patient megatrial known as STABILITY.
That being said, darapladib, the oral inhibitor of lipoprotein-associated phospholipase A2 (Lp-PLA2) under study, did significantly reduce the rate of the prespecified secondary endpoint of major coronary events, compared with placebo. And that’s an encouraging finding, Dr. Harvey D. White said at the annual meeting of the American College of Cardiology.

"We believe that there is a signal here on coronary events. This is the first large trial of a new treatment addressing a novel mechanism of inhibition of inflammation in the atherosclerotic plaque, and we believe this signal may be very important for patient care," said Dr. White, director of coronary care and cardiovascular research at Auckland (New Zealand) City Hospital.
Patients with stable coronary heart disease (CHD) constitute a population with an unmet need, as evidenced by the substantial clinical event rate in the placebo group, he added.
Lp-PLA2 is produced by white blood cells and macrophages and carried by low-density lipoprotein (LDL) cholesterol. Within an atheroma, Lp-PLA2 increases production of inflammatory products associated with necrotic core expansion. Stable atheromatous plaques contain little Lp-PLA2, but vulnerable or ruptured plaques contain it in high concentrations. A strong association between Lp-PLA2 activity and the risk of CHD has been shown in several dozen studies (Lancet 2010;375:1536-44). And darapladib reduces Lp-PLA2 levels by roughly 60%. In coronary imaging studies, the drug halts progression of coronary artery necrotic plaque core volume, the cardiologist noted.
STABILITY (the Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) was a 39-nation clinical trial that included 15,828 patients with stable CHD who were randomized to receive once-daily darapladib at 160 mg or placebo and then followed prospectively for a median of 3.7 years. The combined rate of cardiovascular death, myocardial infarction, or stroke in patients randomized to darapladib was 9.7%, yielding a nonsignificant 6% relative risk reduction, compared with placebo. There was a consistent benefit favoring darapladib across all coronary-specific endpoints (see chart).
Diarrhea was significantly more common in the darapladib group. Three percent of patients randomized to darapladib discontinued treatment due to diarrhea, another 2% because of abnormal feces, 2% due to abnormal skin odor, and 1% for abnormal-smelling urine. This is believed to be a consequence of the sulfhydryl group in the drug molecule.
The STABILITY trial was hampered in that the study was designed before epidemiologic evidence showed Lp-PLA2 levels are unrelated to stroke risk. Yet stroke was a component of the primary endpoint. Also, the rate of background guideline-directed medical therapy in STABILITY participants was greater than in any previous secondary prevention trial: 93% of subjects were on aspirin and 97% on a statin – and statin therapy in and of itself has been shown to reduce Lp-PLA2 by up to 35%. One-third of STABILITY patients had a baseline LDL below 70 mg/dL, and only one-quarter had an LDL above 100 mg/dL.
In addition, three-quarters of subjects had previously undergone coronary revascularization, with one-third of participants having had coronary artery bypass surgery.
"This high rate of optimized background therapy may have made it hard to modulate events with darapladib," Dr. White observed.
Baseline levels of Lp-PLA2 are still being collected and will be reported later. Planned secondary analyses will assess whether darapladib has a more pronounced benefit in patients with high baseline Lp-PLA2.
A sister study known as SOLID, being led by the Harvard University–based TIMI group, will report results later this year. SOLID is evaluating darapladib for the prevention of ischemic events in patients started on the drug within 1 month after an acute coronary syndrome rather than in patients with stable coronary disease as in STABILITY.
"We can’t guess the results, but you could hypothesize that since there’s more inflammation in the cardiac core when the plaque is disrupted, SOLID will provide very interesting data," the cardiologist said.
Simultaneous with Dr. White’s presentation at ACC 14, the STABILITY results were published online at NEJM.org (doi: 10.1056/NEJMoa1315878).
STABILITY was sponsored by GlaxoSmithKline. Dr. White reported receiving research grant and travel support from the company.
WASHINGTON – A novel pharmacologic approach to the prevention of ischemic events in patients with stable coronary heart disease failed to reduce the combined risk of cardiovascular death, acute myocardial infarction, or stroke in a nearly 16,000-patient megatrial known as STABILITY.
That being said, darapladib, the oral inhibitor of lipoprotein-associated phospholipase A2 (Lp-PLA2) under study, did significantly reduce the rate of the prespecified secondary endpoint of major coronary events, compared with placebo. And that’s an encouraging finding, Dr. Harvey D. White said at the annual meeting of the American College of Cardiology.

"We believe that there is a signal here on coronary events. This is the first large trial of a new treatment addressing a novel mechanism of inhibition of inflammation in the atherosclerotic plaque, and we believe this signal may be very important for patient care," said Dr. White, director of coronary care and cardiovascular research at Auckland (New Zealand) City Hospital.
Patients with stable coronary heart disease (CHD) constitute a population with an unmet need, as evidenced by the substantial clinical event rate in the placebo group, he added.
Lp-PLA2 is produced by white blood cells and macrophages and carried by low-density lipoprotein (LDL) cholesterol. Within an atheroma, Lp-PLA2 increases production of inflammatory products associated with necrotic core expansion. Stable atheromatous plaques contain little Lp-PLA2, but vulnerable or ruptured plaques contain it in high concentrations. A strong association between Lp-PLA2 activity and the risk of CHD has been shown in several dozen studies (Lancet 2010;375:1536-44). And darapladib reduces Lp-PLA2 levels by roughly 60%. In coronary imaging studies, the drug halts progression of coronary artery necrotic plaque core volume, the cardiologist noted.
STABILITY (the Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) was a 39-nation clinical trial that included 15,828 patients with stable CHD who were randomized to receive once-daily darapladib at 160 mg or placebo and then followed prospectively for a median of 3.7 years. The combined rate of cardiovascular death, myocardial infarction, or stroke in patients randomized to darapladib was 9.7%, yielding a nonsignificant 6% relative risk reduction, compared with placebo. There was a consistent benefit favoring darapladib across all coronary-specific endpoints (see chart).
Diarrhea was significantly more common in the darapladib group. Three percent of patients randomized to darapladib discontinued treatment due to diarrhea, another 2% because of abnormal feces, 2% due to abnormal skin odor, and 1% for abnormal-smelling urine. This is believed to be a consequence of the sulfhydryl group in the drug molecule.
The STABILITY trial was hampered in that the study was designed before epidemiologic evidence showed Lp-PLA2 levels are unrelated to stroke risk. Yet stroke was a component of the primary endpoint. Also, the rate of background guideline-directed medical therapy in STABILITY participants was greater than in any previous secondary prevention trial: 93% of subjects were on aspirin and 97% on a statin – and statin therapy in and of itself has been shown to reduce Lp-PLA2 by up to 35%. One-third of STABILITY patients had a baseline LDL below 70 mg/dL, and only one-quarter had an LDL above 100 mg/dL.
In addition, three-quarters of subjects had previously undergone coronary revascularization, with one-third of participants having had coronary artery bypass surgery.
"This high rate of optimized background therapy may have made it hard to modulate events with darapladib," Dr. White observed.
Baseline levels of Lp-PLA2 are still being collected and will be reported later. Planned secondary analyses will assess whether darapladib has a more pronounced benefit in patients with high baseline Lp-PLA2.
A sister study known as SOLID, being led by the Harvard University–based TIMI group, will report results later this year. SOLID is evaluating darapladib for the prevention of ischemic events in patients started on the drug within 1 month after an acute coronary syndrome rather than in patients with stable coronary disease as in STABILITY.
"We can’t guess the results, but you could hypothesize that since there’s more inflammation in the cardiac core when the plaque is disrupted, SOLID will provide very interesting data," the cardiologist said.
Simultaneous with Dr. White’s presentation at ACC 14, the STABILITY results were published online at NEJM.org (doi: 10.1056/NEJMoa1315878).
STABILITY was sponsored by GlaxoSmithKline. Dr. White reported receiving research grant and travel support from the company.
AT ACC 14
Major finding: The combined rate of cardiovascular death, myocardial infarction, or stroke in patients randomized to darapladib was 9.7%, yielding a nonsignificant 6% relative risk reduction, compared with placebo. But a significant reduction was seen in major coronary events.
Data source: STABILITY, a randomized, double-blind study in which 15,828 patients with stable coronary heart disease were followed on darapladib or placebo for a median of 3.7 years.
Disclosures: STABILITY was sponsored by GlaxoSmithKline. Dr. White reported receiving research grant and travel support from the company.
Symple is as simple does
After the surprising, top-line result from the SYMPLICITY HTN-3 trial came out in a press release from Medtronic last January, the question remained of what went wrong: Why did renal denervation fail to outperform a sham procedure in reducing blood pressure?
The apparent answer emerged in late March when the full results finally went public in a report at the annual meeting of the American College of Cardiology and in an article in the New England Journal of Medicine. Many of the 364 patients who underwent active denervation – which involves zapping the efferent nerves that run along the outer wall of the renal arteries with a few brief pulses of radiofrequency energy – probably failed to receive adequate treatment, so their renal innervation remained mostly intact. There’s no proof that’s what happened, but it seems plausible given that all the U.S. operators in the trial had no prior experience performing the procedure, as well as the observation several years ago that denervation can produce highly variable results and is very operator dependent.
This variability in success had been documented back in the 2000s by one of the pioneers of renal denervation, Dr. Murray Esler of Melbourne, yet the people who designed SYMPLICITY HTN-3 didn’t pay attention. Their failure to apply what earlier findings had taught about the variability of denervation proved especially egregious, as the interventionalists also couldn’t gauge their procedural success because no easy way exists right now to do this.
But these details didn’t slow the first controlled clinical trial. The concept was so ... simple: Insert catheter into renal artery, throw switch and zap, remove catheter. Easy peasy.
I first heard about renal denervation more than 2 years ago, and marveled at the unmitigated hubris to name the first catheter developed for denervation Symplicity, as well as giving that moniker to a series of uncontrolled and controlled studies that tested the technique. The Symplicity crowd seemed very sure of themselves, of this catheter, and of this procedure.
Fast forward a couple of years and the name morphs into the ironic butt of an expensive, failed trial.
If there is anything I’ve learned during more than 3 decades of covering medicine, it’s that the discipline is hardly ever simple. Think of signaling-pathway diagrams, the ones with all the arrows, boxes, and small fonts. The most reliable reaction when confronted in medicine by something that appears simple is to ask: What am I missing here? Hopefully, when this still-promising technology resurrects, its developers will have learned that lesson.
On Twitter @mitchelzoler
After the surprising, top-line result from the SYMPLICITY HTN-3 trial came out in a press release from Medtronic last January, the question remained of what went wrong: Why did renal denervation fail to outperform a sham procedure in reducing blood pressure?
The apparent answer emerged in late March when the full results finally went public in a report at the annual meeting of the American College of Cardiology and in an article in the New England Journal of Medicine. Many of the 364 patients who underwent active denervation – which involves zapping the efferent nerves that run along the outer wall of the renal arteries with a few brief pulses of radiofrequency energy – probably failed to receive adequate treatment, so their renal innervation remained mostly intact. There’s no proof that’s what happened, but it seems plausible given that all the U.S. operators in the trial had no prior experience performing the procedure, as well as the observation several years ago that denervation can produce highly variable results and is very operator dependent.
This variability in success had been documented back in the 2000s by one of the pioneers of renal denervation, Dr. Murray Esler of Melbourne, yet the people who designed SYMPLICITY HTN-3 didn’t pay attention. Their failure to apply what earlier findings had taught about the variability of denervation proved especially egregious, as the interventionalists also couldn’t gauge their procedural success because no easy way exists right now to do this.
But these details didn’t slow the first controlled clinical trial. The concept was so ... simple: Insert catheter into renal artery, throw switch and zap, remove catheter. Easy peasy.
I first heard about renal denervation more than 2 years ago, and marveled at the unmitigated hubris to name the first catheter developed for denervation Symplicity, as well as giving that moniker to a series of uncontrolled and controlled studies that tested the technique. The Symplicity crowd seemed very sure of themselves, of this catheter, and of this procedure.
Fast forward a couple of years and the name morphs into the ironic butt of an expensive, failed trial.
If there is anything I’ve learned during more than 3 decades of covering medicine, it’s that the discipline is hardly ever simple. Think of signaling-pathway diagrams, the ones with all the arrows, boxes, and small fonts. The most reliable reaction when confronted in medicine by something that appears simple is to ask: What am I missing here? Hopefully, when this still-promising technology resurrects, its developers will have learned that lesson.
On Twitter @mitchelzoler
After the surprising, top-line result from the SYMPLICITY HTN-3 trial came out in a press release from Medtronic last January, the question remained of what went wrong: Why did renal denervation fail to outperform a sham procedure in reducing blood pressure?
The apparent answer emerged in late March when the full results finally went public in a report at the annual meeting of the American College of Cardiology and in an article in the New England Journal of Medicine. Many of the 364 patients who underwent active denervation – which involves zapping the efferent nerves that run along the outer wall of the renal arteries with a few brief pulses of radiofrequency energy – probably failed to receive adequate treatment, so their renal innervation remained mostly intact. There’s no proof that’s what happened, but it seems plausible given that all the U.S. operators in the trial had no prior experience performing the procedure, as well as the observation several years ago that denervation can produce highly variable results and is very operator dependent.
This variability in success had been documented back in the 2000s by one of the pioneers of renal denervation, Dr. Murray Esler of Melbourne, yet the people who designed SYMPLICITY HTN-3 didn’t pay attention. Their failure to apply what earlier findings had taught about the variability of denervation proved especially egregious, as the interventionalists also couldn’t gauge their procedural success because no easy way exists right now to do this.
But these details didn’t slow the first controlled clinical trial. The concept was so ... simple: Insert catheter into renal artery, throw switch and zap, remove catheter. Easy peasy.
I first heard about renal denervation more than 2 years ago, and marveled at the unmitigated hubris to name the first catheter developed for denervation Symplicity, as well as giving that moniker to a series of uncontrolled and controlled studies that tested the technique. The Symplicity crowd seemed very sure of themselves, of this catheter, and of this procedure.
Fast forward a couple of years and the name morphs into the ironic butt of an expensive, failed trial.
If there is anything I’ve learned during more than 3 decades of covering medicine, it’s that the discipline is hardly ever simple. Think of signaling-pathway diagrams, the ones with all the arrows, boxes, and small fonts. The most reliable reaction when confronted in medicine by something that appears simple is to ask: What am I missing here? Hopefully, when this still-promising technology resurrects, its developers will have learned that lesson.
On Twitter @mitchelzoler
VIDEO: PCSK9 inhibitors placed in perspective
WASHINGTON – "We’re at the dawn of an era of biological therapeutics in cardiology," Dr. Peter Libby, chief of cardiovascular medicine at the Brigham and Women’s Hospital in Boston, told us at the annual meeting of the American College of Cardiology, as he provided the lowdown on the potentially game-changing novel class of LDL cholesterol–lowering medications known as PCSK9 inhibitors.
One such agent, evolocumab, was the focus of five highly positive phase III clinical trials presented at ACC 14.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WASHINGTON – "We’re at the dawn of an era of biological therapeutics in cardiology," Dr. Peter Libby, chief of cardiovascular medicine at the Brigham and Women’s Hospital in Boston, told us at the annual meeting of the American College of Cardiology, as he provided the lowdown on the potentially game-changing novel class of LDL cholesterol–lowering medications known as PCSK9 inhibitors.
One such agent, evolocumab, was the focus of five highly positive phase III clinical trials presented at ACC 14.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WASHINGTON – "We’re at the dawn of an era of biological therapeutics in cardiology," Dr. Peter Libby, chief of cardiovascular medicine at the Brigham and Women’s Hospital in Boston, told us at the annual meeting of the American College of Cardiology, as he provided the lowdown on the potentially game-changing novel class of LDL cholesterol–lowering medications known as PCSK9 inhibitors.
One such agent, evolocumab, was the focus of five highly positive phase III clinical trials presented at ACC 14.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ACC 14
VIDEO: Exploring SYMPLICITY’s failure, Part 2
WASHINGTON – Far from being doomed after the failure SYMPLICITY HTN-3, renal denervation for refractory hypertension will continue to be explored. But it can’t go very far without finding a way to measure its effectiveness.
In Part 2 of our interview, Dr. Prakash Deedwania and Dr. George Bakris explore the options, and don’t miss the chance to talk about the controversial new hypertension guidelines.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
WASHINGTON – Far from being doomed after the failure SYMPLICITY HTN-3, renal denervation for refractory hypertension will continue to be explored. But it can’t go very far without finding a way to measure its effectiveness.
In Part 2 of our interview, Dr. Prakash Deedwania and Dr. George Bakris explore the options, and don’t miss the chance to talk about the controversial new hypertension guidelines.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
WASHINGTON – Far from being doomed after the failure SYMPLICITY HTN-3, renal denervation for refractory hypertension will continue to be explored. But it can’t go very far without finding a way to measure its effectiveness.
In Part 2 of our interview, Dr. Prakash Deedwania and Dr. George Bakris explore the options, and don’t miss the chance to talk about the controversial new hypertension guidelines.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
AT ACC 14
How to spend 2 days as a cognitive cardiologist
WASHINGTON – Another annual meeting of the American College of Cardiology has drawn to a close, and aside from the seemingly endless rain (and brief snow flurries), a lot of interesting discourse took place.
In the heart failure discipline, we have to a large degree become incrementalists: Although there were no blockbusters, important data on renal denervation were released, and corridor discussions about serelaxin and ivabradine were animated. At a time of ICD-10 rollouts, electronic health record (EHR) struggles, board recertification challenges, accreditation, precertification, compliance mandates, and more, it was a delight to be a cognitive cardiologist again. For a few precious moments, we could own our profession again.
We saw no hospital administrators; we did not receive any missives about the (lack of) timeliness of closing encounters in EHRs; we engaged in real peer-to-peer interactions with real peers, unlike the shadow variant we deal with during an appeal of an insurance coverage decision.
And then it was over, ironically with the sun shining. It’s now back to work, but perhaps with renewed purpose. Let’s find ways to reestablish facts on the ground: Our patients and the decisions we make about our patients come first. Research and teaching do, too. Hospital administrators? Send them to a very long annual meeting. And hope that they forget to pack umbrellas.
Dr. Hauptman is professor of internal medicine and assistant dean of clinical-translational research at Saint Louis University and director of heart failure at Saint Louis University Hospital.
WASHINGTON – Another annual meeting of the American College of Cardiology has drawn to a close, and aside from the seemingly endless rain (and brief snow flurries), a lot of interesting discourse took place.
In the heart failure discipline, we have to a large degree become incrementalists: Although there were no blockbusters, important data on renal denervation were released, and corridor discussions about serelaxin and ivabradine were animated. At a time of ICD-10 rollouts, electronic health record (EHR) struggles, board recertification challenges, accreditation, precertification, compliance mandates, and more, it was a delight to be a cognitive cardiologist again. For a few precious moments, we could own our profession again.
We saw no hospital administrators; we did not receive any missives about the (lack of) timeliness of closing encounters in EHRs; we engaged in real peer-to-peer interactions with real peers, unlike the shadow variant we deal with during an appeal of an insurance coverage decision.
And then it was over, ironically with the sun shining. It’s now back to work, but perhaps with renewed purpose. Let’s find ways to reestablish facts on the ground: Our patients and the decisions we make about our patients come first. Research and teaching do, too. Hospital administrators? Send them to a very long annual meeting. And hope that they forget to pack umbrellas.
Dr. Hauptman is professor of internal medicine and assistant dean of clinical-translational research at Saint Louis University and director of heart failure at Saint Louis University Hospital.
WASHINGTON – Another annual meeting of the American College of Cardiology has drawn to a close, and aside from the seemingly endless rain (and brief snow flurries), a lot of interesting discourse took place.
In the heart failure discipline, we have to a large degree become incrementalists: Although there were no blockbusters, important data on renal denervation were released, and corridor discussions about serelaxin and ivabradine were animated. At a time of ICD-10 rollouts, electronic health record (EHR) struggles, board recertification challenges, accreditation, precertification, compliance mandates, and more, it was a delight to be a cognitive cardiologist again. For a few precious moments, we could own our profession again.
We saw no hospital administrators; we did not receive any missives about the (lack of) timeliness of closing encounters in EHRs; we engaged in real peer-to-peer interactions with real peers, unlike the shadow variant we deal with during an appeal of an insurance coverage decision.
And then it was over, ironically with the sun shining. It’s now back to work, but perhaps with renewed purpose. Let’s find ways to reestablish facts on the ground: Our patients and the decisions we make about our patients come first. Research and teaching do, too. Hospital administrators? Send them to a very long annual meeting. And hope that they forget to pack umbrellas.
Dr. Hauptman is professor of internal medicine and assistant dean of clinical-translational research at Saint Louis University and director of heart failure at Saint Louis University Hospital.