User login
Point/Counterpoint: Does Surgery Improve Survival in Stage IV Breast Cancer?
Yes – It Is Time to Consider a Paradigm Shift.
By Dr. Seema Khan
In stage IV breast cancer, local therapy is not considered important except for controlling symptoms. But recent large-scale retrospective studies tell us that, if you follow enough women long enough, you see a difference in survival related to control of local disease. These emerging data tell us it’s time to consider a paradigm shift in the treatment of stage IV breast cancer.
Researchers are finding that primary site treatment regimens are important because an intact primary tumor can serve as a continued source of new metastatic lesions. There is reason, therefore, to believe that treating these can prolong life.
First explored by Dr. Larry Norton and Joan Massagué, Ph.D., (Nature Med. 2006;12:875-8), the concept of cancer self-seeding holds that cells from the primary tumor not only travel unidirectionally to seed metastases but also return to the primary tumor, self-seeding it and helping it to grow even more.
This view incorporates the microenvironment of the primary tumor. Here, fibroblasts and other cells – particularly mesenchymal stem cells derived from bone marrow – may play a very important role, creating a reactive stroma that seems to release growth-promoting substances that increase the metastatic efficiency of the circulating cells.
Robert A. Weinberg, Ph.D., published data showing that these mesenchymal stem cells are pluripotent progenitor cells. When weakly metastatic human breast cancer cells were mixed with these stem cells, the cancer cells’ metastatic potential greatly increased. The breast cancer cells then stimulated the stem cells to secrete a chemokine which – in turn – enhanced the cancer cells’ motility and their ability to invade and metastasize (Nature 2007;449:557-63). In effect, the primary tumor acts like a filling station, giving these tumor cells more energy to go out and metastasize in different sites.
Although such studies remain animal models, I think we can say there is a basis for primary tumor therapy in the metastatic setting. A dozen or more retrospective studies of large institutional series, cancer databases, and population-based studies have examined outcomes in women treated with and without surgical resection. Data on about 30,000 women have been published, with about 50% receiving some form of primary tumor therapy. The survival benefit across them is quite consistent, with the hazard ratio of death over a given time period reduced from 30% to 50%.
These studies do demonstrate selection bias: Women treated with surgical removal were more likely to be young, white, married, with smaller tumors and a lower burden of disease, and it is possible that they would have done better anyway. Studies have also shown that better survival, even in stage IV cancer, occurs in women who have a therapeutic target (that is, hormone receptors or HER2). But there was still a significant survival benefit for surgery.
Surgical resection may also confer benefit by preventing the primary tumor in the breast from becoming a quality of-life problem. At Northwestern Memorial Hospital, Chicago, we conducted a study that showed the odds of symptomatic chest wall disease were far lower in the surgical group, compared with the nonsurgical group. In this 2008 study, we found that women with good local control– surgical or systemic - did better. Time to first progression was prolonged by 50% in the surgical group, while chest wall control was associated with a 60% improvement in overall survival, regardless of whether surgical resection was performed (Cancer 2008;113:2011-9).
So the question is, do we need a randomized trial? My unbiased answer is that we do – and I am the principal investigator of such a trial open to all U.S. and Canadian institutions (NCT01242800). The end points are survival, local control, and quality of life. In addition, the trial will provide an opportunity to answer biological questions regarding the relationship between the primary tumor and metastatic site. Only when we have samples of primary and metastatic tumors can we settle this continuing debate.
Dr. Seema Khan is the Bluhm Family Professor of Cancer Research at the Lurie Comprehensive Cancer Center, Chicago.
No – Case Selection Bias Is Behind the Survival Advantage.
By Dr. Blake Cady
It’s true that some studies show an increase in stage IV breast cancer survival after primary site resection – but this more likely reflects a bias of case selection than a true benefit of local therapy.
Any findings of positive benefit other than case selection must be biologically plausible. The suggestion that the primary tumor releases some sort of growth-promoting substance that is gone when you remove the tumor, inhibiting metastatic disease has not yet been proven in humans; nor has the proposition that continued cell shedding might further metastasis.
Decreased tumor burden has sometimes been shown to increase the usefulness of systemic therapy, but I think that, all in all, case selection simply promotes breast surgery in patients with a better prognosis.
To show improved survival, we must find causality and not simply coincidence. Findings from large case series or cancer databases are unable to provide the answer. Although registry interpretations can provide basic data, they often break down when you attempt to extract detailed data.
Sometimes a stage IV cancer is registered initially on the basis of a pulmonary or liver shadow on imaging, which later is proven not to have been metastatic disease – and the case is never amended in the registry. There are also registry misinterpretations of the type of surgery that’s been done.
We performed a matched pair analysis of stage IV breast cancer patients with and without resection of the primary tumor. This was based on the Partners Health Care Consortium cancer database, which included information on 19,464 invasive breast cancers treated between 1970 and 2002; 808 of these (4%) were recorded as stage IV. Of these, 622 were analyzed by case matching that considered age, diagnostic date, metastatic disease location, estrogen-receptor status, and systemic therapy (Ann. Surg. Oncol. 2008; 15:3384-95).
It’s absolutely true that there was a statistically significant difference in survival in the overall comparison of surgery vs. no surgery. With bone metastases only, it was still statistically significant, but with a substantially lower difference in survival. In visceral metastases, the difference became nonsignificant.
Looking at therapy sequence in those who had the same type of systemic therapy, but with chemotherapy before or after surgery, you see a suggestion that giving systemic therapy first with a clinical response, which is a favorable prognostic indication, leads to a selection bias in choosing patients for surgery.
I reviewed the 86 cases who survived 5 years with records available. In those records, 22 were actually not stage IV disease, leaving 64 with confirmed stage IV (M1) – an overall 10% 5-year survival rate. Patients who survived were significantly younger than those who did not survive, significantly more likely to have estrogen receptor-positive tumors, and significantly more likely to have bone rather than visceral metastases or oligometastatic disease – all of which are good prognostic factors.
Among the 64 5-year survivors, 25 had therapeutic breast surgery. Of these, 8 had an excellent chemotherapy response followed by breast surgery; 8 had delayed surgery following a failure of systemic therapy; 7 had an initial therapeutic and curative surgery, but staging revealed metastases because of unexpected positive nodes; and 2 had initial surgery with oligometastatic resection.
Thirty-nine patients had no therapeutic breast surgery, and, of these, 16 had an excellent response to systemic therapy; 15 had slowly progressive disease; and 8 had oligometastatic disease – a positive prognostic factor. Thus, favorable prognostic factors led to a selection of primary-site surgery, whereas poor prognostic factors mean that primary-site surgery was avoided, contradicting the assumption of its therapeutic benefit.
There were also surgical discrepancies among the 5-year survivors. The registry listed 11 as having had breast surgery, while a chart review showed that surgery was not therapeutic. Five were listed as having no breast surgery, while they had indeed undergone a therapeutic operation.
Cancer registry data are not reliable for defining stage IV disease, discriminating advanced local disease with bone involvement from true distant, imaging-confirmed metastasis, or assessing surgical procedures. Case selection bias accounts for most, if not all, of the apparent survival advantage.
Dr. Blake Cady is professor of surgery (emeritus) at Harvard Medical School, Boston, and at Brown University, Providence, R.I.
Yes – It Is Time to Consider a Paradigm Shift.
By Dr. Seema Khan
In stage IV breast cancer, local therapy is not considered important except for controlling symptoms. But recent large-scale retrospective studies tell us that, if you follow enough women long enough, you see a difference in survival related to control of local disease. These emerging data tell us it’s time to consider a paradigm shift in the treatment of stage IV breast cancer.
Researchers are finding that primary site treatment regimens are important because an intact primary tumor can serve as a continued source of new metastatic lesions. There is reason, therefore, to believe that treating these can prolong life.
First explored by Dr. Larry Norton and Joan Massagué, Ph.D., (Nature Med. 2006;12:875-8), the concept of cancer self-seeding holds that cells from the primary tumor not only travel unidirectionally to seed metastases but also return to the primary tumor, self-seeding it and helping it to grow even more.
This view incorporates the microenvironment of the primary tumor. Here, fibroblasts and other cells – particularly mesenchymal stem cells derived from bone marrow – may play a very important role, creating a reactive stroma that seems to release growth-promoting substances that increase the metastatic efficiency of the circulating cells.
Robert A. Weinberg, Ph.D., published data showing that these mesenchymal stem cells are pluripotent progenitor cells. When weakly metastatic human breast cancer cells were mixed with these stem cells, the cancer cells’ metastatic potential greatly increased. The breast cancer cells then stimulated the stem cells to secrete a chemokine which – in turn – enhanced the cancer cells’ motility and their ability to invade and metastasize (Nature 2007;449:557-63). In effect, the primary tumor acts like a filling station, giving these tumor cells more energy to go out and metastasize in different sites.
Although such studies remain animal models, I think we can say there is a basis for primary tumor therapy in the metastatic setting. A dozen or more retrospective studies of large institutional series, cancer databases, and population-based studies have examined outcomes in women treated with and without surgical resection. Data on about 30,000 women have been published, with about 50% receiving some form of primary tumor therapy. The survival benefit across them is quite consistent, with the hazard ratio of death over a given time period reduced from 30% to 50%.
These studies do demonstrate selection bias: Women treated with surgical removal were more likely to be young, white, married, with smaller tumors and a lower burden of disease, and it is possible that they would have done better anyway. Studies have also shown that better survival, even in stage IV cancer, occurs in women who have a therapeutic target (that is, hormone receptors or HER2). But there was still a significant survival benefit for surgery.
Surgical resection may also confer benefit by preventing the primary tumor in the breast from becoming a quality of-life problem. At Northwestern Memorial Hospital, Chicago, we conducted a study that showed the odds of symptomatic chest wall disease were far lower in the surgical group, compared with the nonsurgical group. In this 2008 study, we found that women with good local control– surgical or systemic - did better. Time to first progression was prolonged by 50% in the surgical group, while chest wall control was associated with a 60% improvement in overall survival, regardless of whether surgical resection was performed (Cancer 2008;113:2011-9).
So the question is, do we need a randomized trial? My unbiased answer is that we do – and I am the principal investigator of such a trial open to all U.S. and Canadian institutions (NCT01242800). The end points are survival, local control, and quality of life. In addition, the trial will provide an opportunity to answer biological questions regarding the relationship between the primary tumor and metastatic site. Only when we have samples of primary and metastatic tumors can we settle this continuing debate.
Dr. Seema Khan is the Bluhm Family Professor of Cancer Research at the Lurie Comprehensive Cancer Center, Chicago.
No – Case Selection Bias Is Behind the Survival Advantage.
By Dr. Blake Cady
It’s true that some studies show an increase in stage IV breast cancer survival after primary site resection – but this more likely reflects a bias of case selection than a true benefit of local therapy.
Any findings of positive benefit other than case selection must be biologically plausible. The suggestion that the primary tumor releases some sort of growth-promoting substance that is gone when you remove the tumor, inhibiting metastatic disease has not yet been proven in humans; nor has the proposition that continued cell shedding might further metastasis.
Decreased tumor burden has sometimes been shown to increase the usefulness of systemic therapy, but I think that, all in all, case selection simply promotes breast surgery in patients with a better prognosis.
To show improved survival, we must find causality and not simply coincidence. Findings from large case series or cancer databases are unable to provide the answer. Although registry interpretations can provide basic data, they often break down when you attempt to extract detailed data.
Sometimes a stage IV cancer is registered initially on the basis of a pulmonary or liver shadow on imaging, which later is proven not to have been metastatic disease – and the case is never amended in the registry. There are also registry misinterpretations of the type of surgery that’s been done.
We performed a matched pair analysis of stage IV breast cancer patients with and without resection of the primary tumor. This was based on the Partners Health Care Consortium cancer database, which included information on 19,464 invasive breast cancers treated between 1970 and 2002; 808 of these (4%) were recorded as stage IV. Of these, 622 were analyzed by case matching that considered age, diagnostic date, metastatic disease location, estrogen-receptor status, and systemic therapy (Ann. Surg. Oncol. 2008; 15:3384-95).
It’s absolutely true that there was a statistically significant difference in survival in the overall comparison of surgery vs. no surgery. With bone metastases only, it was still statistically significant, but with a substantially lower difference in survival. In visceral metastases, the difference became nonsignificant.
Looking at therapy sequence in those who had the same type of systemic therapy, but with chemotherapy before or after surgery, you see a suggestion that giving systemic therapy first with a clinical response, which is a favorable prognostic indication, leads to a selection bias in choosing patients for surgery.
I reviewed the 86 cases who survived 5 years with records available. In those records, 22 were actually not stage IV disease, leaving 64 with confirmed stage IV (M1) – an overall 10% 5-year survival rate. Patients who survived were significantly younger than those who did not survive, significantly more likely to have estrogen receptor-positive tumors, and significantly more likely to have bone rather than visceral metastases or oligometastatic disease – all of which are good prognostic factors.
Among the 64 5-year survivors, 25 had therapeutic breast surgery. Of these, 8 had an excellent chemotherapy response followed by breast surgery; 8 had delayed surgery following a failure of systemic therapy; 7 had an initial therapeutic and curative surgery, but staging revealed metastases because of unexpected positive nodes; and 2 had initial surgery with oligometastatic resection.
Thirty-nine patients had no therapeutic breast surgery, and, of these, 16 had an excellent response to systemic therapy; 15 had slowly progressive disease; and 8 had oligometastatic disease – a positive prognostic factor. Thus, favorable prognostic factors led to a selection of primary-site surgery, whereas poor prognostic factors mean that primary-site surgery was avoided, contradicting the assumption of its therapeutic benefit.
There were also surgical discrepancies among the 5-year survivors. The registry listed 11 as having had breast surgery, while a chart review showed that surgery was not therapeutic. Five were listed as having no breast surgery, while they had indeed undergone a therapeutic operation.
Cancer registry data are not reliable for defining stage IV disease, discriminating advanced local disease with bone involvement from true distant, imaging-confirmed metastasis, or assessing surgical procedures. Case selection bias accounts for most, if not all, of the apparent survival advantage.
Dr. Blake Cady is professor of surgery (emeritus) at Harvard Medical School, Boston, and at Brown University, Providence, R.I.
Yes – It Is Time to Consider a Paradigm Shift.
By Dr. Seema Khan
In stage IV breast cancer, local therapy is not considered important except for controlling symptoms. But recent large-scale retrospective studies tell us that, if you follow enough women long enough, you see a difference in survival related to control of local disease. These emerging data tell us it’s time to consider a paradigm shift in the treatment of stage IV breast cancer.
Researchers are finding that primary site treatment regimens are important because an intact primary tumor can serve as a continued source of new metastatic lesions. There is reason, therefore, to believe that treating these can prolong life.
First explored by Dr. Larry Norton and Joan Massagué, Ph.D., (Nature Med. 2006;12:875-8), the concept of cancer self-seeding holds that cells from the primary tumor not only travel unidirectionally to seed metastases but also return to the primary tumor, self-seeding it and helping it to grow even more.
This view incorporates the microenvironment of the primary tumor. Here, fibroblasts and other cells – particularly mesenchymal stem cells derived from bone marrow – may play a very important role, creating a reactive stroma that seems to release growth-promoting substances that increase the metastatic efficiency of the circulating cells.
Robert A. Weinberg, Ph.D., published data showing that these mesenchymal stem cells are pluripotent progenitor cells. When weakly metastatic human breast cancer cells were mixed with these stem cells, the cancer cells’ metastatic potential greatly increased. The breast cancer cells then stimulated the stem cells to secrete a chemokine which – in turn – enhanced the cancer cells’ motility and their ability to invade and metastasize (Nature 2007;449:557-63). In effect, the primary tumor acts like a filling station, giving these tumor cells more energy to go out and metastasize in different sites.
Although such studies remain animal models, I think we can say there is a basis for primary tumor therapy in the metastatic setting. A dozen or more retrospective studies of large institutional series, cancer databases, and population-based studies have examined outcomes in women treated with and without surgical resection. Data on about 30,000 women have been published, with about 50% receiving some form of primary tumor therapy. The survival benefit across them is quite consistent, with the hazard ratio of death over a given time period reduced from 30% to 50%.
These studies do demonstrate selection bias: Women treated with surgical removal were more likely to be young, white, married, with smaller tumors and a lower burden of disease, and it is possible that they would have done better anyway. Studies have also shown that better survival, even in stage IV cancer, occurs in women who have a therapeutic target (that is, hormone receptors or HER2). But there was still a significant survival benefit for surgery.
Surgical resection may also confer benefit by preventing the primary tumor in the breast from becoming a quality of-life problem. At Northwestern Memorial Hospital, Chicago, we conducted a study that showed the odds of symptomatic chest wall disease were far lower in the surgical group, compared with the nonsurgical group. In this 2008 study, we found that women with good local control– surgical or systemic - did better. Time to first progression was prolonged by 50% in the surgical group, while chest wall control was associated with a 60% improvement in overall survival, regardless of whether surgical resection was performed (Cancer 2008;113:2011-9).
So the question is, do we need a randomized trial? My unbiased answer is that we do – and I am the principal investigator of such a trial open to all U.S. and Canadian institutions (NCT01242800). The end points are survival, local control, and quality of life. In addition, the trial will provide an opportunity to answer biological questions regarding the relationship between the primary tumor and metastatic site. Only when we have samples of primary and metastatic tumors can we settle this continuing debate.
Dr. Seema Khan is the Bluhm Family Professor of Cancer Research at the Lurie Comprehensive Cancer Center, Chicago.
No – Case Selection Bias Is Behind the Survival Advantage.
By Dr. Blake Cady
It’s true that some studies show an increase in stage IV breast cancer survival after primary site resection – but this more likely reflects a bias of case selection than a true benefit of local therapy.
Any findings of positive benefit other than case selection must be biologically plausible. The suggestion that the primary tumor releases some sort of growth-promoting substance that is gone when you remove the tumor, inhibiting metastatic disease has not yet been proven in humans; nor has the proposition that continued cell shedding might further metastasis.
Decreased tumor burden has sometimes been shown to increase the usefulness of systemic therapy, but I think that, all in all, case selection simply promotes breast surgery in patients with a better prognosis.
To show improved survival, we must find causality and not simply coincidence. Findings from large case series or cancer databases are unable to provide the answer. Although registry interpretations can provide basic data, they often break down when you attempt to extract detailed data.
Sometimes a stage IV cancer is registered initially on the basis of a pulmonary or liver shadow on imaging, which later is proven not to have been metastatic disease – and the case is never amended in the registry. There are also registry misinterpretations of the type of surgery that’s been done.
We performed a matched pair analysis of stage IV breast cancer patients with and without resection of the primary tumor. This was based on the Partners Health Care Consortium cancer database, which included information on 19,464 invasive breast cancers treated between 1970 and 2002; 808 of these (4%) were recorded as stage IV. Of these, 622 were analyzed by case matching that considered age, diagnostic date, metastatic disease location, estrogen-receptor status, and systemic therapy (Ann. Surg. Oncol. 2008; 15:3384-95).
It’s absolutely true that there was a statistically significant difference in survival in the overall comparison of surgery vs. no surgery. With bone metastases only, it was still statistically significant, but with a substantially lower difference in survival. In visceral metastases, the difference became nonsignificant.
Looking at therapy sequence in those who had the same type of systemic therapy, but with chemotherapy before or after surgery, you see a suggestion that giving systemic therapy first with a clinical response, which is a favorable prognostic indication, leads to a selection bias in choosing patients for surgery.
I reviewed the 86 cases who survived 5 years with records available. In those records, 22 were actually not stage IV disease, leaving 64 with confirmed stage IV (M1) – an overall 10% 5-year survival rate. Patients who survived were significantly younger than those who did not survive, significantly more likely to have estrogen receptor-positive tumors, and significantly more likely to have bone rather than visceral metastases or oligometastatic disease – all of which are good prognostic factors.
Among the 64 5-year survivors, 25 had therapeutic breast surgery. Of these, 8 had an excellent chemotherapy response followed by breast surgery; 8 had delayed surgery following a failure of systemic therapy; 7 had an initial therapeutic and curative surgery, but staging revealed metastases because of unexpected positive nodes; and 2 had initial surgery with oligometastatic resection.
Thirty-nine patients had no therapeutic breast surgery, and, of these, 16 had an excellent response to systemic therapy; 15 had slowly progressive disease; and 8 had oligometastatic disease – a positive prognostic factor. Thus, favorable prognostic factors led to a selection of primary-site surgery, whereas poor prognostic factors mean that primary-site surgery was avoided, contradicting the assumption of its therapeutic benefit.
There were also surgical discrepancies among the 5-year survivors. The registry listed 11 as having had breast surgery, while a chart review showed that surgery was not therapeutic. Five were listed as having no breast surgery, while they had indeed undergone a therapeutic operation.
Cancer registry data are not reliable for defining stage IV disease, discriminating advanced local disease with bone involvement from true distant, imaging-confirmed metastasis, or assessing surgical procedures. Case selection bias accounts for most, if not all, of the apparent survival advantage.
Dr. Blake Cady is professor of surgery (emeritus) at Harvard Medical School, Boston, and at Brown University, Providence, R.I.
Point/Counterpoint: Does Surgery Improve Survival in Stage IV Breast Cancer?
Yes – It Is Time to Consider a Paradigm Shift.
In stage IV breast cancer, local therapy is not considered important except for controlling symptoms. But recent large-scale retrospective studies tell us that, if you follow enough women long enough, you see a difference in survival related to control of local disease. These emerging data tell us it’s time to consider a paradigm shift in the treatment of stage IV breast cancer.
Researchers are finding that primary site treatment regimens are important because an intact primary tumor can serve as a continued source of new metastatic lesions. There is reason, therefore, to believe that treating these can prolong life.
First explored by Dr. Larry Norton and Joan Massagué, Ph.D., (Nature Med. 2006;12:875-8), the concept of cancer self-seeding holds that cells from the primary tumor not only travel unidirectionally to seed metastases but also return to the primary tumor, self-seeding it and helping it to grow even more.
This view incorporates the microenvironment of the primary tumor. Here, fibroblasts and other cells – particularly mesenchymal stem cells derived from bone marrow – may play a very important role, creating a reactive stroma that seems to release growth-promoting substances that increase the metastatic efficiency of the circulating cells.
Robert A. Weinberg, Ph.D., published data showing that these mesenchymal stem cells are pluripotent progenitor cells. When weakly metastatic human breast cancer cells were mixed with these stem cells, the cancer cells’ metastatic potential greatly increased. The breast cancer cells then stimulated the stem cells to secrete a chemokine which – in turn – enhanced the cancer cells’ motility and their ability to invade and metastasize (Nature 2007;449:557-63). In effect, the primary tumor acts like a filling station, giving these tumor cells more energy to go out and metastasize in different sites.
Although such studies remain animal models, I think we can say there is a basis for primary tumor therapy in the metastatic setting. A dozen or more retrospective studies of large institutional series, cancer databases, and population-based studies have examined outcomes in women treated with and without surgical resection. Data on about 30,000 women have been published, with about 50% receiving some form of primary tumor therapy. The survival benefit across them is quite consistent, with the hazard ratio of death over a given time period reduced from 30% to 50%.
These studies do demonstrate selection bias: Women treated with surgical removal were more likely to be young, white, married, with smaller tumors and a lower burden of disease, and it is possible that they would have done better anyway. Studies have also shown that better survival, even in stage IV cancer, occurs in women who have a therapeutic target (that is, hormone receptors or HER2). But there was still a significant survival benefit for surgery.
Surgical resection may also confer benefit by preventing the primary tumor in the breast from becoming a quality of-life problem. At Northwestern Memorial Hospital, Chicago, we conducted a study that showed the odds of symptomatic chest wall disease were far lower in the surgical group, compared with the nonsurgical group. In this 2008 study, we found that women with good local control– surgical or systemic - did better. Time to first progression was prolonged by 50% in the surgical group, while chest wall control was associated with a 60% improvement in overall survival, regardless of whether surgical resection was performed (Cancer 2008;113:2011-9).
So the question is, do we need a randomized trial? My unbiased answer is that we do – and I am the principal investigator of such a trial open to all U.S. and Canadian institutions (NCT01242800). The end points are survival, local control, and quality of life. In addition, the trial will provide an opportunity to answer biological questions regarding the relationship between the primary tumor and metastatic site. Only when we have samples of primary and metastatic tumors can we settle this continuing debate.
Dr. Khan is the Bluhm Family Professor of Cancer Research at the Lurie Comprehensive Cancer Center, Chicago.
No – Case Selection Bias Is Behind the Survival Advantage.
It’s true that some studies show an increase in stage IV breast cancer survival after primary site resection – but this more likely reflects a bias of case selection than a true benefit of local therapy.
Any findings of positive benefit other than case selection must be biologically plausible. The suggestion that the primary tumor releases some sort of growth-promoting substance that is gone when you remove the tumor, inhibiting metastatic disease has not yet been proven in humans; nor has the proposition that continued cell shedding might further metastasis.
Decreased tumor burden has sometimes been shown to increase the usefulness of systemic therapy, but I think that, all in all, case selection simply promotes breast surgery in patients with a better prognosis.
To show improved survival, we must find causality and not simply coincidence. Findings from large case series or cancer databases are unable to provide the answer. Although registry interpretations can provide basic data, they often break down when you attempt to extract detailed data.
Sometimes a stage IV cancer is registered initially on the basis of a pulmonary or liver shadow on imaging, which later is proven not to have been metastatic disease – and the case is never amended in the registry. There are also registry misinterpretations of the type of surgery that’s been done.
We performed a matched pair analysis of stage IV breast cancer patients with and without resection of the primary tumor. This was based on the Partners Health Care Consortium cancer database, which included information on 19,464 invasive breast cancers treated between 1970 and 2002; 808 of these (4%) were recorded as stage IV. Of these, 622 were analyzed by case matching that considered age, diagnostic date, metastatic disease location, estrogen-receptor status, and systemic therapy (Ann. Surg. Oncol. 2008; 15:3384-95).
It’s absolutely true that there was a statistically significant difference in survival in the overall comparison of surgery vs. no surgery. With bone metastases only, it was still statistically significant, but with a substantially lower difference in survival. In visceral metastases, the difference became nonsignificant.
Looking at therapy sequence in those who had the same type of systemic therapy, but with chemotherapy before or after surgery, you see a suggestion that giving systemic therapy first with a clinical response, which is a favorable prognostic indication, leads to a selection bias in choosing patients for surgery.
I reviewed the 86 cases who survived 5 years with records available. In those records, 22 were actually not stage IV disease, leaving 64 with confirmed stage IV (M1) – an overall 10% 5-year survival rate. Patients who survived were significantly younger than those who did not survive, significantly more likely to have estrogen receptor-positive tumors, and significantly more likely to have bone rather than visceral metastases or oligometastatic disease – all of which are good prognostic factors.
Among the 64 5-year survivors, 25 had therapeutic breast surgery. Of these, 8 had an excellent chemotherapy response followed by breast surgery; 8 had delayed surgery following a failure of systemic therapy; 7 had an initial therapeutic and curative surgery, but staging revealed metastases because of unexpected positive nodes; and 2 had initial surgery with oligometastatic resection.
Thirty-nine patients had no therapeutic breast surgery, and, of these, 16 had an excellent response to systemic therapy; 15 had slowly progressive disease; and 8 had oligometastatic disease – a positive prognostic factor. Thus, favorable prognostic factors led to a selection of primary-site surgery, whereas poor prognostic factors mean that primary-site surgery was avoided, contradicting the assumption of its therapeutic benefit.
There were also surgical discrepancies among the 5-year survivors. The registry listed 11 as having had breast surgery, while a chart review showed that surgery was not therapeutic. Five were listed as having no breast surgery, while they had indeed undergone a therapeutic operation.
Cancer registry data are not reliable for defining stage IV disease, discriminating advanced local disease with bone involvement from true distant, imaging-confirmed metastasis, or assessing surgical procedures. Case selection bias accounts for most, if not all, of the apparent survival advantage.
Dr. Blake Cady is professor of surgery (emeritus) at Harvard Medical School, Boston, and at Brown University, Providence, R.I.
Yes – It Is Time to Consider a Paradigm Shift.
In stage IV breast cancer, local therapy is not considered important except for controlling symptoms. But recent large-scale retrospective studies tell us that, if you follow enough women long enough, you see a difference in survival related to control of local disease. These emerging data tell us it’s time to consider a paradigm shift in the treatment of stage IV breast cancer.
Researchers are finding that primary site treatment regimens are important because an intact primary tumor can serve as a continued source of new metastatic lesions. There is reason, therefore, to believe that treating these can prolong life.
First explored by Dr. Larry Norton and Joan Massagué, Ph.D., (Nature Med. 2006;12:875-8), the concept of cancer self-seeding holds that cells from the primary tumor not only travel unidirectionally to seed metastases but also return to the primary tumor, self-seeding it and helping it to grow even more.
This view incorporates the microenvironment of the primary tumor. Here, fibroblasts and other cells – particularly mesenchymal stem cells derived from bone marrow – may play a very important role, creating a reactive stroma that seems to release growth-promoting substances that increase the metastatic efficiency of the circulating cells.
Robert A. Weinberg, Ph.D., published data showing that these mesenchymal stem cells are pluripotent progenitor cells. When weakly metastatic human breast cancer cells were mixed with these stem cells, the cancer cells’ metastatic potential greatly increased. The breast cancer cells then stimulated the stem cells to secrete a chemokine which – in turn – enhanced the cancer cells’ motility and their ability to invade and metastasize (Nature 2007;449:557-63). In effect, the primary tumor acts like a filling station, giving these tumor cells more energy to go out and metastasize in different sites.
Although such studies remain animal models, I think we can say there is a basis for primary tumor therapy in the metastatic setting. A dozen or more retrospective studies of large institutional series, cancer databases, and population-based studies have examined outcomes in women treated with and without surgical resection. Data on about 30,000 women have been published, with about 50% receiving some form of primary tumor therapy. The survival benefit across them is quite consistent, with the hazard ratio of death over a given time period reduced from 30% to 50%.
These studies do demonstrate selection bias: Women treated with surgical removal were more likely to be young, white, married, with smaller tumors and a lower burden of disease, and it is possible that they would have done better anyway. Studies have also shown that better survival, even in stage IV cancer, occurs in women who have a therapeutic target (that is, hormone receptors or HER2). But there was still a significant survival benefit for surgery.
Surgical resection may also confer benefit by preventing the primary tumor in the breast from becoming a quality of-life problem. At Northwestern Memorial Hospital, Chicago, we conducted a study that showed the odds of symptomatic chest wall disease were far lower in the surgical group, compared with the nonsurgical group. In this 2008 study, we found that women with good local control– surgical or systemic - did better. Time to first progression was prolonged by 50% in the surgical group, while chest wall control was associated with a 60% improvement in overall survival, regardless of whether surgical resection was performed (Cancer 2008;113:2011-9).
So the question is, do we need a randomized trial? My unbiased answer is that we do – and I am the principal investigator of such a trial open to all U.S. and Canadian institutions (NCT01242800). The end points are survival, local control, and quality of life. In addition, the trial will provide an opportunity to answer biological questions regarding the relationship between the primary tumor and metastatic site. Only when we have samples of primary and metastatic tumors can we settle this continuing debate.
Dr. Khan is the Bluhm Family Professor of Cancer Research at the Lurie Comprehensive Cancer Center, Chicago.
No – Case Selection Bias Is Behind the Survival Advantage.
It’s true that some studies show an increase in stage IV breast cancer survival after primary site resection – but this more likely reflects a bias of case selection than a true benefit of local therapy.
Any findings of positive benefit other than case selection must be biologically plausible. The suggestion that the primary tumor releases some sort of growth-promoting substance that is gone when you remove the tumor, inhibiting metastatic disease has not yet been proven in humans; nor has the proposition that continued cell shedding might further metastasis.
Decreased tumor burden has sometimes been shown to increase the usefulness of systemic therapy, but I think that, all in all, case selection simply promotes breast surgery in patients with a better prognosis.
To show improved survival, we must find causality and not simply coincidence. Findings from large case series or cancer databases are unable to provide the answer. Although registry interpretations can provide basic data, they often break down when you attempt to extract detailed data.
Sometimes a stage IV cancer is registered initially on the basis of a pulmonary or liver shadow on imaging, which later is proven not to have been metastatic disease – and the case is never amended in the registry. There are also registry misinterpretations of the type of surgery that’s been done.
We performed a matched pair analysis of stage IV breast cancer patients with and without resection of the primary tumor. This was based on the Partners Health Care Consortium cancer database, which included information on 19,464 invasive breast cancers treated between 1970 and 2002; 808 of these (4%) were recorded as stage IV. Of these, 622 were analyzed by case matching that considered age, diagnostic date, metastatic disease location, estrogen-receptor status, and systemic therapy (Ann. Surg. Oncol. 2008; 15:3384-95).
It’s absolutely true that there was a statistically significant difference in survival in the overall comparison of surgery vs. no surgery. With bone metastases only, it was still statistically significant, but with a substantially lower difference in survival. In visceral metastases, the difference became nonsignificant.
Looking at therapy sequence in those who had the same type of systemic therapy, but with chemotherapy before or after surgery, you see a suggestion that giving systemic therapy first with a clinical response, which is a favorable prognostic indication, leads to a selection bias in choosing patients for surgery.
I reviewed the 86 cases who survived 5 years with records available. In those records, 22 were actually not stage IV disease, leaving 64 with confirmed stage IV (M1) – an overall 10% 5-year survival rate. Patients who survived were significantly younger than those who did not survive, significantly more likely to have estrogen receptor-positive tumors, and significantly more likely to have bone rather than visceral metastases or oligometastatic disease – all of which are good prognostic factors.
Among the 64 5-year survivors, 25 had therapeutic breast surgery. Of these, 8 had an excellent chemotherapy response followed by breast surgery; 8 had delayed surgery following a failure of systemic therapy; 7 had an initial therapeutic and curative surgery, but staging revealed metastases because of unexpected positive nodes; and 2 had initial surgery with oligometastatic resection.
Thirty-nine patients had no therapeutic breast surgery, and, of these, 16 had an excellent response to systemic therapy; 15 had slowly progressive disease; and 8 had oligometastatic disease – a positive prognostic factor. Thus, favorable prognostic factors led to a selection of primary-site surgery, whereas poor prognostic factors mean that primary-site surgery was avoided, contradicting the assumption of its therapeutic benefit.
There were also surgical discrepancies among the 5-year survivors. The registry listed 11 as having had breast surgery, while a chart review showed that surgery was not therapeutic. Five were listed as having no breast surgery, while they had indeed undergone a therapeutic operation.
Cancer registry data are not reliable for defining stage IV disease, discriminating advanced local disease with bone involvement from true distant, imaging-confirmed metastasis, or assessing surgical procedures. Case selection bias accounts for most, if not all, of the apparent survival advantage.
Dr. Blake Cady is professor of surgery (emeritus) at Harvard Medical School, Boston, and at Brown University, Providence, R.I.
Yes – It Is Time to Consider a Paradigm Shift.
In stage IV breast cancer, local therapy is not considered important except for controlling symptoms. But recent large-scale retrospective studies tell us that, if you follow enough women long enough, you see a difference in survival related to control of local disease. These emerging data tell us it’s time to consider a paradigm shift in the treatment of stage IV breast cancer.
Researchers are finding that primary site treatment regimens are important because an intact primary tumor can serve as a continued source of new metastatic lesions. There is reason, therefore, to believe that treating these can prolong life.
First explored by Dr. Larry Norton and Joan Massagué, Ph.D., (Nature Med. 2006;12:875-8), the concept of cancer self-seeding holds that cells from the primary tumor not only travel unidirectionally to seed metastases but also return to the primary tumor, self-seeding it and helping it to grow even more.
This view incorporates the microenvironment of the primary tumor. Here, fibroblasts and other cells – particularly mesenchymal stem cells derived from bone marrow – may play a very important role, creating a reactive stroma that seems to release growth-promoting substances that increase the metastatic efficiency of the circulating cells.
Robert A. Weinberg, Ph.D., published data showing that these mesenchymal stem cells are pluripotent progenitor cells. When weakly metastatic human breast cancer cells were mixed with these stem cells, the cancer cells’ metastatic potential greatly increased. The breast cancer cells then stimulated the stem cells to secrete a chemokine which – in turn – enhanced the cancer cells’ motility and their ability to invade and metastasize (Nature 2007;449:557-63). In effect, the primary tumor acts like a filling station, giving these tumor cells more energy to go out and metastasize in different sites.
Although such studies remain animal models, I think we can say there is a basis for primary tumor therapy in the metastatic setting. A dozen or more retrospective studies of large institutional series, cancer databases, and population-based studies have examined outcomes in women treated with and without surgical resection. Data on about 30,000 women have been published, with about 50% receiving some form of primary tumor therapy. The survival benefit across them is quite consistent, with the hazard ratio of death over a given time period reduced from 30% to 50%.
These studies do demonstrate selection bias: Women treated with surgical removal were more likely to be young, white, married, with smaller tumors and a lower burden of disease, and it is possible that they would have done better anyway. Studies have also shown that better survival, even in stage IV cancer, occurs in women who have a therapeutic target (that is, hormone receptors or HER2). But there was still a significant survival benefit for surgery.
Surgical resection may also confer benefit by preventing the primary tumor in the breast from becoming a quality of-life problem. At Northwestern Memorial Hospital, Chicago, we conducted a study that showed the odds of symptomatic chest wall disease were far lower in the surgical group, compared with the nonsurgical group. In this 2008 study, we found that women with good local control– surgical or systemic - did better. Time to first progression was prolonged by 50% in the surgical group, while chest wall control was associated with a 60% improvement in overall survival, regardless of whether surgical resection was performed (Cancer 2008;113:2011-9).
So the question is, do we need a randomized trial? My unbiased answer is that we do – and I am the principal investigator of such a trial open to all U.S. and Canadian institutions (NCT01242800). The end points are survival, local control, and quality of life. In addition, the trial will provide an opportunity to answer biological questions regarding the relationship between the primary tumor and metastatic site. Only when we have samples of primary and metastatic tumors can we settle this continuing debate.
Dr. Khan is the Bluhm Family Professor of Cancer Research at the Lurie Comprehensive Cancer Center, Chicago.
No – Case Selection Bias Is Behind the Survival Advantage.
It’s true that some studies show an increase in stage IV breast cancer survival after primary site resection – but this more likely reflects a bias of case selection than a true benefit of local therapy.
Any findings of positive benefit other than case selection must be biologically plausible. The suggestion that the primary tumor releases some sort of growth-promoting substance that is gone when you remove the tumor, inhibiting metastatic disease has not yet been proven in humans; nor has the proposition that continued cell shedding might further metastasis.
Decreased tumor burden has sometimes been shown to increase the usefulness of systemic therapy, but I think that, all in all, case selection simply promotes breast surgery in patients with a better prognosis.
To show improved survival, we must find causality and not simply coincidence. Findings from large case series or cancer databases are unable to provide the answer. Although registry interpretations can provide basic data, they often break down when you attempt to extract detailed data.
Sometimes a stage IV cancer is registered initially on the basis of a pulmonary or liver shadow on imaging, which later is proven not to have been metastatic disease – and the case is never amended in the registry. There are also registry misinterpretations of the type of surgery that’s been done.
We performed a matched pair analysis of stage IV breast cancer patients with and without resection of the primary tumor. This was based on the Partners Health Care Consortium cancer database, which included information on 19,464 invasive breast cancers treated between 1970 and 2002; 808 of these (4%) were recorded as stage IV. Of these, 622 were analyzed by case matching that considered age, diagnostic date, metastatic disease location, estrogen-receptor status, and systemic therapy (Ann. Surg. Oncol. 2008; 15:3384-95).
It’s absolutely true that there was a statistically significant difference in survival in the overall comparison of surgery vs. no surgery. With bone metastases only, it was still statistically significant, but with a substantially lower difference in survival. In visceral metastases, the difference became nonsignificant.
Looking at therapy sequence in those who had the same type of systemic therapy, but with chemotherapy before or after surgery, you see a suggestion that giving systemic therapy first with a clinical response, which is a favorable prognostic indication, leads to a selection bias in choosing patients for surgery.
I reviewed the 86 cases who survived 5 years with records available. In those records, 22 were actually not stage IV disease, leaving 64 with confirmed stage IV (M1) – an overall 10% 5-year survival rate. Patients who survived were significantly younger than those who did not survive, significantly more likely to have estrogen receptor-positive tumors, and significantly more likely to have bone rather than visceral metastases or oligometastatic disease – all of which are good prognostic factors.
Among the 64 5-year survivors, 25 had therapeutic breast surgery. Of these, 8 had an excellent chemotherapy response followed by breast surgery; 8 had delayed surgery following a failure of systemic therapy; 7 had an initial therapeutic and curative surgery, but staging revealed metastases because of unexpected positive nodes; and 2 had initial surgery with oligometastatic resection.
Thirty-nine patients had no therapeutic breast surgery, and, of these, 16 had an excellent response to systemic therapy; 15 had slowly progressive disease; and 8 had oligometastatic disease – a positive prognostic factor. Thus, favorable prognostic factors led to a selection of primary-site surgery, whereas poor prognostic factors mean that primary-site surgery was avoided, contradicting the assumption of its therapeutic benefit.
There were also surgical discrepancies among the 5-year survivors. The registry listed 11 as having had breast surgery, while a chart review showed that surgery was not therapeutic. Five were listed as having no breast surgery, while they had indeed undergone a therapeutic operation.
Cancer registry data are not reliable for defining stage IV disease, discriminating advanced local disease with bone involvement from true distant, imaging-confirmed metastasis, or assessing surgical procedures. Case selection bias accounts for most, if not all, of the apparent survival advantage.
Dr. Blake Cady is professor of surgery (emeritus) at Harvard Medical School, Boston, and at Brown University, Providence, R.I.
Low Vitamin D Associated With Poor Prognostic Features in Breast Cancer
WASHINGTON – Vitamin D deficiency was not only twice as common in women undergoing breast cancer surgery, but it also was associated with poor-prognosis tumors in a case-control study that compared cancer patients with cancer-free women who had been tested for vitamin D.
The breast cancer patients had significantly lower mean vitamin D levels than did controls (33 ng/mL vs. 37 ng/mL), Dr. Kristin A. Skinner reported at the annual meeting of the American Association of Breast Surgeons. Patients were also more than twice as likely to have deficient levels (odds ratio, 2.4; P less than .01), she said.
Analyses presented by Dr. Skinner showed that mean vitamin D levels were significantly lower in the following subgroups of breast cancer patients:
• Those with estrogen receptornegative cancers vs. those with ER-positive cancers (28 ng/mL vs. 33 ng/mL; P = .04).
• Those with triple-negative cancers vs. those with cancers that were not triple negative (26 ng/mL vs.33 ng/mL; P -= .02).
• Those of the basal-like phenotype vs. those of the luminal A phenotype (24 ng/mL vs. 33 ng/mL; P = .04).
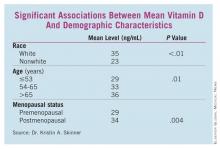
Some patient characteristics also carried significant associations with decreased vitamin D. White women, women aged 65 years and older, and those who were postmenopausal had significantly higher vitamin D levels than did nonwhite, younger, and premenopausal women, respectively. (See box.)
Although vitamin D levels were lower in patients with high Oncotype DX recurrence scores, progesterone receptornegative tumors, and invasive tumors, these differences were not statistically significant. Nor were family history or HER2, tumor, or nodal status significantly related to vitamin D levels, according to Dr. Skinner, a surgical oncologist and breast specialist at the cancer center of the University of Rochester (N.Y.).
In the case-control study, Dr. Skinner and her colleagues selected 194 women who were treated for breast cancer (stage 0-III) at the center and had total 25-hydroxy vitamin D levels drawn in the 3 months before or after their cancer surgery; the mean time of the blood draw was 30 days before surgery.
The patients were matched 1:1 with cancer-free controls who were drawn from a pool of more than 37,000 women who also underwent vitamin D testing in the university’s clinical labs in 2009-2010, the same time the cases were treated. The women were matched for age and the season of testing, since vitamin D levels can change as sun exposure varies.
The researchers divided vitamin D levels into tertiles: Optimal level was considered at least 32 ng/mL, suboptimal was 20-31 ng/mL, and deficient was less than 20 ng/mL.
The findings may argue for vitamin D testing and supplementation either in a primary care setting or in one devoted to breast health, Dr. Skinner said during a press briefing.
"At our institution, we routinely check vitamin D levels and replace them until they are well into the normal range," which is greater than 32 ng/mL, she said. "We really aim for a level of about 50 ng/mL, and titrate their replacement to those levels. In terms of taking supplements, we usually recommend starting at 1,000-2,000 IU daily, but the most effective way is to check levels, and replace accordingly."
Extant epidemiologic data have consistently found a link between more aggressive breast cancers and low vitamin D levels, she said, describing the relationship as biologically plausible. "The vitamin D receptor appears to modulate cell cycles, including the proliferation and differentiation of cells and the activation of apoptosis. Some studies have shown that vitamin D supplementation reduces the risk of breast cancer and improves survival outcomes, but very little is known about vitamin D levels and standard prognostic factors in breast cancer," she said.
"These findings may explain the associations seen in the epidemiologic studies, and may help explain why the black and other nonwhite populations tend to get more-aggressive breast cancer, and get breast cancer at a younger age," Dr. Skinner said.
She had no financial declarations with regard to the work.
WASHINGTON – Vitamin D deficiency was not only twice as common in women undergoing breast cancer surgery, but it also was associated with poor-prognosis tumors in a case-control study that compared cancer patients with cancer-free women who had been tested for vitamin D.
The breast cancer patients had significantly lower mean vitamin D levels than did controls (33 ng/mL vs. 37 ng/mL), Dr. Kristin A. Skinner reported at the annual meeting of the American Association of Breast Surgeons. Patients were also more than twice as likely to have deficient levels (odds ratio, 2.4; P less than .01), she said.
Analyses presented by Dr. Skinner showed that mean vitamin D levels were significantly lower in the following subgroups of breast cancer patients:
• Those with estrogen receptornegative cancers vs. those with ER-positive cancers (28 ng/mL vs. 33 ng/mL; P = .04).
• Those with triple-negative cancers vs. those with cancers that were not triple negative (26 ng/mL vs.33 ng/mL; P -= .02).
• Those of the basal-like phenotype vs. those of the luminal A phenotype (24 ng/mL vs. 33 ng/mL; P = .04).
Some patient characteristics also carried significant associations with decreased vitamin D. White women, women aged 65 years and older, and those who were postmenopausal had significantly higher vitamin D levels than did nonwhite, younger, and premenopausal women, respectively. (See box.)
Although vitamin D levels were lower in patients with high Oncotype DX recurrence scores, progesterone receptornegative tumors, and invasive tumors, these differences were not statistically significant. Nor were family history or HER2, tumor, or nodal status significantly related to vitamin D levels, according to Dr. Skinner, a surgical oncologist and breast specialist at the cancer center of the University of Rochester (N.Y.).
In the case-control study, Dr. Skinner and her colleagues selected 194 women who were treated for breast cancer (stage 0-III) at the center and had total 25-hydroxy vitamin D levels drawn in the 3 months before or after their cancer surgery; the mean time of the blood draw was 30 days before surgery.
The patients were matched 1:1 with cancer-free controls who were drawn from a pool of more than 37,000 women who also underwent vitamin D testing in the university’s clinical labs in 2009-2010, the same time the cases were treated. The women were matched for age and the season of testing, since vitamin D levels can change as sun exposure varies.
The researchers divided vitamin D levels into tertiles: Optimal level was considered at least 32 ng/mL, suboptimal was 20-31 ng/mL, and deficient was less than 20 ng/mL.
The findings may argue for vitamin D testing and supplementation either in a primary care setting or in one devoted to breast health, Dr. Skinner said during a press briefing.
"At our institution, we routinely check vitamin D levels and replace them until they are well into the normal range," which is greater than 32 ng/mL, she said. "We really aim for a level of about 50 ng/mL, and titrate their replacement to those levels. In terms of taking supplements, we usually recommend starting at 1,000-2,000 IU daily, but the most effective way is to check levels, and replace accordingly."
Extant epidemiologic data have consistently found a link between more aggressive breast cancers and low vitamin D levels, she said, describing the relationship as biologically plausible. "The vitamin D receptor appears to modulate cell cycles, including the proliferation and differentiation of cells and the activation of apoptosis. Some studies have shown that vitamin D supplementation reduces the risk of breast cancer and improves survival outcomes, but very little is known about vitamin D levels and standard prognostic factors in breast cancer," she said.
"These findings may explain the associations seen in the epidemiologic studies, and may help explain why the black and other nonwhite populations tend to get more-aggressive breast cancer, and get breast cancer at a younger age," Dr. Skinner said.
She had no financial declarations with regard to the work.
WASHINGTON – Vitamin D deficiency was not only twice as common in women undergoing breast cancer surgery, but it also was associated with poor-prognosis tumors in a case-control study that compared cancer patients with cancer-free women who had been tested for vitamin D.
The breast cancer patients had significantly lower mean vitamin D levels than did controls (33 ng/mL vs. 37 ng/mL), Dr. Kristin A. Skinner reported at the annual meeting of the American Association of Breast Surgeons. Patients were also more than twice as likely to have deficient levels (odds ratio, 2.4; P less than .01), she said.
Analyses presented by Dr. Skinner showed that mean vitamin D levels were significantly lower in the following subgroups of breast cancer patients:
• Those with estrogen receptornegative cancers vs. those with ER-positive cancers (28 ng/mL vs. 33 ng/mL; P = .04).
• Those with triple-negative cancers vs. those with cancers that were not triple negative (26 ng/mL vs.33 ng/mL; P -= .02).
• Those of the basal-like phenotype vs. those of the luminal A phenotype (24 ng/mL vs. 33 ng/mL; P = .04).
Some patient characteristics also carried significant associations with decreased vitamin D. White women, women aged 65 years and older, and those who were postmenopausal had significantly higher vitamin D levels than did nonwhite, younger, and premenopausal women, respectively. (See box.)
Although vitamin D levels were lower in patients with high Oncotype DX recurrence scores, progesterone receptornegative tumors, and invasive tumors, these differences were not statistically significant. Nor were family history or HER2, tumor, or nodal status significantly related to vitamin D levels, according to Dr. Skinner, a surgical oncologist and breast specialist at the cancer center of the University of Rochester (N.Y.).
In the case-control study, Dr. Skinner and her colleagues selected 194 women who were treated for breast cancer (stage 0-III) at the center and had total 25-hydroxy vitamin D levels drawn in the 3 months before or after their cancer surgery; the mean time of the blood draw was 30 days before surgery.
The patients were matched 1:1 with cancer-free controls who were drawn from a pool of more than 37,000 women who also underwent vitamin D testing in the university’s clinical labs in 2009-2010, the same time the cases were treated. The women were matched for age and the season of testing, since vitamin D levels can change as sun exposure varies.
The researchers divided vitamin D levels into tertiles: Optimal level was considered at least 32 ng/mL, suboptimal was 20-31 ng/mL, and deficient was less than 20 ng/mL.
The findings may argue for vitamin D testing and supplementation either in a primary care setting or in one devoted to breast health, Dr. Skinner said during a press briefing.
"At our institution, we routinely check vitamin D levels and replace them until they are well into the normal range," which is greater than 32 ng/mL, she said. "We really aim for a level of about 50 ng/mL, and titrate their replacement to those levels. In terms of taking supplements, we usually recommend starting at 1,000-2,000 IU daily, but the most effective way is to check levels, and replace accordingly."
Extant epidemiologic data have consistently found a link between more aggressive breast cancers and low vitamin D levels, she said, describing the relationship as biologically plausible. "The vitamin D receptor appears to modulate cell cycles, including the proliferation and differentiation of cells and the activation of apoptosis. Some studies have shown that vitamin D supplementation reduces the risk of breast cancer and improves survival outcomes, but very little is known about vitamin D levels and standard prognostic factors in breast cancer," she said.
"These findings may explain the associations seen in the epidemiologic studies, and may help explain why the black and other nonwhite populations tend to get more-aggressive breast cancer, and get breast cancer at a younger age," Dr. Skinner said.
She had no financial declarations with regard to the work.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF BREAST SURGEONS
Finding: Vitamin D deficiency was more than twice as common as normal levels in women undergoing surgery for breast cancer (OR, 2.4).
Data Source: A case-control study of vitamin D levels in 194 women with breast cancer matched 1:1 to a control population.
Disclosures: Dr. Skinner said she had no relevant disclosures.
Low Vitamin D Associated With Poor Prognostic Features in Breast Cancer
WASHINGTON – Vitamin D deficiency was not only twice as common in women undergoing breast cancer surgery, but it also was associated with poor-prognosis tumors in a case-control study that compared cancer patients with cancer-free women who had been tested for vitamin D.
The breast cancer patients had significantly lower mean vitamin D levels than did controls (33 ng/mL vs. 37 ng/mL), Dr. Kristin A. Skinner reported at the annual meeting of the American Association of Breast Surgeons. Patients were also more than twice as likely to have deficient levels (odds ratio, 2.4; P less than .01), she said.
Analyses presented by Dr. Skinner showed that mean vitamin D levels were significantly lower in the following subgroups of breast cancer patients:
• Those with estrogen receptornegative cancers vs. those with ER-positive cancers (28 ng/mL vs. 33 ng/mL; P = .04).
• Those with triple-negative cancers vs. those with cancers that were not triple negative (26 ng/mL vs.33 ng/mL; P -= .02).
• Those of the basal-like phenotype vs. those of the luminal A phenotype (24 ng/mL vs. 33 ng/mL; P = .04).
Some patient characteristics also carried significant associations with decreased vitamin D. White women, women aged 65 years and older, and those who were postmenopausal had significantly higher vitamin D levels than did nonwhite, younger, and premenopausal women, respectively. (See box.)
Although vitamin D levels were lower in patients with high Oncotype DX recurrence scores, progesterone receptornegative tumors, and invasive tumors, these differences were not statistically significant. Nor were family history or HER2, tumor, or nodal status significantly related to vitamin D levels, according to Dr. Skinner, a surgical oncologist and breast specialist at the cancer center of the University of Rochester (N.Y.).
In the case-control study, Dr. Skinner and her colleagues selected 194 women who were treated for breast cancer (stage 0-III) at the center and had total 25-hydroxy vitamin D levels drawn in the 3 months before or after their cancer surgery; the mean time of the blood draw was 30 days before surgery.
The patients were matched 1:1 with cancer-free controls who were drawn from a pool of more than 37,000 women who also underwent vitamin D testing in the university’s clinical labs in 2009-2010, the same time the cases were treated. The women were matched for age and the season of testing, since vitamin D levels can change as sun exposure varies.
The researchers divided vitamin D levels into tertiles: Optimal level was considered at least 32 ng/mL, suboptimal was 20-31 ng/mL, and deficient was less than 20 ng/mL.
The findings may argue for vitamin D testing and supplementation either in a primary care setting or in one devoted to breast health, Dr. Skinner said during a press briefing.
"At our institution, we routinely check vitamin D levels and replace them until they are well into the normal range," which is greater than 32 ng/mL, she said. "We really aim for a level of about 50 ng/mL, and titrate their replacement to those levels. In terms of taking supplements, we usually recommend starting at 1,000-2,000 IU daily, but the most effective way is to check levels, and replace accordingly."
Extant epidemiologic data have consistently found a link between more aggressive breast cancers and low vitamin D levels, she said, describing the relationship as biologically plausible. "The vitamin D receptor appears to modulate cell cycles, including the proliferation and differentiation of cells and the activation of apoptosis. Some studies have shown that vitamin D supplementation reduces the risk of breast cancer and improves survival outcomes, but very little is known about vitamin D levels and standard prognostic factors in breast cancer," she said.
"These findings may explain the associations seen in the epidemiologic studies, and may help explain why the black and other nonwhite populations tend to get more-aggressive breast cancer, and get breast cancer at a younger age," Dr. Skinner said.
She had no financial declarations with regard to the work.
WASHINGTON – Vitamin D deficiency was not only twice as common in women undergoing breast cancer surgery, but it also was associated with poor-prognosis tumors in a case-control study that compared cancer patients with cancer-free women who had been tested for vitamin D.
The breast cancer patients had significantly lower mean vitamin D levels than did controls (33 ng/mL vs. 37 ng/mL), Dr. Kristin A. Skinner reported at the annual meeting of the American Association of Breast Surgeons. Patients were also more than twice as likely to have deficient levels (odds ratio, 2.4; P less than .01), she said.
Analyses presented by Dr. Skinner showed that mean vitamin D levels were significantly lower in the following subgroups of breast cancer patients:
• Those with estrogen receptornegative cancers vs. those with ER-positive cancers (28 ng/mL vs. 33 ng/mL; P = .04).
• Those with triple-negative cancers vs. those with cancers that were not triple negative (26 ng/mL vs.33 ng/mL; P -= .02).
• Those of the basal-like phenotype vs. those of the luminal A phenotype (24 ng/mL vs. 33 ng/mL; P = .04).
Some patient characteristics also carried significant associations with decreased vitamin D. White women, women aged 65 years and older, and those who were postmenopausal had significantly higher vitamin D levels than did nonwhite, younger, and premenopausal women, respectively. (See box.)
Although vitamin D levels were lower in patients with high Oncotype DX recurrence scores, progesterone receptornegative tumors, and invasive tumors, these differences were not statistically significant. Nor were family history or HER2, tumor, or nodal status significantly related to vitamin D levels, according to Dr. Skinner, a surgical oncologist and breast specialist at the cancer center of the University of Rochester (N.Y.).
In the case-control study, Dr. Skinner and her colleagues selected 194 women who were treated for breast cancer (stage 0-III) at the center and had total 25-hydroxy vitamin D levels drawn in the 3 months before or after their cancer surgery; the mean time of the blood draw was 30 days before surgery.
The patients were matched 1:1 with cancer-free controls who were drawn from a pool of more than 37,000 women who also underwent vitamin D testing in the university’s clinical labs in 2009-2010, the same time the cases were treated. The women were matched for age and the season of testing, since vitamin D levels can change as sun exposure varies.
The researchers divided vitamin D levels into tertiles: Optimal level was considered at least 32 ng/mL, suboptimal was 20-31 ng/mL, and deficient was less than 20 ng/mL.
The findings may argue for vitamin D testing and supplementation either in a primary care setting or in one devoted to breast health, Dr. Skinner said during a press briefing.
"At our institution, we routinely check vitamin D levels and replace them until they are well into the normal range," which is greater than 32 ng/mL, she said. "We really aim for a level of about 50 ng/mL, and titrate their replacement to those levels. In terms of taking supplements, we usually recommend starting at 1,000-2,000 IU daily, but the most effective way is to check levels, and replace accordingly."
Extant epidemiologic data have consistently found a link between more aggressive breast cancers and low vitamin D levels, she said, describing the relationship as biologically plausible. "The vitamin D receptor appears to modulate cell cycles, including the proliferation and differentiation of cells and the activation of apoptosis. Some studies have shown that vitamin D supplementation reduces the risk of breast cancer and improves survival outcomes, but very little is known about vitamin D levels and standard prognostic factors in breast cancer," she said.
"These findings may explain the associations seen in the epidemiologic studies, and may help explain why the black and other nonwhite populations tend to get more-aggressive breast cancer, and get breast cancer at a younger age," Dr. Skinner said.
She had no financial declarations with regard to the work.
WASHINGTON – Vitamin D deficiency was not only twice as common in women undergoing breast cancer surgery, but it also was associated with poor-prognosis tumors in a case-control study that compared cancer patients with cancer-free women who had been tested for vitamin D.
The breast cancer patients had significantly lower mean vitamin D levels than did controls (33 ng/mL vs. 37 ng/mL), Dr. Kristin A. Skinner reported at the annual meeting of the American Association of Breast Surgeons. Patients were also more than twice as likely to have deficient levels (odds ratio, 2.4; P less than .01), she said.
Analyses presented by Dr. Skinner showed that mean vitamin D levels were significantly lower in the following subgroups of breast cancer patients:
• Those with estrogen receptornegative cancers vs. those with ER-positive cancers (28 ng/mL vs. 33 ng/mL; P = .04).
• Those with triple-negative cancers vs. those with cancers that were not triple negative (26 ng/mL vs.33 ng/mL; P -= .02).
• Those of the basal-like phenotype vs. those of the luminal A phenotype (24 ng/mL vs. 33 ng/mL; P = .04).
Some patient characteristics also carried significant associations with decreased vitamin D. White women, women aged 65 years and older, and those who were postmenopausal had significantly higher vitamin D levels than did nonwhite, younger, and premenopausal women, respectively. (See box.)
Although vitamin D levels were lower in patients with high Oncotype DX recurrence scores, progesterone receptornegative tumors, and invasive tumors, these differences were not statistically significant. Nor were family history or HER2, tumor, or nodal status significantly related to vitamin D levels, according to Dr. Skinner, a surgical oncologist and breast specialist at the cancer center of the University of Rochester (N.Y.).
In the case-control study, Dr. Skinner and her colleagues selected 194 women who were treated for breast cancer (stage 0-III) at the center and had total 25-hydroxy vitamin D levels drawn in the 3 months before or after their cancer surgery; the mean time of the blood draw was 30 days before surgery.
The patients were matched 1:1 with cancer-free controls who were drawn from a pool of more than 37,000 women who also underwent vitamin D testing in the university’s clinical labs in 2009-2010, the same time the cases were treated. The women were matched for age and the season of testing, since vitamin D levels can change as sun exposure varies.
The researchers divided vitamin D levels into tertiles: Optimal level was considered at least 32 ng/mL, suboptimal was 20-31 ng/mL, and deficient was less than 20 ng/mL.
The findings may argue for vitamin D testing and supplementation either in a primary care setting or in one devoted to breast health, Dr. Skinner said during a press briefing.
"At our institution, we routinely check vitamin D levels and replace them until they are well into the normal range," which is greater than 32 ng/mL, she said. "We really aim for a level of about 50 ng/mL, and titrate their replacement to those levels. In terms of taking supplements, we usually recommend starting at 1,000-2,000 IU daily, but the most effective way is to check levels, and replace accordingly."
Extant epidemiologic data have consistently found a link between more aggressive breast cancers and low vitamin D levels, she said, describing the relationship as biologically plausible. "The vitamin D receptor appears to modulate cell cycles, including the proliferation and differentiation of cells and the activation of apoptosis. Some studies have shown that vitamin D supplementation reduces the risk of breast cancer and improves survival outcomes, but very little is known about vitamin D levels and standard prognostic factors in breast cancer," she said.
"These findings may explain the associations seen in the epidemiologic studies, and may help explain why the black and other nonwhite populations tend to get more-aggressive breast cancer, and get breast cancer at a younger age," Dr. Skinner said.
She had no financial declarations with regard to the work.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF BREAST SURGEONS
Finding: Vitamin D deficiency was more than twice as common as normal levels in women undergoing surgery for breast cancer (OR, 2.4).
Data Source: A case-control study of vitamin D levels in 194 women with breast cancer matched 1:1 to a control population.
Disclosures: Dr. Skinner said she had no relevant disclosures.
Low Vitamin D Associated With Poor Prognostic Features in Breast Cancer
WASHINGTON – Vitamin D deficiency was not only twice as common in women undergoing breast cancer surgery, but it also was associated with poor-prognosis tumors in a case-control study that compared cancer patients with cancer-free women who had been tested for vitamin D.
The breast cancer patients had significantly lower mean vitamin D levels than did controls (33 ng/mL vs. 37 ng/mL), Dr. Kristin A. Skinner reported at the annual meeting of the American Association of Breast Surgeons. Patients were also more than twice as likely to have deficient levels (odds ratio, 2.4; P less than .01), she said.
Analyses presented by Dr. Skinner showed that mean vitamin D levels were significantly lower in the following subgroups of breast cancer patients:
• Those with estrogen receptornegative cancers vs. those with ER-positive cancers (28 ng/mL vs. 33 ng/mL; P = .04).
• Those with triple-negative cancers vs. those with cancers that were not triple negative (26 ng/mL vs.33 ng/mL; P -= .02).
• Those of the basal-like phenotype vs. those of the luminal A phenotype (24 ng/mL vs. 33 ng/mL; P = .04).
Some patient characteristics also carried significant associations with decreased vitamin D. White women, women aged 65 years and older, and those who were postmenopausal had significantly higher vitamin D levels than did nonwhite, younger, and premenopausal women, respectively. (See box.)
Although vitamin D levels were lower in patients with high Oncotype DX recurrence scores, progesterone receptornegative tumors, and invasive tumors, these differences were not statistically significant. Nor were family history or HER2, tumor, or nodal status significantly related to vitamin D levels, according to Dr. Skinner, a surgical oncologist and breast specialist at the cancer center of the University of Rochester (N.Y.).
In the case-control study, Dr. Skinner and her colleagues selected 194 women who were treated for breast cancer (stage 0-III) at the center and had total 25-hydroxy vitamin D levels drawn in the 3 months before or after their cancer surgery; the mean time of the blood draw was 30 days before surgery.
The patients were matched 1:1 with cancer-free controls who were drawn from a pool of more than 37,000 women who also underwent vitamin D testing in the university’s clinical labs in 2009-2010, the same time the cases were treated. The women were matched for age and the season of testing, since vitamin D levels can change as sun exposure varies.
The researchers divided vitamin D levels into tertiles: Optimal level was considered at least 32 ng/mL, suboptimal was 20-31 ng/mL, and deficient was less than 20 ng/mL.
The findings may argue for vitamin D testing and supplementation either in a primary care setting or in one devoted to breast health, Dr. Skinner said during a press briefing.
"At our institution, we routinely check vitamin D levels and replace them until they are well into the normal range," which is greater than 32 ng/mL, she said. "We really aim for a level of about 50 ng/mL, and titrate their replacement to those levels. In terms of taking supplements, we usually recommend starting at 1,000-2,000 IU daily, but the most effective way is to check levels, and replace accordingly."
Extant epidemiologic data have consistently found a link between more aggressive breast cancers and low vitamin D levels, she said, describing the relationship as biologically plausible. "The vitamin D receptor appears to modulate cell cycles, including the proliferation and differentiation of cells and the activation of apoptosis. Some studies have shown that vitamin D supplementation reduces the risk of breast cancer and improves survival outcomes, but very little is known about vitamin D levels and standard prognostic factors in breast cancer," she said.
"These findings may explain the associations seen in the epidemiologic studies, and may help explain why the black and other nonwhite populations tend to get more-aggressive breast cancer, and get breast cancer at a younger age," Dr. Skinner said.
She had no financial declarations with regard to the work.
WASHINGTON – Vitamin D deficiency was not only twice as common in women undergoing breast cancer surgery, but it also was associated with poor-prognosis tumors in a case-control study that compared cancer patients with cancer-free women who had been tested for vitamin D.
The breast cancer patients had significantly lower mean vitamin D levels than did controls (33 ng/mL vs. 37 ng/mL), Dr. Kristin A. Skinner reported at the annual meeting of the American Association of Breast Surgeons. Patients were also more than twice as likely to have deficient levels (odds ratio, 2.4; P less than .01), she said.
Analyses presented by Dr. Skinner showed that mean vitamin D levels were significantly lower in the following subgroups of breast cancer patients:
• Those with estrogen receptornegative cancers vs. those with ER-positive cancers (28 ng/mL vs. 33 ng/mL; P = .04).
• Those with triple-negative cancers vs. those with cancers that were not triple negative (26 ng/mL vs.33 ng/mL; P -= .02).
• Those of the basal-like phenotype vs. those of the luminal A phenotype (24 ng/mL vs. 33 ng/mL; P = .04).
Some patient characteristics also carried significant associations with decreased vitamin D. White women, women aged 65 years and older, and those who were postmenopausal had significantly higher vitamin D levels than did nonwhite, younger, and premenopausal women, respectively. (See box.)
Although vitamin D levels were lower in patients with high Oncotype DX recurrence scores, progesterone receptornegative tumors, and invasive tumors, these differences were not statistically significant. Nor were family history or HER2, tumor, or nodal status significantly related to vitamin D levels, according to Dr. Skinner, a surgical oncologist and breast specialist at the cancer center of the University of Rochester (N.Y.).
In the case-control study, Dr. Skinner and her colleagues selected 194 women who were treated for breast cancer (stage 0-III) at the center and had total 25-hydroxy vitamin D levels drawn in the 3 months before or after their cancer surgery; the mean time of the blood draw was 30 days before surgery.
The patients were matched 1:1 with cancer-free controls who were drawn from a pool of more than 37,000 women who also underwent vitamin D testing in the university’s clinical labs in 2009-2010, the same time the cases were treated. The women were matched for age and the season of testing, since vitamin D levels can change as sun exposure varies.
The researchers divided vitamin D levels into tertiles: Optimal level was considered at least 32 ng/mL, suboptimal was 20-31 ng/mL, and deficient was less than 20 ng/mL.
The findings may argue for vitamin D testing and supplementation either in a primary care setting or in one devoted to breast health, Dr. Skinner said during a press briefing.
"At our institution, we routinely check vitamin D levels and replace them until they are well into the normal range," which is greater than 32 ng/mL, she said. "We really aim for a level of about 50 ng/mL, and titrate their replacement to those levels. In terms of taking supplements, we usually recommend starting at 1,000-2,000 IU daily, but the most effective way is to check levels, and replace accordingly."
Extant epidemiologic data have consistently found a link between more aggressive breast cancers and low vitamin D levels, she said, describing the relationship as biologically plausible. "The vitamin D receptor appears to modulate cell cycles, including the proliferation and differentiation of cells and the activation of apoptosis. Some studies have shown that vitamin D supplementation reduces the risk of breast cancer and improves survival outcomes, but very little is known about vitamin D levels and standard prognostic factors in breast cancer," she said.
"These findings may explain the associations seen in the epidemiologic studies, and may help explain why the black and other nonwhite populations tend to get more-aggressive breast cancer, and get breast cancer at a younger age," Dr. Skinner said.
She had no financial declarations with regard to the work.
WASHINGTON – Vitamin D deficiency was not only twice as common in women undergoing breast cancer surgery, but it also was associated with poor-prognosis tumors in a case-control study that compared cancer patients with cancer-free women who had been tested for vitamin D.
The breast cancer patients had significantly lower mean vitamin D levels than did controls (33 ng/mL vs. 37 ng/mL), Dr. Kristin A. Skinner reported at the annual meeting of the American Association of Breast Surgeons. Patients were also more than twice as likely to have deficient levels (odds ratio, 2.4; P less than .01), she said.
Analyses presented by Dr. Skinner showed that mean vitamin D levels were significantly lower in the following subgroups of breast cancer patients:
• Those with estrogen receptornegative cancers vs. those with ER-positive cancers (28 ng/mL vs. 33 ng/mL; P = .04).
• Those with triple-negative cancers vs. those with cancers that were not triple negative (26 ng/mL vs.33 ng/mL; P -= .02).
• Those of the basal-like phenotype vs. those of the luminal A phenotype (24 ng/mL vs. 33 ng/mL; P = .04).
Some patient characteristics also carried significant associations with decreased vitamin D. White women, women aged 65 years and older, and those who were postmenopausal had significantly higher vitamin D levels than did nonwhite, younger, and premenopausal women, respectively. (See box.)
Although vitamin D levels were lower in patients with high Oncotype DX recurrence scores, progesterone receptornegative tumors, and invasive tumors, these differences were not statistically significant. Nor were family history or HER2, tumor, or nodal status significantly related to vitamin D levels, according to Dr. Skinner, a surgical oncologist and breast specialist at the cancer center of the University of Rochester (N.Y.).
In the case-control study, Dr. Skinner and her colleagues selected 194 women who were treated for breast cancer (stage 0-III) at the center and had total 25-hydroxy vitamin D levels drawn in the 3 months before or after their cancer surgery; the mean time of the blood draw was 30 days before surgery.
The patients were matched 1:1 with cancer-free controls who were drawn from a pool of more than 37,000 women who also underwent vitamin D testing in the university’s clinical labs in 2009-2010, the same time the cases were treated. The women were matched for age and the season of testing, since vitamin D levels can change as sun exposure varies.
The researchers divided vitamin D levels into tertiles: Optimal level was considered at least 32 ng/mL, suboptimal was 20-31 ng/mL, and deficient was less than 20 ng/mL.
The findings may argue for vitamin D testing and supplementation either in a primary care setting or in one devoted to breast health, Dr. Skinner said during a press briefing.
"At our institution, we routinely check vitamin D levels and replace them until they are well into the normal range," which is greater than 32 ng/mL, she said. "We really aim for a level of about 50 ng/mL, and titrate their replacement to those levels. In terms of taking supplements, we usually recommend starting at 1,000-2,000 IU daily, but the most effective way is to check levels, and replace accordingly."
Extant epidemiologic data have consistently found a link between more aggressive breast cancers and low vitamin D levels, she said, describing the relationship as biologically plausible. "The vitamin D receptor appears to modulate cell cycles, including the proliferation and differentiation of cells and the activation of apoptosis. Some studies have shown that vitamin D supplementation reduces the risk of breast cancer and improves survival outcomes, but very little is known about vitamin D levels and standard prognostic factors in breast cancer," she said.
"These findings may explain the associations seen in the epidemiologic studies, and may help explain why the black and other nonwhite populations tend to get more-aggressive breast cancer, and get breast cancer at a younger age," Dr. Skinner said.
She had no financial declarations with regard to the work.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF BREAST SURGEONS
Finding: Vitamin D deficiency was more than twice as common as normal levels in women undergoing surgery for breast cancer (OR, 2.4).
Data Source: A case-control study of vitamin D levels in 194 women with breast cancer matched 1:1 to a control population.
Disclosures: Dr. Skinner said she had no relevant disclosures.
Radioactive Seeds Guide Surgeons to Nonpalpable Breast Lesions
WASHINGTON – Radioactive seeds were a safe and effective method of pinpointing nonpalpable breast lesions for surgery, with an 85% rate of negative margins on the first excision and an ipsilateral recurrence rate of less than 2% over 33 months, according to a large retrospective study.
Close margins occurred in 12% of the 767 patients with nonpalpable breast lesions, and positive margins occurred in 3%. The overall re-excision rate was 15%, Dr. Lee McGhan of the Mayo Clinic, Scottsdale, Ariz., said at the annual meeting of the American Society of Breast Surgeons.
Performing the procedure is "almost intuitive," with a very low learning curve, he said. "It is now our method of choice when dealing with preoperative localization of nonpalpable breast lesions."
His retrospective review of 978 prospectively collected patient records comprised 1,000 radioactive in 2003-2010. Almost 1,150 seeds were deployed.
The patients’ mean age was 65 years; their mean lesion size was 1.2 cm. Most (550) had an invasive carcinoma; 217 had DCIS (ductal carcinoma in situ); 115 had atypical hyperplasia, and the remainder, uncertain or suspicious percutaneous biopsy results.
Dr. McGhan reported 33-month follow-up results on the 767 women with either invasive carcinoma or DCIS. Most patients (910) received just one seed; 84 received two seeds; 5 received three seeds; and 1 patient received four of the devices.
Most (76%) underwent the procedure at least 1 day before surgery. Typically, Dr. McGhan said, patients came to the clinic a few days before surgery for an evaluation. Many chose to have the localization on a Friday, stayed over the weekend, and had the seeds removed early on Monday morning.
The 4- to 5-mm seeds containing radioactive iodine-125 can be placed up to 5 days before surgery. They were deployed through an 18-gauge spinal needle under image guidance; post deployment, a mammogram or ultrasound confirmed their position near the lesion. "We used a handheld gamma probe to identify the area of greatest radioactivity at the skin surface, marking the optimal site of skin incision," Dr. McGhan said.
Intraoperative complications included 30 displaced seeds – including 3 suctioned up by operative tubing and 3 that were improperly deployed during radiology – as well as one instance of an incorrect incision site resulting from a miscommunication between the radiologist and the surgeon, Dr. McGhan said. All of these seeds were retrieved with no patient harm.
All of the localized lesions were successfully removed, along with their associated seeds; the specimens were sent to pathology. Among the 550 invasive cancers, margins were negative in 87%, close in 9%, and positive in 3%. Re-excision was required in 13% (69).
Among the 217 DCIS lesions, margins were negative in 77%, close in 19%, and positive in 3%. Re-excision was necessary in 23% (49).
Sentinel node biopsies occurred in 544 cases, and were successful in all but one, Dr. McGhan said. "There was no blue dye detected, which was determined to be due to tumor invasion of the lymphatics rather than a direct complication of the procedure."
The mean follow-up period was 33 months. Over this time, the overall ipsilateral recurrence rate was 1.6% (12 patients). The rate was slightly higher among patients with DCIS (3%; seven patients). The local recurrence rate was 1% (five patients) among those with invasive cancer. By the end of the follow-up period, there were six mastectomies secondary to recurrence: three (0.5%) in the invasive cancer group and three (1%) in the DCIS group.
Dr. McGhan had no financial declarations.
WASHINGTON – Radioactive seeds were a safe and effective method of pinpointing nonpalpable breast lesions for surgery, with an 85% rate of negative margins on the first excision and an ipsilateral recurrence rate of less than 2% over 33 months, according to a large retrospective study.
Close margins occurred in 12% of the 767 patients with nonpalpable breast lesions, and positive margins occurred in 3%. The overall re-excision rate was 15%, Dr. Lee McGhan of the Mayo Clinic, Scottsdale, Ariz., said at the annual meeting of the American Society of Breast Surgeons.
Performing the procedure is "almost intuitive," with a very low learning curve, he said. "It is now our method of choice when dealing with preoperative localization of nonpalpable breast lesions."
His retrospective review of 978 prospectively collected patient records comprised 1,000 radioactive in 2003-2010. Almost 1,150 seeds were deployed.
The patients’ mean age was 65 years; their mean lesion size was 1.2 cm. Most (550) had an invasive carcinoma; 217 had DCIS (ductal carcinoma in situ); 115 had atypical hyperplasia, and the remainder, uncertain or suspicious percutaneous biopsy results.
Dr. McGhan reported 33-month follow-up results on the 767 women with either invasive carcinoma or DCIS. Most patients (910) received just one seed; 84 received two seeds; 5 received three seeds; and 1 patient received four of the devices.
Most (76%) underwent the procedure at least 1 day before surgery. Typically, Dr. McGhan said, patients came to the clinic a few days before surgery for an evaluation. Many chose to have the localization on a Friday, stayed over the weekend, and had the seeds removed early on Monday morning.
The 4- to 5-mm seeds containing radioactive iodine-125 can be placed up to 5 days before surgery. They were deployed through an 18-gauge spinal needle under image guidance; post deployment, a mammogram or ultrasound confirmed their position near the lesion. "We used a handheld gamma probe to identify the area of greatest radioactivity at the skin surface, marking the optimal site of skin incision," Dr. McGhan said.
Intraoperative complications included 30 displaced seeds – including 3 suctioned up by operative tubing and 3 that were improperly deployed during radiology – as well as one instance of an incorrect incision site resulting from a miscommunication between the radiologist and the surgeon, Dr. McGhan said. All of these seeds were retrieved with no patient harm.
All of the localized lesions were successfully removed, along with their associated seeds; the specimens were sent to pathology. Among the 550 invasive cancers, margins were negative in 87%, close in 9%, and positive in 3%. Re-excision was required in 13% (69).
Among the 217 DCIS lesions, margins were negative in 77%, close in 19%, and positive in 3%. Re-excision was necessary in 23% (49).
Sentinel node biopsies occurred in 544 cases, and were successful in all but one, Dr. McGhan said. "There was no blue dye detected, which was determined to be due to tumor invasion of the lymphatics rather than a direct complication of the procedure."
The mean follow-up period was 33 months. Over this time, the overall ipsilateral recurrence rate was 1.6% (12 patients). The rate was slightly higher among patients with DCIS (3%; seven patients). The local recurrence rate was 1% (five patients) among those with invasive cancer. By the end of the follow-up period, there were six mastectomies secondary to recurrence: three (0.5%) in the invasive cancer group and three (1%) in the DCIS group.
Dr. McGhan had no financial declarations.
WASHINGTON – Radioactive seeds were a safe and effective method of pinpointing nonpalpable breast lesions for surgery, with an 85% rate of negative margins on the first excision and an ipsilateral recurrence rate of less than 2% over 33 months, according to a large retrospective study.
Close margins occurred in 12% of the 767 patients with nonpalpable breast lesions, and positive margins occurred in 3%. The overall re-excision rate was 15%, Dr. Lee McGhan of the Mayo Clinic, Scottsdale, Ariz., said at the annual meeting of the American Society of Breast Surgeons.
Performing the procedure is "almost intuitive," with a very low learning curve, he said. "It is now our method of choice when dealing with preoperative localization of nonpalpable breast lesions."
His retrospective review of 978 prospectively collected patient records comprised 1,000 radioactive in 2003-2010. Almost 1,150 seeds were deployed.
The patients’ mean age was 65 years; their mean lesion size was 1.2 cm. Most (550) had an invasive carcinoma; 217 had DCIS (ductal carcinoma in situ); 115 had atypical hyperplasia, and the remainder, uncertain or suspicious percutaneous biopsy results.
Dr. McGhan reported 33-month follow-up results on the 767 women with either invasive carcinoma or DCIS. Most patients (910) received just one seed; 84 received two seeds; 5 received three seeds; and 1 patient received four of the devices.
Most (76%) underwent the procedure at least 1 day before surgery. Typically, Dr. McGhan said, patients came to the clinic a few days before surgery for an evaluation. Many chose to have the localization on a Friday, stayed over the weekend, and had the seeds removed early on Monday morning.
The 4- to 5-mm seeds containing radioactive iodine-125 can be placed up to 5 days before surgery. They were deployed through an 18-gauge spinal needle under image guidance; post deployment, a mammogram or ultrasound confirmed their position near the lesion. "We used a handheld gamma probe to identify the area of greatest radioactivity at the skin surface, marking the optimal site of skin incision," Dr. McGhan said.
Intraoperative complications included 30 displaced seeds – including 3 suctioned up by operative tubing and 3 that were improperly deployed during radiology – as well as one instance of an incorrect incision site resulting from a miscommunication between the radiologist and the surgeon, Dr. McGhan said. All of these seeds were retrieved with no patient harm.
All of the localized lesions were successfully removed, along with their associated seeds; the specimens were sent to pathology. Among the 550 invasive cancers, margins were negative in 87%, close in 9%, and positive in 3%. Re-excision was required in 13% (69).
Among the 217 DCIS lesions, margins were negative in 77%, close in 19%, and positive in 3%. Re-excision was necessary in 23% (49).
Sentinel node biopsies occurred in 544 cases, and were successful in all but one, Dr. McGhan said. "There was no blue dye detected, which was determined to be due to tumor invasion of the lymphatics rather than a direct complication of the procedure."
The mean follow-up period was 33 months. Over this time, the overall ipsilateral recurrence rate was 1.6% (12 patients). The rate was slightly higher among patients with DCIS (3%; seven patients). The local recurrence rate was 1% (five patients) among those with invasive cancer. By the end of the follow-up period, there were six mastectomies secondary to recurrence: three (0.5%) in the invasive cancer group and three (1%) in the DCIS group.
Dr. McGhan had no financial declarations.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF BREAST SURGEONS
Major Finding: Radioactive seed localization resulted in initial negative margins in 85% of cases and an overall re-excision rate of 2%.
Data Source: A retrospective study of 767 women with invasive cancer or DCIS who were followed for a mean of 33 months.
Disclosures: Dr. McGhan had no financial declarations.
Mixed Outcomes When Radiation Is Added to Excision for DCIS
WASHINGTON – Five years might not be the magic number for a small group of women who are treated for the most curable form of breast cancer: ductal carcinoma in situ.
In a prospective study, women who underwent excision plus radiotherapy did have a significantly lower rate of local recurrence than did women who were treated with surgery alone. But among those who had a recurrence, it was almost twice as likely to be invasive. Additionally, these recurrences took almost twice as long to present: up to 105 months.
"This shows there is definitely an added benefit from radiation, but at the same time, we must be aware that patients who have radiation can still recur, and much later," Dr. Janie Weng Grumley said at a press briefing during the annual meeting of the American Society of Breast Surgeons.
"Many patients think, ‘after 5 years, I’m safe.’ This study really highlights the fact that there is a very different pattern of recurrence [in those who receive one of the two treatments]. We must be aware of this, and follow these women in a different way."
Dr. Grumley, a fellow at the University of Southern California in Los Angeles, presented a prospective study of 1,014 women with ductal carcinoma in situ (DCIS) who were treated with either excision alone (651) or excision plus radiotherapy (363). The average follow-up was significantly longer in the dual-therapy group (average, 109 months) than in the excision-only group (average, 72 months), reflecting the recent increase in lumpectomy-only treatment, Dr. Grumley said. The groups’ average age was 54 years.
The probability of any local recurrence was significantly lower in the dual-therapy group than in the lumpectomy group (18% vs. 30%; P = .0102). But invasive disease was significantly more common among those in the dual-therapy group who did recur (57% vs. 37%). The cancers also took significantly longer to recur, whether the study examined the mean time to any local recurrence (90 vs. 53 months; P less than or equal to .001), the mean time to DCIS recurrence (73 vs. 41 months; P = .002), or the mean time to invasive recurrence (105 vs. 72 months; P = .017).
Women who had surgery and radiation also experienced a significantly different pattern of recurrence, Dr. Grumley said. Among women treated only with surgery, 90% of recurrences were in the same quadrant, compared with 72% of recurrences in women who were treated with dual therapy (P = .0016). Conversely, 28% of recurrences after dual therapy occurred in a different quadrant, compared with 10% of those in the surgery-alone group. Lesions in different quadrants likely reflect new primary tumors, she said.
The increased rate of invasive, recurrent disease in the dual-therapy group was probably responsible for a small but statistically significant mortality difference (98% vs. 100%), Dr. Grumley said.
"This study shows that a small subgroup of irradiated DCIS patients may not be deriving maximum benefit from radiation therapy, or not benefiting from it at all," she said.
She was only able to speculate on the reason for the different recurrence patterns. "Radiation is a hypothesis, but we have nothing here that we can connect [as a] cause and effect. We do know that radiation does affect local recurrence, and we would never tell a patient not to get radiation. But this pattern is definitely something that should be further examined.
Dr. Grumley had no financial conflicts to declare.
WASHINGTON – Five years might not be the magic number for a small group of women who are treated for the most curable form of breast cancer: ductal carcinoma in situ.
In a prospective study, women who underwent excision plus radiotherapy did have a significantly lower rate of local recurrence than did women who were treated with surgery alone. But among those who had a recurrence, it was almost twice as likely to be invasive. Additionally, these recurrences took almost twice as long to present: up to 105 months.
"This shows there is definitely an added benefit from radiation, but at the same time, we must be aware that patients who have radiation can still recur, and much later," Dr. Janie Weng Grumley said at a press briefing during the annual meeting of the American Society of Breast Surgeons.
"Many patients think, ‘after 5 years, I’m safe.’ This study really highlights the fact that there is a very different pattern of recurrence [in those who receive one of the two treatments]. We must be aware of this, and follow these women in a different way."
Dr. Grumley, a fellow at the University of Southern California in Los Angeles, presented a prospective study of 1,014 women with ductal carcinoma in situ (DCIS) who were treated with either excision alone (651) or excision plus radiotherapy (363). The average follow-up was significantly longer in the dual-therapy group (average, 109 months) than in the excision-only group (average, 72 months), reflecting the recent increase in lumpectomy-only treatment, Dr. Grumley said. The groups’ average age was 54 years.
The probability of any local recurrence was significantly lower in the dual-therapy group than in the lumpectomy group (18% vs. 30%; P = .0102). But invasive disease was significantly more common among those in the dual-therapy group who did recur (57% vs. 37%). The cancers also took significantly longer to recur, whether the study examined the mean time to any local recurrence (90 vs. 53 months; P less than or equal to .001), the mean time to DCIS recurrence (73 vs. 41 months; P = .002), or the mean time to invasive recurrence (105 vs. 72 months; P = .017).
Women who had surgery and radiation also experienced a significantly different pattern of recurrence, Dr. Grumley said. Among women treated only with surgery, 90% of recurrences were in the same quadrant, compared with 72% of recurrences in women who were treated with dual therapy (P = .0016). Conversely, 28% of recurrences after dual therapy occurred in a different quadrant, compared with 10% of those in the surgery-alone group. Lesions in different quadrants likely reflect new primary tumors, she said.
The increased rate of invasive, recurrent disease in the dual-therapy group was probably responsible for a small but statistically significant mortality difference (98% vs. 100%), Dr. Grumley said.
"This study shows that a small subgroup of irradiated DCIS patients may not be deriving maximum benefit from radiation therapy, or not benefiting from it at all," she said.
She was only able to speculate on the reason for the different recurrence patterns. "Radiation is a hypothesis, but we have nothing here that we can connect [as a] cause and effect. We do know that radiation does affect local recurrence, and we would never tell a patient not to get radiation. But this pattern is definitely something that should be further examined.
Dr. Grumley had no financial conflicts to declare.
WASHINGTON – Five years might not be the magic number for a small group of women who are treated for the most curable form of breast cancer: ductal carcinoma in situ.
In a prospective study, women who underwent excision plus radiotherapy did have a significantly lower rate of local recurrence than did women who were treated with surgery alone. But among those who had a recurrence, it was almost twice as likely to be invasive. Additionally, these recurrences took almost twice as long to present: up to 105 months.
"This shows there is definitely an added benefit from radiation, but at the same time, we must be aware that patients who have radiation can still recur, and much later," Dr. Janie Weng Grumley said at a press briefing during the annual meeting of the American Society of Breast Surgeons.
"Many patients think, ‘after 5 years, I’m safe.’ This study really highlights the fact that there is a very different pattern of recurrence [in those who receive one of the two treatments]. We must be aware of this, and follow these women in a different way."
Dr. Grumley, a fellow at the University of Southern California in Los Angeles, presented a prospective study of 1,014 women with ductal carcinoma in situ (DCIS) who were treated with either excision alone (651) or excision plus radiotherapy (363). The average follow-up was significantly longer in the dual-therapy group (average, 109 months) than in the excision-only group (average, 72 months), reflecting the recent increase in lumpectomy-only treatment, Dr. Grumley said. The groups’ average age was 54 years.
The probability of any local recurrence was significantly lower in the dual-therapy group than in the lumpectomy group (18% vs. 30%; P = .0102). But invasive disease was significantly more common among those in the dual-therapy group who did recur (57% vs. 37%). The cancers also took significantly longer to recur, whether the study examined the mean time to any local recurrence (90 vs. 53 months; P less than or equal to .001), the mean time to DCIS recurrence (73 vs. 41 months; P = .002), or the mean time to invasive recurrence (105 vs. 72 months; P = .017).
Women who had surgery and radiation also experienced a significantly different pattern of recurrence, Dr. Grumley said. Among women treated only with surgery, 90% of recurrences were in the same quadrant, compared with 72% of recurrences in women who were treated with dual therapy (P = .0016). Conversely, 28% of recurrences after dual therapy occurred in a different quadrant, compared with 10% of those in the surgery-alone group. Lesions in different quadrants likely reflect new primary tumors, she said.
The increased rate of invasive, recurrent disease in the dual-therapy group was probably responsible for a small but statistically significant mortality difference (98% vs. 100%), Dr. Grumley said.
"This study shows that a small subgroup of irradiated DCIS patients may not be deriving maximum benefit from radiation therapy, or not benefiting from it at all," she said.
She was only able to speculate on the reason for the different recurrence patterns. "Radiation is a hypothesis, but we have nothing here that we can connect [as a] cause and effect. We do know that radiation does affect local recurrence, and we would never tell a patient not to get radiation. But this pattern is definitely something that should be further examined.
Dr. Grumley had no financial conflicts to declare.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF BREAST SURGEONS
Major Finding: Invasive cancer appeared in 57% of recurrences among women whose DCIS was treated with excision and radiation, compared with 37% of those in women treated with surgery alone.
Data Source: A prospective study of 1,014 women who were followed for up to 10 years.
Disclosures: Dr. Grumley had no financial conflicts to declare.
Mixed Outcomes When Radiation Is Added to Excision for DCIS
WASHINGTON – Five years might not be the magic number for a small group of women who are treated for the most curable form of breast cancer: ductal carcinoma in situ.
In a prospective study, women who underwent excision plus radiotherapy did have a significantly lower rate of local recurrence than did women who were treated with surgery alone. But among those who had a recurrence, it was almost twice as likely to be invasive. Additionally, these recurrences took almost twice as long to present: up to 105 months.
"This shows there is definitely an added benefit from radiation, but at the same time, we must be aware that patients who have radiation can still recur, and much later," Dr. Janie Weng Grumley said at a press briefing during the annual meeting of the American Society of Breast Surgeons.
"Many patients think, ‘after 5 years, I’m safe.’ This study really highlights the fact that there is a very different pattern of recurrence [in those who receive one of the two treatments]. We must be aware of this, and follow these women in a different way."
Dr. Grumley, a fellow at the University of Southern California in Los Angeles, presented a prospective study of 1,014 women with ductal carcinoma in situ (DCIS) who were treated with either excision alone (651) or excision plus radiotherapy (363). The average follow-up was significantly longer in the dual-therapy group (average, 109 months) than in the excision-only group (average, 72 months), reflecting the recent increase in lumpectomy-only treatment, Dr. Grumley said. The groups’ average age was 54 years.
The probability of any local recurrence was significantly lower in the dual-therapy group than in the lumpectomy group (18% vs. 30%; P = .0102). But invasive disease was significantly more common among those in the dual-therapy group who did recur (57% vs. 37%). The cancers also took significantly longer to recur, whether the study examined the mean time to any local recurrence (90 vs. 53 months; P less than or equal to .001), the mean time to DCIS recurrence (73 vs. 41 months; P = .002), or the mean time to invasive recurrence (105 vs. 72 months; P = .017).
Women who had surgery and radiation also experienced a significantly different pattern of recurrence, Dr. Grumley said. Among women treated only with surgery, 90% of recurrences were in the same quadrant, compared with 72% of recurrences in women who were treated with dual therapy (P = .0016). Conversely, 28% of recurrences after dual therapy occurred in a different quadrant, compared with 10% of those in the surgery-alone group. Lesions in different quadrants likely reflect new primary tumors, she said.
The increased rate of invasive, recurrent disease in the dual-therapy group was probably responsible for a small but statistically significant mortality difference (98% vs. 100%), Dr. Grumley said.
"This study shows that a small subgroup of irradiated DCIS patients may not be deriving maximum benefit from radiation therapy, or not benefiting from it at all," she said.
She was only able to speculate on the reason for the different recurrence patterns. "Radiation is a hypothesis, but we have nothing here that we can connect [as a] cause and effect. We do know that radiation does affect local recurrence, and we would never tell a patient not to get radiation. But this pattern is definitely something that should be further examined.
Dr. Grumley had no financial conflicts to declare.
WASHINGTON – Five years might not be the magic number for a small group of women who are treated for the most curable form of breast cancer: ductal carcinoma in situ.
In a prospective study, women who underwent excision plus radiotherapy did have a significantly lower rate of local recurrence than did women who were treated with surgery alone. But among those who had a recurrence, it was almost twice as likely to be invasive. Additionally, these recurrences took almost twice as long to present: up to 105 months.
"This shows there is definitely an added benefit from radiation, but at the same time, we must be aware that patients who have radiation can still recur, and much later," Dr. Janie Weng Grumley said at a press briefing during the annual meeting of the American Society of Breast Surgeons.
"Many patients think, ‘after 5 years, I’m safe.’ This study really highlights the fact that there is a very different pattern of recurrence [in those who receive one of the two treatments]. We must be aware of this, and follow these women in a different way."
Dr. Grumley, a fellow at the University of Southern California in Los Angeles, presented a prospective study of 1,014 women with ductal carcinoma in situ (DCIS) who were treated with either excision alone (651) or excision plus radiotherapy (363). The average follow-up was significantly longer in the dual-therapy group (average, 109 months) than in the excision-only group (average, 72 months), reflecting the recent increase in lumpectomy-only treatment, Dr. Grumley said. The groups’ average age was 54 years.
The probability of any local recurrence was significantly lower in the dual-therapy group than in the lumpectomy group (18% vs. 30%; P = .0102). But invasive disease was significantly more common among those in the dual-therapy group who did recur (57% vs. 37%). The cancers also took significantly longer to recur, whether the study examined the mean time to any local recurrence (90 vs. 53 months; P less than or equal to .001), the mean time to DCIS recurrence (73 vs. 41 months; P = .002), or the mean time to invasive recurrence (105 vs. 72 months; P = .017).
Women who had surgery and radiation also experienced a significantly different pattern of recurrence, Dr. Grumley said. Among women treated only with surgery, 90% of recurrences were in the same quadrant, compared with 72% of recurrences in women who were treated with dual therapy (P = .0016). Conversely, 28% of recurrences after dual therapy occurred in a different quadrant, compared with 10% of those in the surgery-alone group. Lesions in different quadrants likely reflect new primary tumors, she said.
The increased rate of invasive, recurrent disease in the dual-therapy group was probably responsible for a small but statistically significant mortality difference (98% vs. 100%), Dr. Grumley said.
"This study shows that a small subgroup of irradiated DCIS patients may not be deriving maximum benefit from radiation therapy, or not benefiting from it at all," she said.
She was only able to speculate on the reason for the different recurrence patterns. "Radiation is a hypothesis, but we have nothing here that we can connect [as a] cause and effect. We do know that radiation does affect local recurrence, and we would never tell a patient not to get radiation. But this pattern is definitely something that should be further examined.
Dr. Grumley had no financial conflicts to declare.
WASHINGTON – Five years might not be the magic number for a small group of women who are treated for the most curable form of breast cancer: ductal carcinoma in situ.
In a prospective study, women who underwent excision plus radiotherapy did have a significantly lower rate of local recurrence than did women who were treated with surgery alone. But among those who had a recurrence, it was almost twice as likely to be invasive. Additionally, these recurrences took almost twice as long to present: up to 105 months.
"This shows there is definitely an added benefit from radiation, but at the same time, we must be aware that patients who have radiation can still recur, and much later," Dr. Janie Weng Grumley said at a press briefing during the annual meeting of the American Society of Breast Surgeons.
"Many patients think, ‘after 5 years, I’m safe.’ This study really highlights the fact that there is a very different pattern of recurrence [in those who receive one of the two treatments]. We must be aware of this, and follow these women in a different way."
Dr. Grumley, a fellow at the University of Southern California in Los Angeles, presented a prospective study of 1,014 women with ductal carcinoma in situ (DCIS) who were treated with either excision alone (651) or excision plus radiotherapy (363). The average follow-up was significantly longer in the dual-therapy group (average, 109 months) than in the excision-only group (average, 72 months), reflecting the recent increase in lumpectomy-only treatment, Dr. Grumley said. The groups’ average age was 54 years.
The probability of any local recurrence was significantly lower in the dual-therapy group than in the lumpectomy group (18% vs. 30%; P = .0102). But invasive disease was significantly more common among those in the dual-therapy group who did recur (57% vs. 37%). The cancers also took significantly longer to recur, whether the study examined the mean time to any local recurrence (90 vs. 53 months; P less than or equal to .001), the mean time to DCIS recurrence (73 vs. 41 months; P = .002), or the mean time to invasive recurrence (105 vs. 72 months; P = .017).
Women who had surgery and radiation also experienced a significantly different pattern of recurrence, Dr. Grumley said. Among women treated only with surgery, 90% of recurrences were in the same quadrant, compared with 72% of recurrences in women who were treated with dual therapy (P = .0016). Conversely, 28% of recurrences after dual therapy occurred in a different quadrant, compared with 10% of those in the surgery-alone group. Lesions in different quadrants likely reflect new primary tumors, she said.
The increased rate of invasive, recurrent disease in the dual-therapy group was probably responsible for a small but statistically significant mortality difference (98% vs. 100%), Dr. Grumley said.
"This study shows that a small subgroup of irradiated DCIS patients may not be deriving maximum benefit from radiation therapy, or not benefiting from it at all," she said.
She was only able to speculate on the reason for the different recurrence patterns. "Radiation is a hypothesis, but we have nothing here that we can connect [as a] cause and effect. We do know that radiation does affect local recurrence, and we would never tell a patient not to get radiation. But this pattern is definitely something that should be further examined.
Dr. Grumley had no financial conflicts to declare.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF BREAST SURGEONS
Major Finding: Invasive cancer appeared in 57% of recurrences among women whose DCIS was treated with excision and radiation, compared with 37% of those in women treated with surgery alone.
Data Source: A prospective study of 1,014 women who were followed for up to 10 years.
Disclosures: Dr. Grumley had no financial conflicts to declare.
Change in Mammography Guidelines May Adversely Affect Young Minority Women
WASHINGTON – The revised national screening mammography guidelines may especially impact the health of younger minority women for whom annual screening is no longer recommended, investigators suggested.
A retrospective study derived from a large state cancer registry found that Hispanic, Asian, and black women aged 40-49 years were up to 60% more likely to be diagnosed with ductal cancer in situ (DCIS) and up to 80% more like to have small invasive breast tumors (T1N0) than were their white counterparts.
These women were significantly more likely to have tumors that respond best to very early therapy, Dr. Sharon Lum said at the annual meeting of the American Society of Breast Surgeons. But if their cancers are not detected through mammography, women in these groups might not receive such therapy.
"We already know that breast cancer occurs at a younger age in minorities, and that minority women present with later-stage breast tumors and they have poorer survival, "said Dr. Lum of Loma Linda (Calif.) University. "Yet under the new guidelines, the diagnosis of patients such as these would be delayed until they developed larger tumors evident though manual breast exams. Now, through our study, we know that minority women fall into these categories in a higher percentage" than do white women.
Dr. Lum presented an analysis of the California Cancer Registry, focusing on 46,691 women aged 40-74 years who were diagnosed with DCIS or T1N0 tumors in 2004-2008. She and her colleagues divided the women into two age groups: 40-49 years (23%), for whom annual screening mammograms are no longer recommended by the U.S. Preventive Services Task Force (USPSTF), and 50-74 years (77%), for whom the annual screening recommendation has not changed.
The patients were further subdivided into four race/ethnicity groups: white (65%), Hispanic (15%), Asian/Pacific Islander (13%) and black (5%). Ethnicity was not specified for the remainder of the study group.
Overall, there were 16,067 cases of DCIS and 30,624 T1N0 tumors in the group. Compared with white women, Hispanic women were significantly more likely to have DCIS (odds ratio, 1.62) and T1N0 tumors (OR, 1.82). Women of Asian/Pacific Island descent had a 50% increased risk of DCIS and a 66% increased risk of T1N0 disease, compared with white women. Black women were significantly more likely than whites to have T1N0 cancers (OR, 1.44), but not more likely to have DCIS (OR, 0.0.91).
Age also made a difference, Dr. Lum found. Compared with older women, the younger women were significantly more likely to have hormone receptor–positive DCIS (OR, 1.85) and T1N0 disease (OR, 1.43). Younger women were also more likely to have HER2-positive T1N0 tumors (OR, 1.46), and were 67% more likely to have the difficult-to-treat triple-negative tumors.
The molecular findings argue strongly in favor of regular screening mammograms for younger women – especially minority women, Dr. Lum said. Hormone receptor–positive tumors and HER2-positive tumors have highly effective, targeted therapies. And although triple-negative tumors are a therapeutic challenge, the best curative chance lies with early treatment, she said.
"By excluding these younger women from mammographic screening, you may be relatively diminishing the benefits of these targeted therapies," she said. "And while younger women do have lower cancer rates than older women, under these USPSTF guidelines, we could miss tremendous opportunities to improve outcomes in specific racial and disease groups."
Dr. Lum had no financial declarations with regard to her study.
breast cancer, Hispanic, Asian, black, ductal cancer in situ, DCIS, breast tumors, Dr. Sharon Lum, American Society of Breast Surgeons,
WASHINGTON – The revised national screening mammography guidelines may especially impact the health of younger minority women for whom annual screening is no longer recommended, investigators suggested.
A retrospective study derived from a large state cancer registry found that Hispanic, Asian, and black women aged 40-49 years were up to 60% more likely to be diagnosed with ductal cancer in situ (DCIS) and up to 80% more like to have small invasive breast tumors (T1N0) than were their white counterparts.
These women were significantly more likely to have tumors that respond best to very early therapy, Dr. Sharon Lum said at the annual meeting of the American Society of Breast Surgeons. But if their cancers are not detected through mammography, women in these groups might not receive such therapy.
"We already know that breast cancer occurs at a younger age in minorities, and that minority women present with later-stage breast tumors and they have poorer survival, "said Dr. Lum of Loma Linda (Calif.) University. "Yet under the new guidelines, the diagnosis of patients such as these would be delayed until they developed larger tumors evident though manual breast exams. Now, through our study, we know that minority women fall into these categories in a higher percentage" than do white women.
Dr. Lum presented an analysis of the California Cancer Registry, focusing on 46,691 women aged 40-74 years who were diagnosed with DCIS or T1N0 tumors in 2004-2008. She and her colleagues divided the women into two age groups: 40-49 years (23%), for whom annual screening mammograms are no longer recommended by the U.S. Preventive Services Task Force (USPSTF), and 50-74 years (77%), for whom the annual screening recommendation has not changed.
The patients were further subdivided into four race/ethnicity groups: white (65%), Hispanic (15%), Asian/Pacific Islander (13%) and black (5%). Ethnicity was not specified for the remainder of the study group.
Overall, there were 16,067 cases of DCIS and 30,624 T1N0 tumors in the group. Compared with white women, Hispanic women were significantly more likely to have DCIS (odds ratio, 1.62) and T1N0 tumors (OR, 1.82). Women of Asian/Pacific Island descent had a 50% increased risk of DCIS and a 66% increased risk of T1N0 disease, compared with white women. Black women were significantly more likely than whites to have T1N0 cancers (OR, 1.44), but not more likely to have DCIS (OR, 0.0.91).
Age also made a difference, Dr. Lum found. Compared with older women, the younger women were significantly more likely to have hormone receptor–positive DCIS (OR, 1.85) and T1N0 disease (OR, 1.43). Younger women were also more likely to have HER2-positive T1N0 tumors (OR, 1.46), and were 67% more likely to have the difficult-to-treat triple-negative tumors.
The molecular findings argue strongly in favor of regular screening mammograms for younger women – especially minority women, Dr. Lum said. Hormone receptor–positive tumors and HER2-positive tumors have highly effective, targeted therapies. And although triple-negative tumors are a therapeutic challenge, the best curative chance lies with early treatment, she said.
"By excluding these younger women from mammographic screening, you may be relatively diminishing the benefits of these targeted therapies," she said. "And while younger women do have lower cancer rates than older women, under these USPSTF guidelines, we could miss tremendous opportunities to improve outcomes in specific racial and disease groups."
Dr. Lum had no financial declarations with regard to her study.
WASHINGTON – The revised national screening mammography guidelines may especially impact the health of younger minority women for whom annual screening is no longer recommended, investigators suggested.
A retrospective study derived from a large state cancer registry found that Hispanic, Asian, and black women aged 40-49 years were up to 60% more likely to be diagnosed with ductal cancer in situ (DCIS) and up to 80% more like to have small invasive breast tumors (T1N0) than were their white counterparts.
These women were significantly more likely to have tumors that respond best to very early therapy, Dr. Sharon Lum said at the annual meeting of the American Society of Breast Surgeons. But if their cancers are not detected through mammography, women in these groups might not receive such therapy.
"We already know that breast cancer occurs at a younger age in minorities, and that minority women present with later-stage breast tumors and they have poorer survival, "said Dr. Lum of Loma Linda (Calif.) University. "Yet under the new guidelines, the diagnosis of patients such as these would be delayed until they developed larger tumors evident though manual breast exams. Now, through our study, we know that minority women fall into these categories in a higher percentage" than do white women.
Dr. Lum presented an analysis of the California Cancer Registry, focusing on 46,691 women aged 40-74 years who were diagnosed with DCIS or T1N0 tumors in 2004-2008. She and her colleagues divided the women into two age groups: 40-49 years (23%), for whom annual screening mammograms are no longer recommended by the U.S. Preventive Services Task Force (USPSTF), and 50-74 years (77%), for whom the annual screening recommendation has not changed.
The patients were further subdivided into four race/ethnicity groups: white (65%), Hispanic (15%), Asian/Pacific Islander (13%) and black (5%). Ethnicity was not specified for the remainder of the study group.
Overall, there were 16,067 cases of DCIS and 30,624 T1N0 tumors in the group. Compared with white women, Hispanic women were significantly more likely to have DCIS (odds ratio, 1.62) and T1N0 tumors (OR, 1.82). Women of Asian/Pacific Island descent had a 50% increased risk of DCIS and a 66% increased risk of T1N0 disease, compared with white women. Black women were significantly more likely than whites to have T1N0 cancers (OR, 1.44), but not more likely to have DCIS (OR, 0.0.91).
Age also made a difference, Dr. Lum found. Compared with older women, the younger women were significantly more likely to have hormone receptor–positive DCIS (OR, 1.85) and T1N0 disease (OR, 1.43). Younger women were also more likely to have HER2-positive T1N0 tumors (OR, 1.46), and were 67% more likely to have the difficult-to-treat triple-negative tumors.
The molecular findings argue strongly in favor of regular screening mammograms for younger women – especially minority women, Dr. Lum said. Hormone receptor–positive tumors and HER2-positive tumors have highly effective, targeted therapies. And although triple-negative tumors are a therapeutic challenge, the best curative chance lies with early treatment, she said.
"By excluding these younger women from mammographic screening, you may be relatively diminishing the benefits of these targeted therapies," she said. "And while younger women do have lower cancer rates than older women, under these USPSTF guidelines, we could miss tremendous opportunities to improve outcomes in specific racial and disease groups."
Dr. Lum had no financial declarations with regard to her study.
breast cancer, Hispanic, Asian, black, ductal cancer in situ, DCIS, breast tumors, Dr. Sharon Lum, American Society of Breast Surgeons,
breast cancer, Hispanic, Asian, black, ductal cancer in situ, DCIS, breast tumors, Dr. Sharon Lum, American Society of Breast Surgeons,
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF BREAST SURGEONS
Major Finding: Compared with white women, Hispanic women were significantly more likely to have DCIS (OR, 1.62) and T1N0 tumors (OR, 1.82).
Data Source: A retrospective review of almost 47,000 cancers among women aged 40-74 included in a California cancer database.
Disclosures: Dr. Lum declared no financial conflicts.
Change in Mammography Guidelines May Adversely Affect Young Minority Women
WASHINGTON – The revised national screening mammography guidelines may especially impact the health of younger minority women for whom annual screening is no longer recommended, investigators suggested.
A retrospective study derived from a large state cancer registry found that Hispanic, Asian, and black women aged 40-49 years were up to 60% more likely to be diagnosed with ductal cancer in situ (DCIS) and up to 80% more like to have small invasive breast tumors (T1N0) than were their white counterparts.
These women were significantly more likely to have tumors that respond best to very early therapy, Dr. Sharon Lum said at the annual meeting of the American Society of Breast Surgeons. But if their cancers are not detected through mammography, women in these groups might not receive such therapy.
"We already know that breast cancer occurs at a younger age in minorities, and that minority women present with later-stage breast tumors and they have poorer survival, "said Dr. Lum of Loma Linda (Calif.) University. "Yet under the new guidelines, the diagnosis of patients such as these would be delayed until they developed larger tumors evident though manual breast exams. Now, through our study, we know that minority women fall into these categories in a higher percentage" than do white women.
Dr. Lum presented an analysis of the California Cancer Registry, focusing on 46,691 women aged 40-74 years who were diagnosed with DCIS or T1N0 tumors in 2004-2008. She and her colleagues divided the women into two age groups: 40-49 years (23%), for whom annual screening mammograms are no longer recommended by the U.S. Preventive Services Task Force (USPSTF), and 50-74 years (77%), for whom the annual screening recommendation has not changed.
The patients were further subdivided into four race/ethnicity groups: white (65%), Hispanic (15%), Asian/Pacific Islander (13%) and black (5%). Ethnicity was not specified for the remainder of the study group.
Overall, there were 16,067 cases of DCIS and 30,624 T1N0 tumors in the group. Compared with white women, Hispanic women were significantly more likely to have DCIS (odds ratio, 1.62) and T1N0 tumors (OR, 1.82). Women of Asian/Pacific Island descent had a 50% increased risk of DCIS and a 66% increased risk of T1N0 disease, compared with white women. Black women were significantly more likely than whites to have T1N0 cancers (OR, 1.44), but not more likely to have DCIS (OR, 0.0.91).
Age also made a difference, Dr. Lum found. Compared with older women, the younger women were significantly more likely to have hormone receptor–positive DCIS (OR, 1.85) and T1N0 disease (OR, 1.43). Younger women were also more likely to have HER2-positive T1N0 tumors (OR, 1.46), and were 67% more likely to have the difficult-to-treat triple-negative tumors.
The molecular findings argue strongly in favor of regular screening mammograms for younger women – especially minority women, Dr. Lum said. Hormone receptor–positive tumors and HER2-positive tumors have highly effective, targeted therapies. And although triple-negative tumors are a therapeutic challenge, the best curative chance lies with early treatment, she said.
"By excluding these younger women from mammographic screening, you may be relatively diminishing the benefits of these targeted therapies," she said. "And while younger women do have lower cancer rates than older women, under these USPSTF guidelines, we could miss tremendous opportunities to improve outcomes in specific racial and disease groups."
Dr. Lum had no financial declarations with regard to her study.
breast cancer, Hispanic, Asian, black, ductal cancer in situ, DCIS, breast tumors, Dr. Sharon Lum, American Society of Breast Surgeons,
WASHINGTON – The revised national screening mammography guidelines may especially impact the health of younger minority women for whom annual screening is no longer recommended, investigators suggested.
A retrospective study derived from a large state cancer registry found that Hispanic, Asian, and black women aged 40-49 years were up to 60% more likely to be diagnosed with ductal cancer in situ (DCIS) and up to 80% more like to have small invasive breast tumors (T1N0) than were their white counterparts.
These women were significantly more likely to have tumors that respond best to very early therapy, Dr. Sharon Lum said at the annual meeting of the American Society of Breast Surgeons. But if their cancers are not detected through mammography, women in these groups might not receive such therapy.
"We already know that breast cancer occurs at a younger age in minorities, and that minority women present with later-stage breast tumors and they have poorer survival, "said Dr. Lum of Loma Linda (Calif.) University. "Yet under the new guidelines, the diagnosis of patients such as these would be delayed until they developed larger tumors evident though manual breast exams. Now, through our study, we know that minority women fall into these categories in a higher percentage" than do white women.
Dr. Lum presented an analysis of the California Cancer Registry, focusing on 46,691 women aged 40-74 years who were diagnosed with DCIS or T1N0 tumors in 2004-2008. She and her colleagues divided the women into two age groups: 40-49 years (23%), for whom annual screening mammograms are no longer recommended by the U.S. Preventive Services Task Force (USPSTF), and 50-74 years (77%), for whom the annual screening recommendation has not changed.
The patients were further subdivided into four race/ethnicity groups: white (65%), Hispanic (15%), Asian/Pacific Islander (13%) and black (5%). Ethnicity was not specified for the remainder of the study group.
Overall, there were 16,067 cases of DCIS and 30,624 T1N0 tumors in the group. Compared with white women, Hispanic women were significantly more likely to have DCIS (odds ratio, 1.62) and T1N0 tumors (OR, 1.82). Women of Asian/Pacific Island descent had a 50% increased risk of DCIS and a 66% increased risk of T1N0 disease, compared with white women. Black women were significantly more likely than whites to have T1N0 cancers (OR, 1.44), but not more likely to have DCIS (OR, 0.0.91).
Age also made a difference, Dr. Lum found. Compared with older women, the younger women were significantly more likely to have hormone receptor–positive DCIS (OR, 1.85) and T1N0 disease (OR, 1.43). Younger women were also more likely to have HER2-positive T1N0 tumors (OR, 1.46), and were 67% more likely to have the difficult-to-treat triple-negative tumors.
The molecular findings argue strongly in favor of regular screening mammograms for younger women – especially minority women, Dr. Lum said. Hormone receptor–positive tumors and HER2-positive tumors have highly effective, targeted therapies. And although triple-negative tumors are a therapeutic challenge, the best curative chance lies with early treatment, she said.
"By excluding these younger women from mammographic screening, you may be relatively diminishing the benefits of these targeted therapies," she said. "And while younger women do have lower cancer rates than older women, under these USPSTF guidelines, we could miss tremendous opportunities to improve outcomes in specific racial and disease groups."
Dr. Lum had no financial declarations with regard to her study.
WASHINGTON – The revised national screening mammography guidelines may especially impact the health of younger minority women for whom annual screening is no longer recommended, investigators suggested.
A retrospective study derived from a large state cancer registry found that Hispanic, Asian, and black women aged 40-49 years were up to 60% more likely to be diagnosed with ductal cancer in situ (DCIS) and up to 80% more like to have small invasive breast tumors (T1N0) than were their white counterparts.
These women were significantly more likely to have tumors that respond best to very early therapy, Dr. Sharon Lum said at the annual meeting of the American Society of Breast Surgeons. But if their cancers are not detected through mammography, women in these groups might not receive such therapy.
"We already know that breast cancer occurs at a younger age in minorities, and that minority women present with later-stage breast tumors and they have poorer survival, "said Dr. Lum of Loma Linda (Calif.) University. "Yet under the new guidelines, the diagnosis of patients such as these would be delayed until they developed larger tumors evident though manual breast exams. Now, through our study, we know that minority women fall into these categories in a higher percentage" than do white women.
Dr. Lum presented an analysis of the California Cancer Registry, focusing on 46,691 women aged 40-74 years who were diagnosed with DCIS or T1N0 tumors in 2004-2008. She and her colleagues divided the women into two age groups: 40-49 years (23%), for whom annual screening mammograms are no longer recommended by the U.S. Preventive Services Task Force (USPSTF), and 50-74 years (77%), for whom the annual screening recommendation has not changed.
The patients were further subdivided into four race/ethnicity groups: white (65%), Hispanic (15%), Asian/Pacific Islander (13%) and black (5%). Ethnicity was not specified for the remainder of the study group.
Overall, there were 16,067 cases of DCIS and 30,624 T1N0 tumors in the group. Compared with white women, Hispanic women were significantly more likely to have DCIS (odds ratio, 1.62) and T1N0 tumors (OR, 1.82). Women of Asian/Pacific Island descent had a 50% increased risk of DCIS and a 66% increased risk of T1N0 disease, compared with white women. Black women were significantly more likely than whites to have T1N0 cancers (OR, 1.44), but not more likely to have DCIS (OR, 0.0.91).
Age also made a difference, Dr. Lum found. Compared with older women, the younger women were significantly more likely to have hormone receptor–positive DCIS (OR, 1.85) and T1N0 disease (OR, 1.43). Younger women were also more likely to have HER2-positive T1N0 tumors (OR, 1.46), and were 67% more likely to have the difficult-to-treat triple-negative tumors.
The molecular findings argue strongly in favor of regular screening mammograms for younger women – especially minority women, Dr. Lum said. Hormone receptor–positive tumors and HER2-positive tumors have highly effective, targeted therapies. And although triple-negative tumors are a therapeutic challenge, the best curative chance lies with early treatment, she said.
"By excluding these younger women from mammographic screening, you may be relatively diminishing the benefits of these targeted therapies," she said. "And while younger women do have lower cancer rates than older women, under these USPSTF guidelines, we could miss tremendous opportunities to improve outcomes in specific racial and disease groups."
Dr. Lum had no financial declarations with regard to her study.
breast cancer, Hispanic, Asian, black, ductal cancer in situ, DCIS, breast tumors, Dr. Sharon Lum, American Society of Breast Surgeons,
breast cancer, Hispanic, Asian, black, ductal cancer in situ, DCIS, breast tumors, Dr. Sharon Lum, American Society of Breast Surgeons,
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF BREAST SURGEONS
Major Finding: Compared with white women, Hispanic women were significantly more likely to have DCIS (OR, 1.62) and T1N0 tumors (OR, 1.82).
Data Source: A retrospective review of almost 47,000 cancers among women aged 40-74 included in a California cancer database.
Disclosures: Dr. Lum declared no financial conflicts.