User login
Beyond PSA: New prostate cancer screening options
Two noninvasive tests — an assessment of spermine levels in urine and a blood test that combines free and total PSA and the (-2) pro-PSA isoform (p2PSA) — are much safer than historically risky biopsy and what is now considered to have been unnecessary surgery.
“We’ve ‘cured’ a lot of men,” Franklin Gaylis, MD, from the University of California, San Diego, told Medscape Medical News. “Even some who didn’t need to be cured.” Now, we are working to solve this dilemma, he said. “It’s time we determine who do you screen, [who do you] not screen, and how aggressively?”
Urine Spermine Test More Accurate Than PSA
Data from a highly predictive test that assesses spermine levels in urine were presented by Peter Ka-Fung Chiu, MD, from the University of Hong Kong, at the virtual annual congress of the European Association of Urology. Normal spermine levels are inversely associated with both prostate cancer (PCa) and high-grade prostate cancer (HGPCa).
To investigate the predictive value of spermine for any PCa or HGPCa (Gleason 7 or above), the researchers recruited 556 men from two centers and collected 30 mL of urine prior to prostate biopsy.
They analyzed data from 390 men and used decision-curve analyses for PCa and for HGPCa. The multivariate spermine score — which takes into account age, prostate volume, PSA level, and spermine level — provided net clinical benefit over PSA alone and over spermine score alone.
“At 90% sensitivity, this risk score actually had a negative predictive value of 96.7% and avoided about 50% of unnecessary biopsies,” Chiu explained. “This test predicts prostate cancer and high-grade prostate cancer well, without the need for prior prostate massage, offering improved predictive performance.”
PHI Reduces Need for MRI Screening
Another test, the PHI prostate cancer biomarker, is as predictive as multiparametric (mp)MRI, both with and without PSA scoring.
PHI scores from 554 men from five centers added to either PSA density or mpMRI improved the prediction of risk for ≥GG2 cancers to more than 0.81 and for ≥CPG3 cancers to more than 0.85, according to data from the multicenter PRIM (PHI to Refine MRI) study group recently published in BMC Medicine and presented at EAU.
With a PHI cut-off of 30, mpMRI referrals could be cut by 25%, and unnecessary biopsies could be cut by 40%, the PRIM group reports. PHI misses 8% of ≥GG2 cancers, whereas mpMRI misses 9%.
The PHI strategy reduces “mpMRI and biopsies without compromising detection of significant prostate cancers,” and also reduces costs, Nicholas Boxall, MB ChB, from Cambridge University Hospitals NHS Foundation Trust in the United Kingdom, explained during his presentation
“Instead of screening everyone, we’re risk-adapting who needs to be screened, identifying the right population and defaulting to MRI as an alternative to invasive biopsy, and doing secondary tests to look at biomarkers,” said Gerald Andriole, MD, from the Washington University School of Medicine in St. Louis, Missouri.
“We don’t have to auto-toggle to aggressive treatment,” he told Medscape Medical News. “We’re getting better than we were 10 years ago, but we need slightly better tests, and we also need better biopsies; urologists must be more careful.”
Chiu and Boxall report no relevant financial relationships. Gaylis is a scientific advisor for Stratify Genomics. Andriole is on the advisory board of Stratify Genomics.
This article first appeared on Medscape.com.
Two noninvasive tests — an assessment of spermine levels in urine and a blood test that combines free and total PSA and the (-2) pro-PSA isoform (p2PSA) — are much safer than historically risky biopsy and what is now considered to have been unnecessary surgery.
“We’ve ‘cured’ a lot of men,” Franklin Gaylis, MD, from the University of California, San Diego, told Medscape Medical News. “Even some who didn’t need to be cured.” Now, we are working to solve this dilemma, he said. “It’s time we determine who do you screen, [who do you] not screen, and how aggressively?”
Urine Spermine Test More Accurate Than PSA
Data from a highly predictive test that assesses spermine levels in urine were presented by Peter Ka-Fung Chiu, MD, from the University of Hong Kong, at the virtual annual congress of the European Association of Urology. Normal spermine levels are inversely associated with both prostate cancer (PCa) and high-grade prostate cancer (HGPCa).
To investigate the predictive value of spermine for any PCa or HGPCa (Gleason 7 or above), the researchers recruited 556 men from two centers and collected 30 mL of urine prior to prostate biopsy.
They analyzed data from 390 men and used decision-curve analyses for PCa and for HGPCa. The multivariate spermine score — which takes into account age, prostate volume, PSA level, and spermine level — provided net clinical benefit over PSA alone and over spermine score alone.
“At 90% sensitivity, this risk score actually had a negative predictive value of 96.7% and avoided about 50% of unnecessary biopsies,” Chiu explained. “This test predicts prostate cancer and high-grade prostate cancer well, without the need for prior prostate massage, offering improved predictive performance.”
PHI Reduces Need for MRI Screening
Another test, the PHI prostate cancer biomarker, is as predictive as multiparametric (mp)MRI, both with and without PSA scoring.
PHI scores from 554 men from five centers added to either PSA density or mpMRI improved the prediction of risk for ≥GG2 cancers to more than 0.81 and for ≥CPG3 cancers to more than 0.85, according to data from the multicenter PRIM (PHI to Refine MRI) study group recently published in BMC Medicine and presented at EAU.
With a PHI cut-off of 30, mpMRI referrals could be cut by 25%, and unnecessary biopsies could be cut by 40%, the PRIM group reports. PHI misses 8% of ≥GG2 cancers, whereas mpMRI misses 9%.
The PHI strategy reduces “mpMRI and biopsies without compromising detection of significant prostate cancers,” and also reduces costs, Nicholas Boxall, MB ChB, from Cambridge University Hospitals NHS Foundation Trust in the United Kingdom, explained during his presentation
“Instead of screening everyone, we’re risk-adapting who needs to be screened, identifying the right population and defaulting to MRI as an alternative to invasive biopsy, and doing secondary tests to look at biomarkers,” said Gerald Andriole, MD, from the Washington University School of Medicine in St. Louis, Missouri.
“We don’t have to auto-toggle to aggressive treatment,” he told Medscape Medical News. “We’re getting better than we were 10 years ago, but we need slightly better tests, and we also need better biopsies; urologists must be more careful.”
Chiu and Boxall report no relevant financial relationships. Gaylis is a scientific advisor for Stratify Genomics. Andriole is on the advisory board of Stratify Genomics.
This article first appeared on Medscape.com.
Two noninvasive tests — an assessment of spermine levels in urine and a blood test that combines free and total PSA and the (-2) pro-PSA isoform (p2PSA) — are much safer than historically risky biopsy and what is now considered to have been unnecessary surgery.
“We’ve ‘cured’ a lot of men,” Franklin Gaylis, MD, from the University of California, San Diego, told Medscape Medical News. “Even some who didn’t need to be cured.” Now, we are working to solve this dilemma, he said. “It’s time we determine who do you screen, [who do you] not screen, and how aggressively?”
Urine Spermine Test More Accurate Than PSA
Data from a highly predictive test that assesses spermine levels in urine were presented by Peter Ka-Fung Chiu, MD, from the University of Hong Kong, at the virtual annual congress of the European Association of Urology. Normal spermine levels are inversely associated with both prostate cancer (PCa) and high-grade prostate cancer (HGPCa).
To investigate the predictive value of spermine for any PCa or HGPCa (Gleason 7 or above), the researchers recruited 556 men from two centers and collected 30 mL of urine prior to prostate biopsy.
They analyzed data from 390 men and used decision-curve analyses for PCa and for HGPCa. The multivariate spermine score — which takes into account age, prostate volume, PSA level, and spermine level — provided net clinical benefit over PSA alone and over spermine score alone.
“At 90% sensitivity, this risk score actually had a negative predictive value of 96.7% and avoided about 50% of unnecessary biopsies,” Chiu explained. “This test predicts prostate cancer and high-grade prostate cancer well, without the need for prior prostate massage, offering improved predictive performance.”
PHI Reduces Need for MRI Screening
Another test, the PHI prostate cancer biomarker, is as predictive as multiparametric (mp)MRI, both with and without PSA scoring.
PHI scores from 554 men from five centers added to either PSA density or mpMRI improved the prediction of risk for ≥GG2 cancers to more than 0.81 and for ≥CPG3 cancers to more than 0.85, according to data from the multicenter PRIM (PHI to Refine MRI) study group recently published in BMC Medicine and presented at EAU.
With a PHI cut-off of 30, mpMRI referrals could be cut by 25%, and unnecessary biopsies could be cut by 40%, the PRIM group reports. PHI misses 8% of ≥GG2 cancers, whereas mpMRI misses 9%.
The PHI strategy reduces “mpMRI and biopsies without compromising detection of significant prostate cancers,” and also reduces costs, Nicholas Boxall, MB ChB, from Cambridge University Hospitals NHS Foundation Trust in the United Kingdom, explained during his presentation
“Instead of screening everyone, we’re risk-adapting who needs to be screened, identifying the right population and defaulting to MRI as an alternative to invasive biopsy, and doing secondary tests to look at biomarkers,” said Gerald Andriole, MD, from the Washington University School of Medicine in St. Louis, Missouri.
“We don’t have to auto-toggle to aggressive treatment,” he told Medscape Medical News. “We’re getting better than we were 10 years ago, but we need slightly better tests, and we also need better biopsies; urologists must be more careful.”
Chiu and Boxall report no relevant financial relationships. Gaylis is a scientific advisor for Stratify Genomics. Andriole is on the advisory board of Stratify Genomics.
This article first appeared on Medscape.com.
How prostate cancer treatments affect quality of life
according to a presentation at the virtual annual congress of the European Association of Urology (EAU).
Results of EUPROMS – the first patient-driven, international, prostate cancer quality of life study – showed that fatigue, insomnia, urinary incontinence, and sexual function were worse with certain types of treatments.
“Quality of life is negatively impacted by any treatment for prostate cancer other than active surveillance,” said André Deschamps, the chairman of the patient advocacy movement Europa Uomo, which conducted the study with support from Erasmus University Medical Center in Rotterdam, the Netherlands.
Active surveillance “should be promoted as the first option for treatment for those men where it can be offered safely,” Mr. Deschamps said when presenting the study at the EAU congress.
The study showed that quality of life related to urinary incontinence was lowest in patients who had undergone radical prostatectomy, and sexual function was greatly affected by radiotherapy. Radiotherapy and chemotherapy had the greatest impact on patients’ levels of fatigue, and chemotherapy was associated with “the worst possible outcomes in quality of life,” Mr. Deschamps said.
Conversely, “reported quality of life scores are the best in patients where the cancer is discovered in an early, curable stage. Hence, efforts toward early detection and awareness are essential to avoid unnecessary deterioration in quality of life,” Mr. Deschamps said.
About the survey and respondents
Between August and November 2019, 2,943 prostate cancer patients from 24 European countries completed a web-based survey made available via the Europa Uomo website. The survey took around 20 minutes to complete and used three validated quality of life questionnaires, the EORTC-QLQ-C30, the EQ-5D-5L, and EPIC-26.
“The questionnaires were available in 19 languages, so every patient could answer in their mother tongue,” Mr. Deschamps pointed out, highlighting that this was a Europe-wide survey and was estimated to account for 0.1% of the patient population in Europe.
Countries with the highest number of respondents were Norway (n = 506), Sweden (n = 386), Belgium (n = 339), Germany (n = 253), Netherlands (n = 244), France (n = 234), Denmark (n = 188), the United Kingdom (n = 187), and Poland (n = 109).
The average age of respondents was 70 years at the time of the survey and 64 years at the time of diagnosis. Most patients (82%) were living with a partner.
Two-thirds of patients had received only one treatment for prostate cancer. This was most often radical prostatectomy, external beam radiotherapy, or active surveillance. Among the 22% of patients who had received two treatments, the therapies were most often a combination of surgery and radiotherapy, androgen deprivation therapy (ADT) and radiotherapy or chemotherapy, and active surveillance and surgery.
Fatigue and insomnia
According to the EORTC-QLQ-C30 symptoms questionnaire, fatigue and insomnia were particular problems for men with prostate cancer, as denoted by scores of 25 and 24, respectively, out of a possible 100. Low scores are associated with worse fatigue and insomnia.
The researchers focused their attention on how specific cancer treatments might influence fatigue. They found that radiotherapy doubled and chemotherapy tripled the number of patients reporting fatigue, when compared with active surveillance. The incidence of fatigue was 22% (n = 304), 33% (n = 246), and 11% (n = 179), respectively.
As for insomnia, “it’s bit of a mixed view,” Mr. Deschamps said. “We believe that the progression of disease is more important for insomnia. The only thing you can say is that chemotherapy leads to an increase in reported insomnia.”
Urinary continence and sexual function
The EPIC-26 questionnaire was used to look at the health-related quality of life domains of urinary and sexual function. Sexual function was the most impacted area.
“We often hear that decline in sexual functioning is a relatively small problem for prostate cancer patients, and the effect on their quality of life should not be exaggerated,” Mr. Deschamps said in a press statement.
“We also hear that prostate cancer is typically a disease of ‘old men,’ implying that the loss of sexual function is less relevant. This survey paints a different picture,” he added.
Higher EPIC-26 scores signify better function. For urinary incontinence, the score was 100/100 for active surveillance but 65/100 when active surveillance was combined with surgery and 71/100 for surgery alone. The combination of surgery and radiotherapy carried a score of 73/100 for urinary incontinence. Radiotherapy on its own had a score of 92/100, suggesting it was the addition of the surgery that was having a significant effect. The score for radiotherapy plus ADT was 100/100, and the score for chemotherapy was 86/100.
Chemotherapy appeared to have the worst effect on sexual function, with a score of just 12/100. Radiotherapy was not far behind at 17/100, and surgery alone was 21/100. When radiotherapy and surgery were combined, the score was 15/100.
Sexual function scores were also low for all the other treatments considered – 18/100 for radiotherapy and ADT, 26/100 for active surveillance and surgery, and 57/100 for active surveillance alone.
Implications for practice
“The data collected and the analysis done provide patients and healthcare professionals with a ‘snapshot’ on the impact of treatments based on the experience of fellow patients,” Mr. Deschamps said. “We hope these results will be used to establish and disseminate realistic expectations on the effects of different treatments for prostate cancer on [quality of life].”
“This study is important because it was initiated by patients and meant for patients,” noted Monique Roobol, PhD, professor of decision-making in urology at the Erasmus University Medical Center in Rotterdam, the Netherlands, where the survey data were analyzed.
“The questionnaires were completed unrelated to a hospital visit, which means respondents had more freedom to answer and provide insight into the effect of treatment on quality of life over a longer period,” she added.
“For me, the key point is that, as health care professionals, we have underestimated the impact on the quality of life for patients treated for prostate cancer,” said Hein van Poppel, MD, PhD, of University Hospitals Leuven (Belgium), who chaired the session in which the data were presented.
Arnulf Stenzl, MD, of Tübingen (Germany) University said in a statement that the survey provided valuable information. “It uses the same questionnaires used in standard clinical settings, but it is both qualitatively and quantitatively different to the kind of study usually undertaken, so it needs to be read alongside these previous studies,” Dr. Stenzl said.
There were several strong points, he said, such as the fact that EUPROMS was the largest study of its kind and thus would “reflect the impact of treatment on a wide range of patients, with different health systems.”
As an official EAU spokesperson, Dr. Stenzl added, “We completely agree that early detection and treatment is essential if we are to avoid problems with quality of life later on. It shows that, for many men, quality of life can be poor after most prostate cancer treatment, especially in advanced disease. This message is clear, and we need to listen to the voices of these patients.”
EUPROMS was conducted by Europa Uomo in conjunction with the Erasmus University Medical Centre in Rotterdam, the Netherlands. Funding was received from Bayer, Ipsen, and Janssen. The companies had no influence over any aspect of the study. The commentators did not have conflicts of interest to disclose.
according to a presentation at the virtual annual congress of the European Association of Urology (EAU).
Results of EUPROMS – the first patient-driven, international, prostate cancer quality of life study – showed that fatigue, insomnia, urinary incontinence, and sexual function were worse with certain types of treatments.
“Quality of life is negatively impacted by any treatment for prostate cancer other than active surveillance,” said André Deschamps, the chairman of the patient advocacy movement Europa Uomo, which conducted the study with support from Erasmus University Medical Center in Rotterdam, the Netherlands.
Active surveillance “should be promoted as the first option for treatment for those men where it can be offered safely,” Mr. Deschamps said when presenting the study at the EAU congress.
The study showed that quality of life related to urinary incontinence was lowest in patients who had undergone radical prostatectomy, and sexual function was greatly affected by radiotherapy. Radiotherapy and chemotherapy had the greatest impact on patients’ levels of fatigue, and chemotherapy was associated with “the worst possible outcomes in quality of life,” Mr. Deschamps said.
Conversely, “reported quality of life scores are the best in patients where the cancer is discovered in an early, curable stage. Hence, efforts toward early detection and awareness are essential to avoid unnecessary deterioration in quality of life,” Mr. Deschamps said.
About the survey and respondents
Between August and November 2019, 2,943 prostate cancer patients from 24 European countries completed a web-based survey made available via the Europa Uomo website. The survey took around 20 minutes to complete and used three validated quality of life questionnaires, the EORTC-QLQ-C30, the EQ-5D-5L, and EPIC-26.
“The questionnaires were available in 19 languages, so every patient could answer in their mother tongue,” Mr. Deschamps pointed out, highlighting that this was a Europe-wide survey and was estimated to account for 0.1% of the patient population in Europe.
Countries with the highest number of respondents were Norway (n = 506), Sweden (n = 386), Belgium (n = 339), Germany (n = 253), Netherlands (n = 244), France (n = 234), Denmark (n = 188), the United Kingdom (n = 187), and Poland (n = 109).
The average age of respondents was 70 years at the time of the survey and 64 years at the time of diagnosis. Most patients (82%) were living with a partner.
Two-thirds of patients had received only one treatment for prostate cancer. This was most often radical prostatectomy, external beam radiotherapy, or active surveillance. Among the 22% of patients who had received two treatments, the therapies were most often a combination of surgery and radiotherapy, androgen deprivation therapy (ADT) and radiotherapy or chemotherapy, and active surveillance and surgery.
Fatigue and insomnia
According to the EORTC-QLQ-C30 symptoms questionnaire, fatigue and insomnia were particular problems for men with prostate cancer, as denoted by scores of 25 and 24, respectively, out of a possible 100. Low scores are associated with worse fatigue and insomnia.
The researchers focused their attention on how specific cancer treatments might influence fatigue. They found that radiotherapy doubled and chemotherapy tripled the number of patients reporting fatigue, when compared with active surveillance. The incidence of fatigue was 22% (n = 304), 33% (n = 246), and 11% (n = 179), respectively.
As for insomnia, “it’s bit of a mixed view,” Mr. Deschamps said. “We believe that the progression of disease is more important for insomnia. The only thing you can say is that chemotherapy leads to an increase in reported insomnia.”
Urinary continence and sexual function
The EPIC-26 questionnaire was used to look at the health-related quality of life domains of urinary and sexual function. Sexual function was the most impacted area.
“We often hear that decline in sexual functioning is a relatively small problem for prostate cancer patients, and the effect on their quality of life should not be exaggerated,” Mr. Deschamps said in a press statement.
“We also hear that prostate cancer is typically a disease of ‘old men,’ implying that the loss of sexual function is less relevant. This survey paints a different picture,” he added.
Higher EPIC-26 scores signify better function. For urinary incontinence, the score was 100/100 for active surveillance but 65/100 when active surveillance was combined with surgery and 71/100 for surgery alone. The combination of surgery and radiotherapy carried a score of 73/100 for urinary incontinence. Radiotherapy on its own had a score of 92/100, suggesting it was the addition of the surgery that was having a significant effect. The score for radiotherapy plus ADT was 100/100, and the score for chemotherapy was 86/100.
Chemotherapy appeared to have the worst effect on sexual function, with a score of just 12/100. Radiotherapy was not far behind at 17/100, and surgery alone was 21/100. When radiotherapy and surgery were combined, the score was 15/100.
Sexual function scores were also low for all the other treatments considered – 18/100 for radiotherapy and ADT, 26/100 for active surveillance and surgery, and 57/100 for active surveillance alone.
Implications for practice
“The data collected and the analysis done provide patients and healthcare professionals with a ‘snapshot’ on the impact of treatments based on the experience of fellow patients,” Mr. Deschamps said. “We hope these results will be used to establish and disseminate realistic expectations on the effects of different treatments for prostate cancer on [quality of life].”
“This study is important because it was initiated by patients and meant for patients,” noted Monique Roobol, PhD, professor of decision-making in urology at the Erasmus University Medical Center in Rotterdam, the Netherlands, where the survey data were analyzed.
“The questionnaires were completed unrelated to a hospital visit, which means respondents had more freedom to answer and provide insight into the effect of treatment on quality of life over a longer period,” she added.
“For me, the key point is that, as health care professionals, we have underestimated the impact on the quality of life for patients treated for prostate cancer,” said Hein van Poppel, MD, PhD, of University Hospitals Leuven (Belgium), who chaired the session in which the data were presented.
Arnulf Stenzl, MD, of Tübingen (Germany) University said in a statement that the survey provided valuable information. “It uses the same questionnaires used in standard clinical settings, but it is both qualitatively and quantitatively different to the kind of study usually undertaken, so it needs to be read alongside these previous studies,” Dr. Stenzl said.
There were several strong points, he said, such as the fact that EUPROMS was the largest study of its kind and thus would “reflect the impact of treatment on a wide range of patients, with different health systems.”
As an official EAU spokesperson, Dr. Stenzl added, “We completely agree that early detection and treatment is essential if we are to avoid problems with quality of life later on. It shows that, for many men, quality of life can be poor after most prostate cancer treatment, especially in advanced disease. This message is clear, and we need to listen to the voices of these patients.”
EUPROMS was conducted by Europa Uomo in conjunction with the Erasmus University Medical Centre in Rotterdam, the Netherlands. Funding was received from Bayer, Ipsen, and Janssen. The companies had no influence over any aspect of the study. The commentators did not have conflicts of interest to disclose.
according to a presentation at the virtual annual congress of the European Association of Urology (EAU).
Results of EUPROMS – the first patient-driven, international, prostate cancer quality of life study – showed that fatigue, insomnia, urinary incontinence, and sexual function were worse with certain types of treatments.
“Quality of life is negatively impacted by any treatment for prostate cancer other than active surveillance,” said André Deschamps, the chairman of the patient advocacy movement Europa Uomo, which conducted the study with support from Erasmus University Medical Center in Rotterdam, the Netherlands.
Active surveillance “should be promoted as the first option for treatment for those men where it can be offered safely,” Mr. Deschamps said when presenting the study at the EAU congress.
The study showed that quality of life related to urinary incontinence was lowest in patients who had undergone radical prostatectomy, and sexual function was greatly affected by radiotherapy. Radiotherapy and chemotherapy had the greatest impact on patients’ levels of fatigue, and chemotherapy was associated with “the worst possible outcomes in quality of life,” Mr. Deschamps said.
Conversely, “reported quality of life scores are the best in patients where the cancer is discovered in an early, curable stage. Hence, efforts toward early detection and awareness are essential to avoid unnecessary deterioration in quality of life,” Mr. Deschamps said.
About the survey and respondents
Between August and November 2019, 2,943 prostate cancer patients from 24 European countries completed a web-based survey made available via the Europa Uomo website. The survey took around 20 minutes to complete and used three validated quality of life questionnaires, the EORTC-QLQ-C30, the EQ-5D-5L, and EPIC-26.
“The questionnaires were available in 19 languages, so every patient could answer in their mother tongue,” Mr. Deschamps pointed out, highlighting that this was a Europe-wide survey and was estimated to account for 0.1% of the patient population in Europe.
Countries with the highest number of respondents were Norway (n = 506), Sweden (n = 386), Belgium (n = 339), Germany (n = 253), Netherlands (n = 244), France (n = 234), Denmark (n = 188), the United Kingdom (n = 187), and Poland (n = 109).
The average age of respondents was 70 years at the time of the survey and 64 years at the time of diagnosis. Most patients (82%) were living with a partner.
Two-thirds of patients had received only one treatment for prostate cancer. This was most often radical prostatectomy, external beam radiotherapy, or active surveillance. Among the 22% of patients who had received two treatments, the therapies were most often a combination of surgery and radiotherapy, androgen deprivation therapy (ADT) and radiotherapy or chemotherapy, and active surveillance and surgery.
Fatigue and insomnia
According to the EORTC-QLQ-C30 symptoms questionnaire, fatigue and insomnia were particular problems for men with prostate cancer, as denoted by scores of 25 and 24, respectively, out of a possible 100. Low scores are associated with worse fatigue and insomnia.
The researchers focused their attention on how specific cancer treatments might influence fatigue. They found that radiotherapy doubled and chemotherapy tripled the number of patients reporting fatigue, when compared with active surveillance. The incidence of fatigue was 22% (n = 304), 33% (n = 246), and 11% (n = 179), respectively.
As for insomnia, “it’s bit of a mixed view,” Mr. Deschamps said. “We believe that the progression of disease is more important for insomnia. The only thing you can say is that chemotherapy leads to an increase in reported insomnia.”
Urinary continence and sexual function
The EPIC-26 questionnaire was used to look at the health-related quality of life domains of urinary and sexual function. Sexual function was the most impacted area.
“We often hear that decline in sexual functioning is a relatively small problem for prostate cancer patients, and the effect on their quality of life should not be exaggerated,” Mr. Deschamps said in a press statement.
“We also hear that prostate cancer is typically a disease of ‘old men,’ implying that the loss of sexual function is less relevant. This survey paints a different picture,” he added.
Higher EPIC-26 scores signify better function. For urinary incontinence, the score was 100/100 for active surveillance but 65/100 when active surveillance was combined with surgery and 71/100 for surgery alone. The combination of surgery and radiotherapy carried a score of 73/100 for urinary incontinence. Radiotherapy on its own had a score of 92/100, suggesting it was the addition of the surgery that was having a significant effect. The score for radiotherapy plus ADT was 100/100, and the score for chemotherapy was 86/100.
Chemotherapy appeared to have the worst effect on sexual function, with a score of just 12/100. Radiotherapy was not far behind at 17/100, and surgery alone was 21/100. When radiotherapy and surgery were combined, the score was 15/100.
Sexual function scores were also low for all the other treatments considered – 18/100 for radiotherapy and ADT, 26/100 for active surveillance and surgery, and 57/100 for active surveillance alone.
Implications for practice
“The data collected and the analysis done provide patients and healthcare professionals with a ‘snapshot’ on the impact of treatments based on the experience of fellow patients,” Mr. Deschamps said. “We hope these results will be used to establish and disseminate realistic expectations on the effects of different treatments for prostate cancer on [quality of life].”
“This study is important because it was initiated by patients and meant for patients,” noted Monique Roobol, PhD, professor of decision-making in urology at the Erasmus University Medical Center in Rotterdam, the Netherlands, where the survey data were analyzed.
“The questionnaires were completed unrelated to a hospital visit, which means respondents had more freedom to answer and provide insight into the effect of treatment on quality of life over a longer period,” she added.
“For me, the key point is that, as health care professionals, we have underestimated the impact on the quality of life for patients treated for prostate cancer,” said Hein van Poppel, MD, PhD, of University Hospitals Leuven (Belgium), who chaired the session in which the data were presented.
Arnulf Stenzl, MD, of Tübingen (Germany) University said in a statement that the survey provided valuable information. “It uses the same questionnaires used in standard clinical settings, but it is both qualitatively and quantitatively different to the kind of study usually undertaken, so it needs to be read alongside these previous studies,” Dr. Stenzl said.
There were several strong points, he said, such as the fact that EUPROMS was the largest study of its kind and thus would “reflect the impact of treatment on a wide range of patients, with different health systems.”
As an official EAU spokesperson, Dr. Stenzl added, “We completely agree that early detection and treatment is essential if we are to avoid problems with quality of life later on. It shows that, for many men, quality of life can be poor after most prostate cancer treatment, especially in advanced disease. This message is clear, and we need to listen to the voices of these patients.”
EUPROMS was conducted by Europa Uomo in conjunction with the Erasmus University Medical Centre in Rotterdam, the Netherlands. Funding was received from Bayer, Ipsen, and Janssen. The companies had no influence over any aspect of the study. The commentators did not have conflicts of interest to disclose.
FROM EAU20
Better continence rate gives robotic prostatectomy the edge
At 3 months, 54.3% of prostate cancer patients who underwent RARP and 45.6% of those who had LRP were continent after catheter removal (P = .027).
“We did use a very strong definition for continence, meaning no pad or safety pad; patients wearing one pad per day we’re not classified as continent,” said study investigator Jens-Uwe Stolzenburg, MD, PhD, professor and head of urology at the University of Leipzig Hospital in Germany.
Dr. Stolzenburg presented these findings at the European Association of Urology virtual annual congress.
The findings fit with previous research showing higher continence rates with RARP (69%-80%) than with LRP (62%-63%), although those studies did not always find the difference to be statistically significant, and higher quality evidence was needed (J Sex Med. 2011 May;8[5]:1503-12; Eur Urol. 2013 Apr;63[4]:606-14). “Up to now, there are only two randomized studies published in the literature comparing robotic and classical laparoscopic prostatectomy, and my point of view is that there are strong limitations of both studies,” Dr. Stolzenburg said.
“First of all, both studies are based on the single experience of surgeons, so only one surgeon has performed surgery. The second limitation is the limited numbers of patients included,” he observed. One study had 64 patients in each arm, and the other had 60 patients in each arm.
Providing higher quality evidence
Dr. Stolzenburg presented results of the LAP-01 study, which was designed to close the knowledge gap and determine if there really was an advantage for RARP over LRP for preserving continence.
The trial was conducted at three academic centers and one public hospital in Germany. The final analysis included 718 patients with prostate cancer referred for prostate surgery. They were randomized, in a ratio of three to one, to undergo RARP (n = 530) or LRP (n = 188), being unaware themselves of which surgery they would be having until the 3-month primary endpoint.
In addition to improved continence over LRP, RARP was associated with significantly better erectile function at 3 months (P = .016), as measured by the International Index of Erectile Function (IIEF).
That said, erectile function was still severely affected by both surgical procedures. Total IIEF scores were 6.0 with RARP and 4.7 with LRP, compared with 15.9 and 16.2, respectively, at baseline.
A higher percentage of men who had nerve-sparing procedures reported having an erection suitable for sexual intercourse at 2 months in the RARP group than in the LRP group (17.7% vs. 6.7%, P = .007).
The complication rate was “a little bit higher” in the LRP group than in the RARP group, “but the difference was not statistically significant,” Dr. Stolzenburg said. He added that “the most frequent complication was anastomotic leakage, and most complications overall were low-grade complications in both groups.”
Multicenter experience
The potential for prostatectomy to have effects on urinary continence and sexual function are important issues that need to be discussed upfront with patients, observed Alexandre de la Taille, MD, PhD, who was invited to discuss the study.
Current European guidance says “there is no surgical approach – open, laparoscopic, or robotic radical prostatectomy – that has proven superiority in terms of functional or oncological results,” he said. However, the LAP-01 study “found that the continence rate was better when we use a robotic approach compared to a laparoscopic approach.”
Dr. de la Taille, who is professor and chair of the urology service at CHU Mondor in Cretéil, France, also highlighted that this result was achieved with no increase in the morbidity profile or compromise of cancer control.
“My very first impression is that we are missing a little bit, some granularity of the data in terms of one key question, which is volume of surgery,” said the chair of the session Alberto Briganti, MD, PhD, associate professor of urology at Università Vita-Salute San Raffaele, and deputy director of the Urological Research Institute of IRCCS Ospedale San Raffaele, both in Milan.
“We know that recovery of outcomes is volume-dependent, both in the laparoscopic and robotic setting,” Dr. Briganti added.
“This is really a multicenter study including a lot of surgeons,” Dr. de la Taille countered, agreeing that the volume of surgeries might be something the LAP-01 study investigators could look at in a sub-analysis.
“Of course, some of them have a huge experience in the robotic approach and some of them a lower experience of the robotic approach, but when you put all together, there is a better continence recovery at 3 months when compared to the laparoscopic approach,” Dr. de la Taille said.
Calling the study a “real-life practice study,” he noted that urinary continence at 12 months might be a stronger endpoint, and the difference between the two surgical approaches may become less with time.
“But for the patient, again, daily practice, it’s better to have early urinary continence recovery compared to a late recovery,” Dr. de la Taille said.
This study was funded by the University of Leipzig via a German Cancer Aid grant. All speakers declared no conflicts of interest.
SOURCE: Stolzenburg J-E. EAU20, Abstract.
At 3 months, 54.3% of prostate cancer patients who underwent RARP and 45.6% of those who had LRP were continent after catheter removal (P = .027).
“We did use a very strong definition for continence, meaning no pad or safety pad; patients wearing one pad per day we’re not classified as continent,” said study investigator Jens-Uwe Stolzenburg, MD, PhD, professor and head of urology at the University of Leipzig Hospital in Germany.
Dr. Stolzenburg presented these findings at the European Association of Urology virtual annual congress.
The findings fit with previous research showing higher continence rates with RARP (69%-80%) than with LRP (62%-63%), although those studies did not always find the difference to be statistically significant, and higher quality evidence was needed (J Sex Med. 2011 May;8[5]:1503-12; Eur Urol. 2013 Apr;63[4]:606-14). “Up to now, there are only two randomized studies published in the literature comparing robotic and classical laparoscopic prostatectomy, and my point of view is that there are strong limitations of both studies,” Dr. Stolzenburg said.
“First of all, both studies are based on the single experience of surgeons, so only one surgeon has performed surgery. The second limitation is the limited numbers of patients included,” he observed. One study had 64 patients in each arm, and the other had 60 patients in each arm.
Providing higher quality evidence
Dr. Stolzenburg presented results of the LAP-01 study, which was designed to close the knowledge gap and determine if there really was an advantage for RARP over LRP for preserving continence.
The trial was conducted at three academic centers and one public hospital in Germany. The final analysis included 718 patients with prostate cancer referred for prostate surgery. They were randomized, in a ratio of three to one, to undergo RARP (n = 530) or LRP (n = 188), being unaware themselves of which surgery they would be having until the 3-month primary endpoint.
In addition to improved continence over LRP, RARP was associated with significantly better erectile function at 3 months (P = .016), as measured by the International Index of Erectile Function (IIEF).
That said, erectile function was still severely affected by both surgical procedures. Total IIEF scores were 6.0 with RARP and 4.7 with LRP, compared with 15.9 and 16.2, respectively, at baseline.
A higher percentage of men who had nerve-sparing procedures reported having an erection suitable for sexual intercourse at 2 months in the RARP group than in the LRP group (17.7% vs. 6.7%, P = .007).
The complication rate was “a little bit higher” in the LRP group than in the RARP group, “but the difference was not statistically significant,” Dr. Stolzenburg said. He added that “the most frequent complication was anastomotic leakage, and most complications overall were low-grade complications in both groups.”
Multicenter experience
The potential for prostatectomy to have effects on urinary continence and sexual function are important issues that need to be discussed upfront with patients, observed Alexandre de la Taille, MD, PhD, who was invited to discuss the study.
Current European guidance says “there is no surgical approach – open, laparoscopic, or robotic radical prostatectomy – that has proven superiority in terms of functional or oncological results,” he said. However, the LAP-01 study “found that the continence rate was better when we use a robotic approach compared to a laparoscopic approach.”
Dr. de la Taille, who is professor and chair of the urology service at CHU Mondor in Cretéil, France, also highlighted that this result was achieved with no increase in the morbidity profile or compromise of cancer control.
“My very first impression is that we are missing a little bit, some granularity of the data in terms of one key question, which is volume of surgery,” said the chair of the session Alberto Briganti, MD, PhD, associate professor of urology at Università Vita-Salute San Raffaele, and deputy director of the Urological Research Institute of IRCCS Ospedale San Raffaele, both in Milan.
“We know that recovery of outcomes is volume-dependent, both in the laparoscopic and robotic setting,” Dr. Briganti added.
“This is really a multicenter study including a lot of surgeons,” Dr. de la Taille countered, agreeing that the volume of surgeries might be something the LAP-01 study investigators could look at in a sub-analysis.
“Of course, some of them have a huge experience in the robotic approach and some of them a lower experience of the robotic approach, but when you put all together, there is a better continence recovery at 3 months when compared to the laparoscopic approach,” Dr. de la Taille said.
Calling the study a “real-life practice study,” he noted that urinary continence at 12 months might be a stronger endpoint, and the difference between the two surgical approaches may become less with time.
“But for the patient, again, daily practice, it’s better to have early urinary continence recovery compared to a late recovery,” Dr. de la Taille said.
This study was funded by the University of Leipzig via a German Cancer Aid grant. All speakers declared no conflicts of interest.
SOURCE: Stolzenburg J-E. EAU20, Abstract.
At 3 months, 54.3% of prostate cancer patients who underwent RARP and 45.6% of those who had LRP were continent after catheter removal (P = .027).
“We did use a very strong definition for continence, meaning no pad or safety pad; patients wearing one pad per day we’re not classified as continent,” said study investigator Jens-Uwe Stolzenburg, MD, PhD, professor and head of urology at the University of Leipzig Hospital in Germany.
Dr. Stolzenburg presented these findings at the European Association of Urology virtual annual congress.
The findings fit with previous research showing higher continence rates with RARP (69%-80%) than with LRP (62%-63%), although those studies did not always find the difference to be statistically significant, and higher quality evidence was needed (J Sex Med. 2011 May;8[5]:1503-12; Eur Urol. 2013 Apr;63[4]:606-14). “Up to now, there are only two randomized studies published in the literature comparing robotic and classical laparoscopic prostatectomy, and my point of view is that there are strong limitations of both studies,” Dr. Stolzenburg said.
“First of all, both studies are based on the single experience of surgeons, so only one surgeon has performed surgery. The second limitation is the limited numbers of patients included,” he observed. One study had 64 patients in each arm, and the other had 60 patients in each arm.
Providing higher quality evidence
Dr. Stolzenburg presented results of the LAP-01 study, which was designed to close the knowledge gap and determine if there really was an advantage for RARP over LRP for preserving continence.
The trial was conducted at three academic centers and one public hospital in Germany. The final analysis included 718 patients with prostate cancer referred for prostate surgery. They were randomized, in a ratio of three to one, to undergo RARP (n = 530) or LRP (n = 188), being unaware themselves of which surgery they would be having until the 3-month primary endpoint.
In addition to improved continence over LRP, RARP was associated with significantly better erectile function at 3 months (P = .016), as measured by the International Index of Erectile Function (IIEF).
That said, erectile function was still severely affected by both surgical procedures. Total IIEF scores were 6.0 with RARP and 4.7 with LRP, compared with 15.9 and 16.2, respectively, at baseline.
A higher percentage of men who had nerve-sparing procedures reported having an erection suitable for sexual intercourse at 2 months in the RARP group than in the LRP group (17.7% vs. 6.7%, P = .007).
The complication rate was “a little bit higher” in the LRP group than in the RARP group, “but the difference was not statistically significant,” Dr. Stolzenburg said. He added that “the most frequent complication was anastomotic leakage, and most complications overall were low-grade complications in both groups.”
Multicenter experience
The potential for prostatectomy to have effects on urinary continence and sexual function are important issues that need to be discussed upfront with patients, observed Alexandre de la Taille, MD, PhD, who was invited to discuss the study.
Current European guidance says “there is no surgical approach – open, laparoscopic, or robotic radical prostatectomy – that has proven superiority in terms of functional or oncological results,” he said. However, the LAP-01 study “found that the continence rate was better when we use a robotic approach compared to a laparoscopic approach.”
Dr. de la Taille, who is professor and chair of the urology service at CHU Mondor in Cretéil, France, also highlighted that this result was achieved with no increase in the morbidity profile or compromise of cancer control.
“My very first impression is that we are missing a little bit, some granularity of the data in terms of one key question, which is volume of surgery,” said the chair of the session Alberto Briganti, MD, PhD, associate professor of urology at Università Vita-Salute San Raffaele, and deputy director of the Urological Research Institute of IRCCS Ospedale San Raffaele, both in Milan.
“We know that recovery of outcomes is volume-dependent, both in the laparoscopic and robotic setting,” Dr. Briganti added.
“This is really a multicenter study including a lot of surgeons,” Dr. de la Taille countered, agreeing that the volume of surgeries might be something the LAP-01 study investigators could look at in a sub-analysis.
“Of course, some of them have a huge experience in the robotic approach and some of them a lower experience of the robotic approach, but when you put all together, there is a better continence recovery at 3 months when compared to the laparoscopic approach,” Dr. de la Taille said.
Calling the study a “real-life practice study,” he noted that urinary continence at 12 months might be a stronger endpoint, and the difference between the two surgical approaches may become less with time.
“But for the patient, again, daily practice, it’s better to have early urinary continence recovery compared to a late recovery,” Dr. de la Taille said.
This study was funded by the University of Leipzig via a German Cancer Aid grant. All speakers declared no conflicts of interest.
SOURCE: Stolzenburg J-E. EAU20, Abstract.
FROM EAU20
Robotic renal surgery bests open partial nephrectomy
RAPN was associated with a 61% decrease in intraoperative complications and a 71% decrease in overall complications in the IRON study.
Alessandro Larcher, MD, of San Raffaele Hospital and the Urological Research Institute in Milan, presented results from IRON during a live poster session at the virtual annual congress of the European Association of Urology.
The IRON study was performed in nine high-volume centers and involved 3,468 patients with renal cell cancer. Patients were recruited if they had a localized renal cell mass (cT1-2) with no nodal involvement or metastases. There were 2,405 patients who underwent RAPN and 1,063 who underwent OPN.
Intraoperative complications occurred in 5.7% of patients who underwent RAPN and in 9.3% of those who underwent OPN. Overall complications occurred in 33% and 18%, respectively (P < .001 for both).
“The complication profile was invariably in favor of robot-assisted surgery,” Dr. Larcher observed.
Patients who underwent RAPN had less estimated median blood loss (150 mL vs. 180 mL, P < .001) as well as lower rates of hemorrhagic complications (6.4% vs. 9%, P < .01) and urinary leakage (0.8% vs. 4.6%, P < .01).
The operative time was longer with RAPN than with OPN, at a median of 150 minutes and 120 minutes, respectively (P < .001). However, patients remained in the hospital for less time with RAPN than with OPN, at a median of 4 days and 6 days, respectively (P < .01).
RAPN was associated with fewer surgical complications than OPN according to the Clavien-Dindo system. Grade 2 or higher complications occurred in 12% and 20% of patients, respectively (P < .001). Grade 3 or higher complications occurred in 4% and 6.1%, respectively (P < .001).
“The benefit with respect to the complication risk reduction in the case of robot-assisted surgery was not affected by the tumor complexity, by the dimension of the mass, the comorbidities of the patients, or the baseline renal function,” Dr. Larcher said. “[T]he advantage after robot-assisted surgery is consistent regardless of all these features.”
Early renal function was better after OPN, but there was no significant difference between the two groups at 1 year of follow-up. The median ischemia time was 15 minutes with OPN and 16 minutes with RAPN (P < .001).
Postoperatively, the median estimated glomerular filtration rate was 78 mL/min/1.73m2 with OPN and 76 mL/min/1.73m2 with RAPN (P < .001). At 1 year, the median estimated glomerular filtration rate was 68 and 71 mL/min/1.73m2, respectively (P = .5).
Dr. Larcher noted that there was no difference between RAPN and OPN in terms of 5-year oncologic outcomes. Local recurrence occurred in 1.6% and 2.1% of patients, respectively (P = .06); systemic progression was seen in 1.8% and 4.5%, respectively (P = .5); and clinical progression was observed in 3.2% and 6.6%, respectively (P = .9).
“[IRON is] a really powerful study. It’s one of those studies that kind of has to be done,” said Ben Challacombe, MBBS, a consultant urological surgeon at Guy’s Hospital and St. Thomas’ Hospital in London who chaired the poster session during which these findings were presented.
Dr. Challacombe, who specializes in the treatment of kidney and prostatic disease using robotic surgery, noted that about 75% of procedures in the United Kingdom are now being performed with robotic assistance and queried what percentage of procedures should still be done by open surgery.
“I would turn it,” Dr. Larcher said. “What is the percentage of surgeons that should use one technique or the other?” In the IRON study, as well as other studies, surgical expertise, training, and center volumes were important.
“What the data are telling us is that those who are really confident in robotic surgeries can achieve even better outcomes, also in very complex cases,” Dr. Larcher said. “I think it’s not any longer dependent on the tumor factors. The answer to the question is only determined by human factors.”
The IRON study was supported by a grant from Intuitive. Dr. Larcher declared no conflicts of interest. Dr. Challacombe did not present any disclosures.
SOURCE: Larcher A et al. EAU20, Abstract 30. Eur Urol Open Sci 2020;19(Suppl 2):e142.
RAPN was associated with a 61% decrease in intraoperative complications and a 71% decrease in overall complications in the IRON study.
Alessandro Larcher, MD, of San Raffaele Hospital and the Urological Research Institute in Milan, presented results from IRON during a live poster session at the virtual annual congress of the European Association of Urology.
The IRON study was performed in nine high-volume centers and involved 3,468 patients with renal cell cancer. Patients were recruited if they had a localized renal cell mass (cT1-2) with no nodal involvement or metastases. There were 2,405 patients who underwent RAPN and 1,063 who underwent OPN.
Intraoperative complications occurred in 5.7% of patients who underwent RAPN and in 9.3% of those who underwent OPN. Overall complications occurred in 33% and 18%, respectively (P < .001 for both).
“The complication profile was invariably in favor of robot-assisted surgery,” Dr. Larcher observed.
Patients who underwent RAPN had less estimated median blood loss (150 mL vs. 180 mL, P < .001) as well as lower rates of hemorrhagic complications (6.4% vs. 9%, P < .01) and urinary leakage (0.8% vs. 4.6%, P < .01).
The operative time was longer with RAPN than with OPN, at a median of 150 minutes and 120 minutes, respectively (P < .001). However, patients remained in the hospital for less time with RAPN than with OPN, at a median of 4 days and 6 days, respectively (P < .01).
RAPN was associated with fewer surgical complications than OPN according to the Clavien-Dindo system. Grade 2 or higher complications occurred in 12% and 20% of patients, respectively (P < .001). Grade 3 or higher complications occurred in 4% and 6.1%, respectively (P < .001).
“The benefit with respect to the complication risk reduction in the case of robot-assisted surgery was not affected by the tumor complexity, by the dimension of the mass, the comorbidities of the patients, or the baseline renal function,” Dr. Larcher said. “[T]he advantage after robot-assisted surgery is consistent regardless of all these features.”
Early renal function was better after OPN, but there was no significant difference between the two groups at 1 year of follow-up. The median ischemia time was 15 minutes with OPN and 16 minutes with RAPN (P < .001).
Postoperatively, the median estimated glomerular filtration rate was 78 mL/min/1.73m2 with OPN and 76 mL/min/1.73m2 with RAPN (P < .001). At 1 year, the median estimated glomerular filtration rate was 68 and 71 mL/min/1.73m2, respectively (P = .5).
Dr. Larcher noted that there was no difference between RAPN and OPN in terms of 5-year oncologic outcomes. Local recurrence occurred in 1.6% and 2.1% of patients, respectively (P = .06); systemic progression was seen in 1.8% and 4.5%, respectively (P = .5); and clinical progression was observed in 3.2% and 6.6%, respectively (P = .9).
“[IRON is] a really powerful study. It’s one of those studies that kind of has to be done,” said Ben Challacombe, MBBS, a consultant urological surgeon at Guy’s Hospital and St. Thomas’ Hospital in London who chaired the poster session during which these findings were presented.
Dr. Challacombe, who specializes in the treatment of kidney and prostatic disease using robotic surgery, noted that about 75% of procedures in the United Kingdom are now being performed with robotic assistance and queried what percentage of procedures should still be done by open surgery.
“I would turn it,” Dr. Larcher said. “What is the percentage of surgeons that should use one technique or the other?” In the IRON study, as well as other studies, surgical expertise, training, and center volumes were important.
“What the data are telling us is that those who are really confident in robotic surgeries can achieve even better outcomes, also in very complex cases,” Dr. Larcher said. “I think it’s not any longer dependent on the tumor factors. The answer to the question is only determined by human factors.”
The IRON study was supported by a grant from Intuitive. Dr. Larcher declared no conflicts of interest. Dr. Challacombe did not present any disclosures.
SOURCE: Larcher A et al. EAU20, Abstract 30. Eur Urol Open Sci 2020;19(Suppl 2):e142.
RAPN was associated with a 61% decrease in intraoperative complications and a 71% decrease in overall complications in the IRON study.
Alessandro Larcher, MD, of San Raffaele Hospital and the Urological Research Institute in Milan, presented results from IRON during a live poster session at the virtual annual congress of the European Association of Urology.
The IRON study was performed in nine high-volume centers and involved 3,468 patients with renal cell cancer. Patients were recruited if they had a localized renal cell mass (cT1-2) with no nodal involvement or metastases. There were 2,405 patients who underwent RAPN and 1,063 who underwent OPN.
Intraoperative complications occurred in 5.7% of patients who underwent RAPN and in 9.3% of those who underwent OPN. Overall complications occurred in 33% and 18%, respectively (P < .001 for both).
“The complication profile was invariably in favor of robot-assisted surgery,” Dr. Larcher observed.
Patients who underwent RAPN had less estimated median blood loss (150 mL vs. 180 mL, P < .001) as well as lower rates of hemorrhagic complications (6.4% vs. 9%, P < .01) and urinary leakage (0.8% vs. 4.6%, P < .01).
The operative time was longer with RAPN than with OPN, at a median of 150 minutes and 120 minutes, respectively (P < .001). However, patients remained in the hospital for less time with RAPN than with OPN, at a median of 4 days and 6 days, respectively (P < .01).
RAPN was associated with fewer surgical complications than OPN according to the Clavien-Dindo system. Grade 2 or higher complications occurred in 12% and 20% of patients, respectively (P < .001). Grade 3 or higher complications occurred in 4% and 6.1%, respectively (P < .001).
“The benefit with respect to the complication risk reduction in the case of robot-assisted surgery was not affected by the tumor complexity, by the dimension of the mass, the comorbidities of the patients, or the baseline renal function,” Dr. Larcher said. “[T]he advantage after robot-assisted surgery is consistent regardless of all these features.”
Early renal function was better after OPN, but there was no significant difference between the two groups at 1 year of follow-up. The median ischemia time was 15 minutes with OPN and 16 minutes with RAPN (P < .001).
Postoperatively, the median estimated glomerular filtration rate was 78 mL/min/1.73m2 with OPN and 76 mL/min/1.73m2 with RAPN (P < .001). At 1 year, the median estimated glomerular filtration rate was 68 and 71 mL/min/1.73m2, respectively (P = .5).
Dr. Larcher noted that there was no difference between RAPN and OPN in terms of 5-year oncologic outcomes. Local recurrence occurred in 1.6% and 2.1% of patients, respectively (P = .06); systemic progression was seen in 1.8% and 4.5%, respectively (P = .5); and clinical progression was observed in 3.2% and 6.6%, respectively (P = .9).
“[IRON is] a really powerful study. It’s one of those studies that kind of has to be done,” said Ben Challacombe, MBBS, a consultant urological surgeon at Guy’s Hospital and St. Thomas’ Hospital in London who chaired the poster session during which these findings were presented.
Dr. Challacombe, who specializes in the treatment of kidney and prostatic disease using robotic surgery, noted that about 75% of procedures in the United Kingdom are now being performed with robotic assistance and queried what percentage of procedures should still be done by open surgery.
“I would turn it,” Dr. Larcher said. “What is the percentage of surgeons that should use one technique or the other?” In the IRON study, as well as other studies, surgical expertise, training, and center volumes were important.
“What the data are telling us is that those who are really confident in robotic surgeries can achieve even better outcomes, also in very complex cases,” Dr. Larcher said. “I think it’s not any longer dependent on the tumor factors. The answer to the question is only determined by human factors.”
The IRON study was supported by a grant from Intuitive. Dr. Larcher declared no conflicts of interest. Dr. Challacombe did not present any disclosures.
SOURCE: Larcher A et al. EAU20, Abstract 30. Eur Urol Open Sci 2020;19(Suppl 2):e142.
FROM EAU20
Intravesical BCG dosing frequency ‘critical’ in bladder cancer
The rates of recurrence were 27.1% in the reduced dosing frequency arm and 12% in the standard dosing frequency arm. These results were reported at the virtual annual congress of the European Association of Urology.
More patients in the reduced dosing frequency arm than in the standard dosing frequency arm had a shorter time to recurrence, which was the primary endpoint of the trial.
At 6 months, the rate of recurrence was 18% in the reduced frequency arm and 8% in the standard frequency arm. The gap widened further at both 12 months (24% and 11%, respectively) and 24 months (34% and 15%, respectively). The hazard ratio for time to recurrence was 0.403 in favor of the standard dosing frequency arm.
“The recommended dose and schedule of BCG consists of once-weekly installations during 6 weeks of induction, followed by 3 weeks of maintenance at 3, 6, and 12 months,” observed study investigator Marc-Oliver Grimm, MD, of Jena (Germany) University Hospital.
“BCG instillation is, however, frequently associated with adverse events, which may lead to discontinuation, and several attempts have been made to reduce symptom burden associated with BCG,” he added.
Dr. Grimm presented the recently published findings from NIMBUS (Eur Urol. 2020 May 20;S0302-2838[20]30334-1) alongside some new information from a post hoc analysis.
Trial details
NIMBUS was a randomized, unblinded study of 345 patients with high-grade NMIBC who were recruited over a prolonged period, Dr. Grimm said. The long accrual was caused by a shortage of BCG and meant that the statistical assumptions had to be revised to include fewer patients.
The trial was designed to compare induction consisting of three versus six weekly BCG instillations and maintenance consisting of two versus three weekly BCG instillations at 3, 6, and 12 months. The aim had been to show that a reduced dosing frequency of BCG – 9 rather than 15 instillations – was noninferior to the standard dosing frequency of BCG, Dr. Grimm said. However, that was not the case, and the trial had to be stopped prematurely. In October 2019, the study’s sponsor, the EAU Research Foundation, announced that the trial would end.
Despite its unexpected ending, the trial’s data now fill some knowledge gaps, as pointed out by the discussant for the trial, Peter Black, MD, of the University of British Columbia in Vancouver.
Previous studies, such as the SWOG 8507, EORTC 30962, and CUETO 98013 trials, had shown that maintenance treatment works, but the schedule matters, he said. Results have also shown that the duration of maintenance treatment is less important than the dose of BCG given.
“The NIMBUS trial now tells us that dosing frequency is critical,” Dr. Black said.
Not only did the NIMBUS trial alter the maintenance schedule, it also altered the induction course of BCG instillation.
“The dramatic difference in recurrence-free survival, especially with the large separation of K-M [Kaplan-Meier] curves early on, suggests that this change to induction has had a major impact on the outcomes,” Dr. Black observed.
Post hoc analysis
Dr. Grimm presented a post hoc analysis comparing the rates of recurrence in the NIMBUS trial with rates seen in the EORTC 30962 and CUETO 98013 trials. Dr. Black also compared NIMBUS results to results from the SWOG 8507 trial.
The analysis showed lower rates of recurrence in the standard dose frequency arm in the NIMBUS trial than in the EORTC and CUETO trials at both 12 months (11%, 25%, and 18%, respectively) and 24 months (15%, 32%, and 27%, respectively).
However, as Dr. Black pointed out, the SWOG trial had similar recurrence rates as the NIMBUS trial at 12 months (9% and 11%, respectively) and 24 months (19% and 15%, respectively).
Dr. Grimm suggested that the lower rates of recurrence in the standard dosing arm of NIMBUS versus the other trials might have been because 91% of patients in the NIMBUS trial having undergone repeat transurethral resection for bladder tumor before BCG instillation.
Dr. Black said while this might have had an effect, it was probably not the only answer. While it’s true that the other trials had not considered repeat transurethral resection for bladder tumor, there were other confounding factors that might have been important, from patient selection bias to the use of advanced cystoscopy technologies, he said.
“If we really want to discern differences between surgery and intravesical therapy, we need to focus on CIS [carcinoma in situ] patients. Although this has major implications on feasibility since the patient pool is smaller,” Dr. Black said.
“One final point I’d like to make is that we really need to use these trials to understand the biology of non–muscle invasive bladder cancer,” he said. ”We know that BCG induces a cellular response, and we can measure this, as well as cytokine response. We know that the response builds to a plateau over four to six doses of induction and over two to three doses of maintenance therapy. This is perhaps more rapid in patients with pre-existing BCG immune reactivity. But there is biological rationale for the current six-plus-three protocol, and I think the reduced dose frequency in the NIMBUS trial probably failed to achieve the same immune activation as the established protocol.”
“If we were faced with a BCG shortage, it is better to reduce dose or duration of therapy but not the frequency of dosing,” Dr. Black added.
The NIMBUS trial was sponsored by the EAU Research Foundation. Dr. Grimm disclosed ties to Novartis, Bristol-Myers Squibb, Pfizer, AstraZeneca, and many other pharmaceutical companies. Dr. Black had no conflicts of interests relevant to his comments.
SOURCE: Grimm M-O. EAU20. https://urosource.uroweb.org/resource-centre/EAU20V/212877/Abstract/
The rates of recurrence were 27.1% in the reduced dosing frequency arm and 12% in the standard dosing frequency arm. These results were reported at the virtual annual congress of the European Association of Urology.
More patients in the reduced dosing frequency arm than in the standard dosing frequency arm had a shorter time to recurrence, which was the primary endpoint of the trial.
At 6 months, the rate of recurrence was 18% in the reduced frequency arm and 8% in the standard frequency arm. The gap widened further at both 12 months (24% and 11%, respectively) and 24 months (34% and 15%, respectively). The hazard ratio for time to recurrence was 0.403 in favor of the standard dosing frequency arm.
“The recommended dose and schedule of BCG consists of once-weekly installations during 6 weeks of induction, followed by 3 weeks of maintenance at 3, 6, and 12 months,” observed study investigator Marc-Oliver Grimm, MD, of Jena (Germany) University Hospital.
“BCG instillation is, however, frequently associated with adverse events, which may lead to discontinuation, and several attempts have been made to reduce symptom burden associated with BCG,” he added.
Dr. Grimm presented the recently published findings from NIMBUS (Eur Urol. 2020 May 20;S0302-2838[20]30334-1) alongside some new information from a post hoc analysis.
Trial details
NIMBUS was a randomized, unblinded study of 345 patients with high-grade NMIBC who were recruited over a prolonged period, Dr. Grimm said. The long accrual was caused by a shortage of BCG and meant that the statistical assumptions had to be revised to include fewer patients.
The trial was designed to compare induction consisting of three versus six weekly BCG instillations and maintenance consisting of two versus three weekly BCG instillations at 3, 6, and 12 months. The aim had been to show that a reduced dosing frequency of BCG – 9 rather than 15 instillations – was noninferior to the standard dosing frequency of BCG, Dr. Grimm said. However, that was not the case, and the trial had to be stopped prematurely. In October 2019, the study’s sponsor, the EAU Research Foundation, announced that the trial would end.
Despite its unexpected ending, the trial’s data now fill some knowledge gaps, as pointed out by the discussant for the trial, Peter Black, MD, of the University of British Columbia in Vancouver.
Previous studies, such as the SWOG 8507, EORTC 30962, and CUETO 98013 trials, had shown that maintenance treatment works, but the schedule matters, he said. Results have also shown that the duration of maintenance treatment is less important than the dose of BCG given.
“The NIMBUS trial now tells us that dosing frequency is critical,” Dr. Black said.
Not only did the NIMBUS trial alter the maintenance schedule, it also altered the induction course of BCG instillation.
“The dramatic difference in recurrence-free survival, especially with the large separation of K-M [Kaplan-Meier] curves early on, suggests that this change to induction has had a major impact on the outcomes,” Dr. Black observed.
Post hoc analysis
Dr. Grimm presented a post hoc analysis comparing the rates of recurrence in the NIMBUS trial with rates seen in the EORTC 30962 and CUETO 98013 trials. Dr. Black also compared NIMBUS results to results from the SWOG 8507 trial.
The analysis showed lower rates of recurrence in the standard dose frequency arm in the NIMBUS trial than in the EORTC and CUETO trials at both 12 months (11%, 25%, and 18%, respectively) and 24 months (15%, 32%, and 27%, respectively).
However, as Dr. Black pointed out, the SWOG trial had similar recurrence rates as the NIMBUS trial at 12 months (9% and 11%, respectively) and 24 months (19% and 15%, respectively).
Dr. Grimm suggested that the lower rates of recurrence in the standard dosing arm of NIMBUS versus the other trials might have been because 91% of patients in the NIMBUS trial having undergone repeat transurethral resection for bladder tumor before BCG instillation.
Dr. Black said while this might have had an effect, it was probably not the only answer. While it’s true that the other trials had not considered repeat transurethral resection for bladder tumor, there were other confounding factors that might have been important, from patient selection bias to the use of advanced cystoscopy technologies, he said.
“If we really want to discern differences between surgery and intravesical therapy, we need to focus on CIS [carcinoma in situ] patients. Although this has major implications on feasibility since the patient pool is smaller,” Dr. Black said.
“One final point I’d like to make is that we really need to use these trials to understand the biology of non–muscle invasive bladder cancer,” he said. ”We know that BCG induces a cellular response, and we can measure this, as well as cytokine response. We know that the response builds to a plateau over four to six doses of induction and over two to three doses of maintenance therapy. This is perhaps more rapid in patients with pre-existing BCG immune reactivity. But there is biological rationale for the current six-plus-three protocol, and I think the reduced dose frequency in the NIMBUS trial probably failed to achieve the same immune activation as the established protocol.”
“If we were faced with a BCG shortage, it is better to reduce dose or duration of therapy but not the frequency of dosing,” Dr. Black added.
The NIMBUS trial was sponsored by the EAU Research Foundation. Dr. Grimm disclosed ties to Novartis, Bristol-Myers Squibb, Pfizer, AstraZeneca, and many other pharmaceutical companies. Dr. Black had no conflicts of interests relevant to his comments.
SOURCE: Grimm M-O. EAU20. https://urosource.uroweb.org/resource-centre/EAU20V/212877/Abstract/
The rates of recurrence were 27.1% in the reduced dosing frequency arm and 12% in the standard dosing frequency arm. These results were reported at the virtual annual congress of the European Association of Urology.
More patients in the reduced dosing frequency arm than in the standard dosing frequency arm had a shorter time to recurrence, which was the primary endpoint of the trial.
At 6 months, the rate of recurrence was 18% in the reduced frequency arm and 8% in the standard frequency arm. The gap widened further at both 12 months (24% and 11%, respectively) and 24 months (34% and 15%, respectively). The hazard ratio for time to recurrence was 0.403 in favor of the standard dosing frequency arm.
“The recommended dose and schedule of BCG consists of once-weekly installations during 6 weeks of induction, followed by 3 weeks of maintenance at 3, 6, and 12 months,” observed study investigator Marc-Oliver Grimm, MD, of Jena (Germany) University Hospital.
“BCG instillation is, however, frequently associated with adverse events, which may lead to discontinuation, and several attempts have been made to reduce symptom burden associated with BCG,” he added.
Dr. Grimm presented the recently published findings from NIMBUS (Eur Urol. 2020 May 20;S0302-2838[20]30334-1) alongside some new information from a post hoc analysis.
Trial details
NIMBUS was a randomized, unblinded study of 345 patients with high-grade NMIBC who were recruited over a prolonged period, Dr. Grimm said. The long accrual was caused by a shortage of BCG and meant that the statistical assumptions had to be revised to include fewer patients.
The trial was designed to compare induction consisting of three versus six weekly BCG instillations and maintenance consisting of two versus three weekly BCG instillations at 3, 6, and 12 months. The aim had been to show that a reduced dosing frequency of BCG – 9 rather than 15 instillations – was noninferior to the standard dosing frequency of BCG, Dr. Grimm said. However, that was not the case, and the trial had to be stopped prematurely. In October 2019, the study’s sponsor, the EAU Research Foundation, announced that the trial would end.
Despite its unexpected ending, the trial’s data now fill some knowledge gaps, as pointed out by the discussant for the trial, Peter Black, MD, of the University of British Columbia in Vancouver.
Previous studies, such as the SWOG 8507, EORTC 30962, and CUETO 98013 trials, had shown that maintenance treatment works, but the schedule matters, he said. Results have also shown that the duration of maintenance treatment is less important than the dose of BCG given.
“The NIMBUS trial now tells us that dosing frequency is critical,” Dr. Black said.
Not only did the NIMBUS trial alter the maintenance schedule, it also altered the induction course of BCG instillation.
“The dramatic difference in recurrence-free survival, especially with the large separation of K-M [Kaplan-Meier] curves early on, suggests that this change to induction has had a major impact on the outcomes,” Dr. Black observed.
Post hoc analysis
Dr. Grimm presented a post hoc analysis comparing the rates of recurrence in the NIMBUS trial with rates seen in the EORTC 30962 and CUETO 98013 trials. Dr. Black also compared NIMBUS results to results from the SWOG 8507 trial.
The analysis showed lower rates of recurrence in the standard dose frequency arm in the NIMBUS trial than in the EORTC and CUETO trials at both 12 months (11%, 25%, and 18%, respectively) and 24 months (15%, 32%, and 27%, respectively).
However, as Dr. Black pointed out, the SWOG trial had similar recurrence rates as the NIMBUS trial at 12 months (9% and 11%, respectively) and 24 months (19% and 15%, respectively).
Dr. Grimm suggested that the lower rates of recurrence in the standard dosing arm of NIMBUS versus the other trials might have been because 91% of patients in the NIMBUS trial having undergone repeat transurethral resection for bladder tumor before BCG instillation.
Dr. Black said while this might have had an effect, it was probably not the only answer. While it’s true that the other trials had not considered repeat transurethral resection for bladder tumor, there were other confounding factors that might have been important, from patient selection bias to the use of advanced cystoscopy technologies, he said.
“If we really want to discern differences between surgery and intravesical therapy, we need to focus on CIS [carcinoma in situ] patients. Although this has major implications on feasibility since the patient pool is smaller,” Dr. Black said.
“One final point I’d like to make is that we really need to use these trials to understand the biology of non–muscle invasive bladder cancer,” he said. ”We know that BCG induces a cellular response, and we can measure this, as well as cytokine response. We know that the response builds to a plateau over four to six doses of induction and over two to three doses of maintenance therapy. This is perhaps more rapid in patients with pre-existing BCG immune reactivity. But there is biological rationale for the current six-plus-three protocol, and I think the reduced dose frequency in the NIMBUS trial probably failed to achieve the same immune activation as the established protocol.”
“If we were faced with a BCG shortage, it is better to reduce dose or duration of therapy but not the frequency of dosing,” Dr. Black added.
The NIMBUS trial was sponsored by the EAU Research Foundation. Dr. Grimm disclosed ties to Novartis, Bristol-Myers Squibb, Pfizer, AstraZeneca, and many other pharmaceutical companies. Dr. Black had no conflicts of interests relevant to his comments.
SOURCE: Grimm M-O. EAU20. https://urosource.uroweb.org/resource-centre/EAU20V/212877/Abstract/
FROM EAU20
PSMA PET/CT may be new ‘gold standard’ for prostate cancer staging
The accuracy was 92% for PSMA PET/CT and 65% for CT and bone scintigraphy (P < .001), according to data reported at the virtual annual congress of the European Association of Urology and published in The Lancet.
In addition, PSMA PET/CT had greater effects on treatment. First-line imaging led to treatment changes in 28% of the PSMA PET/CT group and 15% of the CT/bone scan group. Second-line imaging led to treatment changes in 27% and 5% of patients, respectively.
“My strong view is that this is practice-changing data,” said study investigator Michael Hofman, MBBS, of the Peter MacCallum Cancer Centre in Melbourne.
Highly relevant secondary outcomes were included in the study, Dr. Hofman said, and results were all in favor of PSMA PET/CT over conventional imaging.
PSMA PET/CT was associated with a lower rate of equivocal or uncertain findings (7% vs. 23%), and half the radiation dose was needed with PSMA PET/CT (8 mSv vs. 19 mSv). Furthermore, PSMA PET/CT was more accurate when used after CT/bone scan than when CT/bone scan was used after PSMA PET/CT (19% vs. 2%).
“PSMA PET/CT has emerged as a potential new gold standard for imaging prostate cancer,” Dr. Hofman said. The images it can produce were “striking” compared to conventional CT, he added. Pelvic and abdominal metastases that are barely visible on CT were “lighting up very brightly” on PSMA PET/CT, he said.
The study also showed that PSMA PET/CT was superior to CT/bone scans for picking up metastases throughout the body. The detection rate was 91% and 59%, respectively, for pelvic nodal metastases and 95% and 74%, respectively, for distant metastases.
Study details
ProPSMA is a multicenter, phase 3 trial directly comparing PSMA PET/CT and the standard of imaging. Of 339 men assessed for inclusion across 10 centers in Australia, 302 were randomized. They had a median age of 69 years. All patients had high-risk prostate cancer, which was defined as a prostate-specific antigen level of 20 ng/mL, Gleason Grade Group 3-5, or clinical stage T3 or higher. They were all about to undergo either surgery or radiotherapy with the intention of curing their prostate cancer.
PSMA PET/CT was performed using the gallium-68-labelled PSMA-11 tracer, but the results would likely be no different if another tracer were used, Dr. Hofman said in the discussion following his talk.
Of the three available tracers, there were minor differences, mostly in how they were excreted. However, “they’re all extremely good. I’m not sure anyone’s ever going to undertake a head-to-head study comparing them,” Dr. Hofman said.
“Whichever one you can access, at the cheapest cost, I think, is going to be the best one in your center,” he added. “That really does vary geographically, but I really don’t think one is better or worse than the other.”
Praise and criticism
The latest European guidelines acknowledge that PSMA PET/CT is more sensitive for detecting lymph node and bone metastases than the classical workup of abdominopelvic CT and bone scintigraphy, according to invited discussant Matthias Heck, PD Dr. med, of the Technical University of Munich in Germany.
“Molecular imaging using PSMA PET/CT facilitates the detection of small lymph node metastasis, with the size of a few millimeters,” Dr. Heck said.
Although he commended the ProPSMA investigators, Dr. Heck had one criticism of the study design that may have resulted in over-sensitivity of PSMA PET/CT.
“As a urologist, I want to address as a discussion point the low number of histopathologic validation in the ProPSMA study,” he said. “Pelvic lymph node sampling was performed only in 66% of patients treated with radical prostatectomy for high-risk prostate cancer. Hard criteria to define the presence of metastasis were only used in 23% of patients with metastases. Therefore, it is possible that the sensitivity was overestimated by using mainly soft criteria.”
The sensitivity of PSMA PET/CT was 85%, while that of CT/bone scan was 38%. The respective specificities were 98% and 91%.
“What I like most about this study is that, when we perform a PSMA PET/CT, you see the whole body; you don’t see only pelvic lymph nodes,” Dr. Heck said. Since it was not possible to validate distant metastasis by histopathology, he added, this imaging method could clearly help determine the best treatment.
“If we have distant metastasis in the bones or in the lymph nodes outside of the pelvis, it’s clearly unnecessary to direct this patient to undergo local treatment, and we need to think about other treatments,” Dr. Heck said. “Therefore, I think it’s a very important question that is being raised by this study, and we all need to look at the whole body of the patient and not focus only on the pelvic lymph nodes.”
The study was funded by the Prostate Cancer Foundation of Australia. Dr. Hofman said he has no relevant conflicts of interest. Dr. Heck disclosed relationships with Astellas, Janssen, Ipsen, Amgen, Bayer, Heise, Merck, Sanofi, and Takeda.
SOURCES: Hofman M et al. Lancet. March 22, doi: https://doi.org/10.1016/S0140-6736(20)30314-7.
The accuracy was 92% for PSMA PET/CT and 65% for CT and bone scintigraphy (P < .001), according to data reported at the virtual annual congress of the European Association of Urology and published in The Lancet.
In addition, PSMA PET/CT had greater effects on treatment. First-line imaging led to treatment changes in 28% of the PSMA PET/CT group and 15% of the CT/bone scan group. Second-line imaging led to treatment changes in 27% and 5% of patients, respectively.
“My strong view is that this is practice-changing data,” said study investigator Michael Hofman, MBBS, of the Peter MacCallum Cancer Centre in Melbourne.
Highly relevant secondary outcomes were included in the study, Dr. Hofman said, and results were all in favor of PSMA PET/CT over conventional imaging.
PSMA PET/CT was associated with a lower rate of equivocal or uncertain findings (7% vs. 23%), and half the radiation dose was needed with PSMA PET/CT (8 mSv vs. 19 mSv). Furthermore, PSMA PET/CT was more accurate when used after CT/bone scan than when CT/bone scan was used after PSMA PET/CT (19% vs. 2%).
“PSMA PET/CT has emerged as a potential new gold standard for imaging prostate cancer,” Dr. Hofman said. The images it can produce were “striking” compared to conventional CT, he added. Pelvic and abdominal metastases that are barely visible on CT were “lighting up very brightly” on PSMA PET/CT, he said.
The study also showed that PSMA PET/CT was superior to CT/bone scans for picking up metastases throughout the body. The detection rate was 91% and 59%, respectively, for pelvic nodal metastases and 95% and 74%, respectively, for distant metastases.
Study details
ProPSMA is a multicenter, phase 3 trial directly comparing PSMA PET/CT and the standard of imaging. Of 339 men assessed for inclusion across 10 centers in Australia, 302 were randomized. They had a median age of 69 years. All patients had high-risk prostate cancer, which was defined as a prostate-specific antigen level of 20 ng/mL, Gleason Grade Group 3-5, or clinical stage T3 or higher. They were all about to undergo either surgery or radiotherapy with the intention of curing their prostate cancer.
PSMA PET/CT was performed using the gallium-68-labelled PSMA-11 tracer, but the results would likely be no different if another tracer were used, Dr. Hofman said in the discussion following his talk.
Of the three available tracers, there were minor differences, mostly in how they were excreted. However, “they’re all extremely good. I’m not sure anyone’s ever going to undertake a head-to-head study comparing them,” Dr. Hofman said.
“Whichever one you can access, at the cheapest cost, I think, is going to be the best one in your center,” he added. “That really does vary geographically, but I really don’t think one is better or worse than the other.”
Praise and criticism
The latest European guidelines acknowledge that PSMA PET/CT is more sensitive for detecting lymph node and bone metastases than the classical workup of abdominopelvic CT and bone scintigraphy, according to invited discussant Matthias Heck, PD Dr. med, of the Technical University of Munich in Germany.
“Molecular imaging using PSMA PET/CT facilitates the detection of small lymph node metastasis, with the size of a few millimeters,” Dr. Heck said.
Although he commended the ProPSMA investigators, Dr. Heck had one criticism of the study design that may have resulted in over-sensitivity of PSMA PET/CT.
“As a urologist, I want to address as a discussion point the low number of histopathologic validation in the ProPSMA study,” he said. “Pelvic lymph node sampling was performed only in 66% of patients treated with radical prostatectomy for high-risk prostate cancer. Hard criteria to define the presence of metastasis were only used in 23% of patients with metastases. Therefore, it is possible that the sensitivity was overestimated by using mainly soft criteria.”
The sensitivity of PSMA PET/CT was 85%, while that of CT/bone scan was 38%. The respective specificities were 98% and 91%.
“What I like most about this study is that, when we perform a PSMA PET/CT, you see the whole body; you don’t see only pelvic lymph nodes,” Dr. Heck said. Since it was not possible to validate distant metastasis by histopathology, he added, this imaging method could clearly help determine the best treatment.
“If we have distant metastasis in the bones or in the lymph nodes outside of the pelvis, it’s clearly unnecessary to direct this patient to undergo local treatment, and we need to think about other treatments,” Dr. Heck said. “Therefore, I think it’s a very important question that is being raised by this study, and we all need to look at the whole body of the patient and not focus only on the pelvic lymph nodes.”
The study was funded by the Prostate Cancer Foundation of Australia. Dr. Hofman said he has no relevant conflicts of interest. Dr. Heck disclosed relationships with Astellas, Janssen, Ipsen, Amgen, Bayer, Heise, Merck, Sanofi, and Takeda.
SOURCES: Hofman M et al. Lancet. March 22, doi: https://doi.org/10.1016/S0140-6736(20)30314-7.
The accuracy was 92% for PSMA PET/CT and 65% for CT and bone scintigraphy (P < .001), according to data reported at the virtual annual congress of the European Association of Urology and published in The Lancet.
In addition, PSMA PET/CT had greater effects on treatment. First-line imaging led to treatment changes in 28% of the PSMA PET/CT group and 15% of the CT/bone scan group. Second-line imaging led to treatment changes in 27% and 5% of patients, respectively.
“My strong view is that this is practice-changing data,” said study investigator Michael Hofman, MBBS, of the Peter MacCallum Cancer Centre in Melbourne.
Highly relevant secondary outcomes were included in the study, Dr. Hofman said, and results were all in favor of PSMA PET/CT over conventional imaging.
PSMA PET/CT was associated with a lower rate of equivocal or uncertain findings (7% vs. 23%), and half the radiation dose was needed with PSMA PET/CT (8 mSv vs. 19 mSv). Furthermore, PSMA PET/CT was more accurate when used after CT/bone scan than when CT/bone scan was used after PSMA PET/CT (19% vs. 2%).
“PSMA PET/CT has emerged as a potential new gold standard for imaging prostate cancer,” Dr. Hofman said. The images it can produce were “striking” compared to conventional CT, he added. Pelvic and abdominal metastases that are barely visible on CT were “lighting up very brightly” on PSMA PET/CT, he said.
The study also showed that PSMA PET/CT was superior to CT/bone scans for picking up metastases throughout the body. The detection rate was 91% and 59%, respectively, for pelvic nodal metastases and 95% and 74%, respectively, for distant metastases.
Study details
ProPSMA is a multicenter, phase 3 trial directly comparing PSMA PET/CT and the standard of imaging. Of 339 men assessed for inclusion across 10 centers in Australia, 302 were randomized. They had a median age of 69 years. All patients had high-risk prostate cancer, which was defined as a prostate-specific antigen level of 20 ng/mL, Gleason Grade Group 3-5, or clinical stage T3 or higher. They were all about to undergo either surgery or radiotherapy with the intention of curing their prostate cancer.
PSMA PET/CT was performed using the gallium-68-labelled PSMA-11 tracer, but the results would likely be no different if another tracer were used, Dr. Hofman said in the discussion following his talk.
Of the three available tracers, there were minor differences, mostly in how they were excreted. However, “they’re all extremely good. I’m not sure anyone’s ever going to undertake a head-to-head study comparing them,” Dr. Hofman said.
“Whichever one you can access, at the cheapest cost, I think, is going to be the best one in your center,” he added. “That really does vary geographically, but I really don’t think one is better or worse than the other.”
Praise and criticism
The latest European guidelines acknowledge that PSMA PET/CT is more sensitive for detecting lymph node and bone metastases than the classical workup of abdominopelvic CT and bone scintigraphy, according to invited discussant Matthias Heck, PD Dr. med, of the Technical University of Munich in Germany.
“Molecular imaging using PSMA PET/CT facilitates the detection of small lymph node metastasis, with the size of a few millimeters,” Dr. Heck said.
Although he commended the ProPSMA investigators, Dr. Heck had one criticism of the study design that may have resulted in over-sensitivity of PSMA PET/CT.
“As a urologist, I want to address as a discussion point the low number of histopathologic validation in the ProPSMA study,” he said. “Pelvic lymph node sampling was performed only in 66% of patients treated with radical prostatectomy for high-risk prostate cancer. Hard criteria to define the presence of metastasis were only used in 23% of patients with metastases. Therefore, it is possible that the sensitivity was overestimated by using mainly soft criteria.”
The sensitivity of PSMA PET/CT was 85%, while that of CT/bone scan was 38%. The respective specificities were 98% and 91%.
“What I like most about this study is that, when we perform a PSMA PET/CT, you see the whole body; you don’t see only pelvic lymph nodes,” Dr. Heck said. Since it was not possible to validate distant metastasis by histopathology, he added, this imaging method could clearly help determine the best treatment.
“If we have distant metastasis in the bones or in the lymph nodes outside of the pelvis, it’s clearly unnecessary to direct this patient to undergo local treatment, and we need to think about other treatments,” Dr. Heck said. “Therefore, I think it’s a very important question that is being raised by this study, and we all need to look at the whole body of the patient and not focus only on the pelvic lymph nodes.”
The study was funded by the Prostate Cancer Foundation of Australia. Dr. Hofman said he has no relevant conflicts of interest. Dr. Heck disclosed relationships with Astellas, Janssen, Ipsen, Amgen, Bayer, Heise, Merck, Sanofi, and Takeda.
SOURCES: Hofman M et al. Lancet. March 22, doi: https://doi.org/10.1016/S0140-6736(20)30314-7.
FROM EAU20
MRI reliably identifies significant prostate cancer
Prostate cancers that are missed on multiparametric (mp) MRI are small and “not life-threatening,” according to an analysis of data from the Prostate MR Imaging Study (PROMIS).
“Our work suggests that MRI scans of the prostate appear to deliver crucial information about a man’s risk of dying from prostate cancer, even before he has a biopsy,” said Joseph Norris, BM BS, from University College London.
“This may mean that we can finally move prostate cancer to a position in which we can use imaging as the primary tool to direct further investigations, treatment, and prediction of risk,” he told Medscape Medical News.
This is “a position that all other solid organ cancers have reached,” said Norris, who will present the findings at the upcoming virtual European Association of Urology 2020 Congress.
All 576 PROMIS participants underwent an mpMRI scan, a transrectal ultrasonography (TRUS)–guided biopsy, and a template prostate mapping (TPM) biopsy taken at 5-mm intervals across the entire prostate.
PROMIS researchers previously showed that mpMRI had a 93% sensitivity for clinically significant cancer, whereas TRUS biopsy had only a 48% sensitivity, as reported by Medscape Medical News. And they concluded that the use of mpMRI as a first-line diagnostic tool could prevent 27% of all biopsies, which can have serious adverse effects, such as pain, urinary problems, infection, bleeding, and erectile dysfunction.
However, in their study looking at the accuracy of mpMRI and TRUS biopsy, the researchers did not investigate the severity of the 7% of cancers that mpMRI missed. “What if those missed cancers are, in fact, aggressive? That’s what we set out to examine,” Norris explained.
So he and his colleagues conducted a post ad hoc analysis of the PROMIS participants in whom clinically significant cancer had been detected with TPM biopsy to see which of those cancers had been detected with mpMRI. The findings were published online in European Urology.
Cancers met the strict definition of clinically significant if they had a Gleason score of at least 4+3 for a tumor of any length, or a maximum cancer core length (MCCL) greater than 6 mm for a cancer of any grade. They met the less-strict definition if they had a Gleason score of at least 3+4 for a tumor of any length, or a MCCL greater than 4 mm for a cancer of any grade.
In PROMIS, TPM biopsy detected 230 cancers that met the strict definition of clinically significant and 331 that met the less-strict definition.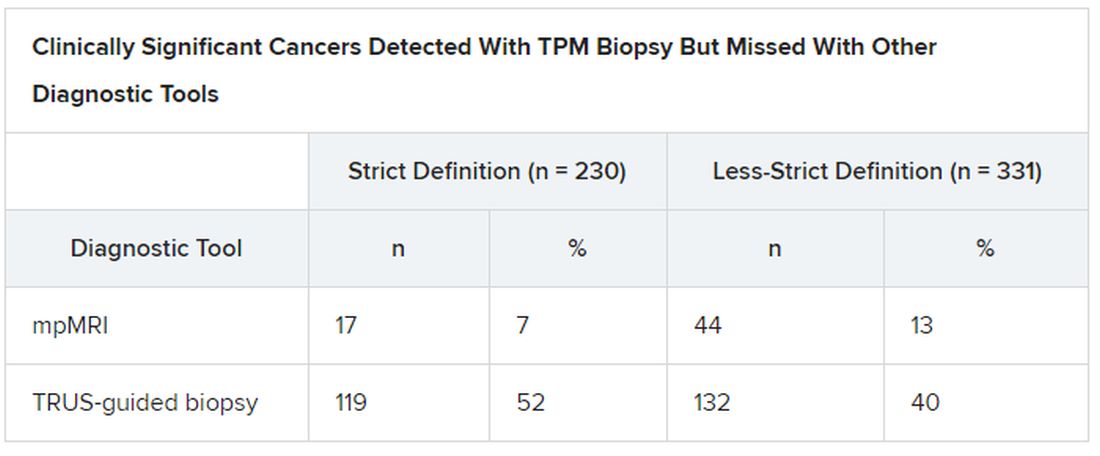
Overall Gleason scores were significantly lower for the 17 strict-definition cancers not detected with 1.5 T mpMRI than for those detected with mpMRI (P = .0007), as were maximum Gleason scores (P < .0001).
Median MCCL was 3 mm shorter for all 17 tumors missed with mpMRI than for those detected with mpMRI (5 vs 8 mm; P < .0001).
mpMRI detected all tumors identified on TPM biopsy that had an overall Gleason score greater than 3+4 (Gleason grades 3 to 5) or a maximum Gleason score greater than 4+3 (Gleason grades 4 and 5).
“This finding is important, given that in PROMIS, no men with an overall Gleason score of 4+3 had cancer missed by MRI, indicating that actually MRI may be able to identify all truly significant cancers,” said Norris.
Adding PSA Density Threshold
To further assess cancers missed on mpMRI, the researchers looked at prostate-specific antigen (PSA) density, calculated as total PSA level (ng/mL) divided by prostate volume (mL).
“We found that if we applied a threshold PSA density to men with normal-looking MRI scans, we could reduce the proportion of missed significant cancer to just 5%. This is exciting; it means we can make MRI an even more effective test for prostate cancer in a very simple way,” Norris reported.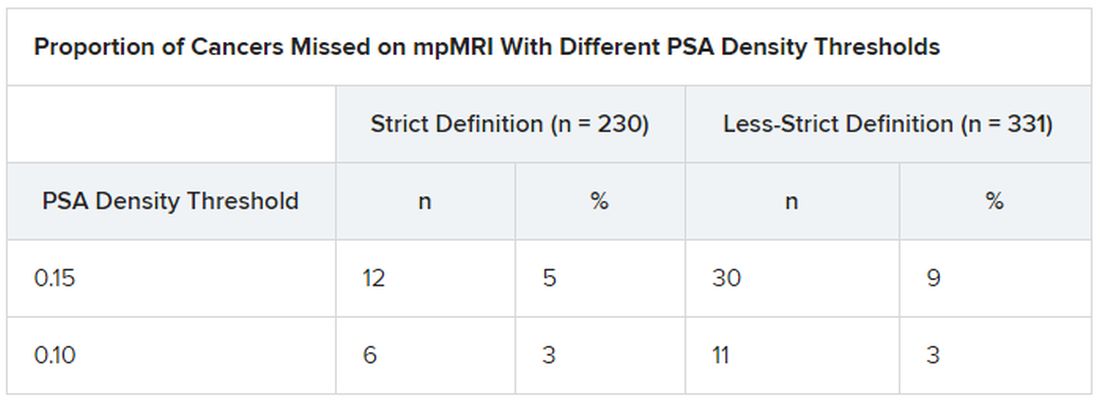
“These data show that no highly aggressive prostate cancers were missed by MRI, either at the level of the whole prostate or at the individual needle level,” said Norris. This should lead to positive outcomes in the long term.
And since the PROMIS data were gathered, MRI technology has improved, he said. The “MRI scanners in PROMIS were 1.5 Tesla,” whereas today’s machines are 3.0 T, which could increase the detection of significant prostate cancer.
In fact, “our analysis here potentially overestimates the amount of undetected disease,” he noted.
Prostate cancer that is not clinically significant is often monitored with active surveillance, so “invisible” cancers missed on mpMRI could actually be looked at in a positive light, he explained.
Variation in technique, interpretation
But the quality of care when it comes to the diagnosis of prostate cancer is not equal everywhere, said Gerald Andriole, MD, from the Washington School of Medicine in St. Louis, Missouri.
“When you get an MRI in a center that doesn’t do a lot of prostate cancer testing, you may not have the best software and you may have a radiologist who is not that experienced,” he told Medscape Medical News. Specialized cancer centers of excellence tend to do a great job finding prostate cancer, “but other centers have high significant-miss rates or high overcall rates.”
“The elephant in the room remains the considerable variation in technique and interobserver interpretation of prostate mpMRI,” write Steven Monda, MD, and Marc Dall’Era, MD, both from UC Davis Health in Sacramento, California, in an editorial that accompanies the new PROMIS analysis.
“These problems must be addressed and remedied in each institution before relying on results from PROMIS to drive changes in clinical practice,” they add.
Norris, Andriole, Monda, and Dall’Era have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Prostate cancers that are missed on multiparametric (mp) MRI are small and “not life-threatening,” according to an analysis of data from the Prostate MR Imaging Study (PROMIS).
“Our work suggests that MRI scans of the prostate appear to deliver crucial information about a man’s risk of dying from prostate cancer, even before he has a biopsy,” said Joseph Norris, BM BS, from University College London.
“This may mean that we can finally move prostate cancer to a position in which we can use imaging as the primary tool to direct further investigations, treatment, and prediction of risk,” he told Medscape Medical News.
This is “a position that all other solid organ cancers have reached,” said Norris, who will present the findings at the upcoming virtual European Association of Urology 2020 Congress.
All 576 PROMIS participants underwent an mpMRI scan, a transrectal ultrasonography (TRUS)–guided biopsy, and a template prostate mapping (TPM) biopsy taken at 5-mm intervals across the entire prostate.
PROMIS researchers previously showed that mpMRI had a 93% sensitivity for clinically significant cancer, whereas TRUS biopsy had only a 48% sensitivity, as reported by Medscape Medical News. And they concluded that the use of mpMRI as a first-line diagnostic tool could prevent 27% of all biopsies, which can have serious adverse effects, such as pain, urinary problems, infection, bleeding, and erectile dysfunction.
However, in their study looking at the accuracy of mpMRI and TRUS biopsy, the researchers did not investigate the severity of the 7% of cancers that mpMRI missed. “What if those missed cancers are, in fact, aggressive? That’s what we set out to examine,” Norris explained.
So he and his colleagues conducted a post ad hoc analysis of the PROMIS participants in whom clinically significant cancer had been detected with TPM biopsy to see which of those cancers had been detected with mpMRI. The findings were published online in European Urology.
Cancers met the strict definition of clinically significant if they had a Gleason score of at least 4+3 for a tumor of any length, or a maximum cancer core length (MCCL) greater than 6 mm for a cancer of any grade. They met the less-strict definition if they had a Gleason score of at least 3+4 for a tumor of any length, or a MCCL greater than 4 mm for a cancer of any grade.
In PROMIS, TPM biopsy detected 230 cancers that met the strict definition of clinically significant and 331 that met the less-strict definition.
Overall Gleason scores were significantly lower for the 17 strict-definition cancers not detected with 1.5 T mpMRI than for those detected with mpMRI (P = .0007), as were maximum Gleason scores (P < .0001).
Median MCCL was 3 mm shorter for all 17 tumors missed with mpMRI than for those detected with mpMRI (5 vs 8 mm; P < .0001).
mpMRI detected all tumors identified on TPM biopsy that had an overall Gleason score greater than 3+4 (Gleason grades 3 to 5) or a maximum Gleason score greater than 4+3 (Gleason grades 4 and 5).
“This finding is important, given that in PROMIS, no men with an overall Gleason score of 4+3 had cancer missed by MRI, indicating that actually MRI may be able to identify all truly significant cancers,” said Norris.
Adding PSA Density Threshold
To further assess cancers missed on mpMRI, the researchers looked at prostate-specific antigen (PSA) density, calculated as total PSA level (ng/mL) divided by prostate volume (mL).
“We found that if we applied a threshold PSA density to men with normal-looking MRI scans, we could reduce the proportion of missed significant cancer to just 5%. This is exciting; it means we can make MRI an even more effective test for prostate cancer in a very simple way,” Norris reported.
“These data show that no highly aggressive prostate cancers were missed by MRI, either at the level of the whole prostate or at the individual needle level,” said Norris. This should lead to positive outcomes in the long term.
And since the PROMIS data were gathered, MRI technology has improved, he said. The “MRI scanners in PROMIS were 1.5 Tesla,” whereas today’s machines are 3.0 T, which could increase the detection of significant prostate cancer.
In fact, “our analysis here potentially overestimates the amount of undetected disease,” he noted.
Prostate cancer that is not clinically significant is often monitored with active surveillance, so “invisible” cancers missed on mpMRI could actually be looked at in a positive light, he explained.
Variation in technique, interpretation
But the quality of care when it comes to the diagnosis of prostate cancer is not equal everywhere, said Gerald Andriole, MD, from the Washington School of Medicine in St. Louis, Missouri.
“When you get an MRI in a center that doesn’t do a lot of prostate cancer testing, you may not have the best software and you may have a radiologist who is not that experienced,” he told Medscape Medical News. Specialized cancer centers of excellence tend to do a great job finding prostate cancer, “but other centers have high significant-miss rates or high overcall rates.”
“The elephant in the room remains the considerable variation in technique and interobserver interpretation of prostate mpMRI,” write Steven Monda, MD, and Marc Dall’Era, MD, both from UC Davis Health in Sacramento, California, in an editorial that accompanies the new PROMIS analysis.
“These problems must be addressed and remedied in each institution before relying on results from PROMIS to drive changes in clinical practice,” they add.
Norris, Andriole, Monda, and Dall’Era have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Prostate cancers that are missed on multiparametric (mp) MRI are small and “not life-threatening,” according to an analysis of data from the Prostate MR Imaging Study (PROMIS).
“Our work suggests that MRI scans of the prostate appear to deliver crucial information about a man’s risk of dying from prostate cancer, even before he has a biopsy,” said Joseph Norris, BM BS, from University College London.
“This may mean that we can finally move prostate cancer to a position in which we can use imaging as the primary tool to direct further investigations, treatment, and prediction of risk,” he told Medscape Medical News.
This is “a position that all other solid organ cancers have reached,” said Norris, who will present the findings at the upcoming virtual European Association of Urology 2020 Congress.
All 576 PROMIS participants underwent an mpMRI scan, a transrectal ultrasonography (TRUS)–guided biopsy, and a template prostate mapping (TPM) biopsy taken at 5-mm intervals across the entire prostate.
PROMIS researchers previously showed that mpMRI had a 93% sensitivity for clinically significant cancer, whereas TRUS biopsy had only a 48% sensitivity, as reported by Medscape Medical News. And they concluded that the use of mpMRI as a first-line diagnostic tool could prevent 27% of all biopsies, which can have serious adverse effects, such as pain, urinary problems, infection, bleeding, and erectile dysfunction.
However, in their study looking at the accuracy of mpMRI and TRUS biopsy, the researchers did not investigate the severity of the 7% of cancers that mpMRI missed. “What if those missed cancers are, in fact, aggressive? That’s what we set out to examine,” Norris explained.
So he and his colleagues conducted a post ad hoc analysis of the PROMIS participants in whom clinically significant cancer had been detected with TPM biopsy to see which of those cancers had been detected with mpMRI. The findings were published online in European Urology.
Cancers met the strict definition of clinically significant if they had a Gleason score of at least 4+3 for a tumor of any length, or a maximum cancer core length (MCCL) greater than 6 mm for a cancer of any grade. They met the less-strict definition if they had a Gleason score of at least 3+4 for a tumor of any length, or a MCCL greater than 4 mm for a cancer of any grade.
In PROMIS, TPM biopsy detected 230 cancers that met the strict definition of clinically significant and 331 that met the less-strict definition.
Overall Gleason scores were significantly lower for the 17 strict-definition cancers not detected with 1.5 T mpMRI than for those detected with mpMRI (P = .0007), as were maximum Gleason scores (P < .0001).
Median MCCL was 3 mm shorter for all 17 tumors missed with mpMRI than for those detected with mpMRI (5 vs 8 mm; P < .0001).
mpMRI detected all tumors identified on TPM biopsy that had an overall Gleason score greater than 3+4 (Gleason grades 3 to 5) or a maximum Gleason score greater than 4+3 (Gleason grades 4 and 5).
“This finding is important, given that in PROMIS, no men with an overall Gleason score of 4+3 had cancer missed by MRI, indicating that actually MRI may be able to identify all truly significant cancers,” said Norris.
Adding PSA Density Threshold
To further assess cancers missed on mpMRI, the researchers looked at prostate-specific antigen (PSA) density, calculated as total PSA level (ng/mL) divided by prostate volume (mL).
“We found that if we applied a threshold PSA density to men with normal-looking MRI scans, we could reduce the proportion of missed significant cancer to just 5%. This is exciting; it means we can make MRI an even more effective test for prostate cancer in a very simple way,” Norris reported.
“These data show that no highly aggressive prostate cancers were missed by MRI, either at the level of the whole prostate or at the individual needle level,” said Norris. This should lead to positive outcomes in the long term.
And since the PROMIS data were gathered, MRI technology has improved, he said. The “MRI scanners in PROMIS were 1.5 Tesla,” whereas today’s machines are 3.0 T, which could increase the detection of significant prostate cancer.
In fact, “our analysis here potentially overestimates the amount of undetected disease,” he noted.
Prostate cancer that is not clinically significant is often monitored with active surveillance, so “invisible” cancers missed on mpMRI could actually be looked at in a positive light, he explained.
Variation in technique, interpretation
But the quality of care when it comes to the diagnosis of prostate cancer is not equal everywhere, said Gerald Andriole, MD, from the Washington School of Medicine in St. Louis, Missouri.
“When you get an MRI in a center that doesn’t do a lot of prostate cancer testing, you may not have the best software and you may have a radiologist who is not that experienced,” he told Medscape Medical News. Specialized cancer centers of excellence tend to do a great job finding prostate cancer, “but other centers have high significant-miss rates or high overcall rates.”
“The elephant in the room remains the considerable variation in technique and interobserver interpretation of prostate mpMRI,” write Steven Monda, MD, and Marc Dall’Era, MD, both from UC Davis Health in Sacramento, California, in an editorial that accompanies the new PROMIS analysis.
“These problems must be addressed and remedied in each institution before relying on results from PROMIS to drive changes in clinical practice,” they add.
Norris, Andriole, Monda, and Dall’Era have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.