User login
Tool predicts lymphoma, death in primary Sjögren’s syndrome patients
The European League Against Rheumatism Sjögren’s Syndrome Disease Activity Index measured at the time of diagnosis predicted the development of lymphoma and death in Spanish patients with severe primary Sjögren’s syndrome in a large, multicenter registry.
"We identified a specific hematological and immunological profile (cytopenias, hypocomplementemia, monoclonal band, and cryoglobulinemia) as laboratory predictors of hematological neoplasia in these patients," said lead study author Dr. Pilar Brito Zerón. "If you have an SS [Sjögren’s syndrome] patient with these features, you have to be very careful because this patient has a higher probability of developing a lymphoma."
"Physicians have had an activity index tool for other diseases for a long time, but there was nothing for SS until recently," when the EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI) was published in 2010, Dr. Brito Zerón said. "In Spain, we have one of the largest cohorts of SS patients in the world," so it was a good opportunity to test the ESSDAI.
Dr. Brito Zerón of Hospital Clinic in Barcelona and her colleagues studied patient records from the GEAS-SS multicenter registry, a cohort of 921 patients with SS from 20 medical centers in Spain, and retrospectively calculated their 2010 ESSDAI. During a mean follow-up period of 75 months, 25 (3%) of 904 patients developed lymphoproliferative disease; 17 were excluded because they had lymphoma before their primary SS diagnosis. Two-thirds were MALT (mucosa-associated lymphoid tissue) lymphomas, 80% of which were located in the parotid glands.
The investigators found that the following baseline features at diagnosis were most associated with lymphoma development: male gender (hazard ratio [HR], 5.78; 95% confidence interval [CI], 2.14-15.63); cryoglobulins (HR, 4.44; 95% CI, 1.86-10.58); monoclonal serum band (HR, 4.23; 95% CI, 1.38-13.02); C3 values less than 0.82 g/L (HR, 3.75; 95% CI, 1.38-10.19); C4 values less than 0.07 g/L (HR, 3.22, 95% CI, 1.08-9.61); and older age (HR, 1.04; 95% CI, 1.00-1.07). Gender, low C3, monoclonal band, and cryoglobulins were significant independent variables related to lymphoma, Dr. Brito Zerón reported at the annual European Congress of Rheumatology.
An ESSDAI score of one or greater in the constitutional (HR, 4.06; 95% CI, 1.54-10.70) and hematologic (HR, 2.59; 95% CI 1.16-5.78) domains was associated with the development of lymphoma, with hematologic activity being independently associated. In the constitutional domain, patients with the highest degree of activity – including fever greater than 38.5° C, night sweats, and/or involuntary weight loss of at least 10% – showed the highest risk of developing lymphoma (HR, 9.11; 95% CI, 2.51-33.12).
At the time of diagnosis with the 2002 primary SS classification criteria, patients had a mean baseline ESSDAI of 5.81. During follow-up, the patients accumulated another mean 3.34 points for a cumulative ESSADI of 9.15. A large majority of patients were women (94%) and had a mean age of nearly 54 years at the time of diagnosis. Most of the 921 patients in the registry had xerostomia (96%), xerophthalmia (95%), positive ocular tests (93%; 805 of 863), grade 3-4 parotid scintigraphy (88%; 598 of 676), and positive salivary gland biopsy (88%; 424 of 482). Cytopenias occurred in 34% overall, including anemia (17%), leucopenia (20%), and thrombocytopenia (9%). Immunologic disease characteristics of the patients included positive autoantibody tests for antinuclear antibodies (90%), anti-Ro (73%), rheumatoid factor (57%), and anti-La (46%). Others had low C4 (12%) or C3 (9%) levels and low cryoglobulins (12%) or monoclonal gammopathy (9%).
The investigators also correlated the baseline ESSDAI score with mortality. After an average follow-up of 75 months, 83 (9%) patients died. Deaths were attributed to causes related to SS (27 patients), cardiovascular disease (20 patients), infections (17 patients), and other causes (11 patients). The cause of death was unknown in eight patients.
The active ESSDAI domains that were associated with death were the constitutional (HR, 2.66; 95% CI, 1.38-5.11), pulmonary (HR, 2.13; 95% CI, 1.09-4.16), and biologic (HR, 3.01; 95% CI, 1.91-4.76), with the pulmonary and biologic domains being independently associated with death.
Further analysis revealed that a score of one or greater in the constitutional, lymphadenopathy, hematologic, and biologic domains was predictive of death related to SS (HRs ranging from 2.59 to 7.88), while activity at the constitutional, cutaneous, pulmonary, renal, neurologic, and hematologic domains predicted mortality related to infection (HRs ranging from 3.7 to 9.29). The investigators found no associations between activity in specific ESSDAI domains and death from cardiovascular disease or other causes.
"Activity of constitutional and lymphadenopathy domains, closely related to lymphoma, correlated with death caused by SS itself, while activity in the main extraglandular sites of involvement (in which high doses of corticosteroids and immunosuppressive agents are used) correlated principally with death caused by infection," Dr. Brito Zerón said. "ESSDAI is a useful tool to score systemic activity in patients with primary SS not only in prospective studies, but also in clinical trials that evaluate the efficacy of a specific drug."
Since the analysis of these 921 patients was completed in January 2013, an additional 124 patients with primary SS have joined the cohort. In this larger cohort, baseline ESDAI score of 14 or higher and presence of more than one laboratory marker (lymphopenia, low cryoglobulins, hypocomplementemia, and monoclonal band) both were significantly associated with SS-related death.
Dr. Brito Zerón noted that the investigators have not analyzed whether treatment influenced outcomes in the cohort, but they plan to.
The investigators had no financial disclosures.
The European League Against Rheumatism Sjögren’s Syndrome Disease Activity Index measured at the time of diagnosis predicted the development of lymphoma and death in Spanish patients with severe primary Sjögren’s syndrome in a large, multicenter registry.
"We identified a specific hematological and immunological profile (cytopenias, hypocomplementemia, monoclonal band, and cryoglobulinemia) as laboratory predictors of hematological neoplasia in these patients," said lead study author Dr. Pilar Brito Zerón. "If you have an SS [Sjögren’s syndrome] patient with these features, you have to be very careful because this patient has a higher probability of developing a lymphoma."
"Physicians have had an activity index tool for other diseases for a long time, but there was nothing for SS until recently," when the EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI) was published in 2010, Dr. Brito Zerón said. "In Spain, we have one of the largest cohorts of SS patients in the world," so it was a good opportunity to test the ESSDAI.
Dr. Brito Zerón of Hospital Clinic in Barcelona and her colleagues studied patient records from the GEAS-SS multicenter registry, a cohort of 921 patients with SS from 20 medical centers in Spain, and retrospectively calculated their 2010 ESSDAI. During a mean follow-up period of 75 months, 25 (3%) of 904 patients developed lymphoproliferative disease; 17 were excluded because they had lymphoma before their primary SS diagnosis. Two-thirds were MALT (mucosa-associated lymphoid tissue) lymphomas, 80% of which were located in the parotid glands.
The investigators found that the following baseline features at diagnosis were most associated with lymphoma development: male gender (hazard ratio [HR], 5.78; 95% confidence interval [CI], 2.14-15.63); cryoglobulins (HR, 4.44; 95% CI, 1.86-10.58); monoclonal serum band (HR, 4.23; 95% CI, 1.38-13.02); C3 values less than 0.82 g/L (HR, 3.75; 95% CI, 1.38-10.19); C4 values less than 0.07 g/L (HR, 3.22, 95% CI, 1.08-9.61); and older age (HR, 1.04; 95% CI, 1.00-1.07). Gender, low C3, monoclonal band, and cryoglobulins were significant independent variables related to lymphoma, Dr. Brito Zerón reported at the annual European Congress of Rheumatology.
An ESSDAI score of one or greater in the constitutional (HR, 4.06; 95% CI, 1.54-10.70) and hematologic (HR, 2.59; 95% CI 1.16-5.78) domains was associated with the development of lymphoma, with hematologic activity being independently associated. In the constitutional domain, patients with the highest degree of activity – including fever greater than 38.5° C, night sweats, and/or involuntary weight loss of at least 10% – showed the highest risk of developing lymphoma (HR, 9.11; 95% CI, 2.51-33.12).
At the time of diagnosis with the 2002 primary SS classification criteria, patients had a mean baseline ESSDAI of 5.81. During follow-up, the patients accumulated another mean 3.34 points for a cumulative ESSADI of 9.15. A large majority of patients were women (94%) and had a mean age of nearly 54 years at the time of diagnosis. Most of the 921 patients in the registry had xerostomia (96%), xerophthalmia (95%), positive ocular tests (93%; 805 of 863), grade 3-4 parotid scintigraphy (88%; 598 of 676), and positive salivary gland biopsy (88%; 424 of 482). Cytopenias occurred in 34% overall, including anemia (17%), leucopenia (20%), and thrombocytopenia (9%). Immunologic disease characteristics of the patients included positive autoantibody tests for antinuclear antibodies (90%), anti-Ro (73%), rheumatoid factor (57%), and anti-La (46%). Others had low C4 (12%) or C3 (9%) levels and low cryoglobulins (12%) or monoclonal gammopathy (9%).
The investigators also correlated the baseline ESSDAI score with mortality. After an average follow-up of 75 months, 83 (9%) patients died. Deaths were attributed to causes related to SS (27 patients), cardiovascular disease (20 patients), infections (17 patients), and other causes (11 patients). The cause of death was unknown in eight patients.
The active ESSDAI domains that were associated with death were the constitutional (HR, 2.66; 95% CI, 1.38-5.11), pulmonary (HR, 2.13; 95% CI, 1.09-4.16), and biologic (HR, 3.01; 95% CI, 1.91-4.76), with the pulmonary and biologic domains being independently associated with death.
Further analysis revealed that a score of one or greater in the constitutional, lymphadenopathy, hematologic, and biologic domains was predictive of death related to SS (HRs ranging from 2.59 to 7.88), while activity at the constitutional, cutaneous, pulmonary, renal, neurologic, and hematologic domains predicted mortality related to infection (HRs ranging from 3.7 to 9.29). The investigators found no associations between activity in specific ESSDAI domains and death from cardiovascular disease or other causes.
"Activity of constitutional and lymphadenopathy domains, closely related to lymphoma, correlated with death caused by SS itself, while activity in the main extraglandular sites of involvement (in which high doses of corticosteroids and immunosuppressive agents are used) correlated principally with death caused by infection," Dr. Brito Zerón said. "ESSDAI is a useful tool to score systemic activity in patients with primary SS not only in prospective studies, but also in clinical trials that evaluate the efficacy of a specific drug."
Since the analysis of these 921 patients was completed in January 2013, an additional 124 patients with primary SS have joined the cohort. In this larger cohort, baseline ESDAI score of 14 or higher and presence of more than one laboratory marker (lymphopenia, low cryoglobulins, hypocomplementemia, and monoclonal band) both were significantly associated with SS-related death.
Dr. Brito Zerón noted that the investigators have not analyzed whether treatment influenced outcomes in the cohort, but they plan to.
The investigators had no financial disclosures.
The European League Against Rheumatism Sjögren’s Syndrome Disease Activity Index measured at the time of diagnosis predicted the development of lymphoma and death in Spanish patients with severe primary Sjögren’s syndrome in a large, multicenter registry.
"We identified a specific hematological and immunological profile (cytopenias, hypocomplementemia, monoclonal band, and cryoglobulinemia) as laboratory predictors of hematological neoplasia in these patients," said lead study author Dr. Pilar Brito Zerón. "If you have an SS [Sjögren’s syndrome] patient with these features, you have to be very careful because this patient has a higher probability of developing a lymphoma."
"Physicians have had an activity index tool for other diseases for a long time, but there was nothing for SS until recently," when the EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI) was published in 2010, Dr. Brito Zerón said. "In Spain, we have one of the largest cohorts of SS patients in the world," so it was a good opportunity to test the ESSDAI.
Dr. Brito Zerón of Hospital Clinic in Barcelona and her colleagues studied patient records from the GEAS-SS multicenter registry, a cohort of 921 patients with SS from 20 medical centers in Spain, and retrospectively calculated their 2010 ESSDAI. During a mean follow-up period of 75 months, 25 (3%) of 904 patients developed lymphoproliferative disease; 17 were excluded because they had lymphoma before their primary SS diagnosis. Two-thirds were MALT (mucosa-associated lymphoid tissue) lymphomas, 80% of which were located in the parotid glands.
The investigators found that the following baseline features at diagnosis were most associated with lymphoma development: male gender (hazard ratio [HR], 5.78; 95% confidence interval [CI], 2.14-15.63); cryoglobulins (HR, 4.44; 95% CI, 1.86-10.58); monoclonal serum band (HR, 4.23; 95% CI, 1.38-13.02); C3 values less than 0.82 g/L (HR, 3.75; 95% CI, 1.38-10.19); C4 values less than 0.07 g/L (HR, 3.22, 95% CI, 1.08-9.61); and older age (HR, 1.04; 95% CI, 1.00-1.07). Gender, low C3, monoclonal band, and cryoglobulins were significant independent variables related to lymphoma, Dr. Brito Zerón reported at the annual European Congress of Rheumatology.
An ESSDAI score of one or greater in the constitutional (HR, 4.06; 95% CI, 1.54-10.70) and hematologic (HR, 2.59; 95% CI 1.16-5.78) domains was associated with the development of lymphoma, with hematologic activity being independently associated. In the constitutional domain, patients with the highest degree of activity – including fever greater than 38.5° C, night sweats, and/or involuntary weight loss of at least 10% – showed the highest risk of developing lymphoma (HR, 9.11; 95% CI, 2.51-33.12).
At the time of diagnosis with the 2002 primary SS classification criteria, patients had a mean baseline ESSDAI of 5.81. During follow-up, the patients accumulated another mean 3.34 points for a cumulative ESSADI of 9.15. A large majority of patients were women (94%) and had a mean age of nearly 54 years at the time of diagnosis. Most of the 921 patients in the registry had xerostomia (96%), xerophthalmia (95%), positive ocular tests (93%; 805 of 863), grade 3-4 parotid scintigraphy (88%; 598 of 676), and positive salivary gland biopsy (88%; 424 of 482). Cytopenias occurred in 34% overall, including anemia (17%), leucopenia (20%), and thrombocytopenia (9%). Immunologic disease characteristics of the patients included positive autoantibody tests for antinuclear antibodies (90%), anti-Ro (73%), rheumatoid factor (57%), and anti-La (46%). Others had low C4 (12%) or C3 (9%) levels and low cryoglobulins (12%) or monoclonal gammopathy (9%).
The investigators also correlated the baseline ESSDAI score with mortality. After an average follow-up of 75 months, 83 (9%) patients died. Deaths were attributed to causes related to SS (27 patients), cardiovascular disease (20 patients), infections (17 patients), and other causes (11 patients). The cause of death was unknown in eight patients.
The active ESSDAI domains that were associated with death were the constitutional (HR, 2.66; 95% CI, 1.38-5.11), pulmonary (HR, 2.13; 95% CI, 1.09-4.16), and biologic (HR, 3.01; 95% CI, 1.91-4.76), with the pulmonary and biologic domains being independently associated with death.
Further analysis revealed that a score of one or greater in the constitutional, lymphadenopathy, hematologic, and biologic domains was predictive of death related to SS (HRs ranging from 2.59 to 7.88), while activity at the constitutional, cutaneous, pulmonary, renal, neurologic, and hematologic domains predicted mortality related to infection (HRs ranging from 3.7 to 9.29). The investigators found no associations between activity in specific ESSDAI domains and death from cardiovascular disease or other causes.
"Activity of constitutional and lymphadenopathy domains, closely related to lymphoma, correlated with death caused by SS itself, while activity in the main extraglandular sites of involvement (in which high doses of corticosteroids and immunosuppressive agents are used) correlated principally with death caused by infection," Dr. Brito Zerón said. "ESSDAI is a useful tool to score systemic activity in patients with primary SS not only in prospective studies, but also in clinical trials that evaluate the efficacy of a specific drug."
Since the analysis of these 921 patients was completed in January 2013, an additional 124 patients with primary SS have joined the cohort. In this larger cohort, baseline ESDAI score of 14 or higher and presence of more than one laboratory marker (lymphopenia, low cryoglobulins, hypocomplementemia, and monoclonal band) both were significantly associated with SS-related death.
Dr. Brito Zerón noted that the investigators have not analyzed whether treatment influenced outcomes in the cohort, but they plan to.
The investigators had no financial disclosures.
FROM THE EULAR CONGRESS 2014
Key clinical point: Patients with specific hematologic and immunologic laboratory markers, as well as high degrees of activity in the constitutional domain of the ESSDAI, should be monitored closely for the development of lymphoma.
Major finding: Male gender (HR, 5.78; 95% CI, 2.14-15.63); low C3 (HR, 3.75; 95% CI, 1.38-10.19); monoclonal band (HR, 4.23; 95% CI, 1.38-13.02); and cryoglobulins (HR, 4.44; 95% CI, 1.86-10.58) were significant independent variables related to lymphoma.
Data source: A retrospective analysis of 921 Spanish patients with primary Sjögren’s syndrome in the GEAS-SS multicenter registry.
Disclosures: The investigators had no financial disclosures.
Giant-cell arteritis patients face high aortic aneurysm dissection risk
PARIS – The usual rule that the larger an aortic aneurysm grows the greater the risk it will undergo dissection or rupture doesn’t work in patients with giant-cell arteritis. Their aortic aneurysms appear liable to dissect or rupture at any size after the diagnosis of giant-cell arteritis occurs, based on a retrospective study of 195 patients followed at a single U.S. center.
"Aortic size at diagnosis or last follow-up did not predict aortic dissection or rupture," Dr. Ashima Makol reported at the annual European Congress of Rheumatology, nor were linear, serial measurements of aortic size able to reliably predict risk for these complications in patients with GCA. Without a reliable way to identify patients with GCA at risk for dissection or rupture, the only management advice remaining is to follow GCA patients annually with imaging, said Dr. Makol, a rheumatologist at the Mayo Clinic in Rochester, Minn.
Positron emission tomography, CT angiography, or MR angiography seem to be the best ways to follow these patients, but if those are too costly to do annually, then transesophageal echocardiography or a chest x-ray are other options, Dr. Makol said in an interview.
Although 30% of patients with GCA have a vasculitis that involves the aorta and its branches and an increased risk for developing aortic aneurysms, the way these aneurysms change over time and the relationship between aneurysm size and the risk for dissection or rupture in GCA patients were not previously reported. To address this, Dr. Makol and her associates reviewed 195 patients with GCA and an aortic aneurysm seen at the Mayo Clinic during 2000-2012.
The aneurysms occurred in the ascending thoracic aorta in 161 patients (83%), the descending thoracic aorta in 21 (11%), and the abdominal aorta in the remaining 13 patients (7%). (Percentages total 101% because of rounding.) The patients averaged 74 years old, 62% were women, and 49% had a history of smoking.
During follow-up, 14 patients (7%) had an aneurysm dissection, and 1 patient (1%) had an aneurysm rupture, the investigators reported. All of the dissections and the rupture occurred in thoracic aorta aneurysms.
At the time of GCA diagnosis, the average aneurysm size in the 15 patients who developed an aneurysm complication was 51 mm, which was very similar to the average size of 49 mm in the 180 patients who did not have an aneurysm dissection or rupture during follow-up.
Patients also showed no clear link between aneurysm size at the time of dissection or rupture and the aneurysm size during follow-up of patients without these complications. The average maximum aneurysm diameter among the 15 patients with a complication at the time of their event was 54 mm, while the average aneurysm size at last follow-up among those without a dissection or rupture during follow-up was 50 mm, a difference that was not statistically significant, Dr. Makol said.
The average rate of aneurysm growth during 3 years of follow-up for all the GCA patients in the analysis was 1.59 mm/year, a rate "somewhat higher" than the average annual growth rate of 1 mm/year reported for aortic aneurysms in patients without inflammatory disease. The 54-mm average aneurysm diameter at the time of dissection or rupture in the CGA patients was "somewhat lower" than the 65-mm average aneurysm diameter seen at the time of dissection or rupture in patients without inflammatory disease, she noted.
Several patients in the series Dr. Makol reviewed who had no aneurysm complications had undergone prophylactic aneurysm repair. Clinicians at the Mayo Clinic follow the usual recommendations, which call for repair of aortic aneurysms when they reach at least 55 mm in diameter in men and 50 mm in women, and repair of thoracic aortic aneurysms that reach at least 55 mm in men and women. Prophylactic repair is also recommended for patients with an aneurysm that grows by more than 5 mm/year or causes symptoms. Many of the GCA patients included in the review therefore did not qualify for repair based on these criteria at the time of their GCA diagnosis or during follow-up. For now, no recommendations suggest that aortic aneurysms in patients in GCA need a different repair approach than patients without inflammatory disease.
The study is the first reported to look at the pattern of aneurysm growth and complications in GCA patients, although it is limited to the retrospective experience at one tertiary referral center and so may reflect a referral bias, Dr. Makol said. But the inability of the analysis to identify aneurysm characteristics in GCA patients that can telegraph an increased risk for complications means that all GCA patients with an aortic aneurysm need careful surveillance by annual imaging, she advised.
Dr. Makol said that she had no disclosures.
*6/24/14: This story was updated.
On Twitter @mitchelzoler
PARIS – The usual rule that the larger an aortic aneurysm grows the greater the risk it will undergo dissection or rupture doesn’t work in patients with giant-cell arteritis. Their aortic aneurysms appear liable to dissect or rupture at any size after the diagnosis of giant-cell arteritis occurs, based on a retrospective study of 195 patients followed at a single U.S. center.
"Aortic size at diagnosis or last follow-up did not predict aortic dissection or rupture," Dr. Ashima Makol reported at the annual European Congress of Rheumatology, nor were linear, serial measurements of aortic size able to reliably predict risk for these complications in patients with GCA. Without a reliable way to identify patients with GCA at risk for dissection or rupture, the only management advice remaining is to follow GCA patients annually with imaging, said Dr. Makol, a rheumatologist at the Mayo Clinic in Rochester, Minn.
Positron emission tomography, CT angiography, or MR angiography seem to be the best ways to follow these patients, but if those are too costly to do annually, then transesophageal echocardiography or a chest x-ray are other options, Dr. Makol said in an interview.
Although 30% of patients with GCA have a vasculitis that involves the aorta and its branches and an increased risk for developing aortic aneurysms, the way these aneurysms change over time and the relationship between aneurysm size and the risk for dissection or rupture in GCA patients were not previously reported. To address this, Dr. Makol and her associates reviewed 195 patients with GCA and an aortic aneurysm seen at the Mayo Clinic during 2000-2012.
The aneurysms occurred in the ascending thoracic aorta in 161 patients (83%), the descending thoracic aorta in 21 (11%), and the abdominal aorta in the remaining 13 patients (7%). (Percentages total 101% because of rounding.) The patients averaged 74 years old, 62% were women, and 49% had a history of smoking.
During follow-up, 14 patients (7%) had an aneurysm dissection, and 1 patient (1%) had an aneurysm rupture, the investigators reported. All of the dissections and the rupture occurred in thoracic aorta aneurysms.
At the time of GCA diagnosis, the average aneurysm size in the 15 patients who developed an aneurysm complication was 51 mm, which was very similar to the average size of 49 mm in the 180 patients who did not have an aneurysm dissection or rupture during follow-up.
Patients also showed no clear link between aneurysm size at the time of dissection or rupture and the aneurysm size during follow-up of patients without these complications. The average maximum aneurysm diameter among the 15 patients with a complication at the time of their event was 54 mm, while the average aneurysm size at last follow-up among those without a dissection or rupture during follow-up was 50 mm, a difference that was not statistically significant, Dr. Makol said.
The average rate of aneurysm growth during 3 years of follow-up for all the GCA patients in the analysis was 1.59 mm/year, a rate "somewhat higher" than the average annual growth rate of 1 mm/year reported for aortic aneurysms in patients without inflammatory disease. The 54-mm average aneurysm diameter at the time of dissection or rupture in the CGA patients was "somewhat lower" than the 65-mm average aneurysm diameter seen at the time of dissection or rupture in patients without inflammatory disease, she noted.
Several patients in the series Dr. Makol reviewed who had no aneurysm complications had undergone prophylactic aneurysm repair. Clinicians at the Mayo Clinic follow the usual recommendations, which call for repair of aortic aneurysms when they reach at least 55 mm in diameter in men and 50 mm in women, and repair of thoracic aortic aneurysms that reach at least 55 mm in men and women. Prophylactic repair is also recommended for patients with an aneurysm that grows by more than 5 mm/year or causes symptoms. Many of the GCA patients included in the review therefore did not qualify for repair based on these criteria at the time of their GCA diagnosis or during follow-up. For now, no recommendations suggest that aortic aneurysms in patients in GCA need a different repair approach than patients without inflammatory disease.
The study is the first reported to look at the pattern of aneurysm growth and complications in GCA patients, although it is limited to the retrospective experience at one tertiary referral center and so may reflect a referral bias, Dr. Makol said. But the inability of the analysis to identify aneurysm characteristics in GCA patients that can telegraph an increased risk for complications means that all GCA patients with an aortic aneurysm need careful surveillance by annual imaging, she advised.
Dr. Makol said that she had no disclosures.
*6/24/14: This story was updated.
On Twitter @mitchelzoler
PARIS – The usual rule that the larger an aortic aneurysm grows the greater the risk it will undergo dissection or rupture doesn’t work in patients with giant-cell arteritis. Their aortic aneurysms appear liable to dissect or rupture at any size after the diagnosis of giant-cell arteritis occurs, based on a retrospective study of 195 patients followed at a single U.S. center.
"Aortic size at diagnosis or last follow-up did not predict aortic dissection or rupture," Dr. Ashima Makol reported at the annual European Congress of Rheumatology, nor were linear, serial measurements of aortic size able to reliably predict risk for these complications in patients with GCA. Without a reliable way to identify patients with GCA at risk for dissection or rupture, the only management advice remaining is to follow GCA patients annually with imaging, said Dr. Makol, a rheumatologist at the Mayo Clinic in Rochester, Minn.
Positron emission tomography, CT angiography, or MR angiography seem to be the best ways to follow these patients, but if those are too costly to do annually, then transesophageal echocardiography or a chest x-ray are other options, Dr. Makol said in an interview.
Although 30% of patients with GCA have a vasculitis that involves the aorta and its branches and an increased risk for developing aortic aneurysms, the way these aneurysms change over time and the relationship between aneurysm size and the risk for dissection or rupture in GCA patients were not previously reported. To address this, Dr. Makol and her associates reviewed 195 patients with GCA and an aortic aneurysm seen at the Mayo Clinic during 2000-2012.
The aneurysms occurred in the ascending thoracic aorta in 161 patients (83%), the descending thoracic aorta in 21 (11%), and the abdominal aorta in the remaining 13 patients (7%). (Percentages total 101% because of rounding.) The patients averaged 74 years old, 62% were women, and 49% had a history of smoking.
During follow-up, 14 patients (7%) had an aneurysm dissection, and 1 patient (1%) had an aneurysm rupture, the investigators reported. All of the dissections and the rupture occurred in thoracic aorta aneurysms.
At the time of GCA diagnosis, the average aneurysm size in the 15 patients who developed an aneurysm complication was 51 mm, which was very similar to the average size of 49 mm in the 180 patients who did not have an aneurysm dissection or rupture during follow-up.
Patients also showed no clear link between aneurysm size at the time of dissection or rupture and the aneurysm size during follow-up of patients without these complications. The average maximum aneurysm diameter among the 15 patients with a complication at the time of their event was 54 mm, while the average aneurysm size at last follow-up among those without a dissection or rupture during follow-up was 50 mm, a difference that was not statistically significant, Dr. Makol said.
The average rate of aneurysm growth during 3 years of follow-up for all the GCA patients in the analysis was 1.59 mm/year, a rate "somewhat higher" than the average annual growth rate of 1 mm/year reported for aortic aneurysms in patients without inflammatory disease. The 54-mm average aneurysm diameter at the time of dissection or rupture in the CGA patients was "somewhat lower" than the 65-mm average aneurysm diameter seen at the time of dissection or rupture in patients without inflammatory disease, she noted.
Several patients in the series Dr. Makol reviewed who had no aneurysm complications had undergone prophylactic aneurysm repair. Clinicians at the Mayo Clinic follow the usual recommendations, which call for repair of aortic aneurysms when they reach at least 55 mm in diameter in men and 50 mm in women, and repair of thoracic aortic aneurysms that reach at least 55 mm in men and women. Prophylactic repair is also recommended for patients with an aneurysm that grows by more than 5 mm/year or causes symptoms. Many of the GCA patients included in the review therefore did not qualify for repair based on these criteria at the time of their GCA diagnosis or during follow-up. For now, no recommendations suggest that aortic aneurysms in patients in GCA need a different repair approach than patients without inflammatory disease.
The study is the first reported to look at the pattern of aneurysm growth and complications in GCA patients, although it is limited to the retrospective experience at one tertiary referral center and so may reflect a referral bias, Dr. Makol said. But the inability of the analysis to identify aneurysm characteristics in GCA patients that can telegraph an increased risk for complications means that all GCA patients with an aortic aneurysm need careful surveillance by annual imaging, she advised.
Dr. Makol said that she had no disclosures.
*6/24/14: This story was updated.
On Twitter @mitchelzoler
AT THE EULAR CONGRESS 2014
Key clinical point: Risk for aortic aneurysm dissection in patients with giant-cell arteritis showed no link to aneurysm size.
Major finding: GCA patients with dissection or rupture of an aortic aneurysm had aneurysms that were similar in size to those of GCA patients without these complications.
Data source: Retrospective study of 195 patients with GCA at one U.S. center.
Disclosures: Dr. Makol said that she had no disclosures.
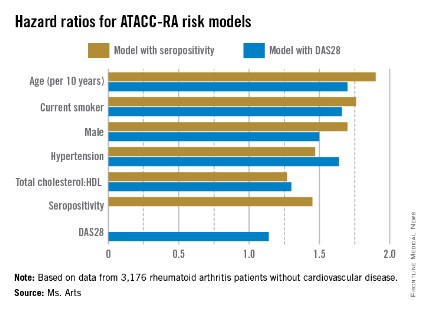
Higher risk of death seen with oral steroids in RA interstitial lung disease
The use of prednisone for 3 or more months at a time was associated with a significantly elevated risk of death in patients with rheumatoid arthritis and interstitial lung disease in a retrospective cohort study.
Interstitial lung disease is present in about 5% of patients with rheumatoid arthritis. For years, oral steroids were commonly used in patients with the disease, but today’s rheumatologists "no longer view oral steroids as optimal treatment in RA-ILD [rheumatoid arthritis–associated interstitial lung disease], and our data now confirm that," said Dr. Clive Kelly of Queen Elizabeth Hospital in Gateshead, England, senior investigator of the study. He added that clinicians should avoid long-term treatment with steroids in this patient group whenever possible.
Dr. Kelly led the British Rheumatoid Interstitial Lung (BRILL) Network’s cohort study of 260 patients with RA-ILD diagnosed over a 25-year period. The BRILL study compared patients with RA-ILD and an equal number of RA controls without lung involvement who were matched for age, sex, and time of diagnosis.
At the annual European Congress of Rheumatology, Dr. Kelly reported that steroid-treated RA-ILD patients, who represented nearly 60% of the cohort, had an elevated relative risk of all-cause death, compared with those who had never been treated with steroids (RR, 1.65; 95% confidence interval, 1.2-2.3; P = .002). Although the relative risk of respiratory death was significantly increased for RA-ILD patients, regardless of treatment, when compared with RA patients without ILD, the risk was higher in those who had been on steroids (RR, 2.75; 95% CI, 1.6-4.7; P = .0002) than in those who had not received steroids (RR, 2.06; 95% CI, 1.1-3.8; P = .02), the investigators found.
The comparison also revealed other important findings related to RA-ILD. Patients with RA-ILD had significantly higher mortality than did those with RA alone. Over the course of the 25-year study period, however, mortality progressively improved among the RA-ILD patients, with median age at death rising from 63 to 76 years. This steady improvement, Dr. Kelly said, is partly the result of better and earlier diagnosis of lung involvement.
"It’s one of my many missions in life to get rheumatologists to listen to the lungs when they examine the joints in patients with rheumatoid arthritis," he said. "I think we are getting better. We’ve persuaded the British Society for Rheumatology to incorporate lung function testing and clinical examination of the chest into their basic assessment of a rheumatoid patient."
Also likely affecting the improved mortality seen over the cohort’s study period is a change in therapeutic approach. While RA-ILD patients diagnosed in the first half of the study period were likely to have been treated with only prednisone and azathioprine, in the latter half they were more likely to have received cyclophosphamide and methylprednisolone or mycophenolate. Over the last 12 years, more were treated with biologics, and in the final 6 years of the cohort, patients requiring biologics tended to be treated with rituximab, a B-cell inhibitor, rather than anti–tumor necrosis factor (anti-TNF) agents, the BRILL investigators found.
Mortality was lower among RA-ILD patients treated with mycophenolate than in those treated with other immunosuppressive agents. Among biologic agents used in the cohort, rituximab treatment was associated with improved mortality, but anti-TNF inhibitors were seen to be associated with elevated risk of death.
About 95% of RA-ILD patients are anticyclic citrullinated peptide (anti-CCP) antibody positive, compared with 55%-60% of the RA population as a whole, Dr. Kelly said, "so there’s a strong statistical association of seropositivity, and in those who are seropositive, rituximab works well."
The finding that rituximab was associated with improved survival in the cohort not only has implications for RA-ILD, he said, but also, potentially, for people with idiopathic pulmonary fibrosis (IPF) who are anti-CCP antibody positive. "What [rheumatologists] have, and chest physicians traditionally don’t, is access to rituximab and mycophenolate. But these might be worth trying in IPF as well," he said.
Dr. Kelly noted that prospective trials in RA-ILD are beginning to enroll patients with progressive disease to compare azathioprine and mycophenolate, allowing for the use of oral steroids, as well as patients with active RA and ILD to compare anti-TNF inhibitors against rituximab, also allowing oral steroids.
Dr. Kelly reported that he had no conflicts of interest related to his findings and that none of his fellow BRILL investigators had conflicts.
The use of prednisone for 3 or more months at a time was associated with a significantly elevated risk of death in patients with rheumatoid arthritis and interstitial lung disease in a retrospective cohort study.
Interstitial lung disease is present in about 5% of patients with rheumatoid arthritis. For years, oral steroids were commonly used in patients with the disease, but today’s rheumatologists "no longer view oral steroids as optimal treatment in RA-ILD [rheumatoid arthritis–associated interstitial lung disease], and our data now confirm that," said Dr. Clive Kelly of Queen Elizabeth Hospital in Gateshead, England, senior investigator of the study. He added that clinicians should avoid long-term treatment with steroids in this patient group whenever possible.
Dr. Kelly led the British Rheumatoid Interstitial Lung (BRILL) Network’s cohort study of 260 patients with RA-ILD diagnosed over a 25-year period. The BRILL study compared patients with RA-ILD and an equal number of RA controls without lung involvement who were matched for age, sex, and time of diagnosis.
At the annual European Congress of Rheumatology, Dr. Kelly reported that steroid-treated RA-ILD patients, who represented nearly 60% of the cohort, had an elevated relative risk of all-cause death, compared with those who had never been treated with steroids (RR, 1.65; 95% confidence interval, 1.2-2.3; P = .002). Although the relative risk of respiratory death was significantly increased for RA-ILD patients, regardless of treatment, when compared with RA patients without ILD, the risk was higher in those who had been on steroids (RR, 2.75; 95% CI, 1.6-4.7; P = .0002) than in those who had not received steroids (RR, 2.06; 95% CI, 1.1-3.8; P = .02), the investigators found.
The comparison also revealed other important findings related to RA-ILD. Patients with RA-ILD had significantly higher mortality than did those with RA alone. Over the course of the 25-year study period, however, mortality progressively improved among the RA-ILD patients, with median age at death rising from 63 to 76 years. This steady improvement, Dr. Kelly said, is partly the result of better and earlier diagnosis of lung involvement.
"It’s one of my many missions in life to get rheumatologists to listen to the lungs when they examine the joints in patients with rheumatoid arthritis," he said. "I think we are getting better. We’ve persuaded the British Society for Rheumatology to incorporate lung function testing and clinical examination of the chest into their basic assessment of a rheumatoid patient."
Also likely affecting the improved mortality seen over the cohort’s study period is a change in therapeutic approach. While RA-ILD patients diagnosed in the first half of the study period were likely to have been treated with only prednisone and azathioprine, in the latter half they were more likely to have received cyclophosphamide and methylprednisolone or mycophenolate. Over the last 12 years, more were treated with biologics, and in the final 6 years of the cohort, patients requiring biologics tended to be treated with rituximab, a B-cell inhibitor, rather than anti–tumor necrosis factor (anti-TNF) agents, the BRILL investigators found.
Mortality was lower among RA-ILD patients treated with mycophenolate than in those treated with other immunosuppressive agents. Among biologic agents used in the cohort, rituximab treatment was associated with improved mortality, but anti-TNF inhibitors were seen to be associated with elevated risk of death.
About 95% of RA-ILD patients are anticyclic citrullinated peptide (anti-CCP) antibody positive, compared with 55%-60% of the RA population as a whole, Dr. Kelly said, "so there’s a strong statistical association of seropositivity, and in those who are seropositive, rituximab works well."
The finding that rituximab was associated with improved survival in the cohort not only has implications for RA-ILD, he said, but also, potentially, for people with idiopathic pulmonary fibrosis (IPF) who are anti-CCP antibody positive. "What [rheumatologists] have, and chest physicians traditionally don’t, is access to rituximab and mycophenolate. But these might be worth trying in IPF as well," he said.
Dr. Kelly noted that prospective trials in RA-ILD are beginning to enroll patients with progressive disease to compare azathioprine and mycophenolate, allowing for the use of oral steroids, as well as patients with active RA and ILD to compare anti-TNF inhibitors against rituximab, also allowing oral steroids.
Dr. Kelly reported that he had no conflicts of interest related to his findings and that none of his fellow BRILL investigators had conflicts.
The use of prednisone for 3 or more months at a time was associated with a significantly elevated risk of death in patients with rheumatoid arthritis and interstitial lung disease in a retrospective cohort study.
Interstitial lung disease is present in about 5% of patients with rheumatoid arthritis. For years, oral steroids were commonly used in patients with the disease, but today’s rheumatologists "no longer view oral steroids as optimal treatment in RA-ILD [rheumatoid arthritis–associated interstitial lung disease], and our data now confirm that," said Dr. Clive Kelly of Queen Elizabeth Hospital in Gateshead, England, senior investigator of the study. He added that clinicians should avoid long-term treatment with steroids in this patient group whenever possible.
Dr. Kelly led the British Rheumatoid Interstitial Lung (BRILL) Network’s cohort study of 260 patients with RA-ILD diagnosed over a 25-year period. The BRILL study compared patients with RA-ILD and an equal number of RA controls without lung involvement who were matched for age, sex, and time of diagnosis.
At the annual European Congress of Rheumatology, Dr. Kelly reported that steroid-treated RA-ILD patients, who represented nearly 60% of the cohort, had an elevated relative risk of all-cause death, compared with those who had never been treated with steroids (RR, 1.65; 95% confidence interval, 1.2-2.3; P = .002). Although the relative risk of respiratory death was significantly increased for RA-ILD patients, regardless of treatment, when compared with RA patients without ILD, the risk was higher in those who had been on steroids (RR, 2.75; 95% CI, 1.6-4.7; P = .0002) than in those who had not received steroids (RR, 2.06; 95% CI, 1.1-3.8; P = .02), the investigators found.
The comparison also revealed other important findings related to RA-ILD. Patients with RA-ILD had significantly higher mortality than did those with RA alone. Over the course of the 25-year study period, however, mortality progressively improved among the RA-ILD patients, with median age at death rising from 63 to 76 years. This steady improvement, Dr. Kelly said, is partly the result of better and earlier diagnosis of lung involvement.
"It’s one of my many missions in life to get rheumatologists to listen to the lungs when they examine the joints in patients with rheumatoid arthritis," he said. "I think we are getting better. We’ve persuaded the British Society for Rheumatology to incorporate lung function testing and clinical examination of the chest into their basic assessment of a rheumatoid patient."
Also likely affecting the improved mortality seen over the cohort’s study period is a change in therapeutic approach. While RA-ILD patients diagnosed in the first half of the study period were likely to have been treated with only prednisone and azathioprine, in the latter half they were more likely to have received cyclophosphamide and methylprednisolone or mycophenolate. Over the last 12 years, more were treated with biologics, and in the final 6 years of the cohort, patients requiring biologics tended to be treated with rituximab, a B-cell inhibitor, rather than anti–tumor necrosis factor (anti-TNF) agents, the BRILL investigators found.
Mortality was lower among RA-ILD patients treated with mycophenolate than in those treated with other immunosuppressive agents. Among biologic agents used in the cohort, rituximab treatment was associated with improved mortality, but anti-TNF inhibitors were seen to be associated with elevated risk of death.
About 95% of RA-ILD patients are anticyclic citrullinated peptide (anti-CCP) antibody positive, compared with 55%-60% of the RA population as a whole, Dr. Kelly said, "so there’s a strong statistical association of seropositivity, and in those who are seropositive, rituximab works well."
The finding that rituximab was associated with improved survival in the cohort not only has implications for RA-ILD, he said, but also, potentially, for people with idiopathic pulmonary fibrosis (IPF) who are anti-CCP antibody positive. "What [rheumatologists] have, and chest physicians traditionally don’t, is access to rituximab and mycophenolate. But these might be worth trying in IPF as well," he said.
Dr. Kelly noted that prospective trials in RA-ILD are beginning to enroll patients with progressive disease to compare azathioprine and mycophenolate, allowing for the use of oral steroids, as well as patients with active RA and ILD to compare anti-TNF inhibitors against rituximab, also allowing oral steroids.
Dr. Kelly reported that he had no conflicts of interest related to his findings and that none of his fellow BRILL investigators had conflicts.
FROM THE EULAR CONGRESS 2014
Key clinical point: Rather than corticosteroids, consider using mycophenolate or rituximab in patients with RA-ILD.
Major finding: Steroid-treated RA-ILD patients had an elevated relative risk of all-cause death, compared with those who had never been treated with steroids (RR, 1.65; 95% confidence interval, 1.2-2.3; P = .002).
Data source: A retrospective study of 260 patients with RA-ILD in the British Rheumatoid Interstitial Lung Network.
Disclosures: Dr. Kelly reported that he had no conflicts of interest related to his findings and that none of his fellow BRILL investigators had conflicts.
VIDEO: Gene profiling could signal start of personalized medicine in RA
PARIS – A set of genetic polymorphisms is beginning to allow researchers to predict which patients with rheumatoid arthritis will have a severe disease course, as well as determine their response to treatment and risk of death.
Changes in amino acids at positions 71 and 74 of the HLA-DRB1 gene, which are a part of the "shared epitope" that is already known to increase genetic susceptibility for rheumatoid arthritis, as well as a new polymorphism at position 11 of the HLA-DRB1 gene that is outside the shared epitope, are key to this effort. These polymorphisms predicted the radiologic outcome of rheumatoid arthritis patients, response to anti-tumor necrosis factor therapy, and mortality in an analysis of blood samples from three independent multicenter, prospective cohort studies. The three polymorphisms defined 16 haplotypes whose effects on RA susceptibility range from protective to increasing risk and were perfectly correlated with the observed levels of disease susceptibility.
Further studies will be necessary to validate the associations observed with the sets of polymorphisms, said Dr. Sebastien Viatte, first author of the study and a research fellow at the Centre for Musculoskeletal Research at the University of Manchester (England). Nonetheless, the results are an important step in showing that "genetics can be used to predict disease outcomes and is ... likely to enter the clinic within 5-10 years," he said in a video interview at the annual European Congress of Rheumatology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PARIS – A set of genetic polymorphisms is beginning to allow researchers to predict which patients with rheumatoid arthritis will have a severe disease course, as well as determine their response to treatment and risk of death.
Changes in amino acids at positions 71 and 74 of the HLA-DRB1 gene, which are a part of the "shared epitope" that is already known to increase genetic susceptibility for rheumatoid arthritis, as well as a new polymorphism at position 11 of the HLA-DRB1 gene that is outside the shared epitope, are key to this effort. These polymorphisms predicted the radiologic outcome of rheumatoid arthritis patients, response to anti-tumor necrosis factor therapy, and mortality in an analysis of blood samples from three independent multicenter, prospective cohort studies. The three polymorphisms defined 16 haplotypes whose effects on RA susceptibility range from protective to increasing risk and were perfectly correlated with the observed levels of disease susceptibility.
Further studies will be necessary to validate the associations observed with the sets of polymorphisms, said Dr. Sebastien Viatte, first author of the study and a research fellow at the Centre for Musculoskeletal Research at the University of Manchester (England). Nonetheless, the results are an important step in showing that "genetics can be used to predict disease outcomes and is ... likely to enter the clinic within 5-10 years," he said in a video interview at the annual European Congress of Rheumatology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PARIS – A set of genetic polymorphisms is beginning to allow researchers to predict which patients with rheumatoid arthritis will have a severe disease course, as well as determine their response to treatment and risk of death.
Changes in amino acids at positions 71 and 74 of the HLA-DRB1 gene, which are a part of the "shared epitope" that is already known to increase genetic susceptibility for rheumatoid arthritis, as well as a new polymorphism at position 11 of the HLA-DRB1 gene that is outside the shared epitope, are key to this effort. These polymorphisms predicted the radiologic outcome of rheumatoid arthritis patients, response to anti-tumor necrosis factor therapy, and mortality in an analysis of blood samples from three independent multicenter, prospective cohort studies. The three polymorphisms defined 16 haplotypes whose effects on RA susceptibility range from protective to increasing risk and were perfectly correlated with the observed levels of disease susceptibility.
Further studies will be necessary to validate the associations observed with the sets of polymorphisms, said Dr. Sebastien Viatte, first author of the study and a research fellow at the Centre for Musculoskeletal Research at the University of Manchester (England). Nonetheless, the results are an important step in showing that "genetics can be used to predict disease outcomes and is ... likely to enter the clinic within 5-10 years," he said in a video interview at the annual European Congress of Rheumatology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE EULAR CONGRESS 2014
Stem-cell transplants growing routine for severe scleroderma
PARIS – Autologous stem-cell transplantation has emerged as an effective and feasible treatment for selected patients with severe systemic sclerosis who inadequately respond to conventional treatments, said two U.S. experts.
In addition, convincing evidence documenting the overall beneficial effect of autologous stem-cell transplantation will soon appear in a published report from the Autologous Stem Cell Transplantation International Scleroderma (ASTIS) trial, Dr. Dinesh Khanna said in a talk at the annual European Congress of Rheumatology.
In his talk, Dr. Khanna gave a short preview of the ASTIS results that he said would appear in a medical journal in the next few weeks. Those results show that among 79 scleroderma patients randomized to treatment with autologous stem-cell transplant, 8 (10%) died from treatment-related causes, compared with none in the control arm of patients who received conventional treatment with cyclophosphamide. But during follow-up, a total of 16 of the 79 (20%) stem-cell transplant patients died, compared with 24 of the 77 (31%) control patients, results that showed an overall mortality benefit from stem-cell transplantation.
The transplanted patients also showed "large improvements" in their skin score and "substantial improvements" in their forced vital capacity and in their activity as measured by the Health Assessment Questionnaire Disability Index (HAQ-DI), noted Dr. Khanna, director of the scleroderma program at the University of Michigan in Ann Arbor.
"Before and after treatment, patients look completely different. Some patients [treated with stem-cell transplants] you can’t tell that they had scleroderma. But you need to select the right patients," he cautioned. "There is high mortality early, but if you select patients correctly the benefits will outweigh the risks. This is what we now offer to patients" who don’t respond to conventional treatments and are suitable for transplantation, Dr. Khanna said.
Researchers had previously reported these ASTIS results during the EULAR 2012 meeting.
Stem-cell treatment is appropriate for "the 10%-15% of patients with scleroderma who are the worst," commented Dr. Daniel Furst, a professor in rheumatology at the University of California, Los Angeles. "The next step is to move this treatment from the worst patients to those who are less severe. In some centers in Europe, stem-cell transplantation is becoming widely used, even for patients with only skin symptoms," Dr. Furst said in an interview.
A major attraction of stem-cell transplantation is that over time it produces regression of fibrosis and collagen deposits and healing of prior organ damage. But the treatment also carries the risk of causing substantial immunosuppression while the immune system repopulates, leaving patients vulnerable to infections and other complications.
The upcoming publication of the ASTIS findings should further cement the role of stem-cell transplantation in scleroderma management, but "I’m more of a skeptic. I would like to see it reproduced" in a second trial, Dr. Furst said. Specifically, he means the North American–based Scleroderma: Cyclophosphamide or Transplantation (SCOT) trial now in progress. A positive result in SCOT is still needed to convince many insurers to cover the cost of stem-cell transplantation, Dr. Furst said. For example, when enrolling patients into SCOT, insurers were willing to reimburse the treatment of about a quarter of the patients who otherwise qualified for enrollment, he said.
Dr. Khanna has received research grants from 14 different drug companies. Dr. Furst has been a consultant to or speaker for 11 different drug companies, and has received research grants from 10 different companies.
mzoler@frontlinemedcom.com On Twitter @mitchelzoler
PARIS – Autologous stem-cell transplantation has emerged as an effective and feasible treatment for selected patients with severe systemic sclerosis who inadequately respond to conventional treatments, said two U.S. experts.
In addition, convincing evidence documenting the overall beneficial effect of autologous stem-cell transplantation will soon appear in a published report from the Autologous Stem Cell Transplantation International Scleroderma (ASTIS) trial, Dr. Dinesh Khanna said in a talk at the annual European Congress of Rheumatology.
In his talk, Dr. Khanna gave a short preview of the ASTIS results that he said would appear in a medical journal in the next few weeks. Those results show that among 79 scleroderma patients randomized to treatment with autologous stem-cell transplant, 8 (10%) died from treatment-related causes, compared with none in the control arm of patients who received conventional treatment with cyclophosphamide. But during follow-up, a total of 16 of the 79 (20%) stem-cell transplant patients died, compared with 24 of the 77 (31%) control patients, results that showed an overall mortality benefit from stem-cell transplantation.
The transplanted patients also showed "large improvements" in their skin score and "substantial improvements" in their forced vital capacity and in their activity as measured by the Health Assessment Questionnaire Disability Index (HAQ-DI), noted Dr. Khanna, director of the scleroderma program at the University of Michigan in Ann Arbor.
"Before and after treatment, patients look completely different. Some patients [treated with stem-cell transplants] you can’t tell that they had scleroderma. But you need to select the right patients," he cautioned. "There is high mortality early, but if you select patients correctly the benefits will outweigh the risks. This is what we now offer to patients" who don’t respond to conventional treatments and are suitable for transplantation, Dr. Khanna said.
Researchers had previously reported these ASTIS results during the EULAR 2012 meeting.
Stem-cell treatment is appropriate for "the 10%-15% of patients with scleroderma who are the worst," commented Dr. Daniel Furst, a professor in rheumatology at the University of California, Los Angeles. "The next step is to move this treatment from the worst patients to those who are less severe. In some centers in Europe, stem-cell transplantation is becoming widely used, even for patients with only skin symptoms," Dr. Furst said in an interview.
A major attraction of stem-cell transplantation is that over time it produces regression of fibrosis and collagen deposits and healing of prior organ damage. But the treatment also carries the risk of causing substantial immunosuppression while the immune system repopulates, leaving patients vulnerable to infections and other complications.
The upcoming publication of the ASTIS findings should further cement the role of stem-cell transplantation in scleroderma management, but "I’m more of a skeptic. I would like to see it reproduced" in a second trial, Dr. Furst said. Specifically, he means the North American–based Scleroderma: Cyclophosphamide or Transplantation (SCOT) trial now in progress. A positive result in SCOT is still needed to convince many insurers to cover the cost of stem-cell transplantation, Dr. Furst said. For example, when enrolling patients into SCOT, insurers were willing to reimburse the treatment of about a quarter of the patients who otherwise qualified for enrollment, he said.
Dr. Khanna has received research grants from 14 different drug companies. Dr. Furst has been a consultant to or speaker for 11 different drug companies, and has received research grants from 10 different companies.
mzoler@frontlinemedcom.com On Twitter @mitchelzoler
PARIS – Autologous stem-cell transplantation has emerged as an effective and feasible treatment for selected patients with severe systemic sclerosis who inadequately respond to conventional treatments, said two U.S. experts.
In addition, convincing evidence documenting the overall beneficial effect of autologous stem-cell transplantation will soon appear in a published report from the Autologous Stem Cell Transplantation International Scleroderma (ASTIS) trial, Dr. Dinesh Khanna said in a talk at the annual European Congress of Rheumatology.
In his talk, Dr. Khanna gave a short preview of the ASTIS results that he said would appear in a medical journal in the next few weeks. Those results show that among 79 scleroderma patients randomized to treatment with autologous stem-cell transplant, 8 (10%) died from treatment-related causes, compared with none in the control arm of patients who received conventional treatment with cyclophosphamide. But during follow-up, a total of 16 of the 79 (20%) stem-cell transplant patients died, compared with 24 of the 77 (31%) control patients, results that showed an overall mortality benefit from stem-cell transplantation.
The transplanted patients also showed "large improvements" in their skin score and "substantial improvements" in their forced vital capacity and in their activity as measured by the Health Assessment Questionnaire Disability Index (HAQ-DI), noted Dr. Khanna, director of the scleroderma program at the University of Michigan in Ann Arbor.
"Before and after treatment, patients look completely different. Some patients [treated with stem-cell transplants] you can’t tell that they had scleroderma. But you need to select the right patients," he cautioned. "There is high mortality early, but if you select patients correctly the benefits will outweigh the risks. This is what we now offer to patients" who don’t respond to conventional treatments and are suitable for transplantation, Dr. Khanna said.
Researchers had previously reported these ASTIS results during the EULAR 2012 meeting.
Stem-cell treatment is appropriate for "the 10%-15% of patients with scleroderma who are the worst," commented Dr. Daniel Furst, a professor in rheumatology at the University of California, Los Angeles. "The next step is to move this treatment from the worst patients to those who are less severe. In some centers in Europe, stem-cell transplantation is becoming widely used, even for patients with only skin symptoms," Dr. Furst said in an interview.
A major attraction of stem-cell transplantation is that over time it produces regression of fibrosis and collagen deposits and healing of prior organ damage. But the treatment also carries the risk of causing substantial immunosuppression while the immune system repopulates, leaving patients vulnerable to infections and other complications.
The upcoming publication of the ASTIS findings should further cement the role of stem-cell transplantation in scleroderma management, but "I’m more of a skeptic. I would like to see it reproduced" in a second trial, Dr. Furst said. Specifically, he means the North American–based Scleroderma: Cyclophosphamide or Transplantation (SCOT) trial now in progress. A positive result in SCOT is still needed to convince many insurers to cover the cost of stem-cell transplantation, Dr. Furst said. For example, when enrolling patients into SCOT, insurers were willing to reimburse the treatment of about a quarter of the patients who otherwise qualified for enrollment, he said.
Dr. Khanna has received research grants from 14 different drug companies. Dr. Furst has been a consultant to or speaker for 11 different drug companies, and has received research grants from 10 different companies.
mzoler@frontlinemedcom.com On Twitter @mitchelzoler
EXPERT ANALYSIS FROM THE EULAR CONGRESS 2014
Key clinical point: Use of autologous stem-cell transplants is spreading for intractable, severe scleroderma as the evidence base expands.
Major finding: Patients who were randomized to autologous stem-cell transplant showed a survival benefit, compared with control patients who received conventional treatment with cyclophosphamide. Transplanted patients also showed "large improvements" in their skin score and "substantial improvements" in forced vital capacity and HAQ-DI.
Data source: Results from 79 patients in the ASTIS trial who were randomized to stem-cell transplant or to cyclophosphamide.
Disclosures: Dr. Khanna has received research grants from 14 different drug companies. Dr. Furst has been a consultant to or speaker for 11 different drug companies, and has received research grants from 10 different companies.
FVC inadequate when assessing scleroderma lung disease
PARIS – The traditional way to assess the status of interstitial lung disease in patients with systemic sclerosis, forced vital capacity, may not be the best way, based on a new analysis of 83 patients enrolled in a scleroderma-treatment trial.
"A structural, physiologic, and patient-oriented composite outcome may be a more comprehensive measure of treatment response" for patients with systemic sclerosis (SSc) and interstitial lung disease, Dr. Elizabeth Volkmann said at the annual European Congress of Rheumatology. "The most robust" association seen in her analysis did not include forced vital capacity (FVC) but instead focused on the Transition Dyspnea Index (TDI), the scleroderma modified Health Assessment Questionnaire Disability Index (HAQ-DI), and quantitative, serial assessment of high-resolution CT (HRCT) images of the patient’s lungs, said Dr. Volkmann, a rheumatologist at the University of California, Los Angeles.
Although her main goal in this analysis was to identify the best assessment of lung disease in SSc patients enrolled in clinical trials, the findings also have implications for managing patients with SSc, also known as scleroderma, in routine practice, Dr. Volkmann said in an interview.
Many physicians "rely solely on FVC for following patients, and I think this may not be the best measure. Now that we have great imaging options we should use them. And the strongest correlates [in the new analysis] were with the HAQ-DI, a measure of what patients can do, and the TDI, in which patients say how much their disease has progressed. They are both patient oriented and tell you how the patient is doing," Dr. Volkmann said.
"The three were more robust and comprehensive than FVC," which can be influenced by many factors and has a variability of 10%. Dr. Volkmann conceded that the quantitative assessment of annual HRCT scans done in the study is not widely available, but she said that visual assessment of HRCT scans highly correlates with quantitative assessment and hence likely makes a reasonable substitute.
A senior collaborator on the study, Dr. Daniel Furst, said that in his opinion it was premature to completely abandon FVC for assessing SSc patients, but it was clearly useful to add the two patient-oriented questionnaires and HRCT imaging.
"We haven’t discarded FVC, but we’ve added the other things," he said in an interview. "Five years ago we only did FVC, 3 years ago we added the scleroderma HAQ-DI," and now he and his associates also use annual HRCT imaging as well as the TDI. The two questionnaires are administered every 3-6 months, said Dr. Furst, professor of rheumatology at UCLA.
"Using all four of these tools is not being widely done" right now in U.S. rheumatology practice. "I really think it’s a step forward." However, Dr. Furst also cautioned that for adoption into routine practice he would like to see evidence documenting that this approach has a positive impact on patient outcomes.
Dr. Volkmann’s analysis involved the 158 U.S. patients enrolled in the first Scleroderma Lung Study, run in 2000-2004 at 13 U.S. centers to compare treatment with oral cyclophosphamide against placebo in patients with active SSC and interstitial lung disease (N. Engl. J. Med. 2006;354:2655-66). Of the 158 patients enrolled, 125 had an HRCT scan at baseline, and among those, 83 also had a HRCT scan after 12 months. These 83 patients formed the basis for Dr. Volkmann’s analysis, including 41 patients randomized to cyclophosphamide treatment and 42 randomized to placebo.
Multivariate analysis identified the HAQ-DI, TDI, FVC, and a quantitative lung fibrosis score derived from analysis of the serial HRCT images as collectively predicting best the outcomes of these patients. A second model that eliminated FVC was "slightly stronger," and both of these combined assessments were each "more robust than FVC alone," Dr. Volkmann said.
Dr. Volkmann said that she had no disclosures. Dr. Furst said that he had no relevant disclosures.
On Twitter @mitchelzoler
PARIS – The traditional way to assess the status of interstitial lung disease in patients with systemic sclerosis, forced vital capacity, may not be the best way, based on a new analysis of 83 patients enrolled in a scleroderma-treatment trial.
"A structural, physiologic, and patient-oriented composite outcome may be a more comprehensive measure of treatment response" for patients with systemic sclerosis (SSc) and interstitial lung disease, Dr. Elizabeth Volkmann said at the annual European Congress of Rheumatology. "The most robust" association seen in her analysis did not include forced vital capacity (FVC) but instead focused on the Transition Dyspnea Index (TDI), the scleroderma modified Health Assessment Questionnaire Disability Index (HAQ-DI), and quantitative, serial assessment of high-resolution CT (HRCT) images of the patient’s lungs, said Dr. Volkmann, a rheumatologist at the University of California, Los Angeles.
Although her main goal in this analysis was to identify the best assessment of lung disease in SSc patients enrolled in clinical trials, the findings also have implications for managing patients with SSc, also known as scleroderma, in routine practice, Dr. Volkmann said in an interview.
Many physicians "rely solely on FVC for following patients, and I think this may not be the best measure. Now that we have great imaging options we should use them. And the strongest correlates [in the new analysis] were with the HAQ-DI, a measure of what patients can do, and the TDI, in which patients say how much their disease has progressed. They are both patient oriented and tell you how the patient is doing," Dr. Volkmann said.
"The three were more robust and comprehensive than FVC," which can be influenced by many factors and has a variability of 10%. Dr. Volkmann conceded that the quantitative assessment of annual HRCT scans done in the study is not widely available, but she said that visual assessment of HRCT scans highly correlates with quantitative assessment and hence likely makes a reasonable substitute.
A senior collaborator on the study, Dr. Daniel Furst, said that in his opinion it was premature to completely abandon FVC for assessing SSc patients, but it was clearly useful to add the two patient-oriented questionnaires and HRCT imaging.
"We haven’t discarded FVC, but we’ve added the other things," he said in an interview. "Five years ago we only did FVC, 3 years ago we added the scleroderma HAQ-DI," and now he and his associates also use annual HRCT imaging as well as the TDI. The two questionnaires are administered every 3-6 months, said Dr. Furst, professor of rheumatology at UCLA.
"Using all four of these tools is not being widely done" right now in U.S. rheumatology practice. "I really think it’s a step forward." However, Dr. Furst also cautioned that for adoption into routine practice he would like to see evidence documenting that this approach has a positive impact on patient outcomes.
Dr. Volkmann’s analysis involved the 158 U.S. patients enrolled in the first Scleroderma Lung Study, run in 2000-2004 at 13 U.S. centers to compare treatment with oral cyclophosphamide against placebo in patients with active SSC and interstitial lung disease (N. Engl. J. Med. 2006;354:2655-66). Of the 158 patients enrolled, 125 had an HRCT scan at baseline, and among those, 83 also had a HRCT scan after 12 months. These 83 patients formed the basis for Dr. Volkmann’s analysis, including 41 patients randomized to cyclophosphamide treatment and 42 randomized to placebo.
Multivariate analysis identified the HAQ-DI, TDI, FVC, and a quantitative lung fibrosis score derived from analysis of the serial HRCT images as collectively predicting best the outcomes of these patients. A second model that eliminated FVC was "slightly stronger," and both of these combined assessments were each "more robust than FVC alone," Dr. Volkmann said.
Dr. Volkmann said that she had no disclosures. Dr. Furst said that he had no relevant disclosures.
On Twitter @mitchelzoler
PARIS – The traditional way to assess the status of interstitial lung disease in patients with systemic sclerosis, forced vital capacity, may not be the best way, based on a new analysis of 83 patients enrolled in a scleroderma-treatment trial.
"A structural, physiologic, and patient-oriented composite outcome may be a more comprehensive measure of treatment response" for patients with systemic sclerosis (SSc) and interstitial lung disease, Dr. Elizabeth Volkmann said at the annual European Congress of Rheumatology. "The most robust" association seen in her analysis did not include forced vital capacity (FVC) but instead focused on the Transition Dyspnea Index (TDI), the scleroderma modified Health Assessment Questionnaire Disability Index (HAQ-DI), and quantitative, serial assessment of high-resolution CT (HRCT) images of the patient’s lungs, said Dr. Volkmann, a rheumatologist at the University of California, Los Angeles.
Although her main goal in this analysis was to identify the best assessment of lung disease in SSc patients enrolled in clinical trials, the findings also have implications for managing patients with SSc, also known as scleroderma, in routine practice, Dr. Volkmann said in an interview.
Many physicians "rely solely on FVC for following patients, and I think this may not be the best measure. Now that we have great imaging options we should use them. And the strongest correlates [in the new analysis] were with the HAQ-DI, a measure of what patients can do, and the TDI, in which patients say how much their disease has progressed. They are both patient oriented and tell you how the patient is doing," Dr. Volkmann said.
"The three were more robust and comprehensive than FVC," which can be influenced by many factors and has a variability of 10%. Dr. Volkmann conceded that the quantitative assessment of annual HRCT scans done in the study is not widely available, but she said that visual assessment of HRCT scans highly correlates with quantitative assessment and hence likely makes a reasonable substitute.
A senior collaborator on the study, Dr. Daniel Furst, said that in his opinion it was premature to completely abandon FVC for assessing SSc patients, but it was clearly useful to add the two patient-oriented questionnaires and HRCT imaging.
"We haven’t discarded FVC, but we’ve added the other things," he said in an interview. "Five years ago we only did FVC, 3 years ago we added the scleroderma HAQ-DI," and now he and his associates also use annual HRCT imaging as well as the TDI. The two questionnaires are administered every 3-6 months, said Dr. Furst, professor of rheumatology at UCLA.
"Using all four of these tools is not being widely done" right now in U.S. rheumatology practice. "I really think it’s a step forward." However, Dr. Furst also cautioned that for adoption into routine practice he would like to see evidence documenting that this approach has a positive impact on patient outcomes.
Dr. Volkmann’s analysis involved the 158 U.S. patients enrolled in the first Scleroderma Lung Study, run in 2000-2004 at 13 U.S. centers to compare treatment with oral cyclophosphamide against placebo in patients with active SSC and interstitial lung disease (N. Engl. J. Med. 2006;354:2655-66). Of the 158 patients enrolled, 125 had an HRCT scan at baseline, and among those, 83 also had a HRCT scan after 12 months. These 83 patients formed the basis for Dr. Volkmann’s analysis, including 41 patients randomized to cyclophosphamide treatment and 42 randomized to placebo.
Multivariate analysis identified the HAQ-DI, TDI, FVC, and a quantitative lung fibrosis score derived from analysis of the serial HRCT images as collectively predicting best the outcomes of these patients. A second model that eliminated FVC was "slightly stronger," and both of these combined assessments were each "more robust than FVC alone," Dr. Volkmann said.
Dr. Volkmann said that she had no disclosures. Dr. Furst said that he had no relevant disclosures.
On Twitter @mitchelzoler
AT THE EULAR CONGRESS 2014
Key clinical point: Patient-oriented questionnaires and lung imaging are key tools to assess systemic sclerosis patients with interstitial lung disease.
Major finding: Combined assessment by patient-oriented questionnaires, CT imaging, and forced vital capacity better predicted patient outcomes than did FVC alone.
Data source: Retrospective analysis of data collected from 83 systemic sclerosis patients enrolled at any of 13 U.S. centers.
Disclosures: Dr. Volkmann said that she had no disclosures. Dr. Furst said that he had no relevant disclosures.
Gut inflammation linked to worsening spondyloarthritis
Spondyloarthritis patients with microscopic gut inflammation had to start anti–tumor necrosis factor treatment sooner in the course of their disease than patients without gut inflammation in a prospective, observational study of 63 Belgian patients with the axial form of spondyloarthritis, the peripheral form, or both.
The finding "is in line with the hypothesis that in SpA [spondyloarthritis] gut inflammation may drive disease," Dr. Heleen Cypers said at the annual European Congress of Rheumatology.
SpA patients with microscopic gut inflammation initiated anti–tumor necrosis factor (TNF) therapy roughly twice as quickly as those without gut inflammation at each time period examined – at 6, 12, and 18 months after their initial diagnosis. After 18 months, 56% of patients with inflammation were on treatment with an anti-TNF agent, compared with almost 29% of the followed patients without microscopic gut inflammation, said Dr. Cypers, a rheumatologist at Ghent (Belgium) University.
The link between microscopic gut inflammation and a faster need to start on biologic treatment occurred at nearly identical rates in both the subset of 11 patients with acute gut inflammation and the subset of 21 patients with chronic inflammation.
Previous research by Dr. Cypers’ group had shown that chronic inflammation in the gut was associated with more extensive bone marrow edema of the sacroiliac joints, a higher risk of progression to ankylosing spondylitis, and a higher risk of developing Crohn’s disease. No information existed on the impact of gut inflammation on therapeutic decision-making in SpA, however.
The current study followed the Ghent Inflammatory Arthritis and Spondylitis Cohort (GIANT) of 63 newly diagnosed patients who underwent an ileocolonoscopy at baseline. Patients were not taking anti-TNF agents and were followed for 18 months. The decision to start anti-TNF treatment was made by a rheumatologist who was unaware of the patient’s gut history.
SpA patients who had microscopic gut inflammation at baseline were more likely to start anti-TNF treatment sooner than were patients without gut inflammation. During follow-up, just under a third of patients (9 of 31) with normal gut histology started anti-TNFs, compared with over half (18 of 32) of the patients with gut inflammation at baseline.
In most cases, anti-TNF drugs were started because first-line therapy had failed, Dr. Cypers reported. Her analysis also showed that initiation of an anti-TNF drug during follow-up was significantly linked with a higher level of C-reactive protein at baseline.
According to Dr. Cypers, the findings support the hypothesis that bowel inflammation in SpA induces a more chronic, persevering disease.
"The findings identify gut inflammation as a marker of a poor prognosis and have relevance for therapeutic decision-making in daily clinical practice. It is conceivable that assessment of gut inflammation will be included in future models for risk stratification of SpA," she said in an interview.
The underlying mechanisms explaining the link are still under investigation, but several studies have suggested that inflammation in the gut may be a triggering factor for SpA development. Interactions in the intestinal immune system also may play an important role, she said.
Future research by the group will focus on ruling out the interdependency of all of these factors, predicting response to biologic therapy based on gut histology, and determining whether therapies aiming at treating microscopic gut inflammation can affect the evolution of the disease.
Dr. Cypers said that she and her associates had no disclosures.
Spondyloarthritis patients with microscopic gut inflammation had to start anti–tumor necrosis factor treatment sooner in the course of their disease than patients without gut inflammation in a prospective, observational study of 63 Belgian patients with the axial form of spondyloarthritis, the peripheral form, or both.
The finding "is in line with the hypothesis that in SpA [spondyloarthritis] gut inflammation may drive disease," Dr. Heleen Cypers said at the annual European Congress of Rheumatology.
SpA patients with microscopic gut inflammation initiated anti–tumor necrosis factor (TNF) therapy roughly twice as quickly as those without gut inflammation at each time period examined – at 6, 12, and 18 months after their initial diagnosis. After 18 months, 56% of patients with inflammation were on treatment with an anti-TNF agent, compared with almost 29% of the followed patients without microscopic gut inflammation, said Dr. Cypers, a rheumatologist at Ghent (Belgium) University.
The link between microscopic gut inflammation and a faster need to start on biologic treatment occurred at nearly identical rates in both the subset of 11 patients with acute gut inflammation and the subset of 21 patients with chronic inflammation.
Previous research by Dr. Cypers’ group had shown that chronic inflammation in the gut was associated with more extensive bone marrow edema of the sacroiliac joints, a higher risk of progression to ankylosing spondylitis, and a higher risk of developing Crohn’s disease. No information existed on the impact of gut inflammation on therapeutic decision-making in SpA, however.
The current study followed the Ghent Inflammatory Arthritis and Spondylitis Cohort (GIANT) of 63 newly diagnosed patients who underwent an ileocolonoscopy at baseline. Patients were not taking anti-TNF agents and were followed for 18 months. The decision to start anti-TNF treatment was made by a rheumatologist who was unaware of the patient’s gut history.
SpA patients who had microscopic gut inflammation at baseline were more likely to start anti-TNF treatment sooner than were patients without gut inflammation. During follow-up, just under a third of patients (9 of 31) with normal gut histology started anti-TNFs, compared with over half (18 of 32) of the patients with gut inflammation at baseline.
In most cases, anti-TNF drugs were started because first-line therapy had failed, Dr. Cypers reported. Her analysis also showed that initiation of an anti-TNF drug during follow-up was significantly linked with a higher level of C-reactive protein at baseline.
According to Dr. Cypers, the findings support the hypothesis that bowel inflammation in SpA induces a more chronic, persevering disease.
"The findings identify gut inflammation as a marker of a poor prognosis and have relevance for therapeutic decision-making in daily clinical practice. It is conceivable that assessment of gut inflammation will be included in future models for risk stratification of SpA," she said in an interview.
The underlying mechanisms explaining the link are still under investigation, but several studies have suggested that inflammation in the gut may be a triggering factor for SpA development. Interactions in the intestinal immune system also may play an important role, she said.
Future research by the group will focus on ruling out the interdependency of all of these factors, predicting response to biologic therapy based on gut histology, and determining whether therapies aiming at treating microscopic gut inflammation can affect the evolution of the disease.
Dr. Cypers said that she and her associates had no disclosures.
Spondyloarthritis patients with microscopic gut inflammation had to start anti–tumor necrosis factor treatment sooner in the course of their disease than patients without gut inflammation in a prospective, observational study of 63 Belgian patients with the axial form of spondyloarthritis, the peripheral form, or both.
The finding "is in line with the hypothesis that in SpA [spondyloarthritis] gut inflammation may drive disease," Dr. Heleen Cypers said at the annual European Congress of Rheumatology.
SpA patients with microscopic gut inflammation initiated anti–tumor necrosis factor (TNF) therapy roughly twice as quickly as those without gut inflammation at each time period examined – at 6, 12, and 18 months after their initial diagnosis. After 18 months, 56% of patients with inflammation were on treatment with an anti-TNF agent, compared with almost 29% of the followed patients without microscopic gut inflammation, said Dr. Cypers, a rheumatologist at Ghent (Belgium) University.
The link between microscopic gut inflammation and a faster need to start on biologic treatment occurred at nearly identical rates in both the subset of 11 patients with acute gut inflammation and the subset of 21 patients with chronic inflammation.
Previous research by Dr. Cypers’ group had shown that chronic inflammation in the gut was associated with more extensive bone marrow edema of the sacroiliac joints, a higher risk of progression to ankylosing spondylitis, and a higher risk of developing Crohn’s disease. No information existed on the impact of gut inflammation on therapeutic decision-making in SpA, however.
The current study followed the Ghent Inflammatory Arthritis and Spondylitis Cohort (GIANT) of 63 newly diagnosed patients who underwent an ileocolonoscopy at baseline. Patients were not taking anti-TNF agents and were followed for 18 months. The decision to start anti-TNF treatment was made by a rheumatologist who was unaware of the patient’s gut history.
SpA patients who had microscopic gut inflammation at baseline were more likely to start anti-TNF treatment sooner than were patients without gut inflammation. During follow-up, just under a third of patients (9 of 31) with normal gut histology started anti-TNFs, compared with over half (18 of 32) of the patients with gut inflammation at baseline.
In most cases, anti-TNF drugs were started because first-line therapy had failed, Dr. Cypers reported. Her analysis also showed that initiation of an anti-TNF drug during follow-up was significantly linked with a higher level of C-reactive protein at baseline.
According to Dr. Cypers, the findings support the hypothesis that bowel inflammation in SpA induces a more chronic, persevering disease.
"The findings identify gut inflammation as a marker of a poor prognosis and have relevance for therapeutic decision-making in daily clinical practice. It is conceivable that assessment of gut inflammation will be included in future models for risk stratification of SpA," she said in an interview.
The underlying mechanisms explaining the link are still under investigation, but several studies have suggested that inflammation in the gut may be a triggering factor for SpA development. Interactions in the intestinal immune system also may play an important role, she said.
Future research by the group will focus on ruling out the interdependency of all of these factors, predicting response to biologic therapy based on gut histology, and determining whether therapies aiming at treating microscopic gut inflammation can affect the evolution of the disease.
Dr. Cypers said that she and her associates had no disclosures.
FROM THE EULAR CONGRESS 2014
Major finding: Among patients with newly diagnosed spondyloarthritis, microscopic gut inflammation at baseline was linked to a two-fold higher rate of anti-TNF treatment initiation.
Data source: A prospective, observational study of 63 Belgian patients with newly diagnosed spondyloarthritis who were followed for 18 months.
Disclosures: Dr. Cypers said that she and her associates had no disclosures.
International RA risk tool for CVD comes closer to reality
An international collaboration of experts has developed a cardiovascular risk calculator specifically for rheumatoid arthritis patients that has the potential to become part of routine rheumatology clinical practice in many parts of the world.
The ATACC-RA (A TransAtlantic Cardiovascular Risk Calculator for Rheumatoid Arthritis) consortium will be constructed from data collected at 13 rheumatology centers in 10 countries, Elke Arts said at the annual European Congress of Rheumatology.
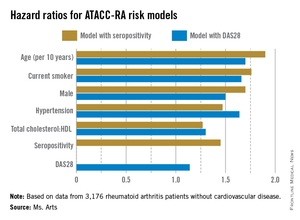
The current version, which is still undergoing validation, contains data from eight rheumatology centers in seven countries (Greece, the Netherlands, Norway, South Africa, Sweden, the United Kingdom, and the United States), said Ms. Arts, a rheumatology researcher at Radboud University Medical Centre in Nijmegen, the Netherlands.
So far, ATACC-RA contains pooled data from 3,176 RA patients without cardiovascular disease. Individual data were collected on CV risk factors and outcomes and then combined with RA-specific information, such as disease duration, seropositivity, rheumatoid factor and/or anti-citrullinated protein antibodies, 28-joint Disease Activity Score (DAS28), and acute phase reactants.
During an average of almost 8 years of follow-up comprising 24,733 patient-years, 314 patients developed cardiovascular disease (CVD).
Two possible models came out of multivariable risk score modeling for predicting the RA-specific 10-year risk of CVD. Each incorporated the traditional risk factors of age, sex, current smoking, presence of hypertension, and ratio of total cholesterol to HDL cholesterol, but also either seropositivity or DAS28. The consortium specifically chose to assess potential risk factors that would be easily available to the health professional regardless of the setting (for example, primary, secondary, and tertiary care, and even private practice).
The models that included seropositivity or DAS28 both demonstrated good discrimination and calibration, when compared with the Framingham or SCORE (Systematic Coronary Risk Evaluation) algorithms. The models also showed good concordance with each other (see chart).
"By pooling data from many centers, it appears possible to develop an RA-specific CVD risk algorithm which is more accurate at predicting CVD in people with RA than the currently available risk algorithms, which have been developed for the general population," members of the consortium wrote in response to e-mailed questions.
The consortium is now working to validate the ATACC-RA calculator, with the ultimate aim of validating it in completely independent cohorts. The continued growth of the consortium will help make this a reality within the next couple of years. The end result may be a risk calculator that can be adapted to account for the underlying risk of each patient population.
Once the algorithm is validated, the consortium hopes it will become part of routine rheumatology clinical practice, much the same as the Framingham or SCORE algorithms are used currently in the general population, but more specifically applied to patients with RA.
"The models proved to be quite robust," Ms. Arts said. "If this holds true through the additional analysis and external validation, we may have one that will be applicable to a wide variety of patients all over the world."
Once fully validated, the investigators plan to produce the tool in a user-friendly application for computers or even smartphones.
The investigators had no conflicts of interest to declare.
An international collaboration of experts has developed a cardiovascular risk calculator specifically for rheumatoid arthritis patients that has the potential to become part of routine rheumatology clinical practice in many parts of the world.
The ATACC-RA (A TransAtlantic Cardiovascular Risk Calculator for Rheumatoid Arthritis) consortium will be constructed from data collected at 13 rheumatology centers in 10 countries, Elke Arts said at the annual European Congress of Rheumatology.

The current version, which is still undergoing validation, contains data from eight rheumatology centers in seven countries (Greece, the Netherlands, Norway, South Africa, Sweden, the United Kingdom, and the United States), said Ms. Arts, a rheumatology researcher at Radboud University Medical Centre in Nijmegen, the Netherlands.
So far, ATACC-RA contains pooled data from 3,176 RA patients without cardiovascular disease. Individual data were collected on CV risk factors and outcomes and then combined with RA-specific information, such as disease duration, seropositivity, rheumatoid factor and/or anti-citrullinated protein antibodies, 28-joint Disease Activity Score (DAS28), and acute phase reactants.
During an average of almost 8 years of follow-up comprising 24,733 patient-years, 314 patients developed cardiovascular disease (CVD).
Two possible models came out of multivariable risk score modeling for predicting the RA-specific 10-year risk of CVD. Each incorporated the traditional risk factors of age, sex, current smoking, presence of hypertension, and ratio of total cholesterol to HDL cholesterol, but also either seropositivity or DAS28. The consortium specifically chose to assess potential risk factors that would be easily available to the health professional regardless of the setting (for example, primary, secondary, and tertiary care, and even private practice).
The models that included seropositivity or DAS28 both demonstrated good discrimination and calibration, when compared with the Framingham or SCORE (Systematic Coronary Risk Evaluation) algorithms. The models also showed good concordance with each other (see chart).
"By pooling data from many centers, it appears possible to develop an RA-specific CVD risk algorithm which is more accurate at predicting CVD in people with RA than the currently available risk algorithms, which have been developed for the general population," members of the consortium wrote in response to e-mailed questions.
The consortium is now working to validate the ATACC-RA calculator, with the ultimate aim of validating it in completely independent cohorts. The continued growth of the consortium will help make this a reality within the next couple of years. The end result may be a risk calculator that can be adapted to account for the underlying risk of each patient population.
Once the algorithm is validated, the consortium hopes it will become part of routine rheumatology clinical practice, much the same as the Framingham or SCORE algorithms are used currently in the general population, but more specifically applied to patients with RA.
"The models proved to be quite robust," Ms. Arts said. "If this holds true through the additional analysis and external validation, we may have one that will be applicable to a wide variety of patients all over the world."
Once fully validated, the investigators plan to produce the tool in a user-friendly application for computers or even smartphones.
The investigators had no conflicts of interest to declare.
An international collaboration of experts has developed a cardiovascular risk calculator specifically for rheumatoid arthritis patients that has the potential to become part of routine rheumatology clinical practice in many parts of the world.
The ATACC-RA (A TransAtlantic Cardiovascular Risk Calculator for Rheumatoid Arthritis) consortium will be constructed from data collected at 13 rheumatology centers in 10 countries, Elke Arts said at the annual European Congress of Rheumatology.

The current version, which is still undergoing validation, contains data from eight rheumatology centers in seven countries (Greece, the Netherlands, Norway, South Africa, Sweden, the United Kingdom, and the United States), said Ms. Arts, a rheumatology researcher at Radboud University Medical Centre in Nijmegen, the Netherlands.
So far, ATACC-RA contains pooled data from 3,176 RA patients without cardiovascular disease. Individual data were collected on CV risk factors and outcomes and then combined with RA-specific information, such as disease duration, seropositivity, rheumatoid factor and/or anti-citrullinated protein antibodies, 28-joint Disease Activity Score (DAS28), and acute phase reactants.
During an average of almost 8 years of follow-up comprising 24,733 patient-years, 314 patients developed cardiovascular disease (CVD).
Two possible models came out of multivariable risk score modeling for predicting the RA-specific 10-year risk of CVD. Each incorporated the traditional risk factors of age, sex, current smoking, presence of hypertension, and ratio of total cholesterol to HDL cholesterol, but also either seropositivity or DAS28. The consortium specifically chose to assess potential risk factors that would be easily available to the health professional regardless of the setting (for example, primary, secondary, and tertiary care, and even private practice).
The models that included seropositivity or DAS28 both demonstrated good discrimination and calibration, when compared with the Framingham or SCORE (Systematic Coronary Risk Evaluation) algorithms. The models also showed good concordance with each other (see chart).
"By pooling data from many centers, it appears possible to develop an RA-specific CVD risk algorithm which is more accurate at predicting CVD in people with RA than the currently available risk algorithms, which have been developed for the general population," members of the consortium wrote in response to e-mailed questions.
The consortium is now working to validate the ATACC-RA calculator, with the ultimate aim of validating it in completely independent cohorts. The continued growth of the consortium will help make this a reality within the next couple of years. The end result may be a risk calculator that can be adapted to account for the underlying risk of each patient population.
Once the algorithm is validated, the consortium hopes it will become part of routine rheumatology clinical practice, much the same as the Framingham or SCORE algorithms are used currently in the general population, but more specifically applied to patients with RA.
"The models proved to be quite robust," Ms. Arts said. "If this holds true through the additional analysis and external validation, we may have one that will be applicable to a wide variety of patients all over the world."
Once fully validated, the investigators plan to produce the tool in a user-friendly application for computers or even smartphones.
The investigators had no conflicts of interest to declare.
FROM THE EULAR CONGRESS 2014
Key clinical point: An RA-specific calculator for 10-year CVD risk may soon be available.
Major finding: The risk factors in models that used seropositivity or DAS28 had similar hazard ratios contributing to CVD risk.
Data source: Pooled data from 3,176 RA patients without CVD who were followed for the development of CVD for a mean of nearly 8 years.
Disclosures: The investigators had no conflicts of interest to declare.
Sarilumab shown safe, effective for RA in phase III trial
PARIS – Rheumatoid arthritis patients who received the interleukin-6–blocking drug sarilumab plus methotrexate had significantly better short-term and 1-year outcomes than did patients on methotrexate alone in a phase III trial with 1,197 patients.
Sarilumab treatment also was relatively safe, with no new safety concerns arising from the study, Dr. Mark Genovese said at the annual European Congress of Rheumatology.
"Both sarilumab-dose groups showed a statistically significant improvement, compared to the placebo group, thereby demonstrating that, in this study, sarilumab plus methotrexate improved the signs and symptoms of rheumatoid arthritis [RA], improved physical function, and inhibited progression of joint damage in adult patients with active RA and inadequate response to methotrexate. Importantly, in this first phase III study with this agent, we did not see any unique safety issues," Dr. Genovese said in an interview.
The SARIL-RA-MOBILITY trial is the first study to finish from a panel of four phase III studies of sarilumab in patients with RA. The three other phase III trials currently in progress have enrolled a total of about 1,400 patients. A submission to the Food and Drug Administration for marketing approval of sarilumab for patients with RA will await completion of these three additional studies, said Dr. Genovese, professor of medicine and cochief of the division of immunology and rheumatology at Stanford (Calif.) University.
The trial enrolled patients with RA and an inadequate response to methotrexate alone at more than 200 centers in more than 30 countries, including the United States. The study randomized patients to treatment with methotrexate plus 150 mg sarilumab administered as a subcutaneous injection every 2 weeks, 200 mg injected every 2 weeks, or placebo.
The study had three primary efficacy endpoints:
• The percentage of patients achieving an American College of Rheumatology 20 response after 24 weeks of treatment, which was achieved by 58% of 400 patients who received 150 mg sarilumab, 66% of 399 patients who received the 200-mg dose, and 33% of the 398 patients who received placebo injections.
• The average change from baseline in the Health Assessment Questionnaire disability index after 16 weeks of treatment, which fell by 0.53 in patients who received the 150-mg dose of sarilumab, by 0.55 in those who received 200-mg sarilumab injections, and by 0.29 in those who received placebo.
• The average change from baseline in the van der Heijde–modified total Sharp score after 52 weeks of treatment, which increased by 0.90 among patients on the lower sarilumab dose, increased by 0.25 in patients on the higher dose, and increased by 2.78 among patients who received placebo.
For all three endpoints, the differences between each of the sarilumab groups and the placebo controls were statistically significant as well as clinically meaningful. "We are encouraged by the potential of sarilumab to offer a new alternative in the treatment armamentarium for patients suffering from RA," Dr. Genovese said. When the two companies developing sarilumab submit their approval application, they will likely initially seek indications for treating adult patients with moderately to severely active RA who had an inadequate response to disease-modifying antirheumatic drugs (DMARDs) or tumor necrosis factor–alpha inhibitors, he predicted.
Serious adverse events during 52 weeks of follow-up occurred in 23 placebo patients, 38 patients on the lower sarilumab dose, and 46 on the higher dose. The most common serious adverse event was infection, which occurred in 10 patients on placebo, 11 patients on low-dose sarilumab, and 17 on the higher dose. No serious infections occurred in patients whose neutrophil level fell below 1,000/mcL.
"The number of serious adverse events thus far remains low, and it will be important to see the integrated safety data across the phase III program before making any judgments regarding the ideal dose and the benefit-to-safety risk ratio of any single dose," Dr. Genovese said.
"Among patients treated with sarilumab, a dose-dependent decrease in mean neutrophil counts was observed. Serious infections were not associated with grades 3 and 4 neutropenia in this study. Increases in mean LDL cholesterol level and transaminases were observed. These safety findings were consistent with those observed in prior investigational studies with sarilumab. The choice of doses will ultimately depend on the integrated safety data across the program and the performance of each dose across a broad range of patient populations," he said.
Sarilumab is the first agent tested from the class of interleukin (IL)-6 blockers. "IL-6 is a key proinflammatory cytokine that impacts inflammation, bone, and metabolism," Dr. Genovese noted.
"Blockade of IL-6 should provide significant improvements in all these areas. This study has demonstrated that inhibition of IL-6 can reduce the signs and symptoms of RA and help to stop the destruction of joints in patients with RA. Additional phase III studies of sarilumab are underway and should provide additional evidence that anti–IL-6 receptor therapy with sarilumab may be a future option for RA patients. It is possibly too early to say if there are any special advantages or opportunities from the strategy of IL-6 blockage, compared with any other class of biologic DMARD," he said.
The trial was sponsored by Sanofi and Regeneron, the companies developing the drug. Dr. Genovese said that he has been a consultant to and received research support from Sanofi. Several coauthors are employees of Sanofi or Regeneron.
On Twitter @mitchelzoler
PARIS – Rheumatoid arthritis patients who received the interleukin-6–blocking drug sarilumab plus methotrexate had significantly better short-term and 1-year outcomes than did patients on methotrexate alone in a phase III trial with 1,197 patients.
Sarilumab treatment also was relatively safe, with no new safety concerns arising from the study, Dr. Mark Genovese said at the annual European Congress of Rheumatology.
"Both sarilumab-dose groups showed a statistically significant improvement, compared to the placebo group, thereby demonstrating that, in this study, sarilumab plus methotrexate improved the signs and symptoms of rheumatoid arthritis [RA], improved physical function, and inhibited progression of joint damage in adult patients with active RA and inadequate response to methotrexate. Importantly, in this first phase III study with this agent, we did not see any unique safety issues," Dr. Genovese said in an interview.
The SARIL-RA-MOBILITY trial is the first study to finish from a panel of four phase III studies of sarilumab in patients with RA. The three other phase III trials currently in progress have enrolled a total of about 1,400 patients. A submission to the Food and Drug Administration for marketing approval of sarilumab for patients with RA will await completion of these three additional studies, said Dr. Genovese, professor of medicine and cochief of the division of immunology and rheumatology at Stanford (Calif.) University.
The trial enrolled patients with RA and an inadequate response to methotrexate alone at more than 200 centers in more than 30 countries, including the United States. The study randomized patients to treatment with methotrexate plus 150 mg sarilumab administered as a subcutaneous injection every 2 weeks, 200 mg injected every 2 weeks, or placebo.
The study had three primary efficacy endpoints:
• The percentage of patients achieving an American College of Rheumatology 20 response after 24 weeks of treatment, which was achieved by 58% of 400 patients who received 150 mg sarilumab, 66% of 399 patients who received the 200-mg dose, and 33% of the 398 patients who received placebo injections.
• The average change from baseline in the Health Assessment Questionnaire disability index after 16 weeks of treatment, which fell by 0.53 in patients who received the 150-mg dose of sarilumab, by 0.55 in those who received 200-mg sarilumab injections, and by 0.29 in those who received placebo.
• The average change from baseline in the van der Heijde–modified total Sharp score after 52 weeks of treatment, which increased by 0.90 among patients on the lower sarilumab dose, increased by 0.25 in patients on the higher dose, and increased by 2.78 among patients who received placebo.
For all three endpoints, the differences between each of the sarilumab groups and the placebo controls were statistically significant as well as clinically meaningful. "We are encouraged by the potential of sarilumab to offer a new alternative in the treatment armamentarium for patients suffering from RA," Dr. Genovese said. When the two companies developing sarilumab submit their approval application, they will likely initially seek indications for treating adult patients with moderately to severely active RA who had an inadequate response to disease-modifying antirheumatic drugs (DMARDs) or tumor necrosis factor–alpha inhibitors, he predicted.
Serious adverse events during 52 weeks of follow-up occurred in 23 placebo patients, 38 patients on the lower sarilumab dose, and 46 on the higher dose. The most common serious adverse event was infection, which occurred in 10 patients on placebo, 11 patients on low-dose sarilumab, and 17 on the higher dose. No serious infections occurred in patients whose neutrophil level fell below 1,000/mcL.
"The number of serious adverse events thus far remains low, and it will be important to see the integrated safety data across the phase III program before making any judgments regarding the ideal dose and the benefit-to-safety risk ratio of any single dose," Dr. Genovese said.
"Among patients treated with sarilumab, a dose-dependent decrease in mean neutrophil counts was observed. Serious infections were not associated with grades 3 and 4 neutropenia in this study. Increases in mean LDL cholesterol level and transaminases were observed. These safety findings were consistent with those observed in prior investigational studies with sarilumab. The choice of doses will ultimately depend on the integrated safety data across the program and the performance of each dose across a broad range of patient populations," he said.
Sarilumab is the first agent tested from the class of interleukin (IL)-6 blockers. "IL-6 is a key proinflammatory cytokine that impacts inflammation, bone, and metabolism," Dr. Genovese noted.
"Blockade of IL-6 should provide significant improvements in all these areas. This study has demonstrated that inhibition of IL-6 can reduce the signs and symptoms of RA and help to stop the destruction of joints in patients with RA. Additional phase III studies of sarilumab are underway and should provide additional evidence that anti–IL-6 receptor therapy with sarilumab may be a future option for RA patients. It is possibly too early to say if there are any special advantages or opportunities from the strategy of IL-6 blockage, compared with any other class of biologic DMARD," he said.
The trial was sponsored by Sanofi and Regeneron, the companies developing the drug. Dr. Genovese said that he has been a consultant to and received research support from Sanofi. Several coauthors are employees of Sanofi or Regeneron.
On Twitter @mitchelzoler
PARIS – Rheumatoid arthritis patients who received the interleukin-6–blocking drug sarilumab plus methotrexate had significantly better short-term and 1-year outcomes than did patients on methotrexate alone in a phase III trial with 1,197 patients.
Sarilumab treatment also was relatively safe, with no new safety concerns arising from the study, Dr. Mark Genovese said at the annual European Congress of Rheumatology.
"Both sarilumab-dose groups showed a statistically significant improvement, compared to the placebo group, thereby demonstrating that, in this study, sarilumab plus methotrexate improved the signs and symptoms of rheumatoid arthritis [RA], improved physical function, and inhibited progression of joint damage in adult patients with active RA and inadequate response to methotrexate. Importantly, in this first phase III study with this agent, we did not see any unique safety issues," Dr. Genovese said in an interview.
The SARIL-RA-MOBILITY trial is the first study to finish from a panel of four phase III studies of sarilumab in patients with RA. The three other phase III trials currently in progress have enrolled a total of about 1,400 patients. A submission to the Food and Drug Administration for marketing approval of sarilumab for patients with RA will await completion of these three additional studies, said Dr. Genovese, professor of medicine and cochief of the division of immunology and rheumatology at Stanford (Calif.) University.
The trial enrolled patients with RA and an inadequate response to methotrexate alone at more than 200 centers in more than 30 countries, including the United States. The study randomized patients to treatment with methotrexate plus 150 mg sarilumab administered as a subcutaneous injection every 2 weeks, 200 mg injected every 2 weeks, or placebo.
The study had three primary efficacy endpoints:
• The percentage of patients achieving an American College of Rheumatology 20 response after 24 weeks of treatment, which was achieved by 58% of 400 patients who received 150 mg sarilumab, 66% of 399 patients who received the 200-mg dose, and 33% of the 398 patients who received placebo injections.
• The average change from baseline in the Health Assessment Questionnaire disability index after 16 weeks of treatment, which fell by 0.53 in patients who received the 150-mg dose of sarilumab, by 0.55 in those who received 200-mg sarilumab injections, and by 0.29 in those who received placebo.
• The average change from baseline in the van der Heijde–modified total Sharp score after 52 weeks of treatment, which increased by 0.90 among patients on the lower sarilumab dose, increased by 0.25 in patients on the higher dose, and increased by 2.78 among patients who received placebo.
For all three endpoints, the differences between each of the sarilumab groups and the placebo controls were statistically significant as well as clinically meaningful. "We are encouraged by the potential of sarilumab to offer a new alternative in the treatment armamentarium for patients suffering from RA," Dr. Genovese said. When the two companies developing sarilumab submit their approval application, they will likely initially seek indications for treating adult patients with moderately to severely active RA who had an inadequate response to disease-modifying antirheumatic drugs (DMARDs) or tumor necrosis factor–alpha inhibitors, he predicted.
Serious adverse events during 52 weeks of follow-up occurred in 23 placebo patients, 38 patients on the lower sarilumab dose, and 46 on the higher dose. The most common serious adverse event was infection, which occurred in 10 patients on placebo, 11 patients on low-dose sarilumab, and 17 on the higher dose. No serious infections occurred in patients whose neutrophil level fell below 1,000/mcL.
"The number of serious adverse events thus far remains low, and it will be important to see the integrated safety data across the phase III program before making any judgments regarding the ideal dose and the benefit-to-safety risk ratio of any single dose," Dr. Genovese said.
"Among patients treated with sarilumab, a dose-dependent decrease in mean neutrophil counts was observed. Serious infections were not associated with grades 3 and 4 neutropenia in this study. Increases in mean LDL cholesterol level and transaminases were observed. These safety findings were consistent with those observed in prior investigational studies with sarilumab. The choice of doses will ultimately depend on the integrated safety data across the program and the performance of each dose across a broad range of patient populations," he said.
Sarilumab is the first agent tested from the class of interleukin (IL)-6 blockers. "IL-6 is a key proinflammatory cytokine that impacts inflammation, bone, and metabolism," Dr. Genovese noted.
"Blockade of IL-6 should provide significant improvements in all these areas. This study has demonstrated that inhibition of IL-6 can reduce the signs and symptoms of RA and help to stop the destruction of joints in patients with RA. Additional phase III studies of sarilumab are underway and should provide additional evidence that anti–IL-6 receptor therapy with sarilumab may be a future option for RA patients. It is possibly too early to say if there are any special advantages or opportunities from the strategy of IL-6 blockage, compared with any other class of biologic DMARD," he said.
The trial was sponsored by Sanofi and Regeneron, the companies developing the drug. Dr. Genovese said that he has been a consultant to and received research support from Sanofi. Several coauthors are employees of Sanofi or Regeneron.
On Twitter @mitchelzoler
AT THE EULAR CONGRESS 2014
Key clinical point: Treatment with the interleukin-6–blocking drug sarilumab showed efficacy and relative safety in the first reported results from a phase III trial.
Major finding: After 24 weeks, 58%-66% of patients on sarilumab had an ACR 20 response, compared with 33% of placebo patients.
Data source: The SARIL-RA-MOBILITY trial, which enrolled 1,197 patients at more than 200 international centers.
Disclosures: SARIL-RA-MOBILITY was sponsored by Sanofi and Regeneron, the companies developing the drug. Dr. Genovese said that he has been a consultant to and received research support from Sanofi. Several coauthors are employees of Sanofi or Regeneron.
Different triggers found for knee and hand OA
Mechanical stress is the most important underlying mechanism in osteoarthritis of the knees, while systemic processes may contribute most to hand osteoarthritis, Dutch researchers have found.
"How obesity results in osteoarthritis [OA] is not quite clear, but several mechanisms are thought to play a role, such as increased mechanical stress and systemic processes that are associated with adipose tissue," said senior study author Dr. Margreet Kloppenburg, professor of rheumatology at Leiden (the Netherlands) University Medical Center.
"Adipose tissue is known to be a source of active mediators, proinflammatory proteins and hormones, influencing inflammation, lipids, glucose metabolism, and insulin resistance," she said. "These systemic processes also could result in the development of OA. Our aim was to understand more about the link between obesity and OA because this insight could help us reach our ultimate goal: to improve treatment for OA patients."
Dr. Kloppenburg and her colleagues reviewed records from 6,628 participants in the Netherlands Epidemiology of Obesity (NEO) study, a population-based cohort of adults aged 45-65 years that is designed to investigate pathways leading to common diseases and conditions in overweight and obese individuals. Participants had a mean age of 56 years and a body mass index of 26 kg/m2; 56% were women. The estimated prevalence of OA was 10% in the knee, 8% in the hand, and 4% in both.
Researchers measured weight and fat mass and calculated fat-free mass. They also calculated odds ratios (ORs) to test for associations between surrogates for mechanical stress (weight and fat-free mass) and systemic processes (metabolic syndrome) with OA in knees alone, hands alone, or both knees and hands. Adjusted ORs were calculated for each OA type in three weight categories: less than 75 kg, 75-90 kg, and more than 90 kg.
After adjustment for metabolic factors, knee OA was significantly associated with weight (OR, 1.49) and fat-free mass (OR, 2.05). Similar results were observed for OA in both the knees and hands. In hand OA, however, investigators found the opposite: Hand OA was significantly associated with metabolic syndrome, independent of weight (OR, 1.46), and had no associations with weight and fat-free mass, Dr. Kloppenburg reported at the annual European Congress of Rheumatology.
"As we hypothesized, knee OA was predominantly associated with surrogates for mechanical stress, whereas hand OA was predominantly associated with surrogates for systemic processes," she said. "But what we had not expected was that surrogates for mechanical stress were predominantly associated with OA in both the knees and hands. This suggests that the co-occurrence of knee and hand OA may not be based on a common underlying pathogenic mechanism, but may represent the presence of two different types of OA."
Adjusted ORs for knee OA and for OA of both the knees and hands were greater in the higher weight categories, but, in hand OA, ORs did not increase with weight.
"Our study supports findings from clinical trials in obese patients with knee OA demonstrating that weight loss, together with exercise, which can potentially modify mechanical stress, is beneficial," she said. "It would be worthwhile to investigate whether weight loss also is beneficial in hand OA."
The NEO study is supported by the Dutch Arthritis Association, Leiden University Medical Center, and Leiden University. The investigators had no conflicts of interest to declare.
Mechanical stress is the most important underlying mechanism in osteoarthritis of the knees, while systemic processes may contribute most to hand osteoarthritis, Dutch researchers have found.
"How obesity results in osteoarthritis [OA] is not quite clear, but several mechanisms are thought to play a role, such as increased mechanical stress and systemic processes that are associated with adipose tissue," said senior study author Dr. Margreet Kloppenburg, professor of rheumatology at Leiden (the Netherlands) University Medical Center.
"Adipose tissue is known to be a source of active mediators, proinflammatory proteins and hormones, influencing inflammation, lipids, glucose metabolism, and insulin resistance," she said. "These systemic processes also could result in the development of OA. Our aim was to understand more about the link between obesity and OA because this insight could help us reach our ultimate goal: to improve treatment for OA patients."
Dr. Kloppenburg and her colleagues reviewed records from 6,628 participants in the Netherlands Epidemiology of Obesity (NEO) study, a population-based cohort of adults aged 45-65 years that is designed to investigate pathways leading to common diseases and conditions in overweight and obese individuals. Participants had a mean age of 56 years and a body mass index of 26 kg/m2; 56% were women. The estimated prevalence of OA was 10% in the knee, 8% in the hand, and 4% in both.
Researchers measured weight and fat mass and calculated fat-free mass. They also calculated odds ratios (ORs) to test for associations between surrogates for mechanical stress (weight and fat-free mass) and systemic processes (metabolic syndrome) with OA in knees alone, hands alone, or both knees and hands. Adjusted ORs were calculated for each OA type in three weight categories: less than 75 kg, 75-90 kg, and more than 90 kg.
After adjustment for metabolic factors, knee OA was significantly associated with weight (OR, 1.49) and fat-free mass (OR, 2.05). Similar results were observed for OA in both the knees and hands. In hand OA, however, investigators found the opposite: Hand OA was significantly associated with metabolic syndrome, independent of weight (OR, 1.46), and had no associations with weight and fat-free mass, Dr. Kloppenburg reported at the annual European Congress of Rheumatology.
"As we hypothesized, knee OA was predominantly associated with surrogates for mechanical stress, whereas hand OA was predominantly associated with surrogates for systemic processes," she said. "But what we had not expected was that surrogates for mechanical stress were predominantly associated with OA in both the knees and hands. This suggests that the co-occurrence of knee and hand OA may not be based on a common underlying pathogenic mechanism, but may represent the presence of two different types of OA."
Adjusted ORs for knee OA and for OA of both the knees and hands were greater in the higher weight categories, but, in hand OA, ORs did not increase with weight.
"Our study supports findings from clinical trials in obese patients with knee OA demonstrating that weight loss, together with exercise, which can potentially modify mechanical stress, is beneficial," she said. "It would be worthwhile to investigate whether weight loss also is beneficial in hand OA."
The NEO study is supported by the Dutch Arthritis Association, Leiden University Medical Center, and Leiden University. The investigators had no conflicts of interest to declare.
Mechanical stress is the most important underlying mechanism in osteoarthritis of the knees, while systemic processes may contribute most to hand osteoarthritis, Dutch researchers have found.
"How obesity results in osteoarthritis [OA] is not quite clear, but several mechanisms are thought to play a role, such as increased mechanical stress and systemic processes that are associated with adipose tissue," said senior study author Dr. Margreet Kloppenburg, professor of rheumatology at Leiden (the Netherlands) University Medical Center.
"Adipose tissue is known to be a source of active mediators, proinflammatory proteins and hormones, influencing inflammation, lipids, glucose metabolism, and insulin resistance," she said. "These systemic processes also could result in the development of OA. Our aim was to understand more about the link between obesity and OA because this insight could help us reach our ultimate goal: to improve treatment for OA patients."
Dr. Kloppenburg and her colleagues reviewed records from 6,628 participants in the Netherlands Epidemiology of Obesity (NEO) study, a population-based cohort of adults aged 45-65 years that is designed to investigate pathways leading to common diseases and conditions in overweight and obese individuals. Participants had a mean age of 56 years and a body mass index of 26 kg/m2; 56% were women. The estimated prevalence of OA was 10% in the knee, 8% in the hand, and 4% in both.
Researchers measured weight and fat mass and calculated fat-free mass. They also calculated odds ratios (ORs) to test for associations between surrogates for mechanical stress (weight and fat-free mass) and systemic processes (metabolic syndrome) with OA in knees alone, hands alone, or both knees and hands. Adjusted ORs were calculated for each OA type in three weight categories: less than 75 kg, 75-90 kg, and more than 90 kg.
After adjustment for metabolic factors, knee OA was significantly associated with weight (OR, 1.49) and fat-free mass (OR, 2.05). Similar results were observed for OA in both the knees and hands. In hand OA, however, investigators found the opposite: Hand OA was significantly associated with metabolic syndrome, independent of weight (OR, 1.46), and had no associations with weight and fat-free mass, Dr. Kloppenburg reported at the annual European Congress of Rheumatology.
"As we hypothesized, knee OA was predominantly associated with surrogates for mechanical stress, whereas hand OA was predominantly associated with surrogates for systemic processes," she said. "But what we had not expected was that surrogates for mechanical stress were predominantly associated with OA in both the knees and hands. This suggests that the co-occurrence of knee and hand OA may not be based on a common underlying pathogenic mechanism, but may represent the presence of two different types of OA."
Adjusted ORs for knee OA and for OA of both the knees and hands were greater in the higher weight categories, but, in hand OA, ORs did not increase with weight.
"Our study supports findings from clinical trials in obese patients with knee OA demonstrating that weight loss, together with exercise, which can potentially modify mechanical stress, is beneficial," she said. "It would be worthwhile to investigate whether weight loss also is beneficial in hand OA."
The NEO study is supported by the Dutch Arthritis Association, Leiden University Medical Center, and Leiden University. The investigators had no conflicts of interest to declare.
FROM THE EULAR CONGRESS 2014
Key clinical point: Weight loss may be a primary target for improving knee OA, but improvements in metabolic factors may most benefit hand OA.
Major finding: Hand OA was significantly associated with metabolic syndrome, independent of weight (OR, 1.46), and knee OA was significantly associated with weight (OR, 1.49) and fat-free mass (OR, 2.05), after adjustment for metabolic factors.
Data source: A population-based study of 6,628 individuals who were monitored for the development of hand or knee OA.
Disclosures: The NEO study is supported by the Dutch Arthritis Association, Leiden University Medical Center, and Leiden University. The investigators had no conflicts of interest to declare.