User login
Society for Pediatric Dermatology (SPD): Annual Meeting
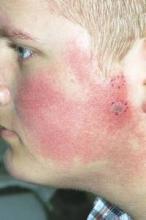
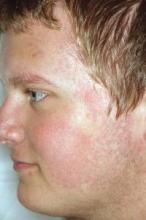
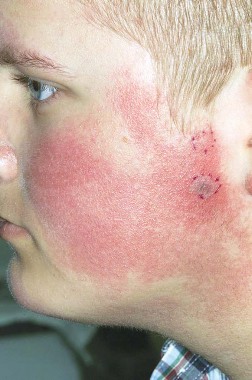
Pulsed dye laser targets keratosis pilaris rubra
MILWAUKEE – Pulsed dye laser therapy reduced the redness associated with chronic keratosis pilaris rubra in as little as one session in a case series of seven adolescents.
The problem, however, is convincing insurers to pay for the procedure.
"That’s part of why we brought this to a forum like this meeting," Dr. Jennifer J. Schoch said at the annual meeting of the Society for Pediatric Dermatology. "These kids are so significantly affected by this and embarrassed [by the condition], that if we can do something in just one treatment and have a good response, it makes sense. But these kids required a lot of letters to the insurance companies, and a lot of them paid out of pocket."
Several attendees at the meeting echoed these comments, and some observed that pulse dye laser (PDL) therapy is not as effective in patients with higher Fitzpatrick skin types or that it was out of reach for their patients at a price tag of $200 or more per session.
"It seems to make a lot of sense, but I don’t think it would be worth the cost for my patients," Dr. Aimee Smidt, University of New Mexico, Albuquerque, said in an interview.
Keratosis pilaris rubra is often viewed as a benign condition, but one patient found it so socially disturbing that he flew from Alabama to Minnesota for treatment, said Dr. Schoch, a dermatologist at the Mayo Clinic in Rochester, Minn.
Dr. Schoch noted that there are few data in the literature on PDL treatment of keratosis pilaris rubra, and the current series is occurring over a 13-year-period at the clinic. In this series, adolescents aged 14-17 years received one to four sessions of PDL at a wavelength of 585 nm or 595 nm, for erythema and hyperkeratotic follicular papules on both cheeks. Two patients also received treatment to the chin, forehead and/or neck.
All patients had Fitzpatrick skin type I or II, five were male, and two also had ulerythema ophryogenes. Some patients had been misdiagnosed with severe acne, and all had failed a range of treatments including emollients, lactic acid, topical retinoids, urea, sulfacetamide lotion, and weak topical corticosteroids, as well as laser therapy with intense pulsed light, Fraxel, and Nd:YAG lasers, she said.
PDL treatment was physician-dependent. Starting fluences ranged from 4-9 J/cm2, with the goal of achieving a mild, bruiselike response. The spot size was predominantly 7 mm, and pulse duration was 1.5, 3, or 10 msec.
"I hypothesize that people are probably undertreating because it’s a benign condition, and you don’t want to cause problems, but to be effective, it seems you have to go for a little more aggressive response," Dr. Schoch said in an interview.
All patients experienced significant improvement after one to four treatments, based on patient report or provider assessment. Bruising resolved in 1-2 weeks. Resolution of erythema was observed, but not specifically measured. Patients also experienced some transient purpura, which was not well documented, said Dr. Schoch.
After 1-19 months follow-up, most patients remained satisfied with the results, although some flushing returned in two patients, she said. Blanching has not been a significant problem, and overlapping the treated areas reduced the risk of a honeycomb pattern developing on the skin.
The investigators are considering a prospective study to more objectively monitor responses. Treatment parameters will depend on patient’s response to test spots, Dr. Schoch said.
Dr. Schoch and her coauthors reported having no financial disclosures.
MILWAUKEE – Pulsed dye laser therapy reduced the redness associated with chronic keratosis pilaris rubra in as little as one session in a case series of seven adolescents.
The problem, however, is convincing insurers to pay for the procedure.
"That’s part of why we brought this to a forum like this meeting," Dr. Jennifer J. Schoch said at the annual meeting of the Society for Pediatric Dermatology. "These kids are so significantly affected by this and embarrassed [by the condition], that if we can do something in just one treatment and have a good response, it makes sense. But these kids required a lot of letters to the insurance companies, and a lot of them paid out of pocket."
Several attendees at the meeting echoed these comments, and some observed that pulse dye laser (PDL) therapy is not as effective in patients with higher Fitzpatrick skin types or that it was out of reach for their patients at a price tag of $200 or more per session.
"It seems to make a lot of sense, but I don’t think it would be worth the cost for my patients," Dr. Aimee Smidt, University of New Mexico, Albuquerque, said in an interview.
Keratosis pilaris rubra is often viewed as a benign condition, but one patient found it so socially disturbing that he flew from Alabama to Minnesota for treatment, said Dr. Schoch, a dermatologist at the Mayo Clinic in Rochester, Minn.
Dr. Schoch noted that there are few data in the literature on PDL treatment of keratosis pilaris rubra, and the current series is occurring over a 13-year-period at the clinic. In this series, adolescents aged 14-17 years received one to four sessions of PDL at a wavelength of 585 nm or 595 nm, for erythema and hyperkeratotic follicular papules on both cheeks. Two patients also received treatment to the chin, forehead and/or neck.
All patients had Fitzpatrick skin type I or II, five were male, and two also had ulerythema ophryogenes. Some patients had been misdiagnosed with severe acne, and all had failed a range of treatments including emollients, lactic acid, topical retinoids, urea, sulfacetamide lotion, and weak topical corticosteroids, as well as laser therapy with intense pulsed light, Fraxel, and Nd:YAG lasers, she said.
PDL treatment was physician-dependent. Starting fluences ranged from 4-9 J/cm2, with the goal of achieving a mild, bruiselike response. The spot size was predominantly 7 mm, and pulse duration was 1.5, 3, or 10 msec.
"I hypothesize that people are probably undertreating because it’s a benign condition, and you don’t want to cause problems, but to be effective, it seems you have to go for a little more aggressive response," Dr. Schoch said in an interview.
All patients experienced significant improvement after one to four treatments, based on patient report or provider assessment. Bruising resolved in 1-2 weeks. Resolution of erythema was observed, but not specifically measured. Patients also experienced some transient purpura, which was not well documented, said Dr. Schoch.
After 1-19 months follow-up, most patients remained satisfied with the results, although some flushing returned in two patients, she said. Blanching has not been a significant problem, and overlapping the treated areas reduced the risk of a honeycomb pattern developing on the skin.
The investigators are considering a prospective study to more objectively monitor responses. Treatment parameters will depend on patient’s response to test spots, Dr. Schoch said.
Dr. Schoch and her coauthors reported having no financial disclosures.
MILWAUKEE – Pulsed dye laser therapy reduced the redness associated with chronic keratosis pilaris rubra in as little as one session in a case series of seven adolescents.
The problem, however, is convincing insurers to pay for the procedure.
"That’s part of why we brought this to a forum like this meeting," Dr. Jennifer J. Schoch said at the annual meeting of the Society for Pediatric Dermatology. "These kids are so significantly affected by this and embarrassed [by the condition], that if we can do something in just one treatment and have a good response, it makes sense. But these kids required a lot of letters to the insurance companies, and a lot of them paid out of pocket."
Several attendees at the meeting echoed these comments, and some observed that pulse dye laser (PDL) therapy is not as effective in patients with higher Fitzpatrick skin types or that it was out of reach for their patients at a price tag of $200 or more per session.
"It seems to make a lot of sense, but I don’t think it would be worth the cost for my patients," Dr. Aimee Smidt, University of New Mexico, Albuquerque, said in an interview.
Keratosis pilaris rubra is often viewed as a benign condition, but one patient found it so socially disturbing that he flew from Alabama to Minnesota for treatment, said Dr. Schoch, a dermatologist at the Mayo Clinic in Rochester, Minn.
Dr. Schoch noted that there are few data in the literature on PDL treatment of keratosis pilaris rubra, and the current series is occurring over a 13-year-period at the clinic. In this series, adolescents aged 14-17 years received one to four sessions of PDL at a wavelength of 585 nm or 595 nm, for erythema and hyperkeratotic follicular papules on both cheeks. Two patients also received treatment to the chin, forehead and/or neck.
All patients had Fitzpatrick skin type I or II, five were male, and two also had ulerythema ophryogenes. Some patients had been misdiagnosed with severe acne, and all had failed a range of treatments including emollients, lactic acid, topical retinoids, urea, sulfacetamide lotion, and weak topical corticosteroids, as well as laser therapy with intense pulsed light, Fraxel, and Nd:YAG lasers, she said.
PDL treatment was physician-dependent. Starting fluences ranged from 4-9 J/cm2, with the goal of achieving a mild, bruiselike response. The spot size was predominantly 7 mm, and pulse duration was 1.5, 3, or 10 msec.
"I hypothesize that people are probably undertreating because it’s a benign condition, and you don’t want to cause problems, but to be effective, it seems you have to go for a little more aggressive response," Dr. Schoch said in an interview.
All patients experienced significant improvement after one to four treatments, based on patient report or provider assessment. Bruising resolved in 1-2 weeks. Resolution of erythema was observed, but not specifically measured. Patients also experienced some transient purpura, which was not well documented, said Dr. Schoch.
After 1-19 months follow-up, most patients remained satisfied with the results, although some flushing returned in two patients, she said. Blanching has not been a significant problem, and overlapping the treated areas reduced the risk of a honeycomb pattern developing on the skin.
The investigators are considering a prospective study to more objectively monitor responses. Treatment parameters will depend on patient’s response to test spots, Dr. Schoch said.
Dr. Schoch and her coauthors reported having no financial disclosures.
AT THE SPD ANNUAL MEETING
Epidermolysis bullosa patients rate itching worse than pain
MILWAUKEE – Itching is more problematic than is pain for patients with epidermolysis bullosa, according to an online survey of 146 patients.
Symptoms reported in conjunction with itching included pain, stinging, burning, and a sensation of ants crawling on the skin, as well as a feeling that the itch was too deep to scratch, Christina Danial and her associates reported at the annual meeting of the Society for Pediatric Dermatology.
Although data from a prior study of 11 children with epidermolysis bullosa (EB) hinted that pruritus is more bothersome than pain is (Acta. Derm. Venereol. 2008;88:143-50), the results are nonetheless startling given that EB is a mutilating disease of the skin and mucosa, Ms. Danial said. Patients with this rare condition cope with blistering of the skin and/or epithelial lining of the organs, GI complications, anemia, and shortened survival due to infection or cancer.
In this study, 216 EB patients and/or caregivers registered in the Epidermolysis Bullosa Clinical Research Consortium received a 42-item online questionnaire, and 146 completed the survey. Responses were based on a 5-point Likert scale in which 1 was "never" and 5 was "always."
Itchiness was rated the most bothersome item (mean 3.3), followed by acute pain (mean 2.9), chronic pain (mean 2.7), problems eating (mean 2.7), stomach problems (mean 2.6), dental problems (mean 2.6), movement (2.5), and surgical procedures (mean 2.4), reported Ms. Danial, a medical student at Stanford (Calif.) University.
Patients with the more severe recessive dystrophic EB subtype had significantly more pruritus than did those with EB simplex (mean 3.9 vs. 3.1; P = .01).
The frequency of itching increased as the day progressed, with bedtime having the highest frequency (mean 3.8). Not surprisingly, itching was found to interfere with sleep (mean 3.1), she noted.
Sweating (mean 3.9) and stress (mean 4.0) increased itching, as did dryness (mean 4.0), heat (mean 3.8), and humidity (mean 3.5).
The 146 respondents comprised 90 patients, 36 caregivers, and 20 patient/caregivers. The average age of the respondents was 22 years; 73 were male and 73 were female.
EB patients reported that pruritus makes them feel frustrated, agitated, and as if they cannot control the itch, although activity was noted to relieve the itching, Ms. Danial reported.
Ms. Danial suggested that further investigation is necessary to determine which treatments are most effective against itching in EB, but recommended that patients avoid dryness, heat, and humidity, and consider nighttime treatment. Treatments to control pain also may help alleviate pruritus, since patients may experience pain while itching, she added.
In all, 66% of patients had dystrophic EB, 21% had EB simplex, 10% had junctional EB, and the EB subtype of 3% was unknown. Severe disease was reported in 38% of those with recessive dystrophic EB, 14% with dominant dystrophic EB, 17% with unknown dystrophic subtype, 3% with EB simplex, 36% with junctional EB, and none with unknown EB.
Ms. Danial and her coauthors reported having no financial disclosures.
MILWAUKEE – Itching is more problematic than is pain for patients with epidermolysis bullosa, according to an online survey of 146 patients.
Symptoms reported in conjunction with itching included pain, stinging, burning, and a sensation of ants crawling on the skin, as well as a feeling that the itch was too deep to scratch, Christina Danial and her associates reported at the annual meeting of the Society for Pediatric Dermatology.
Although data from a prior study of 11 children with epidermolysis bullosa (EB) hinted that pruritus is more bothersome than pain is (Acta. Derm. Venereol. 2008;88:143-50), the results are nonetheless startling given that EB is a mutilating disease of the skin and mucosa, Ms. Danial said. Patients with this rare condition cope with blistering of the skin and/or epithelial lining of the organs, GI complications, anemia, and shortened survival due to infection or cancer.
In this study, 216 EB patients and/or caregivers registered in the Epidermolysis Bullosa Clinical Research Consortium received a 42-item online questionnaire, and 146 completed the survey. Responses were based on a 5-point Likert scale in which 1 was "never" and 5 was "always."
Itchiness was rated the most bothersome item (mean 3.3), followed by acute pain (mean 2.9), chronic pain (mean 2.7), problems eating (mean 2.7), stomach problems (mean 2.6), dental problems (mean 2.6), movement (2.5), and surgical procedures (mean 2.4), reported Ms. Danial, a medical student at Stanford (Calif.) University.
Patients with the more severe recessive dystrophic EB subtype had significantly more pruritus than did those with EB simplex (mean 3.9 vs. 3.1; P = .01).
The frequency of itching increased as the day progressed, with bedtime having the highest frequency (mean 3.8). Not surprisingly, itching was found to interfere with sleep (mean 3.1), she noted.
Sweating (mean 3.9) and stress (mean 4.0) increased itching, as did dryness (mean 4.0), heat (mean 3.8), and humidity (mean 3.5).
The 146 respondents comprised 90 patients, 36 caregivers, and 20 patient/caregivers. The average age of the respondents was 22 years; 73 were male and 73 were female.
EB patients reported that pruritus makes them feel frustrated, agitated, and as if they cannot control the itch, although activity was noted to relieve the itching, Ms. Danial reported.
Ms. Danial suggested that further investigation is necessary to determine which treatments are most effective against itching in EB, but recommended that patients avoid dryness, heat, and humidity, and consider nighttime treatment. Treatments to control pain also may help alleviate pruritus, since patients may experience pain while itching, she added.
In all, 66% of patients had dystrophic EB, 21% had EB simplex, 10% had junctional EB, and the EB subtype of 3% was unknown. Severe disease was reported in 38% of those with recessive dystrophic EB, 14% with dominant dystrophic EB, 17% with unknown dystrophic subtype, 3% with EB simplex, 36% with junctional EB, and none with unknown EB.
Ms. Danial and her coauthors reported having no financial disclosures.
MILWAUKEE – Itching is more problematic than is pain for patients with epidermolysis bullosa, according to an online survey of 146 patients.
Symptoms reported in conjunction with itching included pain, stinging, burning, and a sensation of ants crawling on the skin, as well as a feeling that the itch was too deep to scratch, Christina Danial and her associates reported at the annual meeting of the Society for Pediatric Dermatology.
Although data from a prior study of 11 children with epidermolysis bullosa (EB) hinted that pruritus is more bothersome than pain is (Acta. Derm. Venereol. 2008;88:143-50), the results are nonetheless startling given that EB is a mutilating disease of the skin and mucosa, Ms. Danial said. Patients with this rare condition cope with blistering of the skin and/or epithelial lining of the organs, GI complications, anemia, and shortened survival due to infection or cancer.
In this study, 216 EB patients and/or caregivers registered in the Epidermolysis Bullosa Clinical Research Consortium received a 42-item online questionnaire, and 146 completed the survey. Responses were based on a 5-point Likert scale in which 1 was "never" and 5 was "always."
Itchiness was rated the most bothersome item (mean 3.3), followed by acute pain (mean 2.9), chronic pain (mean 2.7), problems eating (mean 2.7), stomach problems (mean 2.6), dental problems (mean 2.6), movement (2.5), and surgical procedures (mean 2.4), reported Ms. Danial, a medical student at Stanford (Calif.) University.
Patients with the more severe recessive dystrophic EB subtype had significantly more pruritus than did those with EB simplex (mean 3.9 vs. 3.1; P = .01).
The frequency of itching increased as the day progressed, with bedtime having the highest frequency (mean 3.8). Not surprisingly, itching was found to interfere with sleep (mean 3.1), she noted.
Sweating (mean 3.9) and stress (mean 4.0) increased itching, as did dryness (mean 4.0), heat (mean 3.8), and humidity (mean 3.5).
The 146 respondents comprised 90 patients, 36 caregivers, and 20 patient/caregivers. The average age of the respondents was 22 years; 73 were male and 73 were female.
EB patients reported that pruritus makes them feel frustrated, agitated, and as if they cannot control the itch, although activity was noted to relieve the itching, Ms. Danial reported.
Ms. Danial suggested that further investigation is necessary to determine which treatments are most effective against itching in EB, but recommended that patients avoid dryness, heat, and humidity, and consider nighttime treatment. Treatments to control pain also may help alleviate pruritus, since patients may experience pain while itching, she added.
In all, 66% of patients had dystrophic EB, 21% had EB simplex, 10% had junctional EB, and the EB subtype of 3% was unknown. Severe disease was reported in 38% of those with recessive dystrophic EB, 14% with dominant dystrophic EB, 17% with unknown dystrophic subtype, 3% with EB simplex, 36% with junctional EB, and none with unknown EB.
Ms. Danial and her coauthors reported having no financial disclosures.
AT THE SPD ANNUAL MEETING
Major finding: Itchiness was rated the most bothersome symptom (mean 3.3 on a 5-point scale), followed by acute pain (mean 2.9), chronic pain (mean 2.7), and problems eating (mean 2.7).
Data source: Online survey of 146 patients with epidermolysis bullosa and/or their caregivers.
Disclosures: Ms. Danial and her coauthors reported having no financial disclosures.
The chicken or the egg: Obesity or psoriasis?
MILWAUKEE – Excess adiposity occurred prior to psoriasis in 93% of 29 overweight and obese psoriatic children, in a chart review examining the relationship between psoriasis and obesity.
Further, 78% of patients were obese before developing psoriasis, Dr. Lauren Becker reported at the annual meeting of the Society for Pediatric Dermatology.
Although the authors anticipated that most of the children would show increased adiposity before psoriasis based on clinical observations, "none of us predicted that it would be 27 of 29," she said in an interview. "It really was incredible, and even the ones who were normal weight became overweight or obese within a year of their psoriasis."
The review was sparked by a recent international study, led by senior author Dr. Amy Paller of Northwestern University in Chicago, in which 38% of psoriatic children had excess central adiposity (high waist-to-height ratio) compared with 21% of controls. The odds of obesity were also several times higher than those reported for adults with psoriasis, jumping 4.29-fold overall in psoriatic children versus controls, 4.92-fold in those with severe versus mild psoriasis, and 7.6-fold in the United States. (JAMA Dermatol. 2013;149:166-76).
Although other studies in children support an association between obesity and psoriasis, the international study was the first to measure central adiposity, a more sensitive indicator of cardiovascular risk in children than BMI, Dr. Kelly Cordoro remarked in a separate lecture on the comorbidities of pediatric psoriasis at the meeting.
"Adiposity, hypertension, hyperlipidemia, and diabetes are increased in prevalence among pediatric psoriasis patients, but the obesity association is the strongest," she said. "It’s global, and the effect is most pronounced in the United States, where in particular, central obesity is highest and has the accompanying higher cardiovascular risk."
Previous research has shown that obesity is strongly correlated with psoriasis in adults. A recent meta-analysis showed that psoriatic adults were more likely to be obese than those without psoriasis (pooled odds ratio, 1.66), and that the odds were even higher in patients with severe versus mild psoriasis (OR, 2.23, vs. OR, 1.46) (Nutr. Diabetes 2012 ;2:e54).
Both clinicians observed that psoriasis and obesity are chronic inflammatory states, marked by overexpression of circulating proinflammatory cytokines derived from Th1 and Th17 lymphocyte subsets, as well as tumor necrosis factor–alpha and adipokines. In addition, a fat cell is a microenvironment of inflammation, with adipose tissue releasing proinflammatory cytokines such as interleukin-6 and TNF-alpha.
The two diseases drive one another, but the exact relationship remains unclear, said Dr. Becker, a pediatrician and dermatology fellow at Northwestern at the time of the study. In addition, the impact on quality of life and social interactions of being both obese or overweight and having psoriasis may predispose patients to mental and physical health problems.
"Children with excess adiposity or with the highly visible lesions of psoriasis are more often teased or bullied, which may contribute to the tendency to become socially isolated, decrease physical activity, and increase eating," she said. "It really is a vicious circle."
Although the data are clear that psoriasis and obesity are associated, the current chart review suggesting that obesity precedes psoriasis is too small to definitively answer the question, Dr. Cordoro, a pediatric dermatologist at the University of California, San Francisco, said in an interview.
Dr. Becker said obesity clearly came before psoriasis in their study cohort, but agreed that further study is needed to confirm the results. She also stressed the need for biomarker analyses to identify overweight/obese children who are at risk for psoriasis, and studies to address whether weight loss can reverse the severity of pediatric psoriasis.
For example, data from a study of overweight psoriatic adults showed that eating a low-calorie diet improved psoriasis severity and quality of life after 4 months (JAMA Dermatol. 2013;149:795-801).
However, in the absence of adequate data, clinicians should consider metabolic testing to determine whether their overweight pediatric patients are on the path to the metabolic syndrome, Dr. Becker and Dr. Cordoro suggested. Metabolic testing was not performed on any of the 29 children in the chart review, although 48% had a family history of hyperlipidemia and 45% had a family history of obesity.
The average age of the children was 12.6 years; the average age of onset of obesity was 4 years (range, 2-12 years), and the average age of onset of psoriasis was 9 years (range, 2-14 years).
Two obese patients were able to reduce their BMIs from obese to overweight status 1 year after being diagnosed with psoriasis; however, both have remained in the 85th-95th BMI percentile for more than 2 years, Dr. Becker said. Both of the children who had a normal BMI prior to their psoriasis had a BMI in the overweight or obese range within 1 year after diagnosis, Dr. Becker noted.
Dr. Cordoro also made an impassioned plea for clinicians to address the significant psychosocial comorbidity present in psoriatic children.
"You can think about obesity, psoriasis, and depression as being reciprocal exacerbating factors, such that each triggers the other and represents an insult to self-esteem and the overall well-being of these patients," she said. "They end up having high stress levels and really dismal quality of life. These issues are as important in the management of the child as the medical considerations."
Dr. Becker, her coauthors, and Dr. Cordoro reported having no relevant financial disclosures.
MILWAUKEE – Excess adiposity occurred prior to psoriasis in 93% of 29 overweight and obese psoriatic children, in a chart review examining the relationship between psoriasis and obesity.
Further, 78% of patients were obese before developing psoriasis, Dr. Lauren Becker reported at the annual meeting of the Society for Pediatric Dermatology.
Although the authors anticipated that most of the children would show increased adiposity before psoriasis based on clinical observations, "none of us predicted that it would be 27 of 29," she said in an interview. "It really was incredible, and even the ones who were normal weight became overweight or obese within a year of their psoriasis."
The review was sparked by a recent international study, led by senior author Dr. Amy Paller of Northwestern University in Chicago, in which 38% of psoriatic children had excess central adiposity (high waist-to-height ratio) compared with 21% of controls. The odds of obesity were also several times higher than those reported for adults with psoriasis, jumping 4.29-fold overall in psoriatic children versus controls, 4.92-fold in those with severe versus mild psoriasis, and 7.6-fold in the United States. (JAMA Dermatol. 2013;149:166-76).
Although other studies in children support an association between obesity and psoriasis, the international study was the first to measure central adiposity, a more sensitive indicator of cardiovascular risk in children than BMI, Dr. Kelly Cordoro remarked in a separate lecture on the comorbidities of pediatric psoriasis at the meeting.
"Adiposity, hypertension, hyperlipidemia, and diabetes are increased in prevalence among pediatric psoriasis patients, but the obesity association is the strongest," she said. "It’s global, and the effect is most pronounced in the United States, where in particular, central obesity is highest and has the accompanying higher cardiovascular risk."
Previous research has shown that obesity is strongly correlated with psoriasis in adults. A recent meta-analysis showed that psoriatic adults were more likely to be obese than those without psoriasis (pooled odds ratio, 1.66), and that the odds were even higher in patients with severe versus mild psoriasis (OR, 2.23, vs. OR, 1.46) (Nutr. Diabetes 2012 ;2:e54).
Both clinicians observed that psoriasis and obesity are chronic inflammatory states, marked by overexpression of circulating proinflammatory cytokines derived from Th1 and Th17 lymphocyte subsets, as well as tumor necrosis factor–alpha and adipokines. In addition, a fat cell is a microenvironment of inflammation, with adipose tissue releasing proinflammatory cytokines such as interleukin-6 and TNF-alpha.
The two diseases drive one another, but the exact relationship remains unclear, said Dr. Becker, a pediatrician and dermatology fellow at Northwestern at the time of the study. In addition, the impact on quality of life and social interactions of being both obese or overweight and having psoriasis may predispose patients to mental and physical health problems.
"Children with excess adiposity or with the highly visible lesions of psoriasis are more often teased or bullied, which may contribute to the tendency to become socially isolated, decrease physical activity, and increase eating," she said. "It really is a vicious circle."
Although the data are clear that psoriasis and obesity are associated, the current chart review suggesting that obesity precedes psoriasis is too small to definitively answer the question, Dr. Cordoro, a pediatric dermatologist at the University of California, San Francisco, said in an interview.
Dr. Becker said obesity clearly came before psoriasis in their study cohort, but agreed that further study is needed to confirm the results. She also stressed the need for biomarker analyses to identify overweight/obese children who are at risk for psoriasis, and studies to address whether weight loss can reverse the severity of pediatric psoriasis.
For example, data from a study of overweight psoriatic adults showed that eating a low-calorie diet improved psoriasis severity and quality of life after 4 months (JAMA Dermatol. 2013;149:795-801).
However, in the absence of adequate data, clinicians should consider metabolic testing to determine whether their overweight pediatric patients are on the path to the metabolic syndrome, Dr. Becker and Dr. Cordoro suggested. Metabolic testing was not performed on any of the 29 children in the chart review, although 48% had a family history of hyperlipidemia and 45% had a family history of obesity.
The average age of the children was 12.6 years; the average age of onset of obesity was 4 years (range, 2-12 years), and the average age of onset of psoriasis was 9 years (range, 2-14 years).
Two obese patients were able to reduce their BMIs from obese to overweight status 1 year after being diagnosed with psoriasis; however, both have remained in the 85th-95th BMI percentile for more than 2 years, Dr. Becker said. Both of the children who had a normal BMI prior to their psoriasis had a BMI in the overweight or obese range within 1 year after diagnosis, Dr. Becker noted.
Dr. Cordoro also made an impassioned plea for clinicians to address the significant psychosocial comorbidity present in psoriatic children.
"You can think about obesity, psoriasis, and depression as being reciprocal exacerbating factors, such that each triggers the other and represents an insult to self-esteem and the overall well-being of these patients," she said. "They end up having high stress levels and really dismal quality of life. These issues are as important in the management of the child as the medical considerations."
Dr. Becker, her coauthors, and Dr. Cordoro reported having no relevant financial disclosures.
MILWAUKEE – Excess adiposity occurred prior to psoriasis in 93% of 29 overweight and obese psoriatic children, in a chart review examining the relationship between psoriasis and obesity.
Further, 78% of patients were obese before developing psoriasis, Dr. Lauren Becker reported at the annual meeting of the Society for Pediatric Dermatology.
Although the authors anticipated that most of the children would show increased adiposity before psoriasis based on clinical observations, "none of us predicted that it would be 27 of 29," she said in an interview. "It really was incredible, and even the ones who were normal weight became overweight or obese within a year of their psoriasis."
The review was sparked by a recent international study, led by senior author Dr. Amy Paller of Northwestern University in Chicago, in which 38% of psoriatic children had excess central adiposity (high waist-to-height ratio) compared with 21% of controls. The odds of obesity were also several times higher than those reported for adults with psoriasis, jumping 4.29-fold overall in psoriatic children versus controls, 4.92-fold in those with severe versus mild psoriasis, and 7.6-fold in the United States. (JAMA Dermatol. 2013;149:166-76).
Although other studies in children support an association between obesity and psoriasis, the international study was the first to measure central adiposity, a more sensitive indicator of cardiovascular risk in children than BMI, Dr. Kelly Cordoro remarked in a separate lecture on the comorbidities of pediatric psoriasis at the meeting.
"Adiposity, hypertension, hyperlipidemia, and diabetes are increased in prevalence among pediatric psoriasis patients, but the obesity association is the strongest," she said. "It’s global, and the effect is most pronounced in the United States, where in particular, central obesity is highest and has the accompanying higher cardiovascular risk."
Previous research has shown that obesity is strongly correlated with psoriasis in adults. A recent meta-analysis showed that psoriatic adults were more likely to be obese than those without psoriasis (pooled odds ratio, 1.66), and that the odds were even higher in patients with severe versus mild psoriasis (OR, 2.23, vs. OR, 1.46) (Nutr. Diabetes 2012 ;2:e54).
Both clinicians observed that psoriasis and obesity are chronic inflammatory states, marked by overexpression of circulating proinflammatory cytokines derived from Th1 and Th17 lymphocyte subsets, as well as tumor necrosis factor–alpha and adipokines. In addition, a fat cell is a microenvironment of inflammation, with adipose tissue releasing proinflammatory cytokines such as interleukin-6 and TNF-alpha.
The two diseases drive one another, but the exact relationship remains unclear, said Dr. Becker, a pediatrician and dermatology fellow at Northwestern at the time of the study. In addition, the impact on quality of life and social interactions of being both obese or overweight and having psoriasis may predispose patients to mental and physical health problems.
"Children with excess adiposity or with the highly visible lesions of psoriasis are more often teased or bullied, which may contribute to the tendency to become socially isolated, decrease physical activity, and increase eating," she said. "It really is a vicious circle."
Although the data are clear that psoriasis and obesity are associated, the current chart review suggesting that obesity precedes psoriasis is too small to definitively answer the question, Dr. Cordoro, a pediatric dermatologist at the University of California, San Francisco, said in an interview.
Dr. Becker said obesity clearly came before psoriasis in their study cohort, but agreed that further study is needed to confirm the results. She also stressed the need for biomarker analyses to identify overweight/obese children who are at risk for psoriasis, and studies to address whether weight loss can reverse the severity of pediatric psoriasis.
For example, data from a study of overweight psoriatic adults showed that eating a low-calorie diet improved psoriasis severity and quality of life after 4 months (JAMA Dermatol. 2013;149:795-801).
However, in the absence of adequate data, clinicians should consider metabolic testing to determine whether their overweight pediatric patients are on the path to the metabolic syndrome, Dr. Becker and Dr. Cordoro suggested. Metabolic testing was not performed on any of the 29 children in the chart review, although 48% had a family history of hyperlipidemia and 45% had a family history of obesity.
The average age of the children was 12.6 years; the average age of onset of obesity was 4 years (range, 2-12 years), and the average age of onset of psoriasis was 9 years (range, 2-14 years).
Two obese patients were able to reduce their BMIs from obese to overweight status 1 year after being diagnosed with psoriasis; however, both have remained in the 85th-95th BMI percentile for more than 2 years, Dr. Becker said. Both of the children who had a normal BMI prior to their psoriasis had a BMI in the overweight or obese range within 1 year after diagnosis, Dr. Becker noted.
Dr. Cordoro also made an impassioned plea for clinicians to address the significant psychosocial comorbidity present in psoriatic children.
"You can think about obesity, psoriasis, and depression as being reciprocal exacerbating factors, such that each triggers the other and represents an insult to self-esteem and the overall well-being of these patients," she said. "They end up having high stress levels and really dismal quality of life. These issues are as important in the management of the child as the medical considerations."
Dr. Becker, her coauthors, and Dr. Cordoro reported having no relevant financial disclosures.
AT THE SPD ANNUAL MEETING
Major finding: Among 29 children, 93% met criteria for excess adiposity prior to developing psoriasis, and 78% of these were obese before their psoriasis.
Data source: A growth chart review of 29 obese/overweight children with psoriasis.
Disclosures: Dr. Becker, her coauthors, and Dr. Cordoro reported having no relevant financial disclosures.
Tune in to hearing problems in ichthyosis
MILWAUKEE – More than two-thirds of patients with ichthyosis reported hearing problems, based on data from a survey of 135 patients ranging in age from 5 months to 80 years.
Overall, 80% of the survey respondents had ear pruritus, 66% had trouble hearing, 29% reported frequent ear pain, 28% had abnormal hearing test results, and 16% had used hearing aids.
Notably, of the 88 patients who reported trouble hearing, 27% had never seen a hearing specialist, Dr. Jennifer T. Huang reported at the annual meeting of the Society for Pediatric Dermatology.
"Early diagnosis and intervention, by both pediatric dermatologists and pediatricians, may improve quality of life," she said.
She also recommended that "regular ear canal debridement and hearing tests should become routine care for patients reporting ear symptoms."
Ichthyosis includes a heterogeneous array of skin disorders characterized by dry, scaling skin, and otologic issues may be attributed to abnormal keratinization of the external ear canals and tympanic membranes and/or accumulation of emollients within the ear canals, said Dr. Huang of Boston Children’s Hospital, Mass. Sufficient build-up causes the ear canals to become plugged, resulting in conductive (though not permanent) hearing loss. While ear structures can be affected, ear-related symptoms have never been investigated in patients with ichthyosis, according to the authors of the award-winning research poster.
The pilot survey was posted on the Foundation for Ichthyosis & Related Skin Types (FIRST) website for 6 months, and Dr. Huang and her colleagues analyzed the data for 135 of 148 respondents. Patients with keratitis ichthyosis deafness (KID) syndrome and surveys with fewer than two completed items were excluded from the analysis.
Lamellar ichthyosis was the most common type of ichthyosis (30%), followed by unknown (17%), vulgaris (13%), X-linked (12%), congenital ichthyosiform erythroderma (10%), and epidermolytic hyperkeratosis (10%). The patients ranged in age from 5 months to 80 years, with an average age of 27 years; 43% were younger than 18 years.
Adult patients, defined as those 18 years and older, were significantly more likely than pediatric patients to report trouble hearing (74% vs. 53%), Dr. Huang reported.
There were no significant differences, however, between older and younger patients in the reported frequency of rubbing/scratching (79% vs. 74%), ear pain (22% vs. 38%), abnormal hearing test results (30% vs. 26), and use of hearing aids (17% vs. 14%).
However, parents who completed the survey for their infant or child may not have been able to fully report the child’s hearing difficulties, she noted.
Of the 75 patients who had a previous hearing test, half (51%) were found to have hearing deficits. Half of those patients who had abnormal results also reported using hearing aids. Two-thirds of patients reported previous ear cleaning (66%).
Finally, the frequency of trouble hearing, ear pruritus, and ear pain was not significantly different among types of ichthyosis.
"Patients with all forms of ichthyosis, across all age groups, are affected," noted Dr. Huang, who called for further studies using objective tools to measure hearing loss and intervention assessments.
Dr. Huang reported having no financial disclosures, but acknowledged the assistance of the Foundation for Ichthyosis & Related Skin Types in the execution of the survey.
MILWAUKEE – More than two-thirds of patients with ichthyosis reported hearing problems, based on data from a survey of 135 patients ranging in age from 5 months to 80 years.
Overall, 80% of the survey respondents had ear pruritus, 66% had trouble hearing, 29% reported frequent ear pain, 28% had abnormal hearing test results, and 16% had used hearing aids.
Notably, of the 88 patients who reported trouble hearing, 27% had never seen a hearing specialist, Dr. Jennifer T. Huang reported at the annual meeting of the Society for Pediatric Dermatology.
"Early diagnosis and intervention, by both pediatric dermatologists and pediatricians, may improve quality of life," she said.
She also recommended that "regular ear canal debridement and hearing tests should become routine care for patients reporting ear symptoms."
Ichthyosis includes a heterogeneous array of skin disorders characterized by dry, scaling skin, and otologic issues may be attributed to abnormal keratinization of the external ear canals and tympanic membranes and/or accumulation of emollients within the ear canals, said Dr. Huang of Boston Children’s Hospital, Mass. Sufficient build-up causes the ear canals to become plugged, resulting in conductive (though not permanent) hearing loss. While ear structures can be affected, ear-related symptoms have never been investigated in patients with ichthyosis, according to the authors of the award-winning research poster.
The pilot survey was posted on the Foundation for Ichthyosis & Related Skin Types (FIRST) website for 6 months, and Dr. Huang and her colleagues analyzed the data for 135 of 148 respondents. Patients with keratitis ichthyosis deafness (KID) syndrome and surveys with fewer than two completed items were excluded from the analysis.
Lamellar ichthyosis was the most common type of ichthyosis (30%), followed by unknown (17%), vulgaris (13%), X-linked (12%), congenital ichthyosiform erythroderma (10%), and epidermolytic hyperkeratosis (10%). The patients ranged in age from 5 months to 80 years, with an average age of 27 years; 43% were younger than 18 years.
Adult patients, defined as those 18 years and older, were significantly more likely than pediatric patients to report trouble hearing (74% vs. 53%), Dr. Huang reported.
There were no significant differences, however, between older and younger patients in the reported frequency of rubbing/scratching (79% vs. 74%), ear pain (22% vs. 38%), abnormal hearing test results (30% vs. 26), and use of hearing aids (17% vs. 14%).
However, parents who completed the survey for their infant or child may not have been able to fully report the child’s hearing difficulties, she noted.
Of the 75 patients who had a previous hearing test, half (51%) were found to have hearing deficits. Half of those patients who had abnormal results also reported using hearing aids. Two-thirds of patients reported previous ear cleaning (66%).
Finally, the frequency of trouble hearing, ear pruritus, and ear pain was not significantly different among types of ichthyosis.
"Patients with all forms of ichthyosis, across all age groups, are affected," noted Dr. Huang, who called for further studies using objective tools to measure hearing loss and intervention assessments.
Dr. Huang reported having no financial disclosures, but acknowledged the assistance of the Foundation for Ichthyosis & Related Skin Types in the execution of the survey.
MILWAUKEE – More than two-thirds of patients with ichthyosis reported hearing problems, based on data from a survey of 135 patients ranging in age from 5 months to 80 years.
Overall, 80% of the survey respondents had ear pruritus, 66% had trouble hearing, 29% reported frequent ear pain, 28% had abnormal hearing test results, and 16% had used hearing aids.
Notably, of the 88 patients who reported trouble hearing, 27% had never seen a hearing specialist, Dr. Jennifer T. Huang reported at the annual meeting of the Society for Pediatric Dermatology.
"Early diagnosis and intervention, by both pediatric dermatologists and pediatricians, may improve quality of life," she said.
She also recommended that "regular ear canal debridement and hearing tests should become routine care for patients reporting ear symptoms."
Ichthyosis includes a heterogeneous array of skin disorders characterized by dry, scaling skin, and otologic issues may be attributed to abnormal keratinization of the external ear canals and tympanic membranes and/or accumulation of emollients within the ear canals, said Dr. Huang of Boston Children’s Hospital, Mass. Sufficient build-up causes the ear canals to become plugged, resulting in conductive (though not permanent) hearing loss. While ear structures can be affected, ear-related symptoms have never been investigated in patients with ichthyosis, according to the authors of the award-winning research poster.
The pilot survey was posted on the Foundation for Ichthyosis & Related Skin Types (FIRST) website for 6 months, and Dr. Huang and her colleagues analyzed the data for 135 of 148 respondents. Patients with keratitis ichthyosis deafness (KID) syndrome and surveys with fewer than two completed items were excluded from the analysis.
Lamellar ichthyosis was the most common type of ichthyosis (30%), followed by unknown (17%), vulgaris (13%), X-linked (12%), congenital ichthyosiform erythroderma (10%), and epidermolytic hyperkeratosis (10%). The patients ranged in age from 5 months to 80 years, with an average age of 27 years; 43% were younger than 18 years.
Adult patients, defined as those 18 years and older, were significantly more likely than pediatric patients to report trouble hearing (74% vs. 53%), Dr. Huang reported.
There were no significant differences, however, between older and younger patients in the reported frequency of rubbing/scratching (79% vs. 74%), ear pain (22% vs. 38%), abnormal hearing test results (30% vs. 26), and use of hearing aids (17% vs. 14%).
However, parents who completed the survey for their infant or child may not have been able to fully report the child’s hearing difficulties, she noted.
Of the 75 patients who had a previous hearing test, half (51%) were found to have hearing deficits. Half of those patients who had abnormal results also reported using hearing aids. Two-thirds of patients reported previous ear cleaning (66%).
Finally, the frequency of trouble hearing, ear pruritus, and ear pain was not significantly different among types of ichthyosis.
"Patients with all forms of ichthyosis, across all age groups, are affected," noted Dr. Huang, who called for further studies using objective tools to measure hearing loss and intervention assessments.
Dr. Huang reported having no financial disclosures, but acknowledged the assistance of the Foundation for Ichthyosis & Related Skin Types in the execution of the survey.
AT THE ANNUAL MEETING OF THE SOCIETY FOR PEDIATRIC DERMATOLOGY
Major finding: Among 135 respondents, 80% reported ear pruritus, 66% trouble hearing, 29% frequent ear pain, 28% abnormal hearing test results, and 16% had used hearing aids.
Data source: Pilot survey study involving 135 respondents with ichthyosis.
Disclosures: Dr. Huang reported having no financial disclosures, but acknowledged the assistance of the Foundation for Ichthyosis and Related Skin Types in conducting the survey.
High-dose isotretinoin restrains acne relapse
MILWAUKEE– Boosting cumulative isotretinoin doses above 220 mg/kg significantly reduced the risk of relapse without dramatically increasing side effects in patients with severe, treatment-resistant nodulocystic acne in a prospective, observational study.
During the 12-month study, 27% of patients who received more than 220 mg/kg and 47% of those who received less than 220 mg/kg had a relapse, defined as treatment with another prescription acne medicine (P = .029).
The number of patients needing retreatment with isotretinoin was similar in both groups (2 vs. 0; P = .31), Mr. Christopher Stamey reported at the annual meeting of the Society for Pediatric Dermatology.
He acknowledged that the dosing regimen in this study was considerably higher than in previous studies of isotretinoin, which support a cumulative dose of 120-150 mg/kg over conventional dosing of less than 100 mg/kg to decrease the risk of relapse/retrial.
Low-dose regimens also have minimal rates of many side effects, but clinicians are becoming more comfortable giving higher doses, explained Mr. Stamey, a fourth-year medical student* and research fellow in dermatology at the University of North Carolina at Chapel Hill (UNC).
A study from a tertiary care center in the New York reported a 100% cure rate and significantly improved quality of life among 80 patients with nodulocystic acne, who were maintained on a cumulative dose of 290 mg/kg (average daily dose, 1.6 mg/kg per day) for an average of 178 days (Int. J. Dermatol. 2012;51:1123-30).
In the current 116-patient study, the average cumulative dose was 309.8 mg/kg in the high-dose group and 170.8 mg/kg in the low-dose group (P less than .000). The daily dose varied by patient and provider, but averaged 120-160 mg/day in the high-dose group, Mr. Stamey said.
Patients were started on 40 mg/day for the first month and then increased to greater than 1 mg/kg per day to avoid any kind of fulminant acne reaction that can occur with isotretinoin. Eventually, the dose was titrated and the patients in the high-dose group were maintained on 1.5-2 mg/kg per day.
In the low-dose group, treated at a cumulative dose less than 220 mg/kg, patients were given 30 mg twice per day.
Treatment duration was significantly longer in the high-dose group compared with the low-dose group (6.5 vs. 5.8 months).
During a discussion of the study at the meeting, concerns were raised about the dosing and the ability to maintain children who are highly inflammatory on such a high daily dose. By initiating the medication at a dose less than 1 mg/kg per day for the first month, brisk inflammatory reactions were avoided, Mr. Stamey said. No patient in the high-dose group had to discontinue isotretinoin for an inflammatory reaction, although two low-dose patients discontinued treatment for 1 month and 1 week, respectively, because of elevated triglycerides.
"The initiation of dosing was the most important aspect with regard to avoiding inflammatory reactions," Mr. Stamey said in an interview. "This is a general practice when using isotretinoin at UNC dermatology clinics."
Overall, the side effects were similar between the groups. Mr. Stamey said. Only retinoid dermatitis was significantly more common during high-dose treatment than lower-dose treatment (54% vs. 32%).
Laboratory abnormalities were slightly, but not significantly higher in the high-dose group. These values included aspartate aminotransferase greater than 90 U/L (6.41% vs. 0%), alanine aminotransferase greater than 105 U/L (1.3% vs. 0%), a total cholesterol level greater than 300 mg/dL (1.3% vs. 0%), and triglycerides greater than 300 mg/dL (11.5% vs. 5.3%).
No differences were observed in itching, muscle or joint aches, mood, or suicidal ideation, although 13.8% of all patients had nail changes, including paronychia in 7.8%, Mr. Stamey noted.
Three-fourths of the patient cohort were white, approximately half were female, and the average age in the high- and low-dose groups was 21 years and 18 years, respectively.
Mr. Stamey reported having no relevant financial disclosures.
*Correction, 7/26/2013: A previous version of this story misstated Mr. Stamey's position as resident. He is a medical student.
MILWAUKEE– Boosting cumulative isotretinoin doses above 220 mg/kg significantly reduced the risk of relapse without dramatically increasing side effects in patients with severe, treatment-resistant nodulocystic acne in a prospective, observational study.
During the 12-month study, 27% of patients who received more than 220 mg/kg and 47% of those who received less than 220 mg/kg had a relapse, defined as treatment with another prescription acne medicine (P = .029).
The number of patients needing retreatment with isotretinoin was similar in both groups (2 vs. 0; P = .31), Mr. Christopher Stamey reported at the annual meeting of the Society for Pediatric Dermatology.
He acknowledged that the dosing regimen in this study was considerably higher than in previous studies of isotretinoin, which support a cumulative dose of 120-150 mg/kg over conventional dosing of less than 100 mg/kg to decrease the risk of relapse/retrial.
Low-dose regimens also have minimal rates of many side effects, but clinicians are becoming more comfortable giving higher doses, explained Mr. Stamey, a fourth-year medical student* and research fellow in dermatology at the University of North Carolina at Chapel Hill (UNC).
A study from a tertiary care center in the New York reported a 100% cure rate and significantly improved quality of life among 80 patients with nodulocystic acne, who were maintained on a cumulative dose of 290 mg/kg (average daily dose, 1.6 mg/kg per day) for an average of 178 days (Int. J. Dermatol. 2012;51:1123-30).
In the current 116-patient study, the average cumulative dose was 309.8 mg/kg in the high-dose group and 170.8 mg/kg in the low-dose group (P less than .000). The daily dose varied by patient and provider, but averaged 120-160 mg/day in the high-dose group, Mr. Stamey said.
Patients were started on 40 mg/day for the first month and then increased to greater than 1 mg/kg per day to avoid any kind of fulminant acne reaction that can occur with isotretinoin. Eventually, the dose was titrated and the patients in the high-dose group were maintained on 1.5-2 mg/kg per day.
In the low-dose group, treated at a cumulative dose less than 220 mg/kg, patients were given 30 mg twice per day.
Treatment duration was significantly longer in the high-dose group compared with the low-dose group (6.5 vs. 5.8 months).
During a discussion of the study at the meeting, concerns were raised about the dosing and the ability to maintain children who are highly inflammatory on such a high daily dose. By initiating the medication at a dose less than 1 mg/kg per day for the first month, brisk inflammatory reactions were avoided, Mr. Stamey said. No patient in the high-dose group had to discontinue isotretinoin for an inflammatory reaction, although two low-dose patients discontinued treatment for 1 month and 1 week, respectively, because of elevated triglycerides.
"The initiation of dosing was the most important aspect with regard to avoiding inflammatory reactions," Mr. Stamey said in an interview. "This is a general practice when using isotretinoin at UNC dermatology clinics."
Overall, the side effects were similar between the groups. Mr. Stamey said. Only retinoid dermatitis was significantly more common during high-dose treatment than lower-dose treatment (54% vs. 32%).
Laboratory abnormalities were slightly, but not significantly higher in the high-dose group. These values included aspartate aminotransferase greater than 90 U/L (6.41% vs. 0%), alanine aminotransferase greater than 105 U/L (1.3% vs. 0%), a total cholesterol level greater than 300 mg/dL (1.3% vs. 0%), and triglycerides greater than 300 mg/dL (11.5% vs. 5.3%).
No differences were observed in itching, muscle or joint aches, mood, or suicidal ideation, although 13.8% of all patients had nail changes, including paronychia in 7.8%, Mr. Stamey noted.
Three-fourths of the patient cohort were white, approximately half were female, and the average age in the high- and low-dose groups was 21 years and 18 years, respectively.
Mr. Stamey reported having no relevant financial disclosures.
*Correction, 7/26/2013: A previous version of this story misstated Mr. Stamey's position as resident. He is a medical student.
MILWAUKEE– Boosting cumulative isotretinoin doses above 220 mg/kg significantly reduced the risk of relapse without dramatically increasing side effects in patients with severe, treatment-resistant nodulocystic acne in a prospective, observational study.
During the 12-month study, 27% of patients who received more than 220 mg/kg and 47% of those who received less than 220 mg/kg had a relapse, defined as treatment with another prescription acne medicine (P = .029).
The number of patients needing retreatment with isotretinoin was similar in both groups (2 vs. 0; P = .31), Mr. Christopher Stamey reported at the annual meeting of the Society for Pediatric Dermatology.
He acknowledged that the dosing regimen in this study was considerably higher than in previous studies of isotretinoin, which support a cumulative dose of 120-150 mg/kg over conventional dosing of less than 100 mg/kg to decrease the risk of relapse/retrial.
Low-dose regimens also have minimal rates of many side effects, but clinicians are becoming more comfortable giving higher doses, explained Mr. Stamey, a fourth-year medical student* and research fellow in dermatology at the University of North Carolina at Chapel Hill (UNC).
A study from a tertiary care center in the New York reported a 100% cure rate and significantly improved quality of life among 80 patients with nodulocystic acne, who were maintained on a cumulative dose of 290 mg/kg (average daily dose, 1.6 mg/kg per day) for an average of 178 days (Int. J. Dermatol. 2012;51:1123-30).
In the current 116-patient study, the average cumulative dose was 309.8 mg/kg in the high-dose group and 170.8 mg/kg in the low-dose group (P less than .000). The daily dose varied by patient and provider, but averaged 120-160 mg/day in the high-dose group, Mr. Stamey said.
Patients were started on 40 mg/day for the first month and then increased to greater than 1 mg/kg per day to avoid any kind of fulminant acne reaction that can occur with isotretinoin. Eventually, the dose was titrated and the patients in the high-dose group were maintained on 1.5-2 mg/kg per day.
In the low-dose group, treated at a cumulative dose less than 220 mg/kg, patients were given 30 mg twice per day.
Treatment duration was significantly longer in the high-dose group compared with the low-dose group (6.5 vs. 5.8 months).
During a discussion of the study at the meeting, concerns were raised about the dosing and the ability to maintain children who are highly inflammatory on such a high daily dose. By initiating the medication at a dose less than 1 mg/kg per day for the first month, brisk inflammatory reactions were avoided, Mr. Stamey said. No patient in the high-dose group had to discontinue isotretinoin for an inflammatory reaction, although two low-dose patients discontinued treatment for 1 month and 1 week, respectively, because of elevated triglycerides.
"The initiation of dosing was the most important aspect with regard to avoiding inflammatory reactions," Mr. Stamey said in an interview. "This is a general practice when using isotretinoin at UNC dermatology clinics."
Overall, the side effects were similar between the groups. Mr. Stamey said. Only retinoid dermatitis was significantly more common during high-dose treatment than lower-dose treatment (54% vs. 32%).
Laboratory abnormalities were slightly, but not significantly higher in the high-dose group. These values included aspartate aminotransferase greater than 90 U/L (6.41% vs. 0%), alanine aminotransferase greater than 105 U/L (1.3% vs. 0%), a total cholesterol level greater than 300 mg/dL (1.3% vs. 0%), and triglycerides greater than 300 mg/dL (11.5% vs. 5.3%).
No differences were observed in itching, muscle or joint aches, mood, or suicidal ideation, although 13.8% of all patients had nail changes, including paronychia in 7.8%, Mr. Stamey noted.
Three-fourths of the patient cohort were white, approximately half were female, and the average age in the high- and low-dose groups was 21 years and 18 years, respectively.
Mr. Stamey reported having no relevant financial disclosures.
*Correction, 7/26/2013: A previous version of this story misstated Mr. Stamey's position as resident. He is a medical student.
AT THE SPD ANNUAL MEETING
Major finding: The rate of relapse was 27% in patients whose cumulative dose of isotretinoin exceeded 220 mg/kg and 47% in patients with a cumulative dose below 220 mg/kg (P = .029).
Data source: A prospective, observational intervention study of 116 patients with treatment-resistant nodulocystic acne.
Disclosures: Dr. Stamey reported having no relevant financial disclosures.
Wet wipes linked to contact dermatitis in children
MILWAUKEE – Despite extensive testing, seemingly innocuous wet wipes have been linked to allergic contact dermatitis in children.
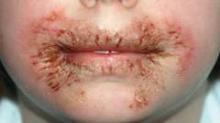
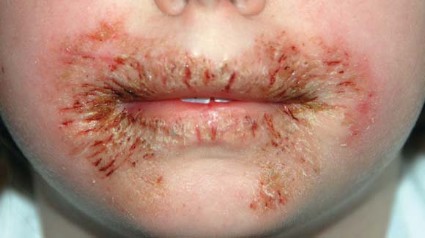
The first reported case occurred in a healthy 8-year-old girl who presented with chronic, recalcitrant erythematous, eczematous patches and plaques with crusting and fissuring around the mouth and perianal area. She had been using wipes containing methylisothiazolinone (MI), without methylchloroisothiazolinone (MCI).
Within 22 months, an additional five children had been diagnosed with allergic contact dermatitis to MI in wet wipes, Dr. Mary Wu Chang and Ms. Radhika Nakrani reported at the annual meeting of the Society for Pediatric Dermatology.
All cases were confirmed on patch testing, and all patients had rapid, complete resolution within about 2 days after discontinuing use of the wipes. Ms. Nakrani also interviewed the parents to determine the type of wipes used, identified as Cottonelle and Huggies brands (both manufactured by Kimberly-Clark). The preservative MI was identified in these brands.
"This is going to explode," Dr. Chang, a pediatric dermatologist at the University of Connecticut, West Hartford, said in an interview. "There’s more marketing [of wipes] now for non–diaper wearing children, and older people use wipes out of convenience."
The case series was described as the first report of pediatric allergic contact dermatitis to MI in wet wipes in the United States.
Although the MCI/MI patch test (T.R.U.E. Test) detected contact allergy to MI in the six children, identifying MI contact sensitization may require specialized MI patch tests with higher concentrations of MI or the use of small squares of the wipes themselves, Dr. Chang observed. Patch testing containing 100 ppm of MCI/MI mixture consists of only 25 ppm of MI and may be inadequate to detect MI alone, missing 33%-60% of cases.
Acute contact dermatitis was suspected in the index case, when the 8-year-old girl presented to pediatric dermatology after suffering for 11 months. She was initially misdiagnosed with impetiginized eczema and had received numerous oral and topical corticosteroids and antibiotics, said Dr. Chang. Questioning revealed that the patient used wet wipes for toileting and facial cleansing, and the rash resolved after the wipes were discontinued. MI sensitivity was confirmed on patch testing. Ms. Nakrani also interviewed the parents to determine the type of wipes used, and the preservative MI was identified in these brands.
"Remember to ask about wet wipe use, even in individuals who do not wear diapers and even if the rash in on the face," Dr. Chang said.
In the five other cases, allergic reactions to MI in the wet wipes were misdiagnosed as impetigo, psoriasis, diaper dermatitis, or atopic dermatitis, and unsuccessfully treated with a multitude of medications including steroids, antifungals, and topical tacrolimus (Protopic). The children, aged 3-8 years, had experienced symptoms for 1-12 months, according to the authors, who earned top honors for their poster presentation at the meeting.
The index patient also suffered from chronic retroauricular dermatitis, which resolved after discontinuing a shampoo containing MI.
MI was named the 2013 contact allergen of the year by the American Contact Dermatitis Society. In another recent study, Australian researchers reported 23 reactions to MI from a variety of personal care products, including 7 cases of hand dermatitis in parents of young children that was caused by an allergic reaction to MI in baby wipes (Australas. J. Dermatol. 2013 May 29 [doi: 10.1111/ajd.12062]).
The preservative MI was previously used only in a 3:1 MCI/MI combination (Kathon CG) that is widely known to cause allergic contact dermatitis. In an attempt to minimize such reactions, MI has been used alone, explained Ms. Nakrani, a medical student at the University of Connecticut.
However, its permitted concentration has increased by more than 25 times – from 3.7 ppm to 100 ppm – because it was thought to be a weaker sensitizer than MCI, she said.
"Once we found out the culprit was MI in the wet wipes and instructed parents to read labels and avoid MI, parents became more vigilant about the other products they were using like shampoos and soaps, and it really turned things right around once they stopped using these products," Ms. Nakrani said. "One mom, very grateful that the rashes finally cleared, said her child was going to school and everyone thought it was contagious."
The investigators have not contacted Kimberly-Clark regarding their findings. Bob Brand, director of external communications for Kimberly-Clark, would not specifically say whether they had received complaints of allergic contact dermatitis to MI in their wet wipes, but said all of their products undergo a thorough safety review prior to commercialization.
"While we understand there might be concern regarding a potential reaction to one of our products, consumers can use our wipes with confidence and know that the concentration levels of MI in our products are considered safe and well within the recommended levels as established by scientific and regulatory bodies such as the Cosmetic Ingredient Review Expert Panel in the U.S.A. and the European Commission Scientific Committee on Consumer Safety," he told Skin & Allergy News.
The formulation of the wipes appears to vary by product. Ms. Dianna Kenneally, principal scientist, baby care scientific communication, for Procter & Gamble, said in a separate interview that Procter & Gamble baby wipe brands do not contain MI.
Dr. Chang and Ms. Nakrani reported no relevant disclosures.
MILWAUKEE – Despite extensive testing, seemingly innocuous wet wipes have been linked to allergic contact dermatitis in children.
The first reported case occurred in a healthy 8-year-old girl who presented with chronic, recalcitrant erythematous, eczematous patches and plaques with crusting and fissuring around the mouth and perianal area. She had been using wipes containing methylisothiazolinone (MI), without methylchloroisothiazolinone (MCI).
Within 22 months, an additional five children had been diagnosed with allergic contact dermatitis to MI in wet wipes, Dr. Mary Wu Chang and Ms. Radhika Nakrani reported at the annual meeting of the Society for Pediatric Dermatology.
All cases were confirmed on patch testing, and all patients had rapid, complete resolution within about 2 days after discontinuing use of the wipes. Ms. Nakrani also interviewed the parents to determine the type of wipes used, identified as Cottonelle and Huggies brands (both manufactured by Kimberly-Clark). The preservative MI was identified in these brands.
"This is going to explode," Dr. Chang, a pediatric dermatologist at the University of Connecticut, West Hartford, said in an interview. "There’s more marketing [of wipes] now for non–diaper wearing children, and older people use wipes out of convenience."
The case series was described as the first report of pediatric allergic contact dermatitis to MI in wet wipes in the United States.
Although the MCI/MI patch test (T.R.U.E. Test) detected contact allergy to MI in the six children, identifying MI contact sensitization may require specialized MI patch tests with higher concentrations of MI or the use of small squares of the wipes themselves, Dr. Chang observed. Patch testing containing 100 ppm of MCI/MI mixture consists of only 25 ppm of MI and may be inadequate to detect MI alone, missing 33%-60% of cases.
Acute contact dermatitis was suspected in the index case, when the 8-year-old girl presented to pediatric dermatology after suffering for 11 months. She was initially misdiagnosed with impetiginized eczema and had received numerous oral and topical corticosteroids and antibiotics, said Dr. Chang. Questioning revealed that the patient used wet wipes for toileting and facial cleansing, and the rash resolved after the wipes were discontinued. MI sensitivity was confirmed on patch testing. Ms. Nakrani also interviewed the parents to determine the type of wipes used, and the preservative MI was identified in these brands.
"Remember to ask about wet wipe use, even in individuals who do not wear diapers and even if the rash in on the face," Dr. Chang said.
In the five other cases, allergic reactions to MI in the wet wipes were misdiagnosed as impetigo, psoriasis, diaper dermatitis, or atopic dermatitis, and unsuccessfully treated with a multitude of medications including steroids, antifungals, and topical tacrolimus (Protopic). The children, aged 3-8 years, had experienced symptoms for 1-12 months, according to the authors, who earned top honors for their poster presentation at the meeting.
The index patient also suffered from chronic retroauricular dermatitis, which resolved after discontinuing a shampoo containing MI.
MI was named the 2013 contact allergen of the year by the American Contact Dermatitis Society. In another recent study, Australian researchers reported 23 reactions to MI from a variety of personal care products, including 7 cases of hand dermatitis in parents of young children that was caused by an allergic reaction to MI in baby wipes (Australas. J. Dermatol. 2013 May 29 [doi: 10.1111/ajd.12062]).
The preservative MI was previously used only in a 3:1 MCI/MI combination (Kathon CG) that is widely known to cause allergic contact dermatitis. In an attempt to minimize such reactions, MI has been used alone, explained Ms. Nakrani, a medical student at the University of Connecticut.
However, its permitted concentration has increased by more than 25 times – from 3.7 ppm to 100 ppm – because it was thought to be a weaker sensitizer than MCI, she said.
"Once we found out the culprit was MI in the wet wipes and instructed parents to read labels and avoid MI, parents became more vigilant about the other products they were using like shampoos and soaps, and it really turned things right around once they stopped using these products," Ms. Nakrani said. "One mom, very grateful that the rashes finally cleared, said her child was going to school and everyone thought it was contagious."
The investigators have not contacted Kimberly-Clark regarding their findings. Bob Brand, director of external communications for Kimberly-Clark, would not specifically say whether they had received complaints of allergic contact dermatitis to MI in their wet wipes, but said all of their products undergo a thorough safety review prior to commercialization.
"While we understand there might be concern regarding a potential reaction to one of our products, consumers can use our wipes with confidence and know that the concentration levels of MI in our products are considered safe and well within the recommended levels as established by scientific and regulatory bodies such as the Cosmetic Ingredient Review Expert Panel in the U.S.A. and the European Commission Scientific Committee on Consumer Safety," he told Skin & Allergy News.
The formulation of the wipes appears to vary by product. Ms. Dianna Kenneally, principal scientist, baby care scientific communication, for Procter & Gamble, said in a separate interview that Procter & Gamble baby wipe brands do not contain MI.
Dr. Chang and Ms. Nakrani reported no relevant disclosures.
MILWAUKEE – Despite extensive testing, seemingly innocuous wet wipes have been linked to allergic contact dermatitis in children.
The first reported case occurred in a healthy 8-year-old girl who presented with chronic, recalcitrant erythematous, eczematous patches and plaques with crusting and fissuring around the mouth and perianal area. She had been using wipes containing methylisothiazolinone (MI), without methylchloroisothiazolinone (MCI).
Within 22 months, an additional five children had been diagnosed with allergic contact dermatitis to MI in wet wipes, Dr. Mary Wu Chang and Ms. Radhika Nakrani reported at the annual meeting of the Society for Pediatric Dermatology.
All cases were confirmed on patch testing, and all patients had rapid, complete resolution within about 2 days after discontinuing use of the wipes. Ms. Nakrani also interviewed the parents to determine the type of wipes used, identified as Cottonelle and Huggies brands (both manufactured by Kimberly-Clark). The preservative MI was identified in these brands.
"This is going to explode," Dr. Chang, a pediatric dermatologist at the University of Connecticut, West Hartford, said in an interview. "There’s more marketing [of wipes] now for non–diaper wearing children, and older people use wipes out of convenience."
The case series was described as the first report of pediatric allergic contact dermatitis to MI in wet wipes in the United States.
Although the MCI/MI patch test (T.R.U.E. Test) detected contact allergy to MI in the six children, identifying MI contact sensitization may require specialized MI patch tests with higher concentrations of MI or the use of small squares of the wipes themselves, Dr. Chang observed. Patch testing containing 100 ppm of MCI/MI mixture consists of only 25 ppm of MI and may be inadequate to detect MI alone, missing 33%-60% of cases.
Acute contact dermatitis was suspected in the index case, when the 8-year-old girl presented to pediatric dermatology after suffering for 11 months. She was initially misdiagnosed with impetiginized eczema and had received numerous oral and topical corticosteroids and antibiotics, said Dr. Chang. Questioning revealed that the patient used wet wipes for toileting and facial cleansing, and the rash resolved after the wipes were discontinued. MI sensitivity was confirmed on patch testing. Ms. Nakrani also interviewed the parents to determine the type of wipes used, and the preservative MI was identified in these brands.
"Remember to ask about wet wipe use, even in individuals who do not wear diapers and even if the rash in on the face," Dr. Chang said.
In the five other cases, allergic reactions to MI in the wet wipes were misdiagnosed as impetigo, psoriasis, diaper dermatitis, or atopic dermatitis, and unsuccessfully treated with a multitude of medications including steroids, antifungals, and topical tacrolimus (Protopic). The children, aged 3-8 years, had experienced symptoms for 1-12 months, according to the authors, who earned top honors for their poster presentation at the meeting.
The index patient also suffered from chronic retroauricular dermatitis, which resolved after discontinuing a shampoo containing MI.
MI was named the 2013 contact allergen of the year by the American Contact Dermatitis Society. In another recent study, Australian researchers reported 23 reactions to MI from a variety of personal care products, including 7 cases of hand dermatitis in parents of young children that was caused by an allergic reaction to MI in baby wipes (Australas. J. Dermatol. 2013 May 29 [doi: 10.1111/ajd.12062]).
The preservative MI was previously used only in a 3:1 MCI/MI combination (Kathon CG) that is widely known to cause allergic contact dermatitis. In an attempt to minimize such reactions, MI has been used alone, explained Ms. Nakrani, a medical student at the University of Connecticut.
However, its permitted concentration has increased by more than 25 times – from 3.7 ppm to 100 ppm – because it was thought to be a weaker sensitizer than MCI, she said.
"Once we found out the culprit was MI in the wet wipes and instructed parents to read labels and avoid MI, parents became more vigilant about the other products they were using like shampoos and soaps, and it really turned things right around once they stopped using these products," Ms. Nakrani said. "One mom, very grateful that the rashes finally cleared, said her child was going to school and everyone thought it was contagious."
The investigators have not contacted Kimberly-Clark regarding their findings. Bob Brand, director of external communications for Kimberly-Clark, would not specifically say whether they had received complaints of allergic contact dermatitis to MI in their wet wipes, but said all of their products undergo a thorough safety review prior to commercialization.
"While we understand there might be concern regarding a potential reaction to one of our products, consumers can use our wipes with confidence and know that the concentration levels of MI in our products are considered safe and well within the recommended levels as established by scientific and regulatory bodies such as the Cosmetic Ingredient Review Expert Panel in the U.S.A. and the European Commission Scientific Committee on Consumer Safety," he told Skin & Allergy News.
The formulation of the wipes appears to vary by product. Ms. Dianna Kenneally, principal scientist, baby care scientific communication, for Procter & Gamble, said in a separate interview that Procter & Gamble baby wipe brands do not contain MI.
Dr. Chang and Ms. Nakrani reported no relevant disclosures.
AT THE SPD ANNUAL MEETING
Major finding: Six cases of pediatric allergic contact dermatitis to methylisothiazolinone in wet wipes were confirmed on patch testing, and resolved within 1-2 days after discontinuing the wipes.
Data source: A case series of six children with allergic contact dermatitis to methylisothiazolinone.
Disclosures: Dr. Chang and Ms. Nakrani reported no relevant disclosures.
Methotrexate assay useful in severe pediatric dermatitis, psoriasis
MILWAUKEE – In children with severe atopic dermatitis or psoriasis, the methotrexate polyglutamate assay appears useful in guiding dose modification in those failing to respond to methotrexate at 12 weeks, a retrospective study has shown.
The methotrexate polyglutamate 3 assay (MTX PG3) measures concentrations of methotrexate polyglutamates, the active metabolites of methotrexate, Syed Rahman said at the annual meeting of the Society for Pediatric Dermatology.
Methotrexate, a prodrug, requires enzymatic conversion via sequential addition of glutamic acid residues. Those residues form a glutamate "tail" that enhances intracellular methotrexate retention. The 3-tail metabolite, MTX PG3, is the predominant species in most patients, explained Mr. Rahman of St. Louis University.
"This study supports the usefulness of a commercially available tool for customizing optimal methotrexate dosing, coauthor Dr. Elaine Siegfried, professor of pediatrics and dermatology at the university, said in an interview.
The MTX PG3 assay has been validated in adults with rheumatoid arthritis where patients with a therapeutic methotrexate polyglutamate level of more than 60 nmol/L were fivefold more likely to have a good response to methotrexate than those with levels below this threshold, according to poster results presented at the 2008 American College of Rheumatology annual meeting (cited on the company website). However, the test was not predictive of response in 36% of patients whose arthritis improved with treatment despite subtherapeutic levels.
For the current analysis, the investigators reviewed the charts of 46 children with severe atopic dermatitis, psoriasis, or atopic dermatitis–psoriasis overlap, who were treated with a starting dose of oral methotrexate 0.5 mg/kg per week (maximum, 15 mg) and assessed by MTX PG3 assay at week 12. In a subset of children who failed to respond, the dose was modified and the level rechecked at 8 weeks. Disease severity was rated using a 5-point physician global assessment scale, with a good to excellent response defined by a 2-point improvement.
In all, 83% of children achieved a good to excellent response, with 27 patients doing so within 12 weeks and 11 responding after dose adjustment involving either a higher oral dose or switching to subcutaneous administration, Mr. Rahman reported.
Response rates to methotrexate were slightly higher in children with psoriasis or overlap (the two groups were combined) than those with atopic dermatitis (94% vs. 77%; no P value given). Overall, 8 children failed to respond.
The mean MTX PG3 level significantly correlated with efficacy in patients with atopic dermatitis (responders, 34.8 nmol/L, vs. 17.8 nmol/L, nonresponders; P = .018), but not in those with psoriasis–atopic dermatitis overlap (responders, 26.5 nmol/L, vs. 20.7, nonresponders; P = .724), Mr. Rahman reported.
The data suggest that compared with the therapeutic level of 60 nmol/L identified in adults, "pediatric patients have a lower therapeutic value," he said.
A subset analysis of MTX PG3 levels based on response identified a significantly higher mean maximum MTX PG3 level for responders than nonresponders (31.5 vs. 18.1 nmol/L; P = .035).
More importantly, late responders had the highest methotrexate levels. All late responders achieved levels greater than 30 nmol/L, and mean levels were significantly higher than for early responders (41.9 vs. 27.3 nmol/L; P = .024), suggesting that the test is most valuable for the subset of children requiring dose adjustment, Mr. Rahman said.
"Patients who respond won’t even need the test, but for those at the 12-week juncture who do not respond and their levels are less than 30 nmol/L, this [study] suggests that if we increase the PO [dose] or change to subcutaneous [administration], we will eventually have a response," he said.
During a discussion of the results, an audience member questioned whether the test, with its added expense, really enhances clinical judgment because most clinicians would already adjust the dose in their patients failing to respond at 12 weeks.
Mr. Rahman replied that not all patients will respond to continued treatment with methotrexate, and that the test could differentiate between responders and nonresponders, thereby directing the patient to alternative therapy earlier.
Session moderator Dr. Amy Paller, chair of dermatology and professor of pediatrics and dermatology at Northwestern University in Chicago, agreed that the test is most valuable for the subset of children who are nonresponders at 12 weeks.
"In these children, this test can guide dose modification," she said in an interview. "Pharmacogenetic variation can require relatively higher oral doses or subcutaneous administration."
In contrast to a therapeutic level of more than 60 nmol/L established for adults, a level of 30 nmol/L may be sufficient in children, Dr. Paller said.
"A MTX PG3 less than 30 nmol/L in a child who has failed to respond within 12 weeks suggests that an increase in oral dose, or even a change to subcutaneous [administration], will boost efficacy," she commented.
Mr. Rahman, Dr. Siegfried, and Dr. Paller reported having no relevant financial disclosures.
MILWAUKEE – In children with severe atopic dermatitis or psoriasis, the methotrexate polyglutamate assay appears useful in guiding dose modification in those failing to respond to methotrexate at 12 weeks, a retrospective study has shown.
The methotrexate polyglutamate 3 assay (MTX PG3) measures concentrations of methotrexate polyglutamates, the active metabolites of methotrexate, Syed Rahman said at the annual meeting of the Society for Pediatric Dermatology.
Methotrexate, a prodrug, requires enzymatic conversion via sequential addition of glutamic acid residues. Those residues form a glutamate "tail" that enhances intracellular methotrexate retention. The 3-tail metabolite, MTX PG3, is the predominant species in most patients, explained Mr. Rahman of St. Louis University.
"This study supports the usefulness of a commercially available tool for customizing optimal methotrexate dosing, coauthor Dr. Elaine Siegfried, professor of pediatrics and dermatology at the university, said in an interview.
The MTX PG3 assay has been validated in adults with rheumatoid arthritis where patients with a therapeutic methotrexate polyglutamate level of more than 60 nmol/L were fivefold more likely to have a good response to methotrexate than those with levels below this threshold, according to poster results presented at the 2008 American College of Rheumatology annual meeting (cited on the company website). However, the test was not predictive of response in 36% of patients whose arthritis improved with treatment despite subtherapeutic levels.
For the current analysis, the investigators reviewed the charts of 46 children with severe atopic dermatitis, psoriasis, or atopic dermatitis–psoriasis overlap, who were treated with a starting dose of oral methotrexate 0.5 mg/kg per week (maximum, 15 mg) and assessed by MTX PG3 assay at week 12. In a subset of children who failed to respond, the dose was modified and the level rechecked at 8 weeks. Disease severity was rated using a 5-point physician global assessment scale, with a good to excellent response defined by a 2-point improvement.
In all, 83% of children achieved a good to excellent response, with 27 patients doing so within 12 weeks and 11 responding after dose adjustment involving either a higher oral dose or switching to subcutaneous administration, Mr. Rahman reported.
Response rates to methotrexate were slightly higher in children with psoriasis or overlap (the two groups were combined) than those with atopic dermatitis (94% vs. 77%; no P value given). Overall, 8 children failed to respond.
The mean MTX PG3 level significantly correlated with efficacy in patients with atopic dermatitis (responders, 34.8 nmol/L, vs. 17.8 nmol/L, nonresponders; P = .018), but not in those with psoriasis–atopic dermatitis overlap (responders, 26.5 nmol/L, vs. 20.7, nonresponders; P = .724), Mr. Rahman reported.
The data suggest that compared with the therapeutic level of 60 nmol/L identified in adults, "pediatric patients have a lower therapeutic value," he said.
A subset analysis of MTX PG3 levels based on response identified a significantly higher mean maximum MTX PG3 level for responders than nonresponders (31.5 vs. 18.1 nmol/L; P = .035).
More importantly, late responders had the highest methotrexate levels. All late responders achieved levels greater than 30 nmol/L, and mean levels were significantly higher than for early responders (41.9 vs. 27.3 nmol/L; P = .024), suggesting that the test is most valuable for the subset of children requiring dose adjustment, Mr. Rahman said.
"Patients who respond won’t even need the test, but for those at the 12-week juncture who do not respond and their levels are less than 30 nmol/L, this [study] suggests that if we increase the PO [dose] or change to subcutaneous [administration], we will eventually have a response," he said.
During a discussion of the results, an audience member questioned whether the test, with its added expense, really enhances clinical judgment because most clinicians would already adjust the dose in their patients failing to respond at 12 weeks.
Mr. Rahman replied that not all patients will respond to continued treatment with methotrexate, and that the test could differentiate between responders and nonresponders, thereby directing the patient to alternative therapy earlier.
Session moderator Dr. Amy Paller, chair of dermatology and professor of pediatrics and dermatology at Northwestern University in Chicago, agreed that the test is most valuable for the subset of children who are nonresponders at 12 weeks.
"In these children, this test can guide dose modification," she said in an interview. "Pharmacogenetic variation can require relatively higher oral doses or subcutaneous administration."
In contrast to a therapeutic level of more than 60 nmol/L established for adults, a level of 30 nmol/L may be sufficient in children, Dr. Paller said.
"A MTX PG3 less than 30 nmol/L in a child who has failed to respond within 12 weeks suggests that an increase in oral dose, or even a change to subcutaneous [administration], will boost efficacy," she commented.
Mr. Rahman, Dr. Siegfried, and Dr. Paller reported having no relevant financial disclosures.
MILWAUKEE – In children with severe atopic dermatitis or psoriasis, the methotrexate polyglutamate assay appears useful in guiding dose modification in those failing to respond to methotrexate at 12 weeks, a retrospective study has shown.
The methotrexate polyglutamate 3 assay (MTX PG3) measures concentrations of methotrexate polyglutamates, the active metabolites of methotrexate, Syed Rahman said at the annual meeting of the Society for Pediatric Dermatology.
Methotrexate, a prodrug, requires enzymatic conversion via sequential addition of glutamic acid residues. Those residues form a glutamate "tail" that enhances intracellular methotrexate retention. The 3-tail metabolite, MTX PG3, is the predominant species in most patients, explained Mr. Rahman of St. Louis University.
"This study supports the usefulness of a commercially available tool for customizing optimal methotrexate dosing, coauthor Dr. Elaine Siegfried, professor of pediatrics and dermatology at the university, said in an interview.
The MTX PG3 assay has been validated in adults with rheumatoid arthritis where patients with a therapeutic methotrexate polyglutamate level of more than 60 nmol/L were fivefold more likely to have a good response to methotrexate than those with levels below this threshold, according to poster results presented at the 2008 American College of Rheumatology annual meeting (cited on the company website). However, the test was not predictive of response in 36% of patients whose arthritis improved with treatment despite subtherapeutic levels.
For the current analysis, the investigators reviewed the charts of 46 children with severe atopic dermatitis, psoriasis, or atopic dermatitis–psoriasis overlap, who were treated with a starting dose of oral methotrexate 0.5 mg/kg per week (maximum, 15 mg) and assessed by MTX PG3 assay at week 12. In a subset of children who failed to respond, the dose was modified and the level rechecked at 8 weeks. Disease severity was rated using a 5-point physician global assessment scale, with a good to excellent response defined by a 2-point improvement.
In all, 83% of children achieved a good to excellent response, with 27 patients doing so within 12 weeks and 11 responding after dose adjustment involving either a higher oral dose or switching to subcutaneous administration, Mr. Rahman reported.
Response rates to methotrexate were slightly higher in children with psoriasis or overlap (the two groups were combined) than those with atopic dermatitis (94% vs. 77%; no P value given). Overall, 8 children failed to respond.
The mean MTX PG3 level significantly correlated with efficacy in patients with atopic dermatitis (responders, 34.8 nmol/L, vs. 17.8 nmol/L, nonresponders; P = .018), but not in those with psoriasis–atopic dermatitis overlap (responders, 26.5 nmol/L, vs. 20.7, nonresponders; P = .724), Mr. Rahman reported.
The data suggest that compared with the therapeutic level of 60 nmol/L identified in adults, "pediatric patients have a lower therapeutic value," he said.
A subset analysis of MTX PG3 levels based on response identified a significantly higher mean maximum MTX PG3 level for responders than nonresponders (31.5 vs. 18.1 nmol/L; P = .035).
More importantly, late responders had the highest methotrexate levels. All late responders achieved levels greater than 30 nmol/L, and mean levels were significantly higher than for early responders (41.9 vs. 27.3 nmol/L; P = .024), suggesting that the test is most valuable for the subset of children requiring dose adjustment, Mr. Rahman said.
"Patients who respond won’t even need the test, but for those at the 12-week juncture who do not respond and their levels are less than 30 nmol/L, this [study] suggests that if we increase the PO [dose] or change to subcutaneous [administration], we will eventually have a response," he said.
During a discussion of the results, an audience member questioned whether the test, with its added expense, really enhances clinical judgment because most clinicians would already adjust the dose in their patients failing to respond at 12 weeks.
Mr. Rahman replied that not all patients will respond to continued treatment with methotrexate, and that the test could differentiate between responders and nonresponders, thereby directing the patient to alternative therapy earlier.
Session moderator Dr. Amy Paller, chair of dermatology and professor of pediatrics and dermatology at Northwestern University in Chicago, agreed that the test is most valuable for the subset of children who are nonresponders at 12 weeks.
"In these children, this test can guide dose modification," she said in an interview. "Pharmacogenetic variation can require relatively higher oral doses or subcutaneous administration."
In contrast to a therapeutic level of more than 60 nmol/L established for adults, a level of 30 nmol/L may be sufficient in children, Dr. Paller said.
"A MTX PG3 less than 30 nmol/L in a child who has failed to respond within 12 weeks suggests that an increase in oral dose, or even a change to subcutaneous [administration], will boost efficacy," she commented.
Mr. Rahman, Dr. Siegfried, and Dr. Paller reported having no relevant financial disclosures.
AT THE ANNUAL MEETING OF THE SOCIETY FOR PEDIATRIC DERMATOLOGY
Major finding: Late responders had the highest methotrexate levels (41.9 nmol/L vs. 27.3 nmol/L in early responders; P = .024) on the MTX PG3 assay.
Data source: A retrospective study of 46 children on methotrexate for atopic dermatitis, psoriasis, or atopic dermatitis–psoriasis overlap who were assessed by MTX PG3 assay.
Disclosures: Mr. Rahman, Dr. Siegfried, and Dr. Paller reported having no relevant financial disclosures.