User login
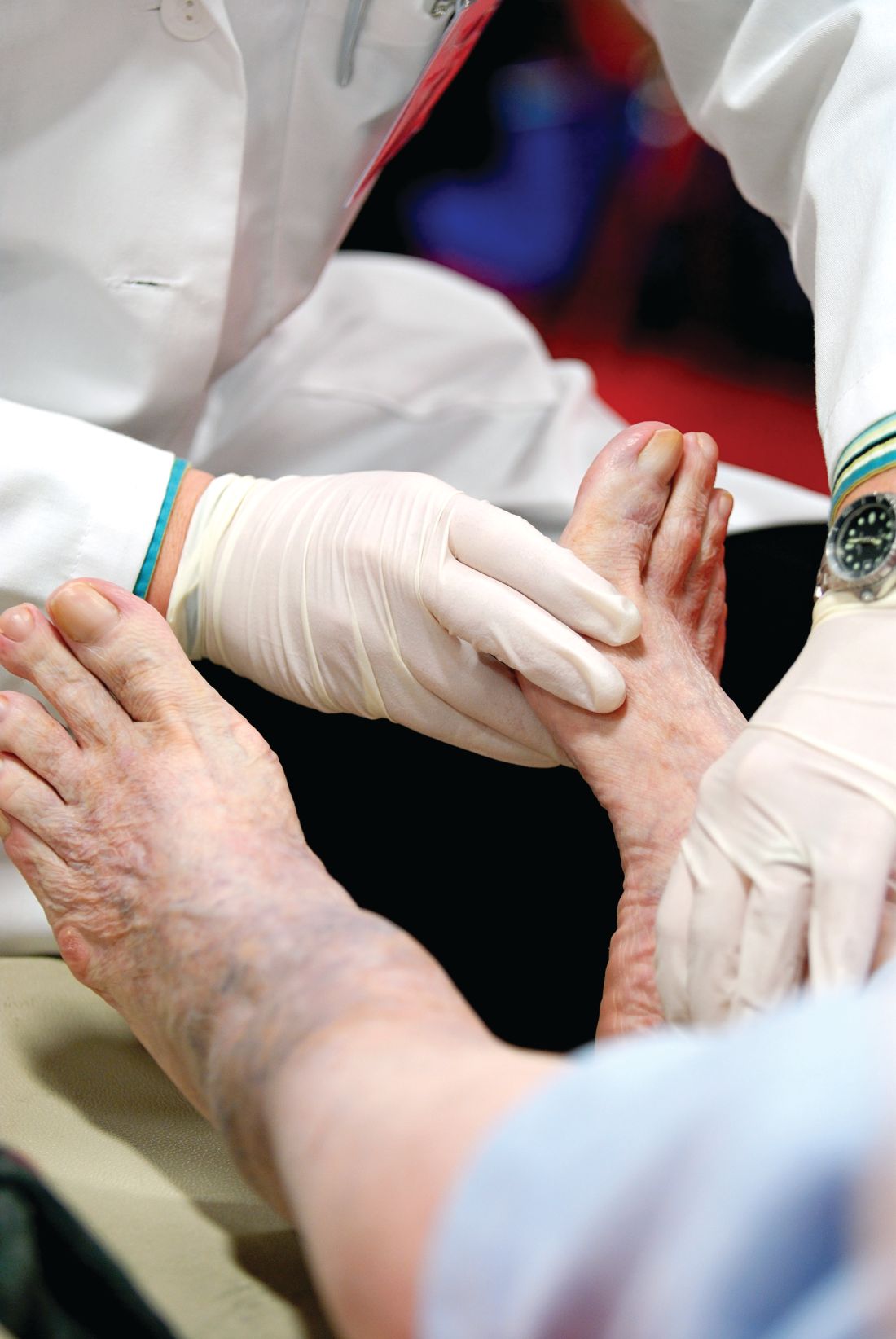
Wound expert: Consider hyperbaric oxygen therapy for diabetic foot ulcers
SAN DIEGO – Hyperbaric oxygen therapy, a mainstay of wound care, has a long and controversial history as a treatment for diabetic foot ulcers. Conflicting studies have spawned plenty of debate, and the most recent Cochrane Library review of existing research didn’t shed much light on the value of the treatment because the evidence was weak (Cochrane Database Syst Rev. 2015 Jun 24;[6]:CD004123).
But William H. Tettelbach, MD, a wound care specialist, told an audience at the annual scientific sessions of the American Diabetic Association that hyperbaric treatments are worth a try in certain cases. And he brought evidence to prove it – a 2015 report he coauthored that reviewed studies and offered clinical practice guidelines for hyperbaric oxygen therapy for the treatment of diabetic foot ulcers (DFUs) (Undersea Hyperb Med. 2015 May-Jun;42[3]:205-47).
In an interview, Dr. Tettelbach discussed ideal candidates for the treatment and offered clinical advice to endocrinologists.
Question: What did your review of research tell you about the value of hyperbaric oxygen treatment for DFUs?
Answer: We came to the same conclusion that most of the papers have indicated over the years: Hyperbaric oxygen is effective and attains goals such as reducing rates of amputation in a select population of diabetic ulcer patients.
Patients who have Wagner grade 3 or greater ulcers or admitted for surgery due to a septic diabetic foot benefit from an evaluation by a hyperbaric medicine–trained physician and treatment when indicated. There is evidence and years of clinical experience indicating that these patients benefit and have improved outcomes when evaluated and treated appropriately with hyperbaric oxygen therapy.
In the United States, hyperbaric oxygen therapy is not indicated in Wagner grade 2, 1 or 0 diabetic foot ulcers, the ulcers that involve soft tissue but not deep structure like bone.
Q: Why has there been so much controversy over the value of this treatment?
A: In the past, there have been problems with commercial outpatient wound centers that are heavily driven by profits. Financial margins in wound care clinics can be tight, and the need to remain profitable has at times resulted in patients being treated inappropriately with hyperbaric oxygen therapy (Adv Skin Wound Care. 2017 Apr;30[4]:181-90).
Q: Why does hyperbaric oxygen treatment work in some cases?
A: When you place a patient in a hyperbaric chamber where they breathe 100% oxygen under pressure, you increase the percentage of oxygen in the blood. At such a high percentage, oxygen saturates the plasma versus just being carried by red blood cells, thereby allowing the oxygen to penetrate farther into hypoxic tissues. By increasing the oxygen, you have the ability to make the environment unfavorable for rapid proliferation of anaerobic or microaerophilic bacteria that do not survive a highly oxygen-rich environment. Increasing tissue oxygen tension to 30 mm Hg or greater increases the macrophages’ ability to have an oxidative burst needed to kill bacteria. Furthermore, there are antibiotics that require certain levels of oxygen for transport across the bacterial cell wall.
Q: What should physicians understand about hyperbaric oxygen therapy for DFUs?
A: Overall, hyperbaric practitioners need to be more selective in identifying and treating patients according to what the evidence supports. Poorly designed trials with misleading results should not drive medical decisions. We should revisit diabetic foot ulcers through well-thought-out studies that target those who would benefit as suggested by current evidence. Prior trials have been heavily weighted with Wagner grade 1 and 2 candidates or ischemic diabetic ulcers that are not revascularized. These are biased toward poor outcomes since the current evidence does not strongly support treating these types of individuals with adjunctive hyperbaric oxygen therapy (Ont Health Technol Assess Ser. 2017 May 12;17[5]:1-142. eCollection 2017).
Q: What conditions should trigger endocrinologists to think about hyperbaric oxygen therapy for their DFU patients?
A: Candidates for the therapy include diabetic ulcers that have persisted for longer than 30 days, since these ulcers are at a significantly higher risk of a complicating infection, along with those that have failed treatment or are becoming more symptomatic over time (Undersea Hyperb Med. 2017 Mar-Apr;44[2]:157-60).
At that point, it might make sense to refer those patients to a wound and hyperbaric specialist for further evaluation and management, especially to a wound center that offers hyperbaric oxygen therapy.
These wound centers can be found in smaller towns. But some folks will have to travel, perhaps to a wound center at a hospital that has room and board like they do for cancer patients.
Q: What about treatment after surgery?
A: Using hyperbariatric oxygen therapy to treat inpatients with septic diabetic foot ulcers – Wagner grade 3 or higher – immediately after surgery may reduce length of stay as well as lower the risk of requiring multiple surgical debridements.
Q: What are the best-case scenarios for treatment?
A: A significant portion of what we do is limb preservation. Hyperbaric oxygen therapy often can help save a digit, forefoot, or even an extremity.
But it’s not something that just happens overnight. It’s a long-term process. Underlying complicating osteomyelitis may require up to 40-60 adjunctive hyperbaric oxygen treatments, 5 days a week with weekends off, along with concurrent antibiotics, wound care, and vascular interventions when indicated.
Q: Is insurance ever an issue for this treatment?
A: Typically, not if one follows the indications set by the Centers for Medicare & Medicaid Services and the Undersea and Hyperbaric Medical Society.
Medicare lists 15 medical indications that it will cover, and a majority of commercial insurers will cover the same 15 indications and possibly more. But commercial insurers may require prior authorization of medical necessity before preceding with hyperbaric oxygen therapy (Diving Hyperb Med. 2016 Sep;46[3]:133-4).
SAN DIEGO – Hyperbaric oxygen therapy, a mainstay of wound care, has a long and controversial history as a treatment for diabetic foot ulcers. Conflicting studies have spawned plenty of debate, and the most recent Cochrane Library review of existing research didn’t shed much light on the value of the treatment because the evidence was weak (Cochrane Database Syst Rev. 2015 Jun 24;[6]:CD004123).
But William H. Tettelbach, MD, a wound care specialist, told an audience at the annual scientific sessions of the American Diabetic Association that hyperbaric treatments are worth a try in certain cases. And he brought evidence to prove it – a 2015 report he coauthored that reviewed studies and offered clinical practice guidelines for hyperbaric oxygen therapy for the treatment of diabetic foot ulcers (DFUs) (Undersea Hyperb Med. 2015 May-Jun;42[3]:205-47).
In an interview, Dr. Tettelbach discussed ideal candidates for the treatment and offered clinical advice to endocrinologists.
Question: What did your review of research tell you about the value of hyperbaric oxygen treatment for DFUs?
Answer: We came to the same conclusion that most of the papers have indicated over the years: Hyperbaric oxygen is effective and attains goals such as reducing rates of amputation in a select population of diabetic ulcer patients.
Patients who have Wagner grade 3 or greater ulcers or admitted for surgery due to a septic diabetic foot benefit from an evaluation by a hyperbaric medicine–trained physician and treatment when indicated. There is evidence and years of clinical experience indicating that these patients benefit and have improved outcomes when evaluated and treated appropriately with hyperbaric oxygen therapy.
In the United States, hyperbaric oxygen therapy is not indicated in Wagner grade 2, 1 or 0 diabetic foot ulcers, the ulcers that involve soft tissue but not deep structure like bone.
Q: Why has there been so much controversy over the value of this treatment?
A: In the past, there have been problems with commercial outpatient wound centers that are heavily driven by profits. Financial margins in wound care clinics can be tight, and the need to remain profitable has at times resulted in patients being treated inappropriately with hyperbaric oxygen therapy (Adv Skin Wound Care. 2017 Apr;30[4]:181-90).
Q: Why does hyperbaric oxygen treatment work in some cases?
A: When you place a patient in a hyperbaric chamber where they breathe 100% oxygen under pressure, you increase the percentage of oxygen in the blood. At such a high percentage, oxygen saturates the plasma versus just being carried by red blood cells, thereby allowing the oxygen to penetrate farther into hypoxic tissues. By increasing the oxygen, you have the ability to make the environment unfavorable for rapid proliferation of anaerobic or microaerophilic bacteria that do not survive a highly oxygen-rich environment. Increasing tissue oxygen tension to 30 mm Hg or greater increases the macrophages’ ability to have an oxidative burst needed to kill bacteria. Furthermore, there are antibiotics that require certain levels of oxygen for transport across the bacterial cell wall.
Q: What should physicians understand about hyperbaric oxygen therapy for DFUs?
A: Overall, hyperbaric practitioners need to be more selective in identifying and treating patients according to what the evidence supports. Poorly designed trials with misleading results should not drive medical decisions. We should revisit diabetic foot ulcers through well-thought-out studies that target those who would benefit as suggested by current evidence. Prior trials have been heavily weighted with Wagner grade 1 and 2 candidates or ischemic diabetic ulcers that are not revascularized. These are biased toward poor outcomes since the current evidence does not strongly support treating these types of individuals with adjunctive hyperbaric oxygen therapy (Ont Health Technol Assess Ser. 2017 May 12;17[5]:1-142. eCollection 2017).
Q: What conditions should trigger endocrinologists to think about hyperbaric oxygen therapy for their DFU patients?
A: Candidates for the therapy include diabetic ulcers that have persisted for longer than 30 days, since these ulcers are at a significantly higher risk of a complicating infection, along with those that have failed treatment or are becoming more symptomatic over time (Undersea Hyperb Med. 2017 Mar-Apr;44[2]:157-60).
At that point, it might make sense to refer those patients to a wound and hyperbaric specialist for further evaluation and management, especially to a wound center that offers hyperbaric oxygen therapy.
These wound centers can be found in smaller towns. But some folks will have to travel, perhaps to a wound center at a hospital that has room and board like they do for cancer patients.
Q: What about treatment after surgery?
A: Using hyperbariatric oxygen therapy to treat inpatients with septic diabetic foot ulcers – Wagner grade 3 or higher – immediately after surgery may reduce length of stay as well as lower the risk of requiring multiple surgical debridements.
Q: What are the best-case scenarios for treatment?
A: A significant portion of what we do is limb preservation. Hyperbaric oxygen therapy often can help save a digit, forefoot, or even an extremity.
But it’s not something that just happens overnight. It’s a long-term process. Underlying complicating osteomyelitis may require up to 40-60 adjunctive hyperbaric oxygen treatments, 5 days a week with weekends off, along with concurrent antibiotics, wound care, and vascular interventions when indicated.
Q: Is insurance ever an issue for this treatment?
A: Typically, not if one follows the indications set by the Centers for Medicare & Medicaid Services and the Undersea and Hyperbaric Medical Society.
Medicare lists 15 medical indications that it will cover, and a majority of commercial insurers will cover the same 15 indications and possibly more. But commercial insurers may require prior authorization of medical necessity before preceding with hyperbaric oxygen therapy (Diving Hyperb Med. 2016 Sep;46[3]:133-4).
SAN DIEGO – Hyperbaric oxygen therapy, a mainstay of wound care, has a long and controversial history as a treatment for diabetic foot ulcers. Conflicting studies have spawned plenty of debate, and the most recent Cochrane Library review of existing research didn’t shed much light on the value of the treatment because the evidence was weak (Cochrane Database Syst Rev. 2015 Jun 24;[6]:CD004123).
But William H. Tettelbach, MD, a wound care specialist, told an audience at the annual scientific sessions of the American Diabetic Association that hyperbaric treatments are worth a try in certain cases. And he brought evidence to prove it – a 2015 report he coauthored that reviewed studies and offered clinical practice guidelines for hyperbaric oxygen therapy for the treatment of diabetic foot ulcers (DFUs) (Undersea Hyperb Med. 2015 May-Jun;42[3]:205-47).
In an interview, Dr. Tettelbach discussed ideal candidates for the treatment and offered clinical advice to endocrinologists.
Question: What did your review of research tell you about the value of hyperbaric oxygen treatment for DFUs?
Answer: We came to the same conclusion that most of the papers have indicated over the years: Hyperbaric oxygen is effective and attains goals such as reducing rates of amputation in a select population of diabetic ulcer patients.
Patients who have Wagner grade 3 or greater ulcers or admitted for surgery due to a septic diabetic foot benefit from an evaluation by a hyperbaric medicine–trained physician and treatment when indicated. There is evidence and years of clinical experience indicating that these patients benefit and have improved outcomes when evaluated and treated appropriately with hyperbaric oxygen therapy.
In the United States, hyperbaric oxygen therapy is not indicated in Wagner grade 2, 1 or 0 diabetic foot ulcers, the ulcers that involve soft tissue but not deep structure like bone.
Q: Why has there been so much controversy over the value of this treatment?
A: In the past, there have been problems with commercial outpatient wound centers that are heavily driven by profits. Financial margins in wound care clinics can be tight, and the need to remain profitable has at times resulted in patients being treated inappropriately with hyperbaric oxygen therapy (Adv Skin Wound Care. 2017 Apr;30[4]:181-90).
Q: Why does hyperbaric oxygen treatment work in some cases?
A: When you place a patient in a hyperbaric chamber where they breathe 100% oxygen under pressure, you increase the percentage of oxygen in the blood. At such a high percentage, oxygen saturates the plasma versus just being carried by red blood cells, thereby allowing the oxygen to penetrate farther into hypoxic tissues. By increasing the oxygen, you have the ability to make the environment unfavorable for rapid proliferation of anaerobic or microaerophilic bacteria that do not survive a highly oxygen-rich environment. Increasing tissue oxygen tension to 30 mm Hg or greater increases the macrophages’ ability to have an oxidative burst needed to kill bacteria. Furthermore, there are antibiotics that require certain levels of oxygen for transport across the bacterial cell wall.
Q: What should physicians understand about hyperbaric oxygen therapy for DFUs?
A: Overall, hyperbaric practitioners need to be more selective in identifying and treating patients according to what the evidence supports. Poorly designed trials with misleading results should not drive medical decisions. We should revisit diabetic foot ulcers through well-thought-out studies that target those who would benefit as suggested by current evidence. Prior trials have been heavily weighted with Wagner grade 1 and 2 candidates or ischemic diabetic ulcers that are not revascularized. These are biased toward poor outcomes since the current evidence does not strongly support treating these types of individuals with adjunctive hyperbaric oxygen therapy (Ont Health Technol Assess Ser. 2017 May 12;17[5]:1-142. eCollection 2017).
Q: What conditions should trigger endocrinologists to think about hyperbaric oxygen therapy for their DFU patients?
A: Candidates for the therapy include diabetic ulcers that have persisted for longer than 30 days, since these ulcers are at a significantly higher risk of a complicating infection, along with those that have failed treatment or are becoming more symptomatic over time (Undersea Hyperb Med. 2017 Mar-Apr;44[2]:157-60).
At that point, it might make sense to refer those patients to a wound and hyperbaric specialist for further evaluation and management, especially to a wound center that offers hyperbaric oxygen therapy.
These wound centers can be found in smaller towns. But some folks will have to travel, perhaps to a wound center at a hospital that has room and board like they do for cancer patients.
Q: What about treatment after surgery?
A: Using hyperbariatric oxygen therapy to treat inpatients with septic diabetic foot ulcers – Wagner grade 3 or higher – immediately after surgery may reduce length of stay as well as lower the risk of requiring multiple surgical debridements.
Q: What are the best-case scenarios for treatment?
A: A significant portion of what we do is limb preservation. Hyperbaric oxygen therapy often can help save a digit, forefoot, or even an extremity.
But it’s not something that just happens overnight. It’s a long-term process. Underlying complicating osteomyelitis may require up to 40-60 adjunctive hyperbaric oxygen treatments, 5 days a week with weekends off, along with concurrent antibiotics, wound care, and vascular interventions when indicated.
Q: Is insurance ever an issue for this treatment?
A: Typically, not if one follows the indications set by the Centers for Medicare & Medicaid Services and the Undersea and Hyperbaric Medical Society.
Medicare lists 15 medical indications that it will cover, and a majority of commercial insurers will cover the same 15 indications and possibly more. But commercial insurers may require prior authorization of medical necessity before preceding with hyperbaric oxygen therapy (Diving Hyperb Med. 2016 Sep;46[3]:133-4).
EXPERT ANALYSIS AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Neurologist to endocrinologists: Listen to your patients’ feet
SAN DIEGO – Listen to the feet of your patients, and don’t just focus on glucose control as a way to combat diabetic neuropathy, according to Eva L. Feldman, MD, PhD.
Regular foot exams are crucial for patients with diabetes because of the high risk of diabetic neuropathy (DN), Dr. Feldman, professor of neurology and director of the Program for Neurology Research and Discovery at University of Michigan, Ann Arbor, said in a presentation at the annual scientific sessions of the American Diabetes Association.
“Diabetic neuropathy has some important clinical consequences in a patient’s life. There’s clearly impaired function, a lower quality of life, and increased mortality. There’s also a high association between DN and cardiovascular disease and a high risk of amputation,” said Dr. Feldman, coauthor of the ADA’s new clinical guidelines for diabetic neuropathy (Diabetes Care. 2017;40[1]:136-54).
And in patients with type 2 diabetes, “diabetic neuropathy is not just hyperglycemia, which is what we’ve focused all our efforts on up until the past 5 years. There is also a role for dyslipidemia and other metabolic impairments,” she said.
In a follow-up interview, Dr. Feldman elaborated on a common misconception about DN, simple tools for foot exams by endocrinologists, and how to know when it’s time for a referral to a neurologist or podiatrist.
Q: What do you think physicians/endocrinologists misunderstand about diabetic neuropathy?
A: Commonly, physicians think if patients with diabetes do not complain of pain or numbness, they do not have DN. This simply isn’t correct. Over 80% of patients with DN have insensate feet – they simply do not have feeling in their feet. Physicians must examine a patient’s foot at least once a year to ensure the patient has not developed DN.
Q: What should endocrinologists understand about how diabetic neuropathy develops?
A: We know that excellent glucose control has a significant impact on DN in patients with type 1 diabetes. In patients with type 2 diabetes, we know that excellent glucose control plays a much less significant role. While it’s important, it must be coupled with control of other components of metabolic syndrome – elevated blood lipids, obesity, and hypertension.
A: Take a 126 Hz tuning fork and determine if the patient can feel vibration on the joint of the great toe for at least 10 seconds. Then take a 10-gram filament and a pin and determine if the patient can feel both of these instruments when they are applied to the joint of the great toe. Some physicians also take a 10-gram filament and apply it to the sole of the foot. I would not suggest using a pin on the sole of the foot.
Q: What else should they look for when they inspect feet? And how often would you recommend that endocrinologists do this per patient?
A: Inspection for callous formation, fissure formation, and fungal infections is important, and a foot exam should be done once yearly.
Q: What about testing whether patents can feel temperature?
A: The pin tests the same class of nerve fibers so this is routinely not done in an endocrinologist’s office.
Q: When should endocrinologists refer out for neuropathy?
A: Endocrinologists can treat DN by treating the diabetic condition and, in type 2 diabetes, the metabolic syndrome. Referral to a neurologist is indicated if there are atypical symptoms or signs, such as motor impairment that’s greater than sensory impairment, a significant asymmetry, or a very rapid onset. All patients with severe DN should be under the care of a podiatrist to prevent the development of nonhealing wounds and ulcers.
Q: What have you learned about how diabetic neuropathy affects the lives of patients?
A: DN definitely affects the quality of a patient’s life, not only in terms of work productivity and ability to perform activities of daily living. Quality of life and general enjoyment of life can frequently be adversely affected.
Q: How successful is treatment for diabetic neuropathy?
A: Treatment for pain can be very successful, and we have outlined a protocol in our recent ADA guidelines. For patients with uncontrollable pain, frequently a referral to a pain clinic is in order.
Dr. Feldman reports no relevant disclosures.
SAN DIEGO – Listen to the feet of your patients, and don’t just focus on glucose control as a way to combat diabetic neuropathy, according to Eva L. Feldman, MD, PhD.
Regular foot exams are crucial for patients with diabetes because of the high risk of diabetic neuropathy (DN), Dr. Feldman, professor of neurology and director of the Program for Neurology Research and Discovery at University of Michigan, Ann Arbor, said in a presentation at the annual scientific sessions of the American Diabetes Association.
“Diabetic neuropathy has some important clinical consequences in a patient’s life. There’s clearly impaired function, a lower quality of life, and increased mortality. There’s also a high association between DN and cardiovascular disease and a high risk of amputation,” said Dr. Feldman, coauthor of the ADA’s new clinical guidelines for diabetic neuropathy (Diabetes Care. 2017;40[1]:136-54).
And in patients with type 2 diabetes, “diabetic neuropathy is not just hyperglycemia, which is what we’ve focused all our efforts on up until the past 5 years. There is also a role for dyslipidemia and other metabolic impairments,” she said.
In a follow-up interview, Dr. Feldman elaborated on a common misconception about DN, simple tools for foot exams by endocrinologists, and how to know when it’s time for a referral to a neurologist or podiatrist.
Q: What do you think physicians/endocrinologists misunderstand about diabetic neuropathy?
A: Commonly, physicians think if patients with diabetes do not complain of pain or numbness, they do not have DN. This simply isn’t correct. Over 80% of patients with DN have insensate feet – they simply do not have feeling in their feet. Physicians must examine a patient’s foot at least once a year to ensure the patient has not developed DN.
Q: What should endocrinologists understand about how diabetic neuropathy develops?
A: We know that excellent glucose control has a significant impact on DN in patients with type 1 diabetes. In patients with type 2 diabetes, we know that excellent glucose control plays a much less significant role. While it’s important, it must be coupled with control of other components of metabolic syndrome – elevated blood lipids, obesity, and hypertension.
A: Take a 126 Hz tuning fork and determine if the patient can feel vibration on the joint of the great toe for at least 10 seconds. Then take a 10-gram filament and a pin and determine if the patient can feel both of these instruments when they are applied to the joint of the great toe. Some physicians also take a 10-gram filament and apply it to the sole of the foot. I would not suggest using a pin on the sole of the foot.
Q: What else should they look for when they inspect feet? And how often would you recommend that endocrinologists do this per patient?
A: Inspection for callous formation, fissure formation, and fungal infections is important, and a foot exam should be done once yearly.
Q: What about testing whether patents can feel temperature?
A: The pin tests the same class of nerve fibers so this is routinely not done in an endocrinologist’s office.
Q: When should endocrinologists refer out for neuropathy?
A: Endocrinologists can treat DN by treating the diabetic condition and, in type 2 diabetes, the metabolic syndrome. Referral to a neurologist is indicated if there are atypical symptoms or signs, such as motor impairment that’s greater than sensory impairment, a significant asymmetry, or a very rapid onset. All patients with severe DN should be under the care of a podiatrist to prevent the development of nonhealing wounds and ulcers.
Q: What have you learned about how diabetic neuropathy affects the lives of patients?
A: DN definitely affects the quality of a patient’s life, not only in terms of work productivity and ability to perform activities of daily living. Quality of life and general enjoyment of life can frequently be adversely affected.
Q: How successful is treatment for diabetic neuropathy?
A: Treatment for pain can be very successful, and we have outlined a protocol in our recent ADA guidelines. For patients with uncontrollable pain, frequently a referral to a pain clinic is in order.
Dr. Feldman reports no relevant disclosures.
SAN DIEGO – Listen to the feet of your patients, and don’t just focus on glucose control as a way to combat diabetic neuropathy, according to Eva L. Feldman, MD, PhD.
Regular foot exams are crucial for patients with diabetes because of the high risk of diabetic neuropathy (DN), Dr. Feldman, professor of neurology and director of the Program for Neurology Research and Discovery at University of Michigan, Ann Arbor, said in a presentation at the annual scientific sessions of the American Diabetes Association.
“Diabetic neuropathy has some important clinical consequences in a patient’s life. There’s clearly impaired function, a lower quality of life, and increased mortality. There’s also a high association between DN and cardiovascular disease and a high risk of amputation,” said Dr. Feldman, coauthor of the ADA’s new clinical guidelines for diabetic neuropathy (Diabetes Care. 2017;40[1]:136-54).
And in patients with type 2 diabetes, “diabetic neuropathy is not just hyperglycemia, which is what we’ve focused all our efforts on up until the past 5 years. There is also a role for dyslipidemia and other metabolic impairments,” she said.
In a follow-up interview, Dr. Feldman elaborated on a common misconception about DN, simple tools for foot exams by endocrinologists, and how to know when it’s time for a referral to a neurologist or podiatrist.
Q: What do you think physicians/endocrinologists misunderstand about diabetic neuropathy?
A: Commonly, physicians think if patients with diabetes do not complain of pain or numbness, they do not have DN. This simply isn’t correct. Over 80% of patients with DN have insensate feet – they simply do not have feeling in their feet. Physicians must examine a patient’s foot at least once a year to ensure the patient has not developed DN.
Q: What should endocrinologists understand about how diabetic neuropathy develops?
A: We know that excellent glucose control has a significant impact on DN in patients with type 1 diabetes. In patients with type 2 diabetes, we know that excellent glucose control plays a much less significant role. While it’s important, it must be coupled with control of other components of metabolic syndrome – elevated blood lipids, obesity, and hypertension.
A: Take a 126 Hz tuning fork and determine if the patient can feel vibration on the joint of the great toe for at least 10 seconds. Then take a 10-gram filament and a pin and determine if the patient can feel both of these instruments when they are applied to the joint of the great toe. Some physicians also take a 10-gram filament and apply it to the sole of the foot. I would not suggest using a pin on the sole of the foot.
Q: What else should they look for when they inspect feet? And how often would you recommend that endocrinologists do this per patient?
A: Inspection for callous formation, fissure formation, and fungal infections is important, and a foot exam should be done once yearly.
Q: What about testing whether patents can feel temperature?
A: The pin tests the same class of nerve fibers so this is routinely not done in an endocrinologist’s office.
Q: When should endocrinologists refer out for neuropathy?
A: Endocrinologists can treat DN by treating the diabetic condition and, in type 2 diabetes, the metabolic syndrome. Referral to a neurologist is indicated if there are atypical symptoms or signs, such as motor impairment that’s greater than sensory impairment, a significant asymmetry, or a very rapid onset. All patients with severe DN should be under the care of a podiatrist to prevent the development of nonhealing wounds and ulcers.
Q: What have you learned about how diabetic neuropathy affects the lives of patients?
A: DN definitely affects the quality of a patient’s life, not only in terms of work productivity and ability to perform activities of daily living. Quality of life and general enjoyment of life can frequently be adversely affected.
Q: How successful is treatment for diabetic neuropathy?
A: Treatment for pain can be very successful, and we have outlined a protocol in our recent ADA guidelines. For patients with uncontrollable pain, frequently a referral to a pain clinic is in order.
Dr. Feldman reports no relevant disclosures.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Risk of sexual dysfunction in diabetes is high, but treatments can help
SAN DIEGO –
This isn’t normal for men of that age, according to Hunter B. Wessells, MD.“It’s not just that they’re aging. It’s a 20-year acceleration of the aging process,” he said at the annual scientific sessions of the American Diabetes Association.
That’s not all. In some cases, men with diabetes may experience decreased libido that’s potentially caused by low testosterone, said Dr. Wessells, professor and Wilma Wise Nelson, Ole A. Nelson, and Mabel Wise Nelson Endowed Chair in Urology at the University of Washington, Seattle
Still, research findings offer useful insights into the frequency of sexual dysfunction in people with diabetes and the potential – and limitations – of available treatments, said Dr. Wessells.
In patients with well-controlled diabetes, “these conditions impact quality of life to a greater degree than complications like nephropathy, neuropathy, and retinopathy,” he said in an interview. “Thus, treatment of urological symptoms can be a high-yield endeavor.”
In both sexes, Dr. Wessells said, diabetes can disrupt the mechanism of desire, arousal, and orgasm by affecting a long list of bodily functions such as central nervous system stimulation, hormone activity, autonomic and somatic nerve activity, and processing of calcium ions and nitric acid.
In men, diabetes boosts the risk of erectile dysfunction to a larger extent than do related conditions such as obesity, heart disease, and depression. “But they are interrelated,” he said. “The primary mechanisms include the metabolic effects of high glucose, autonomic nerve damage, and microvascular disease.”
Low testosterone levels also can cause problems in patients with diabetes, he said. “Type 2 diabetes has greater effects on testosterone than type 1. It is most closely linked to weight in the type 1 population and affects only a small percentage.”
A 2017 systematic review and meta-analysis of 145 studies with more than 88,000 subjects (average age 55.8 ± 7.9 years) suggests that ED was more common in type 2 diabetes (66.3%) than type 1 diabetes (37.5%) after statistical adjustment to account for publication bias (Diabet Med. 2017 Jul 18. doi: 10.1111/dme.13403).
A smaller analysis found that men with diabetes had almost four times the odds (odd ratio = 3.62) of ED compared with healthy controls (Diabet Med. 2017 Jul 18. doi: 10.1111/dme.13403). Phosphodiesterase-5 inhibitors – such as sildenafil, vardenafil, and tadalafil – are one option for men with diabetes and ED, Dr. Wessells said. “They work pretty well, but men with diabetes tend to have more severe ED. They’re going to get better, but will they get better enough to be normal? That’s the question.”
A 2007 Cochrane Library analysis found that men with diabetes and ED gained from PDE5 inhibitors overall (Cochrane Database Syst Rev. 2007 Jan 24[1]:CD002187. doi: 10.1002/14651858.CD002187.pub3).
“They’re not going to do as well as the general population,” Dr. Wessells said, “but we should try these as first-line agents in absence of things like severe unstable cardiovascular disease and other risk factors.”
Second-line therapies, typically offered by urologists, include penile prostheses and injection therapy, he said. A 2014 analysis of previous research found that men with diabetes were “more than 50% more likely to be prescribed secondary ED treatments over the 2-year observation period, and more than twice as likely to undergo penile prosthesis surgery” (Int J Impot Res. 2014 May-Jun;26[3]:112-5).
As for women, a 2009 study found that of 424 sexually active women with type 1 diabetes (97% of whom were white), 35% showed signs of female sexual dysfunction (FSD). Of those with FSD, problems included loss of libido (57%); problems with orgasm (51%), lubrication (47%), and/or arousal (38%); and pain (21%) (Diabetes Care. 2009 May;32[5]:780-5).
Only one drug, flibanserin (Addyi), is approved for FSD in the United States. Its impact on patients with diabetes is unknown, Dr. Wessells said, and the drug has the potential for significant adverse events.
The good news: Research is providing insight into which men and women are more likely to develop sexual dysfunction, Dr. Wessells said.
Age is important in both genders. For women, depression and being married appear to be risk factors, he said. “This needs more exploration to help us understand how to intervene.”
And in men, he said, ED is linked to jumps in hemoglobin A1c, while men on intensive glycemic therapy have a lower risk.
“Maybe we can find out who needs to be targeted for earlier intervention,” he said. This is especially important for men because ED becomes more likely to be irreversible after just a few years, he said.
Dr. Wessells reports no relevant disclosures.
SAN DIEGO –
This isn’t normal for men of that age, according to Hunter B. Wessells, MD.“It’s not just that they’re aging. It’s a 20-year acceleration of the aging process,” he said at the annual scientific sessions of the American Diabetes Association.
That’s not all. In some cases, men with diabetes may experience decreased libido that’s potentially caused by low testosterone, said Dr. Wessells, professor and Wilma Wise Nelson, Ole A. Nelson, and Mabel Wise Nelson Endowed Chair in Urology at the University of Washington, Seattle
Still, research findings offer useful insights into the frequency of sexual dysfunction in people with diabetes and the potential – and limitations – of available treatments, said Dr. Wessells.
In patients with well-controlled diabetes, “these conditions impact quality of life to a greater degree than complications like nephropathy, neuropathy, and retinopathy,” he said in an interview. “Thus, treatment of urological symptoms can be a high-yield endeavor.”
In both sexes, Dr. Wessells said, diabetes can disrupt the mechanism of desire, arousal, and orgasm by affecting a long list of bodily functions such as central nervous system stimulation, hormone activity, autonomic and somatic nerve activity, and processing of calcium ions and nitric acid.
In men, diabetes boosts the risk of erectile dysfunction to a larger extent than do related conditions such as obesity, heart disease, and depression. “But they are interrelated,” he said. “The primary mechanisms include the metabolic effects of high glucose, autonomic nerve damage, and microvascular disease.”
Low testosterone levels also can cause problems in patients with diabetes, he said. “Type 2 diabetes has greater effects on testosterone than type 1. It is most closely linked to weight in the type 1 population and affects only a small percentage.”
A 2017 systematic review and meta-analysis of 145 studies with more than 88,000 subjects (average age 55.8 ± 7.9 years) suggests that ED was more common in type 2 diabetes (66.3%) than type 1 diabetes (37.5%) after statistical adjustment to account for publication bias (Diabet Med. 2017 Jul 18. doi: 10.1111/dme.13403).
A smaller analysis found that men with diabetes had almost four times the odds (odd ratio = 3.62) of ED compared with healthy controls (Diabet Med. 2017 Jul 18. doi: 10.1111/dme.13403). Phosphodiesterase-5 inhibitors – such as sildenafil, vardenafil, and tadalafil – are one option for men with diabetes and ED, Dr. Wessells said. “They work pretty well, but men with diabetes tend to have more severe ED. They’re going to get better, but will they get better enough to be normal? That’s the question.”
A 2007 Cochrane Library analysis found that men with diabetes and ED gained from PDE5 inhibitors overall (Cochrane Database Syst Rev. 2007 Jan 24[1]:CD002187. doi: 10.1002/14651858.CD002187.pub3).
“They’re not going to do as well as the general population,” Dr. Wessells said, “but we should try these as first-line agents in absence of things like severe unstable cardiovascular disease and other risk factors.”
Second-line therapies, typically offered by urologists, include penile prostheses and injection therapy, he said. A 2014 analysis of previous research found that men with diabetes were “more than 50% more likely to be prescribed secondary ED treatments over the 2-year observation period, and more than twice as likely to undergo penile prosthesis surgery” (Int J Impot Res. 2014 May-Jun;26[3]:112-5).
As for women, a 2009 study found that of 424 sexually active women with type 1 diabetes (97% of whom were white), 35% showed signs of female sexual dysfunction (FSD). Of those with FSD, problems included loss of libido (57%); problems with orgasm (51%), lubrication (47%), and/or arousal (38%); and pain (21%) (Diabetes Care. 2009 May;32[5]:780-5).
Only one drug, flibanserin (Addyi), is approved for FSD in the United States. Its impact on patients with diabetes is unknown, Dr. Wessells said, and the drug has the potential for significant adverse events.
The good news: Research is providing insight into which men and women are more likely to develop sexual dysfunction, Dr. Wessells said.
Age is important in both genders. For women, depression and being married appear to be risk factors, he said. “This needs more exploration to help us understand how to intervene.”
And in men, he said, ED is linked to jumps in hemoglobin A1c, while men on intensive glycemic therapy have a lower risk.
“Maybe we can find out who needs to be targeted for earlier intervention,” he said. This is especially important for men because ED becomes more likely to be irreversible after just a few years, he said.
Dr. Wessells reports no relevant disclosures.
SAN DIEGO –
This isn’t normal for men of that age, according to Hunter B. Wessells, MD.“It’s not just that they’re aging. It’s a 20-year acceleration of the aging process,” he said at the annual scientific sessions of the American Diabetes Association.
That’s not all. In some cases, men with diabetes may experience decreased libido that’s potentially caused by low testosterone, said Dr. Wessells, professor and Wilma Wise Nelson, Ole A. Nelson, and Mabel Wise Nelson Endowed Chair in Urology at the University of Washington, Seattle
Still, research findings offer useful insights into the frequency of sexual dysfunction in people with diabetes and the potential – and limitations – of available treatments, said Dr. Wessells.
In patients with well-controlled diabetes, “these conditions impact quality of life to a greater degree than complications like nephropathy, neuropathy, and retinopathy,” he said in an interview. “Thus, treatment of urological symptoms can be a high-yield endeavor.”
In both sexes, Dr. Wessells said, diabetes can disrupt the mechanism of desire, arousal, and orgasm by affecting a long list of bodily functions such as central nervous system stimulation, hormone activity, autonomic and somatic nerve activity, and processing of calcium ions and nitric acid.
In men, diabetes boosts the risk of erectile dysfunction to a larger extent than do related conditions such as obesity, heart disease, and depression. “But they are interrelated,” he said. “The primary mechanisms include the metabolic effects of high glucose, autonomic nerve damage, and microvascular disease.”
Low testosterone levels also can cause problems in patients with diabetes, he said. “Type 2 diabetes has greater effects on testosterone than type 1. It is most closely linked to weight in the type 1 population and affects only a small percentage.”
A 2017 systematic review and meta-analysis of 145 studies with more than 88,000 subjects (average age 55.8 ± 7.9 years) suggests that ED was more common in type 2 diabetes (66.3%) than type 1 diabetes (37.5%) after statistical adjustment to account for publication bias (Diabet Med. 2017 Jul 18. doi: 10.1111/dme.13403).
A smaller analysis found that men with diabetes had almost four times the odds (odd ratio = 3.62) of ED compared with healthy controls (Diabet Med. 2017 Jul 18. doi: 10.1111/dme.13403). Phosphodiesterase-5 inhibitors – such as sildenafil, vardenafil, and tadalafil – are one option for men with diabetes and ED, Dr. Wessells said. “They work pretty well, but men with diabetes tend to have more severe ED. They’re going to get better, but will they get better enough to be normal? That’s the question.”
A 2007 Cochrane Library analysis found that men with diabetes and ED gained from PDE5 inhibitors overall (Cochrane Database Syst Rev. 2007 Jan 24[1]:CD002187. doi: 10.1002/14651858.CD002187.pub3).
“They’re not going to do as well as the general population,” Dr. Wessells said, “but we should try these as first-line agents in absence of things like severe unstable cardiovascular disease and other risk factors.”
Second-line therapies, typically offered by urologists, include penile prostheses and injection therapy, he said. A 2014 analysis of previous research found that men with diabetes were “more than 50% more likely to be prescribed secondary ED treatments over the 2-year observation period, and more than twice as likely to undergo penile prosthesis surgery” (Int J Impot Res. 2014 May-Jun;26[3]:112-5).
As for women, a 2009 study found that of 424 sexually active women with type 1 diabetes (97% of whom were white), 35% showed signs of female sexual dysfunction (FSD). Of those with FSD, problems included loss of libido (57%); problems with orgasm (51%), lubrication (47%), and/or arousal (38%); and pain (21%) (Diabetes Care. 2009 May;32[5]:780-5).
Only one drug, flibanserin (Addyi), is approved for FSD in the United States. Its impact on patients with diabetes is unknown, Dr. Wessells said, and the drug has the potential for significant adverse events.
The good news: Research is providing insight into which men and women are more likely to develop sexual dysfunction, Dr. Wessells said.
Age is important in both genders. For women, depression and being married appear to be risk factors, he said. “This needs more exploration to help us understand how to intervene.”
And in men, he said, ED is linked to jumps in hemoglobin A1c, while men on intensive glycemic therapy have a lower risk.
“Maybe we can find out who needs to be targeted for earlier intervention,” he said. This is especially important for men because ED becomes more likely to be irreversible after just a few years, he said.
Dr. Wessells reports no relevant disclosures.
EXPERT ANALYSIS AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Transplant safety has improved for patients with diabetes
SAN DIEGO – The risks for patients with diabetes who face organ transplants have diminished greatly, according to an endocrinologist.
But there are still many limitations for these patients – some transplants are not appropriate for patients with diabetes – and there are potential complications when they do get transplants.
“While it used to be that outcomes were worse in patients with diabetes, such as more infections and higher mortality, this has become much less so over time, because of team-based care and better focus on diabetes management postoperatively,” said Jennifer Larsen, MD. Still, “the complexities come with the variable other factors and conditions the patient might have, such as autonomic neuropathies, the sudden variations in kidney function that can occur before and after transplant, and the impact of the transplant medications on other aspects of diabetes care such as lipid management and metabolism of other drugs.”
“They also take care of diabetes patients who get other types of transplant such as heart transplant, liver, and lung,” said Dr. Larsen, vice chancellor for research and professor of internal medicine at University of Nebraska Medical Center, Omaha.
And, she added, they take care of patients who develop diabetes after transplants – posttransplant diabetes. “So it’s important to the endocrinologist today to be familiar with the transplant world, the medicines used, and how chronic kidney disease impacts diabetes management,” she said.
Endocrinologists serve in a variety of roles when patients need transplants, she added. “In some cases the transplant surgeon is referring to us, the endocrinologist. If the patient is heading toward kidney transplant in particular, the pancreas and islet options with kidney transplants would all be handled by the transplant nephrologists, who work hand in hand with the transplant surgeons. Some endocrinologists are embedded in these teams, too.”
Patients with diabetes complicated by chronic kidney disease may be eligible for a transplant of a kidney – in line with the adage that “any kidney is better than dialysis,” Dr. Larsen said – or kidney/pancreas or kidney/islet transplants.
Kidney/pancreas and kidney/islet transplants may be performed simultaneously or with the kidney transplant first. However, islet transplants are not appropriate for patients with type 2 diabetes, and these patients also may not be eligible for simultaneous kidney/pancreas transplants.
According to the United Network for Organ Sharing, there were more than 415,075 kidney transplants from Jan. 1, 1988, to June 30, 2017 (www.unos.org/data). The numbers for pancreas and kidney/pancreas transplants are 8,462 and 22,496, respectively. Islet transplant numbers were not available.
Five-year patient survival rates for kidney transplants are 85%, and graft survival rates are 71%, Dr. Larsen said, and they’re similar for kidney/pancreas transplants. According to Dr. Larsen, patient survival is lower after islet transplantation.
Adjusted patient and graft survival rates in kidney transplants are the same among nondiabetic patients and those with diabetes (Nephrol Dial Transplant. 2002.17[9]:1678-83).
Diabetes complications can affect patient eligibility for these kinds of transplants, Dr. Larsen said, and weight can be a complicating factor. The drugs used in transplants in patients with higher body mass indexes exacerbate insulin resistance, Dr. Larsen said, “and that will make it harder to manage afterward. We haven’t worked out if BMI affects graft function over time.”
Reduced cardiac function eliminates simultaneous pancreas/kidney (SPK) transplants as an option for patients with diabetes, while blindness, severe hypoglycemia unawareness, and other autonomic neuropathies can make SPK more appropriate. Gastroparesis, meanwhile, can be an issue for all transplants.
Dr. Larsen’s own research has suggested that SPK transplants can be better than kidney transplant alone in terms of improving neuropathy, symptoms of peripheral neuropathy, and, perhaps, gastroparesis symptoms. However, SPK is not better in terms of improving bladder neuropathy, eye disease, amputations, and cardiac complications of diabetes (Endocr Rev. 2004;25[6]:919-46).
While the prognosis for transplants in diabetes patients is often promising Dr. Larsen cautioned that there can still be a big obstacle: Primary care physicians who fail to act.
“Most diabetes patients are not managed by endocrinologists,” she said, “and there are still many primary care physicians who delay referral to transplant teams for their diabetes patients or even to endocrinologists when they are struggling with diabetes management.”
Dr. Larsen reports no relevant disclosures.
SAN DIEGO – The risks for patients with diabetes who face organ transplants have diminished greatly, according to an endocrinologist.
But there are still many limitations for these patients – some transplants are not appropriate for patients with diabetes – and there are potential complications when they do get transplants.
“While it used to be that outcomes were worse in patients with diabetes, such as more infections and higher mortality, this has become much less so over time, because of team-based care and better focus on diabetes management postoperatively,” said Jennifer Larsen, MD. Still, “the complexities come with the variable other factors and conditions the patient might have, such as autonomic neuropathies, the sudden variations in kidney function that can occur before and after transplant, and the impact of the transplant medications on other aspects of diabetes care such as lipid management and metabolism of other drugs.”
“They also take care of diabetes patients who get other types of transplant such as heart transplant, liver, and lung,” said Dr. Larsen, vice chancellor for research and professor of internal medicine at University of Nebraska Medical Center, Omaha.
And, she added, they take care of patients who develop diabetes after transplants – posttransplant diabetes. “So it’s important to the endocrinologist today to be familiar with the transplant world, the medicines used, and how chronic kidney disease impacts diabetes management,” she said.
Endocrinologists serve in a variety of roles when patients need transplants, she added. “In some cases the transplant surgeon is referring to us, the endocrinologist. If the patient is heading toward kidney transplant in particular, the pancreas and islet options with kidney transplants would all be handled by the transplant nephrologists, who work hand in hand with the transplant surgeons. Some endocrinologists are embedded in these teams, too.”
Patients with diabetes complicated by chronic kidney disease may be eligible for a transplant of a kidney – in line with the adage that “any kidney is better than dialysis,” Dr. Larsen said – or kidney/pancreas or kidney/islet transplants.
Kidney/pancreas and kidney/islet transplants may be performed simultaneously or with the kidney transplant first. However, islet transplants are not appropriate for patients with type 2 diabetes, and these patients also may not be eligible for simultaneous kidney/pancreas transplants.
According to the United Network for Organ Sharing, there were more than 415,075 kidney transplants from Jan. 1, 1988, to June 30, 2017 (www.unos.org/data). The numbers for pancreas and kidney/pancreas transplants are 8,462 and 22,496, respectively. Islet transplant numbers were not available.
Five-year patient survival rates for kidney transplants are 85%, and graft survival rates are 71%, Dr. Larsen said, and they’re similar for kidney/pancreas transplants. According to Dr. Larsen, patient survival is lower after islet transplantation.
Adjusted patient and graft survival rates in kidney transplants are the same among nondiabetic patients and those with diabetes (Nephrol Dial Transplant. 2002.17[9]:1678-83).
Diabetes complications can affect patient eligibility for these kinds of transplants, Dr. Larsen said, and weight can be a complicating factor. The drugs used in transplants in patients with higher body mass indexes exacerbate insulin resistance, Dr. Larsen said, “and that will make it harder to manage afterward. We haven’t worked out if BMI affects graft function over time.”
Reduced cardiac function eliminates simultaneous pancreas/kidney (SPK) transplants as an option for patients with diabetes, while blindness, severe hypoglycemia unawareness, and other autonomic neuropathies can make SPK more appropriate. Gastroparesis, meanwhile, can be an issue for all transplants.
Dr. Larsen’s own research has suggested that SPK transplants can be better than kidney transplant alone in terms of improving neuropathy, symptoms of peripheral neuropathy, and, perhaps, gastroparesis symptoms. However, SPK is not better in terms of improving bladder neuropathy, eye disease, amputations, and cardiac complications of diabetes (Endocr Rev. 2004;25[6]:919-46).
While the prognosis for transplants in diabetes patients is often promising Dr. Larsen cautioned that there can still be a big obstacle: Primary care physicians who fail to act.
“Most diabetes patients are not managed by endocrinologists,” she said, “and there are still many primary care physicians who delay referral to transplant teams for their diabetes patients or even to endocrinologists when they are struggling with diabetes management.”
Dr. Larsen reports no relevant disclosures.
SAN DIEGO – The risks for patients with diabetes who face organ transplants have diminished greatly, according to an endocrinologist.
But there are still many limitations for these patients – some transplants are not appropriate for patients with diabetes – and there are potential complications when they do get transplants.
“While it used to be that outcomes were worse in patients with diabetes, such as more infections and higher mortality, this has become much less so over time, because of team-based care and better focus on diabetes management postoperatively,” said Jennifer Larsen, MD. Still, “the complexities come with the variable other factors and conditions the patient might have, such as autonomic neuropathies, the sudden variations in kidney function that can occur before and after transplant, and the impact of the transplant medications on other aspects of diabetes care such as lipid management and metabolism of other drugs.”
“They also take care of diabetes patients who get other types of transplant such as heart transplant, liver, and lung,” said Dr. Larsen, vice chancellor for research and professor of internal medicine at University of Nebraska Medical Center, Omaha.
And, she added, they take care of patients who develop diabetes after transplants – posttransplant diabetes. “So it’s important to the endocrinologist today to be familiar with the transplant world, the medicines used, and how chronic kidney disease impacts diabetes management,” she said.
Endocrinologists serve in a variety of roles when patients need transplants, she added. “In some cases the transplant surgeon is referring to us, the endocrinologist. If the patient is heading toward kidney transplant in particular, the pancreas and islet options with kidney transplants would all be handled by the transplant nephrologists, who work hand in hand with the transplant surgeons. Some endocrinologists are embedded in these teams, too.”
Patients with diabetes complicated by chronic kidney disease may be eligible for a transplant of a kidney – in line with the adage that “any kidney is better than dialysis,” Dr. Larsen said – or kidney/pancreas or kidney/islet transplants.
Kidney/pancreas and kidney/islet transplants may be performed simultaneously or with the kidney transplant first. However, islet transplants are not appropriate for patients with type 2 diabetes, and these patients also may not be eligible for simultaneous kidney/pancreas transplants.
According to the United Network for Organ Sharing, there were more than 415,075 kidney transplants from Jan. 1, 1988, to June 30, 2017 (www.unos.org/data). The numbers for pancreas and kidney/pancreas transplants are 8,462 and 22,496, respectively. Islet transplant numbers were not available.
Five-year patient survival rates for kidney transplants are 85%, and graft survival rates are 71%, Dr. Larsen said, and they’re similar for kidney/pancreas transplants. According to Dr. Larsen, patient survival is lower after islet transplantation.
Adjusted patient and graft survival rates in kidney transplants are the same among nondiabetic patients and those with diabetes (Nephrol Dial Transplant. 2002.17[9]:1678-83).
Diabetes complications can affect patient eligibility for these kinds of transplants, Dr. Larsen said, and weight can be a complicating factor. The drugs used in transplants in patients with higher body mass indexes exacerbate insulin resistance, Dr. Larsen said, “and that will make it harder to manage afterward. We haven’t worked out if BMI affects graft function over time.”
Reduced cardiac function eliminates simultaneous pancreas/kidney (SPK) transplants as an option for patients with diabetes, while blindness, severe hypoglycemia unawareness, and other autonomic neuropathies can make SPK more appropriate. Gastroparesis, meanwhile, can be an issue for all transplants.
Dr. Larsen’s own research has suggested that SPK transplants can be better than kidney transplant alone in terms of improving neuropathy, symptoms of peripheral neuropathy, and, perhaps, gastroparesis symptoms. However, SPK is not better in terms of improving bladder neuropathy, eye disease, amputations, and cardiac complications of diabetes (Endocr Rev. 2004;25[6]:919-46).
While the prognosis for transplants in diabetes patients is often promising Dr. Larsen cautioned that there can still be a big obstacle: Primary care physicians who fail to act.
“Most diabetes patients are not managed by endocrinologists,” she said, “and there are still many primary care physicians who delay referral to transplant teams for their diabetes patients or even to endocrinologists when they are struggling with diabetes management.”
Dr. Larsen reports no relevant disclosures.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Exercise, CBT linked to higher drop in depression in type 2 diabetes
SAN DIEGO – The odds of full remission from clinically diagnosed major depression greatly improved in patients with type 2 diabetes who took part in 12-week supervised exercise and cognitive behavioral therapy (CBT). By the end of the study, 96% of the CBT participants no longer met diagnostic criteria for major depression, compared with just 65% of those on usual care, judging from the findings of a new study.
Another approach – a combination treatment of both exercise and CBT therapies – did not show a statistically significant effect on full remission rates but showed improvement in some other areas.
Dr. De Groot and her colleagues recruited 140 adults – mean age 57 years, 77% female, 71% white, 52% married – who had a diagnosis of both type 2 diabetes and diagnosed clinical depression. They came from three states and had various levels of income and educational background.
The researchers randomly assigned the participants to usual care, 12 weeks of exercise with a personal trainer, 10 individual CBT sessions, or a combination of both exercise and CBT therapies. There were 34-36 participants in each group.
The researchers found improvements in depressive symptoms (P less than .05); negative automatic thoughts (P less than .03), and diabetes distress (P less than .01) and physical quality of life (P less than .03 for all except P greater than 0.1 for CBT) for all three intervention groups compared with usual care. Diabetes-specific quality of life improved in the exercise and combination groups only (P less than .01).
The researchers calculated odds ratios of full or partial remission as 12.4 (CBT) and 5.8 (exercise) compared to usual care (P less than .03), controlling for changes in antidepressant drugs. However, the odds ratio for combination therapy was 2.3 and not deemed statistically significant (P = .218).
The researchers also examined results in subjects with a baseline hemoglobin A1c of 7% or higher and found evidence linking the exercise therapy to clinically meaningful 0.7% improvements in HbA1c (P less than .04).
It’s also not clear whether the interventions will hold up over the long term.
The National Institutes of Health–National Institute of Diabetes and Digestive and Kidney Diseases funded the study. Dr. De Groot reported no relevant disclosures.
SAN DIEGO – The odds of full remission from clinically diagnosed major depression greatly improved in patients with type 2 diabetes who took part in 12-week supervised exercise and cognitive behavioral therapy (CBT). By the end of the study, 96% of the CBT participants no longer met diagnostic criteria for major depression, compared with just 65% of those on usual care, judging from the findings of a new study.
Another approach – a combination treatment of both exercise and CBT therapies – did not show a statistically significant effect on full remission rates but showed improvement in some other areas.
Dr. De Groot and her colleagues recruited 140 adults – mean age 57 years, 77% female, 71% white, 52% married – who had a diagnosis of both type 2 diabetes and diagnosed clinical depression. They came from three states and had various levels of income and educational background.
The researchers randomly assigned the participants to usual care, 12 weeks of exercise with a personal trainer, 10 individual CBT sessions, or a combination of both exercise and CBT therapies. There were 34-36 participants in each group.
The researchers found improvements in depressive symptoms (P less than .05); negative automatic thoughts (P less than .03), and diabetes distress (P less than .01) and physical quality of life (P less than .03 for all except P greater than 0.1 for CBT) for all three intervention groups compared with usual care. Diabetes-specific quality of life improved in the exercise and combination groups only (P less than .01).
The researchers calculated odds ratios of full or partial remission as 12.4 (CBT) and 5.8 (exercise) compared to usual care (P less than .03), controlling for changes in antidepressant drugs. However, the odds ratio for combination therapy was 2.3 and not deemed statistically significant (P = .218).
The researchers also examined results in subjects with a baseline hemoglobin A1c of 7% or higher and found evidence linking the exercise therapy to clinically meaningful 0.7% improvements in HbA1c (P less than .04).
It’s also not clear whether the interventions will hold up over the long term.
The National Institutes of Health–National Institute of Diabetes and Digestive and Kidney Diseases funded the study. Dr. De Groot reported no relevant disclosures.
SAN DIEGO – The odds of full remission from clinically diagnosed major depression greatly improved in patients with type 2 diabetes who took part in 12-week supervised exercise and cognitive behavioral therapy (CBT). By the end of the study, 96% of the CBT participants no longer met diagnostic criteria for major depression, compared with just 65% of those on usual care, judging from the findings of a new study.
Another approach – a combination treatment of both exercise and CBT therapies – did not show a statistically significant effect on full remission rates but showed improvement in some other areas.
Dr. De Groot and her colleagues recruited 140 adults – mean age 57 years, 77% female, 71% white, 52% married – who had a diagnosis of both type 2 diabetes and diagnosed clinical depression. They came from three states and had various levels of income and educational background.
The researchers randomly assigned the participants to usual care, 12 weeks of exercise with a personal trainer, 10 individual CBT sessions, or a combination of both exercise and CBT therapies. There were 34-36 participants in each group.
The researchers found improvements in depressive symptoms (P less than .05); negative automatic thoughts (P less than .03), and diabetes distress (P less than .01) and physical quality of life (P less than .03 for all except P greater than 0.1 for CBT) for all three intervention groups compared with usual care. Diabetes-specific quality of life improved in the exercise and combination groups only (P less than .01).
The researchers calculated odds ratios of full or partial remission as 12.4 (CBT) and 5.8 (exercise) compared to usual care (P less than .03), controlling for changes in antidepressant drugs. However, the odds ratio for combination therapy was 2.3 and not deemed statistically significant (P = .218).
The researchers also examined results in subjects with a baseline hemoglobin A1c of 7% or higher and found evidence linking the exercise therapy to clinically meaningful 0.7% improvements in HbA1c (P less than .04).
It’s also not clear whether the interventions will hold up over the long term.
The National Institutes of Health–National Institute of Diabetes and Digestive and Kidney Diseases funded the study. Dr. De Groot reported no relevant disclosures.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point: Supervised exercise and cognitive behavioral therapies are linked to higher rates of recovery from major depression at 12 weeks in patients with type 2 diabetes.
Major finding: Full or partial remission was more likely in CBT and exercise groups compared with usual care after researchers controlled for changes in antidepressant drugs.
Data source: Prospective study of 140 adults with type 2 diabetes randomly assigned to 12 weeks of exercise with a physical trainer, 10 individual CBT sessions, a combination of the two therapies, or usual care.
Disclosures: The National Institutes of Health–National Institute of Diabetes and Digestive and Kidney Diseases funded the study.
Statins linked to fewer deaths, slightly higher HbA1c in T2DM
SAN DIEGO – Findings from a study of 12,725 new insulin users with type 2 diabetes mellitus (T2DM) show that those who did not take statins were slightly better able to control their blood sugar, but those who used the cholesterol-lowering drugs lived longer and had fewer cardiac events.
“It is clear that, in this high-risk population, the benefits of statins on cardiovascular outcomes outweigh the small adverse metabolic effects on glycemic control,” study lead author Uche Anyanwagu, MBBS, MSc, a graduate student and research fellow at the University of Nottingham (England), said in an interview about his data presented in a poster at the annual scientific sessions of the American Diabetes Association.
However, “there remain great concerns about statins and their modest adverse effects on glucose and insulin metabolism in increasing the risk of new-onset diabetes or worsening glycemic control, especially in higher doses and with the more potent statins,” Dr. Anyanwagu said. “Very little is known about the relationship between statin use and glycemic control in patients with established T2DM.”
The study authors retrospectively analyzed medical records of 12,725 new insulin users with T2DM from a British primary care database. “The commencement of insulin represents a group of patients with T2DM with longer disease duration and more complex needs, many of whom have other comorbid illnesses,” Dr. Anyanwagu said.
The average age of the subjects was 58.6 years (standard deviation, 13.8), and 50% were female (P less than 0.001 for both). Their average HbA1c level was 8.7 (SD, 1.8; P = .556), and 63% were obese.
The numbers of statin users and nonstatin users were 10,682 and 2,043, respectively. Researchers found that the statin users performed better in measures of all-cause mortality, nonfatal stroke, and a composite outcome measure but not in acute myocardial infarction.
In all-cause mortality, the absolute rate over 5 years was 9.5 deaths per 1,000 person years (95% confidence interval, 8.7-10.5) for statin users and 24.9 (95% CI, 21.5-28.9) for nonusers. After adjustment for age, gender, duration of insulin use, albumin, glomerular filtration rate, lipid profile, and coronary heart disease, the hazard ratio was 1.89 (95% CI, 1.51-2.37) for nonusers, compared with a reference of 1 for users. (P less than .0001).
The absolute rate for a composite outcome – encompassing measures of all-cause mortality, nonfatal acute myocardial infarction, and nonfatal stroke – was 20.7 (95% CI, 19.3-22.1) for statin users and 30.9 (95% CI , 27-35.3) for nonusers (P less than .0001).
As for HbA1c levels, at 12 months, they’d fallen by an average of 0.29% in the statin group and 0.37% in the nonuser group (P = .021). At 36 months, there was a smaller difference (–0.31% vs. –0.35%, respectively), and it wasn’t statistically significant (P = .344).
The study was funded by the University of Nottingham. (England). Dr. Anyanwagu reports no relevant disclosures.
SAN DIEGO – Findings from a study of 12,725 new insulin users with type 2 diabetes mellitus (T2DM) show that those who did not take statins were slightly better able to control their blood sugar, but those who used the cholesterol-lowering drugs lived longer and had fewer cardiac events.
“It is clear that, in this high-risk population, the benefits of statins on cardiovascular outcomes outweigh the small adverse metabolic effects on glycemic control,” study lead author Uche Anyanwagu, MBBS, MSc, a graduate student and research fellow at the University of Nottingham (England), said in an interview about his data presented in a poster at the annual scientific sessions of the American Diabetes Association.
However, “there remain great concerns about statins and their modest adverse effects on glucose and insulin metabolism in increasing the risk of new-onset diabetes or worsening glycemic control, especially in higher doses and with the more potent statins,” Dr. Anyanwagu said. “Very little is known about the relationship between statin use and glycemic control in patients with established T2DM.”
The study authors retrospectively analyzed medical records of 12,725 new insulin users with T2DM from a British primary care database. “The commencement of insulin represents a group of patients with T2DM with longer disease duration and more complex needs, many of whom have other comorbid illnesses,” Dr. Anyanwagu said.
The average age of the subjects was 58.6 years (standard deviation, 13.8), and 50% were female (P less than 0.001 for both). Their average HbA1c level was 8.7 (SD, 1.8; P = .556), and 63% were obese.
The numbers of statin users and nonstatin users were 10,682 and 2,043, respectively. Researchers found that the statin users performed better in measures of all-cause mortality, nonfatal stroke, and a composite outcome measure but not in acute myocardial infarction.
In all-cause mortality, the absolute rate over 5 years was 9.5 deaths per 1,000 person years (95% confidence interval, 8.7-10.5) for statin users and 24.9 (95% CI, 21.5-28.9) for nonusers. After adjustment for age, gender, duration of insulin use, albumin, glomerular filtration rate, lipid profile, and coronary heart disease, the hazard ratio was 1.89 (95% CI, 1.51-2.37) for nonusers, compared with a reference of 1 for users. (P less than .0001).
The absolute rate for a composite outcome – encompassing measures of all-cause mortality, nonfatal acute myocardial infarction, and nonfatal stroke – was 20.7 (95% CI, 19.3-22.1) for statin users and 30.9 (95% CI , 27-35.3) for nonusers (P less than .0001).
As for HbA1c levels, at 12 months, they’d fallen by an average of 0.29% in the statin group and 0.37% in the nonuser group (P = .021). At 36 months, there was a smaller difference (–0.31% vs. –0.35%, respectively), and it wasn’t statistically significant (P = .344).
The study was funded by the University of Nottingham. (England). Dr. Anyanwagu reports no relevant disclosures.
SAN DIEGO – Findings from a study of 12,725 new insulin users with type 2 diabetes mellitus (T2DM) show that those who did not take statins were slightly better able to control their blood sugar, but those who used the cholesterol-lowering drugs lived longer and had fewer cardiac events.
“It is clear that, in this high-risk population, the benefits of statins on cardiovascular outcomes outweigh the small adverse metabolic effects on glycemic control,” study lead author Uche Anyanwagu, MBBS, MSc, a graduate student and research fellow at the University of Nottingham (England), said in an interview about his data presented in a poster at the annual scientific sessions of the American Diabetes Association.
However, “there remain great concerns about statins and their modest adverse effects on glucose and insulin metabolism in increasing the risk of new-onset diabetes or worsening glycemic control, especially in higher doses and with the more potent statins,” Dr. Anyanwagu said. “Very little is known about the relationship between statin use and glycemic control in patients with established T2DM.”
The study authors retrospectively analyzed medical records of 12,725 new insulin users with T2DM from a British primary care database. “The commencement of insulin represents a group of patients with T2DM with longer disease duration and more complex needs, many of whom have other comorbid illnesses,” Dr. Anyanwagu said.
The average age of the subjects was 58.6 years (standard deviation, 13.8), and 50% were female (P less than 0.001 for both). Their average HbA1c level was 8.7 (SD, 1.8; P = .556), and 63% were obese.
The numbers of statin users and nonstatin users were 10,682 and 2,043, respectively. Researchers found that the statin users performed better in measures of all-cause mortality, nonfatal stroke, and a composite outcome measure but not in acute myocardial infarction.
In all-cause mortality, the absolute rate over 5 years was 9.5 deaths per 1,000 person years (95% confidence interval, 8.7-10.5) for statin users and 24.9 (95% CI, 21.5-28.9) for nonusers. After adjustment for age, gender, duration of insulin use, albumin, glomerular filtration rate, lipid profile, and coronary heart disease, the hazard ratio was 1.89 (95% CI, 1.51-2.37) for nonusers, compared with a reference of 1 for users. (P less than .0001).
The absolute rate for a composite outcome – encompassing measures of all-cause mortality, nonfatal acute myocardial infarction, and nonfatal stroke – was 20.7 (95% CI, 19.3-22.1) for statin users and 30.9 (95% CI , 27-35.3) for nonusers (P less than .0001).
As for HbA1c levels, at 12 months, they’d fallen by an average of 0.29% in the statin group and 0.37% in the nonuser group (P = .021). At 36 months, there was a smaller difference (–0.31% vs. –0.35%, respectively), and it wasn’t statistically significant (P = .344).
The study was funded by the University of Nottingham. (England). Dr. Anyanwagu reports no relevant disclosures.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point: In new insulin users with T2DM, statin use appears to slightly lower the decline in HbA1c, compared with nonuse, but the drug improved all-cause mortality.
Major finding: The adjusted hazard ratio of 5-year all-cause mortality was 1.89 in nonstatin users, vs. a reference of 1 for statin users. At 12 months, HbA1c fell by an average of 0.29% in the statin group and 0.37% in the nonuser group, but the difference wasn’t statistically significant at 36 months.
Data source: A retrospective study of 10,682 stain users and 2,043 nonusers, all of whom were new insulin users, from a British primary care database.
Disclosures: The study was funded by the University of Nottingham (England).
Red flags for type 2 diabetes seen 25 years before diagnosis
SAN DIEGO – Based on their analysis of a cohort of more than half a million people, Swedish researchers now believe that mildly elevated fasting plasma glucose and triglyceride levels could indicate an increased risk for type 2 diabetes a quarter-century before diagnosis.
“Previous studies have shown that risk factors for type 2 diabetes, including obesity and elevated fasting glucose, may be present up to 10 years before disease onset. Our study extends this period to more than 20 years before diagnosis,” said the study’s lead author Håkan Malmström, PhD, an epidemiologist with the Karolinska Institute, Stockholm. “Even small elevations in subjects over time early in life may be important to recognize, in particular for people who are overweight or obese.” The study findings were presented in an oral presentation at the scientific sessions of the American Diabetes Association.
The researchers identified 47,997 new type 2 diabetes cases in a Swedish cohort of 537,119 people tracked from 1985-2012. For each case, they compared risk factors from clinical examinations performed from 1985-1996 with those of five matched controls.
They found that on average, several risk factors were more common among individuals with type 2 diabetes, compared with the matched controls “many years before the diagnosis,” Dr. Malmström said. “In particular, BMI [body mass index], fasting triglycerides, fasting glucose, the apo B/apo A-I ratio and inflammatory markers were increased up to 25 years before the diagnosis.”
For example, 25 years before diagnosis, mean fasting plasma glucose in the type 2 diabetes group was higher than controls at 90 mg/dL vs. 86 mg/dL, respectively. By 10 years before diagnosis, that gap had widened to 98 mg/dL vs. 88 mg/dL. At 1 year before diagnosis, the levels were 106 mg/dl vs. 90 mg/dL.
As for fasting triglycerides, high levels earlier in life appeared to be especially risky: Individuals with levels over 124 mg/dL were more likely to develop type 2 diabetes 20 years later, even if they weren’t overweight or had elevated mean fasting glucose levels.
At 25 years before diagnosis, the type 2 diabetes group had mean fasting triglyceride levels of 120 mg/dL vs. 89 mg/dL in the control group. And at 1 year before diagnosis, the difference had widened to 146 mg/dL vs. 106 mg/dL.
Researchers found signs of higher levels of fructosamine – a marker of glycemic levels over an extended period of time (2-3 weeks) – at about 15 years before diagnosis. According to Dr. Malmström, this finding suggests that “glucose metabolism was starting to become more disturbed later than the changes in fasting glucose, but still many years before the type 2 diabetes diagnosis.”
He speculated that early signs of type 2 diabetes revealed by the study are related to genetic predisposition. “The risk of developing the disease presents early with increased BMI and dyslipidemia, which in turn leads to successively decreased insulin sensitivity,” he said.
One step for future research, he added, would be to “elaborate on these early changes in risk factors and create a risk score based on a few easily available factors in clinical settings.”
The study was funded by Sweden’s Gunnar and Ingmar Jungner Foundation for Laboratory Medicine. Dr. Malmström reported no relevant disclosures.
SAN DIEGO – Based on their analysis of a cohort of more than half a million people, Swedish researchers now believe that mildly elevated fasting plasma glucose and triglyceride levels could indicate an increased risk for type 2 diabetes a quarter-century before diagnosis.
“Previous studies have shown that risk factors for type 2 diabetes, including obesity and elevated fasting glucose, may be present up to 10 years before disease onset. Our study extends this period to more than 20 years before diagnosis,” said the study’s lead author Håkan Malmström, PhD, an epidemiologist with the Karolinska Institute, Stockholm. “Even small elevations in subjects over time early in life may be important to recognize, in particular for people who are overweight or obese.” The study findings were presented in an oral presentation at the scientific sessions of the American Diabetes Association.
The researchers identified 47,997 new type 2 diabetes cases in a Swedish cohort of 537,119 people tracked from 1985-2012. For each case, they compared risk factors from clinical examinations performed from 1985-1996 with those of five matched controls.
They found that on average, several risk factors were more common among individuals with type 2 diabetes, compared with the matched controls “many years before the diagnosis,” Dr. Malmström said. “In particular, BMI [body mass index], fasting triglycerides, fasting glucose, the apo B/apo A-I ratio and inflammatory markers were increased up to 25 years before the diagnosis.”
For example, 25 years before diagnosis, mean fasting plasma glucose in the type 2 diabetes group was higher than controls at 90 mg/dL vs. 86 mg/dL, respectively. By 10 years before diagnosis, that gap had widened to 98 mg/dL vs. 88 mg/dL. At 1 year before diagnosis, the levels were 106 mg/dl vs. 90 mg/dL.
As for fasting triglycerides, high levels earlier in life appeared to be especially risky: Individuals with levels over 124 mg/dL were more likely to develop type 2 diabetes 20 years later, even if they weren’t overweight or had elevated mean fasting glucose levels.
At 25 years before diagnosis, the type 2 diabetes group had mean fasting triglyceride levels of 120 mg/dL vs. 89 mg/dL in the control group. And at 1 year before diagnosis, the difference had widened to 146 mg/dL vs. 106 mg/dL.
Researchers found signs of higher levels of fructosamine – a marker of glycemic levels over an extended period of time (2-3 weeks) – at about 15 years before diagnosis. According to Dr. Malmström, this finding suggests that “glucose metabolism was starting to become more disturbed later than the changes in fasting glucose, but still many years before the type 2 diabetes diagnosis.”
He speculated that early signs of type 2 diabetes revealed by the study are related to genetic predisposition. “The risk of developing the disease presents early with increased BMI and dyslipidemia, which in turn leads to successively decreased insulin sensitivity,” he said.
One step for future research, he added, would be to “elaborate on these early changes in risk factors and create a risk score based on a few easily available factors in clinical settings.”
The study was funded by Sweden’s Gunnar and Ingmar Jungner Foundation for Laboratory Medicine. Dr. Malmström reported no relevant disclosures.
SAN DIEGO – Based on their analysis of a cohort of more than half a million people, Swedish researchers now believe that mildly elevated fasting plasma glucose and triglyceride levels could indicate an increased risk for type 2 diabetes a quarter-century before diagnosis.
“Previous studies have shown that risk factors for type 2 diabetes, including obesity and elevated fasting glucose, may be present up to 10 years before disease onset. Our study extends this period to more than 20 years before diagnosis,” said the study’s lead author Håkan Malmström, PhD, an epidemiologist with the Karolinska Institute, Stockholm. “Even small elevations in subjects over time early in life may be important to recognize, in particular for people who are overweight or obese.” The study findings were presented in an oral presentation at the scientific sessions of the American Diabetes Association.
The researchers identified 47,997 new type 2 diabetes cases in a Swedish cohort of 537,119 people tracked from 1985-2012. For each case, they compared risk factors from clinical examinations performed from 1985-1996 with those of five matched controls.
They found that on average, several risk factors were more common among individuals with type 2 diabetes, compared with the matched controls “many years before the diagnosis,” Dr. Malmström said. “In particular, BMI [body mass index], fasting triglycerides, fasting glucose, the apo B/apo A-I ratio and inflammatory markers were increased up to 25 years before the diagnosis.”
For example, 25 years before diagnosis, mean fasting plasma glucose in the type 2 diabetes group was higher than controls at 90 mg/dL vs. 86 mg/dL, respectively. By 10 years before diagnosis, that gap had widened to 98 mg/dL vs. 88 mg/dL. At 1 year before diagnosis, the levels were 106 mg/dl vs. 90 mg/dL.
As for fasting triglycerides, high levels earlier in life appeared to be especially risky: Individuals with levels over 124 mg/dL were more likely to develop type 2 diabetes 20 years later, even if they weren’t overweight or had elevated mean fasting glucose levels.
At 25 years before diagnosis, the type 2 diabetes group had mean fasting triglyceride levels of 120 mg/dL vs. 89 mg/dL in the control group. And at 1 year before diagnosis, the difference had widened to 146 mg/dL vs. 106 mg/dL.
Researchers found signs of higher levels of fructosamine – a marker of glycemic levels over an extended period of time (2-3 weeks) – at about 15 years before diagnosis. According to Dr. Malmström, this finding suggests that “glucose metabolism was starting to become more disturbed later than the changes in fasting glucose, but still many years before the type 2 diabetes diagnosis.”
He speculated that early signs of type 2 diabetes revealed by the study are related to genetic predisposition. “The risk of developing the disease presents early with increased BMI and dyslipidemia, which in turn leads to successively decreased insulin sensitivity,” he said.
One step for future research, he added, would be to “elaborate on these early changes in risk factors and create a risk score based on a few easily available factors in clinical settings.”
The study was funded by Sweden’s Gunnar and Ingmar Jungner Foundation for Laboratory Medicine. Dr. Malmström reported no relevant disclosures.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
LEADER post hoc analysis: Mechanism behind liraglutide’s cardioprotective effects unclear
SAN DIEGO – The glucagon-like peptide-1 (GLP-1) receptor agonist liraglutide significantly reduced the risk of initial and recurrent major cardiovascular events in high-risk patients with type 2 diabetes, according to new posthoc analyses from the LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) trial.
Liraglutide’s cardioprotective effect did not depend on baseline use of insulin or cardiovascular medications, nor whether patients started insulin, sulfonylureas, or thiazolidinediones, or developed severe hypoglycemia during the trial, Richard Pratley, MD, told a packed auditorium at the annual scientific sessions of the American Diabetes Association. “It appears unlikely that the cardiovascular risk reduction with liraglutide can be fully explained by the observed differences in hemoglobin A1c, body weight, systolic blood pressure, and lipids,” he said. Experts have proposed several pathways, “but the bottom line is, in humans, we don’t know the mechanism for liraglutide’s benefit.”
In LEADER, 9,340 older patients with suboptimally controlled type 2 diabetes and additional cardiovascular risk factors were randomly assigned to liraglutide or placebo once daily. Patients were typically male, obese, and hypertensive, and about 18% had prior heart failure. Topline results reported last year at ADA included a 13% reduction in the rate of initial cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke in the liraglutide group, compared with the placebo group (hazard ratio, 0.87; 95% confidence interval, 0.78 to 0.97).
The new posthoc analyses indicate that liraglutide also prevents both initial and recurrent cardiovascular events (HR, 0.86; 95% CI, 0.78 to 0.95) and that its cardioprotective effect spans subgroups of patients stratified according to whether they were on insulin, beta-blockers, ACE inhibitors, statins, and platelet aggregation inhibitors at baseline, said Dr. Pratley, senior investigator at the Translational Research Institute for Metabolism and Diabetes, and medical director of the Florida Hospital Diabetes Institute, Orlando.
Liraglutide also reduced cardiovascular events to about the same extent regardless of whether patients later started insulin, sulfonylureas, or thiazolidinediones or developed severe hypoglycemia.
Such findings signal a fundamental shift in diabetes care, commented Steven Nissen, MD, chair of the department of cardiovascular medicine, at the Cleveland Clinic, Cleveland, Ohio. For decades, patients and clinicians lacked diabetes outcomes trials, and “it took three successive shockwaves to awaken the medical community from its 50-year slumber.”
The wake-up call started when Dr. Nissen and his associates linked muraglitazar (JAMA. 2005 Nov 23;294[20]:2581-6) and rosiglitazone (N Engl J Med 2007; 356:2457-71) to an increased risk of major adverse cardiovascular events. This continued when researchers found that targeting glycated hemoglobin levels below 6.0% increased the risk of death in patients with type 2 diabetes, in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial (N Engl J Med 2008; 358:2545-59).
In response, the Food and Drug Administration began requiring cardiovascular outcomes trials before and after approving new diabetes drugs. “The result has been a new era of research” that has revealed uneven outcomes and a lack of uniform class effects, Dr. Nissen said.
For example, lixisenatide and long-acting exenatide are GLP-1 receptor agonists like liraglutide, but neither of these two drugs were found to prevent cardiovascular outcomes compared with placebo. In addition, the DPP-4 inhibitors “provide no meaningful outcome benefits, minimal glucose lowering, and potential harm at a high cost of about $400 per month,” he said.
This class has suffered “three disappointments,” he added. He referred to findings that alogliptin and sitagliptin did not reduce cardiovascular events compared with placebo in the EXAMINE trial (N Engl J Med 2013; 369:1327-35) and TECOS trial (N Engl J Med 2015; 373:232-42), respectively, while the SAVOR-TIMI 53 trial linked saxagliptin to an increased risk of hospitalization for heart failure (HR, 1.27; 95% CI, 1.07 to 1.51).
In contrast, the sodium-glucose cotransporter-2 (SGLT-2) inhibitor empagliflozin reduced the rate of cardiovascular death, stroke, and MI by about 14% in the EMPA-REG trial (N Engl J Med 2015;373:2117-28).
Based on the LEADER results, the Food and Drug Administration’s Endocrinologic and Metabolic Drugs Advisory Committee voted 17-2 supporting a new cardiovascular indication for liraglutide in type 2 diabetes, at a meeting in June 2017.
“After 60 years of stagnation, we are now witnessing a new era in the pharmacological management of type 2 diabetes, which is allowing a choice of therapies based on actual clinical outcomes – risks and benefits – rather than a surrogate biochemical marker like glucose levels,” Dr. Nissen said.
But current ADA recommendations “only weakly reflect contemporary knowledge,” he added. Although these guidelines do recommend considering empagliflozin or liraglutide for patients with atherosclerotic cardiovascular disease and “long-standing, suboptimally controlled type 2 diabetes,” their guidance on dual therapy does not reflect diverse cardiovascular outcomes data within and between classes, he said. “Just as we observed with statins, adoption of pivotal results is often just too slow.”
Integrating knowledge into practice will require close collaboration between cardiovascular and diabetes practitioners and “leadership from ADA to overcome residual inertia from decades of complacency,” he commented.
Dr. Pratley disclosed ties to AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, and several other pharmaceutical companies. Dr. Nissen disclosed research support from Novo Nordisk, Abbvie, Eli Lilly, and several other pharmaceutical companies, and travel support from Novo Nordisk.
SAN DIEGO – The glucagon-like peptide-1 (GLP-1) receptor agonist liraglutide significantly reduced the risk of initial and recurrent major cardiovascular events in high-risk patients with type 2 diabetes, according to new posthoc analyses from the LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) trial.
Liraglutide’s cardioprotective effect did not depend on baseline use of insulin or cardiovascular medications, nor whether patients started insulin, sulfonylureas, or thiazolidinediones, or developed severe hypoglycemia during the trial, Richard Pratley, MD, told a packed auditorium at the annual scientific sessions of the American Diabetes Association. “It appears unlikely that the cardiovascular risk reduction with liraglutide can be fully explained by the observed differences in hemoglobin A1c, body weight, systolic blood pressure, and lipids,” he said. Experts have proposed several pathways, “but the bottom line is, in humans, we don’t know the mechanism for liraglutide’s benefit.”
In LEADER, 9,340 older patients with suboptimally controlled type 2 diabetes and additional cardiovascular risk factors were randomly assigned to liraglutide or placebo once daily. Patients were typically male, obese, and hypertensive, and about 18% had prior heart failure. Topline results reported last year at ADA included a 13% reduction in the rate of initial cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke in the liraglutide group, compared with the placebo group (hazard ratio, 0.87; 95% confidence interval, 0.78 to 0.97).
The new posthoc analyses indicate that liraglutide also prevents both initial and recurrent cardiovascular events (HR, 0.86; 95% CI, 0.78 to 0.95) and that its cardioprotective effect spans subgroups of patients stratified according to whether they were on insulin, beta-blockers, ACE inhibitors, statins, and platelet aggregation inhibitors at baseline, said Dr. Pratley, senior investigator at the Translational Research Institute for Metabolism and Diabetes, and medical director of the Florida Hospital Diabetes Institute, Orlando.
Liraglutide also reduced cardiovascular events to about the same extent regardless of whether patients later started insulin, sulfonylureas, or thiazolidinediones or developed severe hypoglycemia.
Such findings signal a fundamental shift in diabetes care, commented Steven Nissen, MD, chair of the department of cardiovascular medicine, at the Cleveland Clinic, Cleveland, Ohio. For decades, patients and clinicians lacked diabetes outcomes trials, and “it took three successive shockwaves to awaken the medical community from its 50-year slumber.”
The wake-up call started when Dr. Nissen and his associates linked muraglitazar (JAMA. 2005 Nov 23;294[20]:2581-6) and rosiglitazone (N Engl J Med 2007; 356:2457-71) to an increased risk of major adverse cardiovascular events. This continued when researchers found that targeting glycated hemoglobin levels below 6.0% increased the risk of death in patients with type 2 diabetes, in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial (N Engl J Med 2008; 358:2545-59).
In response, the Food and Drug Administration began requiring cardiovascular outcomes trials before and after approving new diabetes drugs. “The result has been a new era of research” that has revealed uneven outcomes and a lack of uniform class effects, Dr. Nissen said.
For example, lixisenatide and long-acting exenatide are GLP-1 receptor agonists like liraglutide, but neither of these two drugs were found to prevent cardiovascular outcomes compared with placebo. In addition, the DPP-4 inhibitors “provide no meaningful outcome benefits, minimal glucose lowering, and potential harm at a high cost of about $400 per month,” he said.
This class has suffered “three disappointments,” he added. He referred to findings that alogliptin and sitagliptin did not reduce cardiovascular events compared with placebo in the EXAMINE trial (N Engl J Med 2013; 369:1327-35) and TECOS trial (N Engl J Med 2015; 373:232-42), respectively, while the SAVOR-TIMI 53 trial linked saxagliptin to an increased risk of hospitalization for heart failure (HR, 1.27; 95% CI, 1.07 to 1.51).
In contrast, the sodium-glucose cotransporter-2 (SGLT-2) inhibitor empagliflozin reduced the rate of cardiovascular death, stroke, and MI by about 14% in the EMPA-REG trial (N Engl J Med 2015;373:2117-28).
Based on the LEADER results, the Food and Drug Administration’s Endocrinologic and Metabolic Drugs Advisory Committee voted 17-2 supporting a new cardiovascular indication for liraglutide in type 2 diabetes, at a meeting in June 2017.
“After 60 years of stagnation, we are now witnessing a new era in the pharmacological management of type 2 diabetes, which is allowing a choice of therapies based on actual clinical outcomes – risks and benefits – rather than a surrogate biochemical marker like glucose levels,” Dr. Nissen said.
But current ADA recommendations “only weakly reflect contemporary knowledge,” he added. Although these guidelines do recommend considering empagliflozin or liraglutide for patients with atherosclerotic cardiovascular disease and “long-standing, suboptimally controlled type 2 diabetes,” their guidance on dual therapy does not reflect diverse cardiovascular outcomes data within and between classes, he said. “Just as we observed with statins, adoption of pivotal results is often just too slow.”
Integrating knowledge into practice will require close collaboration between cardiovascular and diabetes practitioners and “leadership from ADA to overcome residual inertia from decades of complacency,” he commented.
Dr. Pratley disclosed ties to AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, and several other pharmaceutical companies. Dr. Nissen disclosed research support from Novo Nordisk, Abbvie, Eli Lilly, and several other pharmaceutical companies, and travel support from Novo Nordisk.
SAN DIEGO – The glucagon-like peptide-1 (GLP-1) receptor agonist liraglutide significantly reduced the risk of initial and recurrent major cardiovascular events in high-risk patients with type 2 diabetes, according to new posthoc analyses from the LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) trial.
Liraglutide’s cardioprotective effect did not depend on baseline use of insulin or cardiovascular medications, nor whether patients started insulin, sulfonylureas, or thiazolidinediones, or developed severe hypoglycemia during the trial, Richard Pratley, MD, told a packed auditorium at the annual scientific sessions of the American Diabetes Association. “It appears unlikely that the cardiovascular risk reduction with liraglutide can be fully explained by the observed differences in hemoglobin A1c, body weight, systolic blood pressure, and lipids,” he said. Experts have proposed several pathways, “but the bottom line is, in humans, we don’t know the mechanism for liraglutide’s benefit.”
In LEADER, 9,340 older patients with suboptimally controlled type 2 diabetes and additional cardiovascular risk factors were randomly assigned to liraglutide or placebo once daily. Patients were typically male, obese, and hypertensive, and about 18% had prior heart failure. Topline results reported last year at ADA included a 13% reduction in the rate of initial cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke in the liraglutide group, compared with the placebo group (hazard ratio, 0.87; 95% confidence interval, 0.78 to 0.97).
The new posthoc analyses indicate that liraglutide also prevents both initial and recurrent cardiovascular events (HR, 0.86; 95% CI, 0.78 to 0.95) and that its cardioprotective effect spans subgroups of patients stratified according to whether they were on insulin, beta-blockers, ACE inhibitors, statins, and platelet aggregation inhibitors at baseline, said Dr. Pratley, senior investigator at the Translational Research Institute for Metabolism and Diabetes, and medical director of the Florida Hospital Diabetes Institute, Orlando.
Liraglutide also reduced cardiovascular events to about the same extent regardless of whether patients later started insulin, sulfonylureas, or thiazolidinediones or developed severe hypoglycemia.
Such findings signal a fundamental shift in diabetes care, commented Steven Nissen, MD, chair of the department of cardiovascular medicine, at the Cleveland Clinic, Cleveland, Ohio. For decades, patients and clinicians lacked diabetes outcomes trials, and “it took three successive shockwaves to awaken the medical community from its 50-year slumber.”
The wake-up call started when Dr. Nissen and his associates linked muraglitazar (JAMA. 2005 Nov 23;294[20]:2581-6) and rosiglitazone (N Engl J Med 2007; 356:2457-71) to an increased risk of major adverse cardiovascular events. This continued when researchers found that targeting glycated hemoglobin levels below 6.0% increased the risk of death in patients with type 2 diabetes, in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial (N Engl J Med 2008; 358:2545-59).
In response, the Food and Drug Administration began requiring cardiovascular outcomes trials before and after approving new diabetes drugs. “The result has been a new era of research” that has revealed uneven outcomes and a lack of uniform class effects, Dr. Nissen said.
For example, lixisenatide and long-acting exenatide are GLP-1 receptor agonists like liraglutide, but neither of these two drugs were found to prevent cardiovascular outcomes compared with placebo. In addition, the DPP-4 inhibitors “provide no meaningful outcome benefits, minimal glucose lowering, and potential harm at a high cost of about $400 per month,” he said.
This class has suffered “three disappointments,” he added. He referred to findings that alogliptin and sitagliptin did not reduce cardiovascular events compared with placebo in the EXAMINE trial (N Engl J Med 2013; 369:1327-35) and TECOS trial (N Engl J Med 2015; 373:232-42), respectively, while the SAVOR-TIMI 53 trial linked saxagliptin to an increased risk of hospitalization for heart failure (HR, 1.27; 95% CI, 1.07 to 1.51).
In contrast, the sodium-glucose cotransporter-2 (SGLT-2) inhibitor empagliflozin reduced the rate of cardiovascular death, stroke, and MI by about 14% in the EMPA-REG trial (N Engl J Med 2015;373:2117-28).
Based on the LEADER results, the Food and Drug Administration’s Endocrinologic and Metabolic Drugs Advisory Committee voted 17-2 supporting a new cardiovascular indication for liraglutide in type 2 diabetes, at a meeting in June 2017.
“After 60 years of stagnation, we are now witnessing a new era in the pharmacological management of type 2 diabetes, which is allowing a choice of therapies based on actual clinical outcomes – risks and benefits – rather than a surrogate biochemical marker like glucose levels,” Dr. Nissen said.
But current ADA recommendations “only weakly reflect contemporary knowledge,” he added. Although these guidelines do recommend considering empagliflozin or liraglutide for patients with atherosclerotic cardiovascular disease and “long-standing, suboptimally controlled type 2 diabetes,” their guidance on dual therapy does not reflect diverse cardiovascular outcomes data within and between classes, he said. “Just as we observed with statins, adoption of pivotal results is often just too slow.”
Integrating knowledge into practice will require close collaboration between cardiovascular and diabetes practitioners and “leadership from ADA to overcome residual inertia from decades of complacency,” he commented.
Dr. Pratley disclosed ties to AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, and several other pharmaceutical companies. Dr. Nissen disclosed research support from Novo Nordisk, Abbvie, Eli Lilly, and several other pharmaceutical companies, and travel support from Novo Nordisk.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Oral insulin matches glargine in phase II trial
SAN DIEGO – In an industry-funded phase II trial, researchers say they’ve shown for the first time that oral basal insulin tablets can safely decrease plasma glucose in patients with type 2 diabetes (T2DM).
“Oral basal insulin appears safe and efficacious in insulin-naive patients with type 2 [diabetes] insufficiently controlled with medications. It improves glycemic control to a similar extent as does glargine,” said study lead author Leona Plum-Mörschel, MD, CEO of the German clinical research organization Profil Mainz, in an oral presentation at the scientific sessions of the American Diabetes Association. A statement from Novo Nordisk, the maker of the investigational medication, says the company discontinued development of the product due to its low bioavailability and concerns about the costs of producing it.
According to Dr. Plum-Mörschel, researchers have been searching for an oral insulin treatment for almost a century without success.
In the new phase IIa study, researchers tested a basal insulin analog tablet called OI338GT. It uses a technology platform called Gipet by Merrion Pharmaceuticals that aims to boost the absorption of injectable drugs when they are given in oral form.
Researchers recruited 50 insulin-naive patients with T2DM (mean age 61 ± 7 years, mean BMI 30.5 ± 3.7 kg/m²) whose diabetes wasn’t properly controlled by metformin by itself or in conjunction with other drugs. All had hemoglobin A1c (HbA1c) levels between 7% and 10%.
The patients were randomly assigned (1:1) to the investigational oral medication or subcutaneous insulin glargine (IGlar). They took the drugs once a day for 8 weeks in addition to their existing drug regimen.
The researchers increased the doses on a weekly basis with a goal of reaching fasting plasma glucose (FPG) of 80-126 mg/dL. Daily doses of the investigational drug (nmol/kg) began at a mean 30 ± 11 and reached 114 ± 51 by the end of the study; daily mean doses of IGlar (U/kg) began at 0.11 ± 0.03 and ended at 0.33 ± 0.15.
Both medications boosted glycemic control at about the same level, the researchers report, based on levels of FPG, HbA1c, and fructosamine levels at 8 weeks.
Mean FPG levels (mg/dL), the primary endpoint, declined in investigational medication and IGlar from 175 ± 50 and 164 ± 31, respectively, at baseline to 129 ± 33 and 121 ± 17, respectively, at end of treatment. The treatment difference (investigational medication – IGlar) was 5.2 mg/dL [–8.8;19.1], 95% CI, P = .4567.
In a post hoc analysis, mean HbA1c levels declined from 8.1 ± 0.6% and 8.2 ± 0.8% at baseline in investigational medication and IGlar, respectively, to 7.3 ± 0.8% and 7.1 ± 0.6%, respectively. The treatment difference (investigational medication – IGlar) was 0.30% [–0.03;0.63], 95% CI, P = .0774.
In another post hoc analysis, mean fructosamine levels (micromol/L) dipped from 275 ± 44 and 273 ± 50 in investigational medication and IGlar, respectively, to 235 ± 45 and 223 ± 34, respectively. The treatment difference (investigational medication – IGlar)
was 9.6 micromol/L [–11.7;30.9], 95% CI, P = .3700. None of the patients reported severe hypoglycemia. In the investigational group, 6 subjects suffered 7 events of hypoglycemia that emerged or worsened during treatment; 11 events occurred in 6 subjects in the IGlar group. Researchers report similar levels of adverse events in both groups: 31 in 15 patients treated with the investigational drug and 37 in 17 patients treated with IGlar.
“After termination of treatment, plasma glucose rebounded to baseline levels within a couple of weeks,” Dr. Plum-Mörschel said.
Novo Nordisk funded the trial. Dr. Plum-Mörschel reports no disclosures.
SAN DIEGO – In an industry-funded phase II trial, researchers say they’ve shown for the first time that oral basal insulin tablets can safely decrease plasma glucose in patients with type 2 diabetes (T2DM).
“Oral basal insulin appears safe and efficacious in insulin-naive patients with type 2 [diabetes] insufficiently controlled with medications. It improves glycemic control to a similar extent as does glargine,” said study lead author Leona Plum-Mörschel, MD, CEO of the German clinical research organization Profil Mainz, in an oral presentation at the scientific sessions of the American Diabetes Association. A statement from Novo Nordisk, the maker of the investigational medication, says the company discontinued development of the product due to its low bioavailability and concerns about the costs of producing it.
According to Dr. Plum-Mörschel, researchers have been searching for an oral insulin treatment for almost a century without success.
In the new phase IIa study, researchers tested a basal insulin analog tablet called OI338GT. It uses a technology platform called Gipet by Merrion Pharmaceuticals that aims to boost the absorption of injectable drugs when they are given in oral form.
Researchers recruited 50 insulin-naive patients with T2DM (mean age 61 ± 7 years, mean BMI 30.5 ± 3.7 kg/m²) whose diabetes wasn’t properly controlled by metformin by itself or in conjunction with other drugs. All had hemoglobin A1c (HbA1c) levels between 7% and 10%.
The patients were randomly assigned (1:1) to the investigational oral medication or subcutaneous insulin glargine (IGlar). They took the drugs once a day for 8 weeks in addition to their existing drug regimen.
The researchers increased the doses on a weekly basis with a goal of reaching fasting plasma glucose (FPG) of 80-126 mg/dL. Daily doses of the investigational drug (nmol/kg) began at a mean 30 ± 11 and reached 114 ± 51 by the end of the study; daily mean doses of IGlar (U/kg) began at 0.11 ± 0.03 and ended at 0.33 ± 0.15.
Both medications boosted glycemic control at about the same level, the researchers report, based on levels of FPG, HbA1c, and fructosamine levels at 8 weeks.
Mean FPG levels (mg/dL), the primary endpoint, declined in investigational medication and IGlar from 175 ± 50 and 164 ± 31, respectively, at baseline to 129 ± 33 and 121 ± 17, respectively, at end of treatment. The treatment difference (investigational medication – IGlar) was 5.2 mg/dL [–8.8;19.1], 95% CI, P = .4567.
In a post hoc analysis, mean HbA1c levels declined from 8.1 ± 0.6% and 8.2 ± 0.8% at baseline in investigational medication and IGlar, respectively, to 7.3 ± 0.8% and 7.1 ± 0.6%, respectively. The treatment difference (investigational medication – IGlar) was 0.30% [–0.03;0.63], 95% CI, P = .0774.
In another post hoc analysis, mean fructosamine levels (micromol/L) dipped from 275 ± 44 and 273 ± 50 in investigational medication and IGlar, respectively, to 235 ± 45 and 223 ± 34, respectively. The treatment difference (investigational medication – IGlar)
was 9.6 micromol/L [–11.7;30.9], 95% CI, P = .3700. None of the patients reported severe hypoglycemia. In the investigational group, 6 subjects suffered 7 events of hypoglycemia that emerged or worsened during treatment; 11 events occurred in 6 subjects in the IGlar group. Researchers report similar levels of adverse events in both groups: 31 in 15 patients treated with the investigational drug and 37 in 17 patients treated with IGlar.
“After termination of treatment, plasma glucose rebounded to baseline levels within a couple of weeks,” Dr. Plum-Mörschel said.
Novo Nordisk funded the trial. Dr. Plum-Mörschel reports no disclosures.
SAN DIEGO – In an industry-funded phase II trial, researchers say they’ve shown for the first time that oral basal insulin tablets can safely decrease plasma glucose in patients with type 2 diabetes (T2DM).
“Oral basal insulin appears safe and efficacious in insulin-naive patients with type 2 [diabetes] insufficiently controlled with medications. It improves glycemic control to a similar extent as does glargine,” said study lead author Leona Plum-Mörschel, MD, CEO of the German clinical research organization Profil Mainz, in an oral presentation at the scientific sessions of the American Diabetes Association. A statement from Novo Nordisk, the maker of the investigational medication, says the company discontinued development of the product due to its low bioavailability and concerns about the costs of producing it.
According to Dr. Plum-Mörschel, researchers have been searching for an oral insulin treatment for almost a century without success.
In the new phase IIa study, researchers tested a basal insulin analog tablet called OI338GT. It uses a technology platform called Gipet by Merrion Pharmaceuticals that aims to boost the absorption of injectable drugs when they are given in oral form.
Researchers recruited 50 insulin-naive patients with T2DM (mean age 61 ± 7 years, mean BMI 30.5 ± 3.7 kg/m²) whose diabetes wasn’t properly controlled by metformin by itself or in conjunction with other drugs. All had hemoglobin A1c (HbA1c) levels between 7% and 10%.
The patients were randomly assigned (1:1) to the investigational oral medication or subcutaneous insulin glargine (IGlar). They took the drugs once a day for 8 weeks in addition to their existing drug regimen.
The researchers increased the doses on a weekly basis with a goal of reaching fasting plasma glucose (FPG) of 80-126 mg/dL. Daily doses of the investigational drug (nmol/kg) began at a mean 30 ± 11 and reached 114 ± 51 by the end of the study; daily mean doses of IGlar (U/kg) began at 0.11 ± 0.03 and ended at 0.33 ± 0.15.
Both medications boosted glycemic control at about the same level, the researchers report, based on levels of FPG, HbA1c, and fructosamine levels at 8 weeks.
Mean FPG levels (mg/dL), the primary endpoint, declined in investigational medication and IGlar from 175 ± 50 and 164 ± 31, respectively, at baseline to 129 ± 33 and 121 ± 17, respectively, at end of treatment. The treatment difference (investigational medication – IGlar) was 5.2 mg/dL [–8.8;19.1], 95% CI, P = .4567.
In a post hoc analysis, mean HbA1c levels declined from 8.1 ± 0.6% and 8.2 ± 0.8% at baseline in investigational medication and IGlar, respectively, to 7.3 ± 0.8% and 7.1 ± 0.6%, respectively. The treatment difference (investigational medication – IGlar) was 0.30% [–0.03;0.63], 95% CI, P = .0774.
In another post hoc analysis, mean fructosamine levels (micromol/L) dipped from 275 ± 44 and 273 ± 50 in investigational medication and IGlar, respectively, to 235 ± 45 and 223 ± 34, respectively. The treatment difference (investigational medication – IGlar)
was 9.6 micromol/L [–11.7;30.9], 95% CI, P = .3700. None of the patients reported severe hypoglycemia. In the investigational group, 6 subjects suffered 7 events of hypoglycemia that emerged or worsened during treatment; 11 events occurred in 6 subjects in the IGlar group. Researchers report similar levels of adverse events in both groups: 31 in 15 patients treated with the investigational drug and 37 in 17 patients treated with IGlar.
“After termination of treatment, plasma glucose rebounded to baseline levels within a couple of weeks,” Dr. Plum-Mörschel said.
Novo Nordisk funded the trial. Dr. Plum-Mörschel reports no disclosures.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point: In 8-week trial, oral insulin tablets produce similar glucose control to injectable glargine (IGlar) in patients with type 2 diabetes (T2DM).
Major finding: Mean levels fasting plasma glucose (FPG, mg/dL) declined from 175 ± 50 to 129 ± 33 (investigational medication) and from 164 ± 31 to 121 ± 17 (IGlar). The treatment difference (investigational medication – IGlar) was 5.2 mg/dL [–8.8;19.1], 95% CI, P = .4567.
Data source: 50 insulin-naive patients with T2DM randomly assigned (1:1) to daily doses of investigational oral medication or IGlar for 8 weeks, titrated to reach target FPG of 80-126 mg/dL.
Disclosures: Novo Nordisk funded the trial. Dr. Plum-Mörschel reports no disclosures.
SPRINT: Intensive BP control cut cardiovascular risk in patients with prediabetes
SAN DIEGO – Treating systolic hypertension to a target of 120 mm Hg instead of 140 mm Hg significantly reduced the rate of cardiovascular events in high-risk patients with prediabetes, according to a post-hoc analysis of the multicenter, randomized, controlled, open-label Systolic Blood Pressure Intervention Trial (SPRINT).
Thus, prediabetes did not undercut the cardiovascular benefits of intensive systolic blood pressure (SBP) control in older, high-risk, hypertensive patients, Adam P. Bress, PharmD, MS, and his associates concluded in a late-breaking poster presented at the annual scientific sessions of the American Diabetes Association.
In contrast, intensive SBP control did not prevent cardiovascular events, compared with standard control among diabetic patients in the randomized, nonblinded ACCORD BP trial (N Engl J Med. 2010; 362:1575-85).
To help clarify SBP targets for prediabetic individuals, Dr. Bress and his associates parsed cardiovascular outcomes in SPRINT based on baseline fasting serum glucose (FSG) levels. More than 5,400 normoglycemic (average FSG, 91 mg per dL) patients and more than 3,800 prediabetic (average FSG, 110 mg per dL) patients were followed for a median of 3.3 years, after which 101 (1.6%) prediabetic intensive control patients and 144 (2.3%) prediabetic standard control patients experienced the combined primary endpoint (HR, 0.7; 95% confidence interval, 0.5-0.9). The hazard ratio for normoglycemic patients was similar (0.9) and approached statistical significance.
Intensive SBP control also was associated with lower rates of individual cardiovascular outcomes and all-cause mortality, compared with standard control in both normoglycemic and prediabetic patients, Dr. Bress and his associates reported. Wide confidence intervals precluded statistical significance for most individual outcomes, but intensive control was associated with a significantly lower risk of all-cause mortality in normoglycemic patients (0.7% vs. 1.4% with standard control; HR, 0.7; 95% CI, 0.5-0.9).
Serious adverse events affected about 37% of patients in all subgroups. Hypotension, syncope, bradycardia, and electrolyte abnormalities were slightly more common in the intensive control groups than the standard control groups, regardless of baseline FPG levels.
SPRINT was sponsored by the National Heart, Lung, and Blood Institute. The National Institute on Aging, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Neurological Disorders and Stroke, and Wake Forest University Health Sciences provided additional support. Dr. Bress disclosed research support from Novartis and the National Institutes of Health.
SAN DIEGO – Treating systolic hypertension to a target of 120 mm Hg instead of 140 mm Hg significantly reduced the rate of cardiovascular events in high-risk patients with prediabetes, according to a post-hoc analysis of the multicenter, randomized, controlled, open-label Systolic Blood Pressure Intervention Trial (SPRINT).
Thus, prediabetes did not undercut the cardiovascular benefits of intensive systolic blood pressure (SBP) control in older, high-risk, hypertensive patients, Adam P. Bress, PharmD, MS, and his associates concluded in a late-breaking poster presented at the annual scientific sessions of the American Diabetes Association.
In contrast, intensive SBP control did not prevent cardiovascular events, compared with standard control among diabetic patients in the randomized, nonblinded ACCORD BP trial (N Engl J Med. 2010; 362:1575-85).
To help clarify SBP targets for prediabetic individuals, Dr. Bress and his associates parsed cardiovascular outcomes in SPRINT based on baseline fasting serum glucose (FSG) levels. More than 5,400 normoglycemic (average FSG, 91 mg per dL) patients and more than 3,800 prediabetic (average FSG, 110 mg per dL) patients were followed for a median of 3.3 years, after which 101 (1.6%) prediabetic intensive control patients and 144 (2.3%) prediabetic standard control patients experienced the combined primary endpoint (HR, 0.7; 95% confidence interval, 0.5-0.9). The hazard ratio for normoglycemic patients was similar (0.9) and approached statistical significance.
Intensive SBP control also was associated with lower rates of individual cardiovascular outcomes and all-cause mortality, compared with standard control in both normoglycemic and prediabetic patients, Dr. Bress and his associates reported. Wide confidence intervals precluded statistical significance for most individual outcomes, but intensive control was associated with a significantly lower risk of all-cause mortality in normoglycemic patients (0.7% vs. 1.4% with standard control; HR, 0.7; 95% CI, 0.5-0.9).
Serious adverse events affected about 37% of patients in all subgroups. Hypotension, syncope, bradycardia, and electrolyte abnormalities were slightly more common in the intensive control groups than the standard control groups, regardless of baseline FPG levels.
SPRINT was sponsored by the National Heart, Lung, and Blood Institute. The National Institute on Aging, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Neurological Disorders and Stroke, and Wake Forest University Health Sciences provided additional support. Dr. Bress disclosed research support from Novartis and the National Institutes of Health.
SAN DIEGO – Treating systolic hypertension to a target of 120 mm Hg instead of 140 mm Hg significantly reduced the rate of cardiovascular events in high-risk patients with prediabetes, according to a post-hoc analysis of the multicenter, randomized, controlled, open-label Systolic Blood Pressure Intervention Trial (SPRINT).
Thus, prediabetes did not undercut the cardiovascular benefits of intensive systolic blood pressure (SBP) control in older, high-risk, hypertensive patients, Adam P. Bress, PharmD, MS, and his associates concluded in a late-breaking poster presented at the annual scientific sessions of the American Diabetes Association.
In contrast, intensive SBP control did not prevent cardiovascular events, compared with standard control among diabetic patients in the randomized, nonblinded ACCORD BP trial (N Engl J Med. 2010; 362:1575-85).
To help clarify SBP targets for prediabetic individuals, Dr. Bress and his associates parsed cardiovascular outcomes in SPRINT based on baseline fasting serum glucose (FSG) levels. More than 5,400 normoglycemic (average FSG, 91 mg per dL) patients and more than 3,800 prediabetic (average FSG, 110 mg per dL) patients were followed for a median of 3.3 years, after which 101 (1.6%) prediabetic intensive control patients and 144 (2.3%) prediabetic standard control patients experienced the combined primary endpoint (HR, 0.7; 95% confidence interval, 0.5-0.9). The hazard ratio for normoglycemic patients was similar (0.9) and approached statistical significance.
Intensive SBP control also was associated with lower rates of individual cardiovascular outcomes and all-cause mortality, compared with standard control in both normoglycemic and prediabetic patients, Dr. Bress and his associates reported. Wide confidence intervals precluded statistical significance for most individual outcomes, but intensive control was associated with a significantly lower risk of all-cause mortality in normoglycemic patients (0.7% vs. 1.4% with standard control; HR, 0.7; 95% CI, 0.5-0.9).
Serious adverse events affected about 37% of patients in all subgroups. Hypotension, syncope, bradycardia, and electrolyte abnormalities were slightly more common in the intensive control groups than the standard control groups, regardless of baseline FPG levels.
SPRINT was sponsored by the National Heart, Lung, and Blood Institute. The National Institute on Aging, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Neurological Disorders and Stroke, and Wake Forest University Health Sciences provided additional support. Dr. Bress disclosed research support from Novartis and the National Institutes of Health.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point: Treating systolic hypertension to a target of 120 mm Hg instead of 140 mm Hg significantly reduced the rate of cardiovascular events in high-risk patients with prediabetes.
Major finding: After a median of 3.3 years, 1.6% of patients with prediabetes in the intensive control group and 2.2% of patients with prediabetes in the standard control group experienced myocardial infarction, acute coronary syndrome without myocardial infarction, stroke, acute decompensated heart failure, or cardiovascular death.
Data source: SPRINT, a randomized, controlled, open-label trial comparing systolic blood pressure targets of less than 140 mm Hg (standard control) and less than 120 mm Hg (intensive control) in hypertensive patients without clinical diabetes who were older than 50 years and at high risk for cardiovascular disease.
Disclosures: SPRINT was sponsored by the National Heart, Lung, and Blood Institute. The National Institute on Aging, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Neurological Disorders and Stroke, and Wake Forest University Health Sciences provided additional support. Dr. Bress disclosed research support from Novartis and the National Institutes of Health.