User login
Eversense CGM shown safe, accurate for 180 days in adolescents
ORLANDO – The Eversense continuous glucose monitoring (CGM) system, recently approved for use in adults with diabetes, also provides safe, durable, and accurate monitoring in the pediatric population, according to findings from a prospective single-arm study of 30 children and 6 adults.
Study subjects, who were all over age 11 years, with an average of 14 years, had the fully implantable sensor inserted at day 0 and removed at day 180, and the mean absolute relative difference (MARD) between sensor and true laboratory glucose values showed high device accuracy, Ronnie Aronson, MD, reported at the annual scientific sessions of the American Diabetes Association.
“Anything under 10% is considered good, and ours was 9.4% – and it didn’t deteriorate throughout the duration, so at 180 days it was still at 9.4%; every accuracy measure we looked at showed similar high levels of accuracy,” Dr. Aronson, founder and chief medical officer of LMC Diabetes & Endocrinology in Ontario, Canada said in a video interview.
The sensor, which is roughly 1.5 cm long, is coated with a material that fluoresces when exposed to glucose; the sensor uses the amount of light emitted to calculate blood glucose levels. Patients use an adhesive patch, changed daily, to attach a “smart” transmitter that overlies the area where the sensor is implanted. This rechargeable transmitter sends blood glucose levels to the mobile app every 5 minutes, and also powers the sensor. The Food and Drug Administration approved it for use in adults on June 21.
The system was highly rated by study participants, he said. “What makes it stand out is that it’s implanted, it’s there for at least 180 days, it’s accurate for 180 days,” the transmitter can be taken on and off, and the results can be seen very easily on a smart phone or Apple Watch.
Dr. Aronson said he also hopes to study the device in younger patients and for longer durations.
Dr. Aronson is an advisor for Novo Nordisk and Sanofi. He also receives research support from AstraZeneca, Eli Lilly, Valeant, Janssen, and Senseonics.
SOURCE: Aronson R et al. ADA 2018 Abstract 13-OR.
ORLANDO – The Eversense continuous glucose monitoring (CGM) system, recently approved for use in adults with diabetes, also provides safe, durable, and accurate monitoring in the pediatric population, according to findings from a prospective single-arm study of 30 children and 6 adults.
Study subjects, who were all over age 11 years, with an average of 14 years, had the fully implantable sensor inserted at day 0 and removed at day 180, and the mean absolute relative difference (MARD) between sensor and true laboratory glucose values showed high device accuracy, Ronnie Aronson, MD, reported at the annual scientific sessions of the American Diabetes Association.
“Anything under 10% is considered good, and ours was 9.4% – and it didn’t deteriorate throughout the duration, so at 180 days it was still at 9.4%; every accuracy measure we looked at showed similar high levels of accuracy,” Dr. Aronson, founder and chief medical officer of LMC Diabetes & Endocrinology in Ontario, Canada said in a video interview.
The sensor, which is roughly 1.5 cm long, is coated with a material that fluoresces when exposed to glucose; the sensor uses the amount of light emitted to calculate blood glucose levels. Patients use an adhesive patch, changed daily, to attach a “smart” transmitter that overlies the area where the sensor is implanted. This rechargeable transmitter sends blood glucose levels to the mobile app every 5 minutes, and also powers the sensor. The Food and Drug Administration approved it for use in adults on June 21.
The system was highly rated by study participants, he said. “What makes it stand out is that it’s implanted, it’s there for at least 180 days, it’s accurate for 180 days,” the transmitter can be taken on and off, and the results can be seen very easily on a smart phone or Apple Watch.
Dr. Aronson said he also hopes to study the device in younger patients and for longer durations.
Dr. Aronson is an advisor for Novo Nordisk and Sanofi. He also receives research support from AstraZeneca, Eli Lilly, Valeant, Janssen, and Senseonics.
SOURCE: Aronson R et al. ADA 2018 Abstract 13-OR.
ORLANDO – The Eversense continuous glucose monitoring (CGM) system, recently approved for use in adults with diabetes, also provides safe, durable, and accurate monitoring in the pediatric population, according to findings from a prospective single-arm study of 30 children and 6 adults.
Study subjects, who were all over age 11 years, with an average of 14 years, had the fully implantable sensor inserted at day 0 and removed at day 180, and the mean absolute relative difference (MARD) between sensor and true laboratory glucose values showed high device accuracy, Ronnie Aronson, MD, reported at the annual scientific sessions of the American Diabetes Association.
“Anything under 10% is considered good, and ours was 9.4% – and it didn’t deteriorate throughout the duration, so at 180 days it was still at 9.4%; every accuracy measure we looked at showed similar high levels of accuracy,” Dr. Aronson, founder and chief medical officer of LMC Diabetes & Endocrinology in Ontario, Canada said in a video interview.
The sensor, which is roughly 1.5 cm long, is coated with a material that fluoresces when exposed to glucose; the sensor uses the amount of light emitted to calculate blood glucose levels. Patients use an adhesive patch, changed daily, to attach a “smart” transmitter that overlies the area where the sensor is implanted. This rechargeable transmitter sends blood glucose levels to the mobile app every 5 minutes, and also powers the sensor. The Food and Drug Administration approved it for use in adults on June 21.
The system was highly rated by study participants, he said. “What makes it stand out is that it’s implanted, it’s there for at least 180 days, it’s accurate for 180 days,” the transmitter can be taken on and off, and the results can be seen very easily on a smart phone or Apple Watch.
Dr. Aronson said he also hopes to study the device in younger patients and for longer durations.
Dr. Aronson is an advisor for Novo Nordisk and Sanofi. He also receives research support from AstraZeneca, Eli Lilly, Valeant, Janssen, and Senseonics.
SOURCE: Aronson R et al. ADA 2018 Abstract 13-OR.
REPORTING FROM ADA 2018
Key clinical point: The Eversense fully implantable continuous glucose monitoring device is safe and accurate in adolescents.
Major finding: The MARD between sensor and true laboratory glucose values showed high device accuracy, at 9.4% over 180 days.
Study details: A prospective single-arm study of 30 children and 6 adults.
Disclosures: Dr. Aronson is an advisor for Novo Nordisk and Sanofi. He also receives research support from AstraZeneca, Eli Lilly, Valeant, Janssen, and Senseonics.
Source: Aronson R et al. ADA Abstract 13-OR.
Switch back to human insulin a viable money saver
ORLANDO – It’s safe to switch many Medicare beneficiaries with type 2 diabetes to human insulins to save money on analogues, according to a review of 14,635 members of CareMore, a Medicare Advantage company based in Cerritos, Calif.
The company noticed that it’s spending on analogue insulins had ballooned to over $3 million a month by the end of 2014, in the wake of a more than 300% price increase in analogue insulins in recent years, while copays on analogues rose from nothing to $37.50. In 2015, it launched a program to switch type 2 patients to less costly human insulins. Physicians were counseled to stop secretagogues and move patients to premixed insulins at 80% of their former total daily analogue dose, two-thirds at breakfast, and one-third a dinner, with appropriate follow-up.
Analogue insulins fell from 90% of all insulins dispensed to 30%, with a corresponding rise in human insulin prescriptions. Total plan spending on analogues fell to about a half million dollars a month by the end of 2016. Spending on human insulins rose to just under a million dollars. The risk of patients falling into the Medicare Part D coverage gap – where they assume a greater proportion of their drug costs – was reduced by 55% (P less than .001).
“A lot of money was saved as a result of this intervention,” said lead investigator Jin Luo, MD, an internist and health services researcher at Brigham and Women’s Hospital, Boston.
Mean hemoglobin A1c rose 0.14 % from a baseline of 8.46% in 2014 (P less than 0.01), “but we do not believe that this is clinically important because this value falls within the biological within-subject variation of most modern HbA1c assays,” he said at the annual scientific sessions of the American Diabetes Association.
Meanwhile, there was no statistically significant change in the rate of hospitalizations or emergency department visits for hypoglycemia or hyperglycemia.
“Patients with type 2 diabetes and their clinical providers should strongly consider human insulin as a clinically viable and cost effective option,” Dr. Luo said.
“My personal clinical opinion is that if I have a patient who is really hard to control, and after four or five different regimens, we finally settle on an analogue regimen that [keeps] them under control” and out of the hospital, “I’m not going to switch them just because a health plan tells me I should. They are just too brittle, and I’m not comfortable doing that. Whereas if I have a patient who’d be fine with either option, and I’m not really worried about hypoglycemia, I’ll switch them,” he said.
There was no industry funding. Dr. Luo is a consultant for Alosa Health and Health Action International.
SOURCE: Luo J et al. 2018 American Diabetes Association scientific session abstract 4-OR
ORLANDO – It’s safe to switch many Medicare beneficiaries with type 2 diabetes to human insulins to save money on analogues, according to a review of 14,635 members of CareMore, a Medicare Advantage company based in Cerritos, Calif.
The company noticed that it’s spending on analogue insulins had ballooned to over $3 million a month by the end of 2014, in the wake of a more than 300% price increase in analogue insulins in recent years, while copays on analogues rose from nothing to $37.50. In 2015, it launched a program to switch type 2 patients to less costly human insulins. Physicians were counseled to stop secretagogues and move patients to premixed insulins at 80% of their former total daily analogue dose, two-thirds at breakfast, and one-third a dinner, with appropriate follow-up.
Analogue insulins fell from 90% of all insulins dispensed to 30%, with a corresponding rise in human insulin prescriptions. Total plan spending on analogues fell to about a half million dollars a month by the end of 2016. Spending on human insulins rose to just under a million dollars. The risk of patients falling into the Medicare Part D coverage gap – where they assume a greater proportion of their drug costs – was reduced by 55% (P less than .001).
“A lot of money was saved as a result of this intervention,” said lead investigator Jin Luo, MD, an internist and health services researcher at Brigham and Women’s Hospital, Boston.
Mean hemoglobin A1c rose 0.14 % from a baseline of 8.46% in 2014 (P less than 0.01), “but we do not believe that this is clinically important because this value falls within the biological within-subject variation of most modern HbA1c assays,” he said at the annual scientific sessions of the American Diabetes Association.
Meanwhile, there was no statistically significant change in the rate of hospitalizations or emergency department visits for hypoglycemia or hyperglycemia.
“Patients with type 2 diabetes and their clinical providers should strongly consider human insulin as a clinically viable and cost effective option,” Dr. Luo said.
“My personal clinical opinion is that if I have a patient who is really hard to control, and after four or five different regimens, we finally settle on an analogue regimen that [keeps] them under control” and out of the hospital, “I’m not going to switch them just because a health plan tells me I should. They are just too brittle, and I’m not comfortable doing that. Whereas if I have a patient who’d be fine with either option, and I’m not really worried about hypoglycemia, I’ll switch them,” he said.
There was no industry funding. Dr. Luo is a consultant for Alosa Health and Health Action International.
SOURCE: Luo J et al. 2018 American Diabetes Association scientific session abstract 4-OR
ORLANDO – It’s safe to switch many Medicare beneficiaries with type 2 diabetes to human insulins to save money on analogues, according to a review of 14,635 members of CareMore, a Medicare Advantage company based in Cerritos, Calif.
The company noticed that it’s spending on analogue insulins had ballooned to over $3 million a month by the end of 2014, in the wake of a more than 300% price increase in analogue insulins in recent years, while copays on analogues rose from nothing to $37.50. In 2015, it launched a program to switch type 2 patients to less costly human insulins. Physicians were counseled to stop secretagogues and move patients to premixed insulins at 80% of their former total daily analogue dose, two-thirds at breakfast, and one-third a dinner, with appropriate follow-up.
Analogue insulins fell from 90% of all insulins dispensed to 30%, with a corresponding rise in human insulin prescriptions. Total plan spending on analogues fell to about a half million dollars a month by the end of 2016. Spending on human insulins rose to just under a million dollars. The risk of patients falling into the Medicare Part D coverage gap – where they assume a greater proportion of their drug costs – was reduced by 55% (P less than .001).
“A lot of money was saved as a result of this intervention,” said lead investigator Jin Luo, MD, an internist and health services researcher at Brigham and Women’s Hospital, Boston.
Mean hemoglobin A1c rose 0.14 % from a baseline of 8.46% in 2014 (P less than 0.01), “but we do not believe that this is clinically important because this value falls within the biological within-subject variation of most modern HbA1c assays,” he said at the annual scientific sessions of the American Diabetes Association.
Meanwhile, there was no statistically significant change in the rate of hospitalizations or emergency department visits for hypoglycemia or hyperglycemia.
“Patients with type 2 diabetes and their clinical providers should strongly consider human insulin as a clinically viable and cost effective option,” Dr. Luo said.
“My personal clinical opinion is that if I have a patient who is really hard to control, and after four or five different regimens, we finally settle on an analogue regimen that [keeps] them under control” and out of the hospital, “I’m not going to switch them just because a health plan tells me I should. They are just too brittle, and I’m not comfortable doing that. Whereas if I have a patient who’d be fine with either option, and I’m not really worried about hypoglycemia, I’ll switch them,” he said.
There was no industry funding. Dr. Luo is a consultant for Alosa Health and Health Action International.
SOURCE: Luo J et al. 2018 American Diabetes Association scientific session abstract 4-OR
REPORTING FROM ADA 2018
Key clinical point:
Major finding: Mean HbA1c rose just 0.14% from a baseline of 8.46% (P less than 0.01).
Study details: A review of 14,635 members Medicare patients with type 2 diabetes.
Disclosures: There was no industry funding. The lead investigator is a consultant for Alosa Health and Health Action International.
Source: Luo J et al. ADA 2018, Abstract 4-OR
T1D neuropathy declines as glycemic control improves
ORLANDO – Rates of diabetic peripheral neuropathy (DPN) in U.S. patients with type 1 diabetes (T1D) may have dipped, possibly because of improving clinical care, a new study suggests. Researchers also found evidence that nonglycemic factors may play important roles in the development of the condition.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
There are differences between DPN in T1D and type 2 diabetes: Lifetime incidence in T1D is believed to be 45%, lower than in T2D. However, a 2016 report noted that, “whereas treating hyperglycemia in type 1 DM can significantly reduce the incidence of neuropathy by up to 60 to 70%, glucose control in type 2 DM has only a marginal 5 to 7% reduction in the development of neuropathy.” (F1000Research 2016, 5(F1000 Faculty Rev):738)
Still, DPN is believed to be very common in T1D. According to the new study, previous research has suggested that the DPN rate in this population could be as high as 35%.
For the new study, researchers examined self-reports of DPN from 5,058 patients across 62 sites via the T1D Exchange Registry. All patients were at least 18 years of age and had at least 5 years of T1D. Their mean age was 39 years, the duration of diabetes was 22 years, and their average hemoglobin A1c was 8.1. Over half (56%) were women, and most (88%) were white were white.
A preliminary analysis found that just 10% of the patients had signs of DPN, according to their self-reports. In part, the difference between this number and previous estimates of DPN prevalence may be because previous studies relied on symptoms, exams, and electrophysiologic testing, said study researcher Kara Mizokami-Stout, MD, of the University of Michigan, in an interview.
However, study researcher Rodica Pop-Busui, MD, PhD, noted in an interview that one strength of the new study is that it’s “a broad sample of patients with type 1 diabetes as they are currently treated in clinical care across the United States.”
Versus those without DPN, those with the condition were more likely to be older (mean 52 vs. 37 years), female (61% vs. 55%), and had T1D for a longer period (mean 32 vs. 21 years). They were also poorer and had less education. (All P less than .001)
The DPN group also had slightly higher systolic blood pressure (mean 126 vs. 123), higher triglycerides (117 vs. 95) and more than double the rate of tobacco use (9% vs. 4%), all P less than .001.
Also, cardiovascular disease was more common (26% vs. 6%) even though this group used statins (64% vs. 31%) and ACE inhibitors/ARBs (45% vs. 23%) at much higher levels, all P less than .001.
Researchers also found that this with DPN had higher HbA1c even after controlling for various confounders (8.4% vs. 8.1%, P less than .01).
“We have the ability to prevent neuropathy, and we should do that to our advantage, targeting glycemic control as best as possible without increasing the risk of hypoglycemia,” Dr. Mizokami-Stout said. Targeting nonglycemic factors is also crucial, she said.
The study was funded by the Helmsley Charitable Trust. Dr. Mizokami-Stout and Dr. Pop-Busui report no relevant disclosures. Some of the other authors report various disclosures.
SOURCE: Mizokami-Stout K, et al. ADA 2018, Abstract 62-OR.
ORLANDO – Rates of diabetic peripheral neuropathy (DPN) in U.S. patients with type 1 diabetes (T1D) may have dipped, possibly because of improving clinical care, a new study suggests. Researchers also found evidence that nonglycemic factors may play important roles in the development of the condition.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
There are differences between DPN in T1D and type 2 diabetes: Lifetime incidence in T1D is believed to be 45%, lower than in T2D. However, a 2016 report noted that, “whereas treating hyperglycemia in type 1 DM can significantly reduce the incidence of neuropathy by up to 60 to 70%, glucose control in type 2 DM has only a marginal 5 to 7% reduction in the development of neuropathy.” (F1000Research 2016, 5(F1000 Faculty Rev):738)
Still, DPN is believed to be very common in T1D. According to the new study, previous research has suggested that the DPN rate in this population could be as high as 35%.
For the new study, researchers examined self-reports of DPN from 5,058 patients across 62 sites via the T1D Exchange Registry. All patients were at least 18 years of age and had at least 5 years of T1D. Their mean age was 39 years, the duration of diabetes was 22 years, and their average hemoglobin A1c was 8.1. Over half (56%) were women, and most (88%) were white were white.
A preliminary analysis found that just 10% of the patients had signs of DPN, according to their self-reports. In part, the difference between this number and previous estimates of DPN prevalence may be because previous studies relied on symptoms, exams, and electrophysiologic testing, said study researcher Kara Mizokami-Stout, MD, of the University of Michigan, in an interview.
However, study researcher Rodica Pop-Busui, MD, PhD, noted in an interview that one strength of the new study is that it’s “a broad sample of patients with type 1 diabetes as they are currently treated in clinical care across the United States.”
Versus those without DPN, those with the condition were more likely to be older (mean 52 vs. 37 years), female (61% vs. 55%), and had T1D for a longer period (mean 32 vs. 21 years). They were also poorer and had less education. (All P less than .001)
The DPN group also had slightly higher systolic blood pressure (mean 126 vs. 123), higher triglycerides (117 vs. 95) and more than double the rate of tobacco use (9% vs. 4%), all P less than .001.
Also, cardiovascular disease was more common (26% vs. 6%) even though this group used statins (64% vs. 31%) and ACE inhibitors/ARBs (45% vs. 23%) at much higher levels, all P less than .001.
Researchers also found that this with DPN had higher HbA1c even after controlling for various confounders (8.4% vs. 8.1%, P less than .01).
“We have the ability to prevent neuropathy, and we should do that to our advantage, targeting glycemic control as best as possible without increasing the risk of hypoglycemia,” Dr. Mizokami-Stout said. Targeting nonglycemic factors is also crucial, she said.
The study was funded by the Helmsley Charitable Trust. Dr. Mizokami-Stout and Dr. Pop-Busui report no relevant disclosures. Some of the other authors report various disclosures.
SOURCE: Mizokami-Stout K, et al. ADA 2018, Abstract 62-OR.
ORLANDO – Rates of diabetic peripheral neuropathy (DPN) in U.S. patients with type 1 diabetes (T1D) may have dipped, possibly because of improving clinical care, a new study suggests. Researchers also found evidence that nonglycemic factors may play important roles in the development of the condition.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
There are differences between DPN in T1D and type 2 diabetes: Lifetime incidence in T1D is believed to be 45%, lower than in T2D. However, a 2016 report noted that, “whereas treating hyperglycemia in type 1 DM can significantly reduce the incidence of neuropathy by up to 60 to 70%, glucose control in type 2 DM has only a marginal 5 to 7% reduction in the development of neuropathy.” (F1000Research 2016, 5(F1000 Faculty Rev):738)
Still, DPN is believed to be very common in T1D. According to the new study, previous research has suggested that the DPN rate in this population could be as high as 35%.
For the new study, researchers examined self-reports of DPN from 5,058 patients across 62 sites via the T1D Exchange Registry. All patients were at least 18 years of age and had at least 5 years of T1D. Their mean age was 39 years, the duration of diabetes was 22 years, and their average hemoglobin A1c was 8.1. Over half (56%) were women, and most (88%) were white were white.
A preliminary analysis found that just 10% of the patients had signs of DPN, according to their self-reports. In part, the difference between this number and previous estimates of DPN prevalence may be because previous studies relied on symptoms, exams, and electrophysiologic testing, said study researcher Kara Mizokami-Stout, MD, of the University of Michigan, in an interview.
However, study researcher Rodica Pop-Busui, MD, PhD, noted in an interview that one strength of the new study is that it’s “a broad sample of patients with type 1 diabetes as they are currently treated in clinical care across the United States.”
Versus those without DPN, those with the condition were more likely to be older (mean 52 vs. 37 years), female (61% vs. 55%), and had T1D for a longer period (mean 32 vs. 21 years). They were also poorer and had less education. (All P less than .001)
The DPN group also had slightly higher systolic blood pressure (mean 126 vs. 123), higher triglycerides (117 vs. 95) and more than double the rate of tobacco use (9% vs. 4%), all P less than .001.
Also, cardiovascular disease was more common (26% vs. 6%) even though this group used statins (64% vs. 31%) and ACE inhibitors/ARBs (45% vs. 23%) at much higher levels, all P less than .001.
Researchers also found that this with DPN had higher HbA1c even after controlling for various confounders (8.4% vs. 8.1%, P less than .01).
“We have the ability to prevent neuropathy, and we should do that to our advantage, targeting glycemic control as best as possible without increasing the risk of hypoglycemia,” Dr. Mizokami-Stout said. Targeting nonglycemic factors is also crucial, she said.
The study was funded by the Helmsley Charitable Trust. Dr. Mizokami-Stout and Dr. Pop-Busui report no relevant disclosures. Some of the other authors report various disclosures.
SOURCE: Mizokami-Stout K, et al. ADA 2018, Abstract 62-OR.
REPORTING FROM ADA 2018
Key clinical point: Diabetic peripheral neuropathy (DPN) may be on the decline in type 1 diabetes (T1D), and nonglycemic factors may be crucial.
Major finding: 10% of subjects showed signs of DPN via self-report, and those with DPN had much higher rates of cardiovascular disease.
Study details: Analysis of 5,058 patients across 62 sites via the T1D Exchange Registry.
Disclosures: The study was funded by the Helmsley Charitable Trust. Some of the authors report various disclosures.
Source: Mizokami-Stout K, et al. ADA 2018, Abstract 62-OR.
Average glucose, A1c discordance is common, highlights ADAG equation concerns
ORLANDO – Significant discordance exists between average glucose and hemoglobin A1c (HbA1c) measures in patients with certain comorbidities, according to findings from a retrospective chart review.
For example, there was a complete lack of correlation between average glucose (AG) and A1c measures in patients with advanced renal dysfunction and non-alcoholic fatty liver disease, (NAFLD) Jordan E. Perlman, MD, reported at the annual scientific sessions of the American Diabetes Association.
Unweighted averages of self-monitored blood glucose (SMBG) and continuous glucose monitor (CGM) readings were calculated based on downloads from 1,039 patients who had been prescribed insulin for diabetes mellitus between January 2011 and October 2016 and who had a comorbid condition proven or hypothesized to invalidate A1c, including anemia, chronic kidney disease (CKD), abnormal liver function tests (LFTs), and NAFLD. Predicted AG was also derived from paired A1c using the equation established by the A1c Derived Average Glucose (ADAG) Study Group in a 2013 re-analysis of its 2008 report, which excluded patients with comorbidities.
The averages calculated using downloads were then compared with the averages derived using the ADAG equation to assess concordance.
“The term ‘discordant’ refers to averages that differ by more than 15%,” Dr. Perlman explained.
She and her colleagues found that CGM, compared with SMBG, decreased the odds of discordance after controlling for diabetes type (odds ratio, 0.39).
Additionally, having type 2 vs. type 1 diabetes mellitus increased the odds of discordance, as did renal dysfunction.
“Having CKD stage 3b or worse increases the odds of ADAG discordance (OR, 2.04),” she said. “The relationship demonstrates statistical significance at a P value of 0.004. Unfortunately, we did not have enough patients to analyze stage 4 or 5 CKD alone.”
Poor linear correlation was clearly seen between AG and A1c in patients with NAFLD, she noted.
“The relationship doesn’t reach statistical significance, but the odds ratio of 1.6 is difficult to ignore. The wide confidence interval (0.67-3.58) leads us to believe that this particular analysis is probably underpowered,” Dr. Perlman said.
Factors assessed and found to have no significant effect on ADAG discordance included abnormal LFTs, age, body mass index, and hemoglobin, including by gender.
“These important data suggest that any patient on insulin who comes to diabetes clinic has an automatic 33.5% chance of mismatch between their A1c and average glucose, and this is before you know anything else about them. To go a step further, it seems excluding comorbidities doesn’t really improve the percent discordance,” she said, adding that this suggests comorbidities have less impact than previously thought. “This makes us wonder if maybe there is a problem with our test and not the person having the test.”
It remains unclear what is acceptable in terms of discordance, Dr. Perlman said, noting that using ADAG to interpret A1c yields a wide range of estimated AG.
“Comorbidities alone do not explain this variation,” she said. “Clinicians should not rely on A1c alone to make treatment decisions because it is unclear when discordance gains clinical relevance.”
This study is limited by the retrospective study design and a number of factors, such as the difficulties of confirming or excluding comorbidities based on a single encounter and the limitless potential for unestablished confounders of A1c and AG, Dr. Perlman noted.
Also, fingersticks inflate discordance.
“A better assessment of ADAG would be to measure only CGM averages in comorbities, though this may need to be a prospective trial as only 17% of our patients who have identified comorbidities use CGM,” she said.
Dr. Perlman concluded that fingersticks and CGM can provide important confirmation of A1c, but said this applies only at the population level and not to individual patients.
“For individual patients, any level of A1c can translate to a large range of average glucoses. We see this even in our concordant patients,” she said.
Further, while discordance is increased by some comorbidities, it also occurs absent of comorbidities at a rate of 28.7%.
she said.
Dr. Perlman reported having no disclosures. Senior author Irl B. Hirsch, MD, professor of medicine at the University of Washington, Seattle, disclosed financial relationships diabetes drug and device manufacturers Abbot, ADOCIA, Bigfoot Biomedical, Roche, and Medtronic MiniMed.
SOURCE: Perlman J et al., ADA 2018 Abstract 12-OR.
ORLANDO – Significant discordance exists between average glucose and hemoglobin A1c (HbA1c) measures in patients with certain comorbidities, according to findings from a retrospective chart review.
For example, there was a complete lack of correlation between average glucose (AG) and A1c measures in patients with advanced renal dysfunction and non-alcoholic fatty liver disease, (NAFLD) Jordan E. Perlman, MD, reported at the annual scientific sessions of the American Diabetes Association.
Unweighted averages of self-monitored blood glucose (SMBG) and continuous glucose monitor (CGM) readings were calculated based on downloads from 1,039 patients who had been prescribed insulin for diabetes mellitus between January 2011 and October 2016 and who had a comorbid condition proven or hypothesized to invalidate A1c, including anemia, chronic kidney disease (CKD), abnormal liver function tests (LFTs), and NAFLD. Predicted AG was also derived from paired A1c using the equation established by the A1c Derived Average Glucose (ADAG) Study Group in a 2013 re-analysis of its 2008 report, which excluded patients with comorbidities.
The averages calculated using downloads were then compared with the averages derived using the ADAG equation to assess concordance.
“The term ‘discordant’ refers to averages that differ by more than 15%,” Dr. Perlman explained.
She and her colleagues found that CGM, compared with SMBG, decreased the odds of discordance after controlling for diabetes type (odds ratio, 0.39).
Additionally, having type 2 vs. type 1 diabetes mellitus increased the odds of discordance, as did renal dysfunction.
“Having CKD stage 3b or worse increases the odds of ADAG discordance (OR, 2.04),” she said. “The relationship demonstrates statistical significance at a P value of 0.004. Unfortunately, we did not have enough patients to analyze stage 4 or 5 CKD alone.”
Poor linear correlation was clearly seen between AG and A1c in patients with NAFLD, she noted.
“The relationship doesn’t reach statistical significance, but the odds ratio of 1.6 is difficult to ignore. The wide confidence interval (0.67-3.58) leads us to believe that this particular analysis is probably underpowered,” Dr. Perlman said.
Factors assessed and found to have no significant effect on ADAG discordance included abnormal LFTs, age, body mass index, and hemoglobin, including by gender.
“These important data suggest that any patient on insulin who comes to diabetes clinic has an automatic 33.5% chance of mismatch between their A1c and average glucose, and this is before you know anything else about them. To go a step further, it seems excluding comorbidities doesn’t really improve the percent discordance,” she said, adding that this suggests comorbidities have less impact than previously thought. “This makes us wonder if maybe there is a problem with our test and not the person having the test.”
It remains unclear what is acceptable in terms of discordance, Dr. Perlman said, noting that using ADAG to interpret A1c yields a wide range of estimated AG.
“Comorbidities alone do not explain this variation,” she said. “Clinicians should not rely on A1c alone to make treatment decisions because it is unclear when discordance gains clinical relevance.”
This study is limited by the retrospective study design and a number of factors, such as the difficulties of confirming or excluding comorbidities based on a single encounter and the limitless potential for unestablished confounders of A1c and AG, Dr. Perlman noted.
Also, fingersticks inflate discordance.
“A better assessment of ADAG would be to measure only CGM averages in comorbities, though this may need to be a prospective trial as only 17% of our patients who have identified comorbidities use CGM,” she said.
Dr. Perlman concluded that fingersticks and CGM can provide important confirmation of A1c, but said this applies only at the population level and not to individual patients.
“For individual patients, any level of A1c can translate to a large range of average glucoses. We see this even in our concordant patients,” she said.
Further, while discordance is increased by some comorbidities, it also occurs absent of comorbidities at a rate of 28.7%.
she said.
Dr. Perlman reported having no disclosures. Senior author Irl B. Hirsch, MD, professor of medicine at the University of Washington, Seattle, disclosed financial relationships diabetes drug and device manufacturers Abbot, ADOCIA, Bigfoot Biomedical, Roche, and Medtronic MiniMed.
SOURCE: Perlman J et al., ADA 2018 Abstract 12-OR.
ORLANDO – Significant discordance exists between average glucose and hemoglobin A1c (HbA1c) measures in patients with certain comorbidities, according to findings from a retrospective chart review.
For example, there was a complete lack of correlation between average glucose (AG) and A1c measures in patients with advanced renal dysfunction and non-alcoholic fatty liver disease, (NAFLD) Jordan E. Perlman, MD, reported at the annual scientific sessions of the American Diabetes Association.
Unweighted averages of self-monitored blood glucose (SMBG) and continuous glucose monitor (CGM) readings were calculated based on downloads from 1,039 patients who had been prescribed insulin for diabetes mellitus between January 2011 and October 2016 and who had a comorbid condition proven or hypothesized to invalidate A1c, including anemia, chronic kidney disease (CKD), abnormal liver function tests (LFTs), and NAFLD. Predicted AG was also derived from paired A1c using the equation established by the A1c Derived Average Glucose (ADAG) Study Group in a 2013 re-analysis of its 2008 report, which excluded patients with comorbidities.
The averages calculated using downloads were then compared with the averages derived using the ADAG equation to assess concordance.
“The term ‘discordant’ refers to averages that differ by more than 15%,” Dr. Perlman explained.
She and her colleagues found that CGM, compared with SMBG, decreased the odds of discordance after controlling for diabetes type (odds ratio, 0.39).
Additionally, having type 2 vs. type 1 diabetes mellitus increased the odds of discordance, as did renal dysfunction.
“Having CKD stage 3b or worse increases the odds of ADAG discordance (OR, 2.04),” she said. “The relationship demonstrates statistical significance at a P value of 0.004. Unfortunately, we did not have enough patients to analyze stage 4 or 5 CKD alone.”
Poor linear correlation was clearly seen between AG and A1c in patients with NAFLD, she noted.
“The relationship doesn’t reach statistical significance, but the odds ratio of 1.6 is difficult to ignore. The wide confidence interval (0.67-3.58) leads us to believe that this particular analysis is probably underpowered,” Dr. Perlman said.
Factors assessed and found to have no significant effect on ADAG discordance included abnormal LFTs, age, body mass index, and hemoglobin, including by gender.
“These important data suggest that any patient on insulin who comes to diabetes clinic has an automatic 33.5% chance of mismatch between their A1c and average glucose, and this is before you know anything else about them. To go a step further, it seems excluding comorbidities doesn’t really improve the percent discordance,” she said, adding that this suggests comorbidities have less impact than previously thought. “This makes us wonder if maybe there is a problem with our test and not the person having the test.”
It remains unclear what is acceptable in terms of discordance, Dr. Perlman said, noting that using ADAG to interpret A1c yields a wide range of estimated AG.
“Comorbidities alone do not explain this variation,” she said. “Clinicians should not rely on A1c alone to make treatment decisions because it is unclear when discordance gains clinical relevance.”
This study is limited by the retrospective study design and a number of factors, such as the difficulties of confirming or excluding comorbidities based on a single encounter and the limitless potential for unestablished confounders of A1c and AG, Dr. Perlman noted.
Also, fingersticks inflate discordance.
“A better assessment of ADAG would be to measure only CGM averages in comorbities, though this may need to be a prospective trial as only 17% of our patients who have identified comorbidities use CGM,” she said.
Dr. Perlman concluded that fingersticks and CGM can provide important confirmation of A1c, but said this applies only at the population level and not to individual patients.
“For individual patients, any level of A1c can translate to a large range of average glucoses. We see this even in our concordant patients,” she said.
Further, while discordance is increased by some comorbidities, it also occurs absent of comorbidities at a rate of 28.7%.
she said.
Dr. Perlman reported having no disclosures. Senior author Irl B. Hirsch, MD, professor of medicine at the University of Washington, Seattle, disclosed financial relationships diabetes drug and device manufacturers Abbot, ADOCIA, Bigfoot Biomedical, Roche, and Medtronic MiniMed.
SOURCE: Perlman J et al., ADA 2018 Abstract 12-OR.
REPORTING FROM ADA 2018
Key clinical point: AG and A1c discordance is common, thus professional consensus regarding acceptable discordance is needed before ADAG equation use is broadened.
Major finding: CKD stage 3b or worse increases the odds of ADAG discordance (OR, 2.04).
Study details: A retrospective review of 1,039 patient charts.
Disclosures: Dr. Perlman reported having no disclosures. Senior author Irl B. Hirsch, MD, disclosed financial relationships diabetes drug and device manufacturers Abbot, ADOCIA, Bigfoot Biomedical, Roche, and Medtronic MiniMed.
Source: Perlman J et al. ADA 2018 Abstract 12-OR.
Patients going without as insulin prices skyrocket
ORLANDO – About a quarter of diabetes patients use less insulin than prescribed because they can’t afford it, and they have worse glycemic control because of it, according to a survey of patients at the Yale Diabetes Center in New Haven, Conn.
The soaring cost of insulin – especially analogues – has been in the news following a more than 300% increase from 2004-2018. The cash price for a 10 mL vial of insulin lispro (Humalog), for example, has climbed from $59 to $320. The American Diabetes Association recently released a white paper on the issue, citing a “lack of transparency throughout the insulin supply chain” that obscures the reasons for the surge. It’s working with other groups to ensure affordable access.
Six questions were key: In the past 12 months, did you, because of cost, use less insulin than prescribed; try to stretch out your insulin; take smaller doses of insulin than prescribed; stop insulin; not fill an insulin prescription; or not start insulin?
Fifty-one patients answered “yes” to at least one of those questions, signaling to investigators that they were using less insulin than prescribed because they couldn’t afford it. Compared with other patients, they were three times more likely to have HbA1c levels above 9%, controlling for age, sex, diabetes duration, and income (P = 0.03).
“One in four patients were using less of an essential medication because it costs too much for them to take the prescribed amount,” said investigator Darby Herkert, who participated in the study as an undergraduate at Yale. “It’s having a tangible health effect.”
The problem was greatest among people making less than $100,000 dollars a year, and was not associated with race or the type of diabetes they had. Employer health coverage was not protective, and patients who were covered by a mix of government and employer insurance were at greater risk of underuse, as were those who were unable to work.
The situation is probably common in the United States, Ms. Herkert noted at the American Diabetes Association scientific sessions meeting. “New Haven is a demographic microcosm of the U.S.”
“These results highlight an urgent need to address high insulin prices,” she said. This may be done through greater transparency in pricing, advocacy for patients who can’t afford their prescription, use of alternative insulin options for some patients, and assistance programs,” she said.
The work was funded in part by the National Institutes of Health. The investigators had no disclosures.
SOURCE: Herkert D et al. ADA 2018 abstract 2-OR
ORLANDO – About a quarter of diabetes patients use less insulin than prescribed because they can’t afford it, and they have worse glycemic control because of it, according to a survey of patients at the Yale Diabetes Center in New Haven, Conn.
The soaring cost of insulin – especially analogues – has been in the news following a more than 300% increase from 2004-2018. The cash price for a 10 mL vial of insulin lispro (Humalog), for example, has climbed from $59 to $320. The American Diabetes Association recently released a white paper on the issue, citing a “lack of transparency throughout the insulin supply chain” that obscures the reasons for the surge. It’s working with other groups to ensure affordable access.
Six questions were key: In the past 12 months, did you, because of cost, use less insulin than prescribed; try to stretch out your insulin; take smaller doses of insulin than prescribed; stop insulin; not fill an insulin prescription; or not start insulin?
Fifty-one patients answered “yes” to at least one of those questions, signaling to investigators that they were using less insulin than prescribed because they couldn’t afford it. Compared with other patients, they were three times more likely to have HbA1c levels above 9%, controlling for age, sex, diabetes duration, and income (P = 0.03).
“One in four patients were using less of an essential medication because it costs too much for them to take the prescribed amount,” said investigator Darby Herkert, who participated in the study as an undergraduate at Yale. “It’s having a tangible health effect.”
The problem was greatest among people making less than $100,000 dollars a year, and was not associated with race or the type of diabetes they had. Employer health coverage was not protective, and patients who were covered by a mix of government and employer insurance were at greater risk of underuse, as were those who were unable to work.
The situation is probably common in the United States, Ms. Herkert noted at the American Diabetes Association scientific sessions meeting. “New Haven is a demographic microcosm of the U.S.”
“These results highlight an urgent need to address high insulin prices,” she said. This may be done through greater transparency in pricing, advocacy for patients who can’t afford their prescription, use of alternative insulin options for some patients, and assistance programs,” she said.
The work was funded in part by the National Institutes of Health. The investigators had no disclosures.
SOURCE: Herkert D et al. ADA 2018 abstract 2-OR
ORLANDO – About a quarter of diabetes patients use less insulin than prescribed because they can’t afford it, and they have worse glycemic control because of it, according to a survey of patients at the Yale Diabetes Center in New Haven, Conn.
The soaring cost of insulin – especially analogues – has been in the news following a more than 300% increase from 2004-2018. The cash price for a 10 mL vial of insulin lispro (Humalog), for example, has climbed from $59 to $320. The American Diabetes Association recently released a white paper on the issue, citing a “lack of transparency throughout the insulin supply chain” that obscures the reasons for the surge. It’s working with other groups to ensure affordable access.
Six questions were key: In the past 12 months, did you, because of cost, use less insulin than prescribed; try to stretch out your insulin; take smaller doses of insulin than prescribed; stop insulin; not fill an insulin prescription; or not start insulin?
Fifty-one patients answered “yes” to at least one of those questions, signaling to investigators that they were using less insulin than prescribed because they couldn’t afford it. Compared with other patients, they were three times more likely to have HbA1c levels above 9%, controlling for age, sex, diabetes duration, and income (P = 0.03).
“One in four patients were using less of an essential medication because it costs too much for them to take the prescribed amount,” said investigator Darby Herkert, who participated in the study as an undergraduate at Yale. “It’s having a tangible health effect.”
The problem was greatest among people making less than $100,000 dollars a year, and was not associated with race or the type of diabetes they had. Employer health coverage was not protective, and patients who were covered by a mix of government and employer insurance were at greater risk of underuse, as were those who were unable to work.
The situation is probably common in the United States, Ms. Herkert noted at the American Diabetes Association scientific sessions meeting. “New Haven is a demographic microcosm of the U.S.”
“These results highlight an urgent need to address high insulin prices,” she said. This may be done through greater transparency in pricing, advocacy for patients who can’t afford their prescription, use of alternative insulin options for some patients, and assistance programs,” she said.
The work was funded in part by the National Institutes of Health. The investigators had no disclosures.
SOURCE: Herkert D et al. ADA 2018 abstract 2-OR
REPORTING FROM ADA 2018
Key clinical point: because of it.
Major finding: Compared with other patients, they were three times more likely to have hemoglobin A1c levels above 9%, controlling for age, sex, diabetes duration, and income (P = 0.03).
Study details: A single-center survey of 199 patients with type 1 or type 2 diabetes.
Disclosures: The work was funded in part by the National Institutes of Health. The investigators had no disclosures.
Source: Herkert D et al. ADA 2018, Abstract 2-OR
Preview of ADA/EASD statement on hyperglycemia
A move toward more individualized treatment of hyperglycemia is coming in the next American Diabetes Association/European Association for the Study of Diabetes Consensus Report, according to John B. Buse, MD, PhD, cochair of the committee writing the new consensus statement.
He will present a draft of the statement on the management of hyperglycemia in type 2 diabetes at the ADA’s annual scientific sessions in Orlando.
When finalized – after revisions based on comments and feedback from diabetes care providers – clinical researchers, patient groups, payers, regulators, and stakeholders – the statement will update the last revision, issued in 2015.
“We are taking a new look at hyperglycemia based on the many studies conducted since 2014, particularly the cardiovascular outcomes trials,” Dr. Buse, the Verne S. Caviness Distinguished Professor in the division of endocrinology and metabolism and chief of endocrinology at the University of North Carolina, Chapel Hill, said in a statement.
But it’s a good bet that ADA scientific sessions attendees will see a move toward more specific recommendations based on patient characteristics and fewer one-size-fits-all recommendations. Specific characteristics like obesity, cardiovascular disease, and chronic kidney disease will likely be addressed in the new consensus statement.
One aspect of patient care that will see more attention in the ultimate statement is personalized care. “We will certainly highlight the need to individualize all aspects of care in a patient-centered way, taking into account both specific patient attributes and preferences,” Dr. Buse said.
The draft statement will be presented on Tuesday, June 26, at 8:00 a.m., so it may be worth staying for that last day of the meeting.
The final draft of the new statement will be released in October at the EASD annual meeting in Berlin, noted Dr. Buse, also director of the diabetes center at the university.
A move toward more individualized treatment of hyperglycemia is coming in the next American Diabetes Association/European Association for the Study of Diabetes Consensus Report, according to John B. Buse, MD, PhD, cochair of the committee writing the new consensus statement.
He will present a draft of the statement on the management of hyperglycemia in type 2 diabetes at the ADA’s annual scientific sessions in Orlando.
When finalized – after revisions based on comments and feedback from diabetes care providers – clinical researchers, patient groups, payers, regulators, and stakeholders – the statement will update the last revision, issued in 2015.
“We are taking a new look at hyperglycemia based on the many studies conducted since 2014, particularly the cardiovascular outcomes trials,” Dr. Buse, the Verne S. Caviness Distinguished Professor in the division of endocrinology and metabolism and chief of endocrinology at the University of North Carolina, Chapel Hill, said in a statement.
But it’s a good bet that ADA scientific sessions attendees will see a move toward more specific recommendations based on patient characteristics and fewer one-size-fits-all recommendations. Specific characteristics like obesity, cardiovascular disease, and chronic kidney disease will likely be addressed in the new consensus statement.
One aspect of patient care that will see more attention in the ultimate statement is personalized care. “We will certainly highlight the need to individualize all aspects of care in a patient-centered way, taking into account both specific patient attributes and preferences,” Dr. Buse said.
The draft statement will be presented on Tuesday, June 26, at 8:00 a.m., so it may be worth staying for that last day of the meeting.
The final draft of the new statement will be released in October at the EASD annual meeting in Berlin, noted Dr. Buse, also director of the diabetes center at the university.
A move toward more individualized treatment of hyperglycemia is coming in the next American Diabetes Association/European Association for the Study of Diabetes Consensus Report, according to John B. Buse, MD, PhD, cochair of the committee writing the new consensus statement.
He will present a draft of the statement on the management of hyperglycemia in type 2 diabetes at the ADA’s annual scientific sessions in Orlando.
When finalized – after revisions based on comments and feedback from diabetes care providers – clinical researchers, patient groups, payers, regulators, and stakeholders – the statement will update the last revision, issued in 2015.
“We are taking a new look at hyperglycemia based on the many studies conducted since 2014, particularly the cardiovascular outcomes trials,” Dr. Buse, the Verne S. Caviness Distinguished Professor in the division of endocrinology and metabolism and chief of endocrinology at the University of North Carolina, Chapel Hill, said in a statement.
But it’s a good bet that ADA scientific sessions attendees will see a move toward more specific recommendations based on patient characteristics and fewer one-size-fits-all recommendations. Specific characteristics like obesity, cardiovascular disease, and chronic kidney disease will likely be addressed in the new consensus statement.
One aspect of patient care that will see more attention in the ultimate statement is personalized care. “We will certainly highlight the need to individualize all aspects of care in a patient-centered way, taking into account both specific patient attributes and preferences,” Dr. Buse said.
The draft statement will be presented on Tuesday, June 26, at 8:00 a.m., so it may be worth staying for that last day of the meeting.
The final draft of the new statement will be released in October at the EASD annual meeting in Berlin, noted Dr. Buse, also director of the diabetes center at the university.
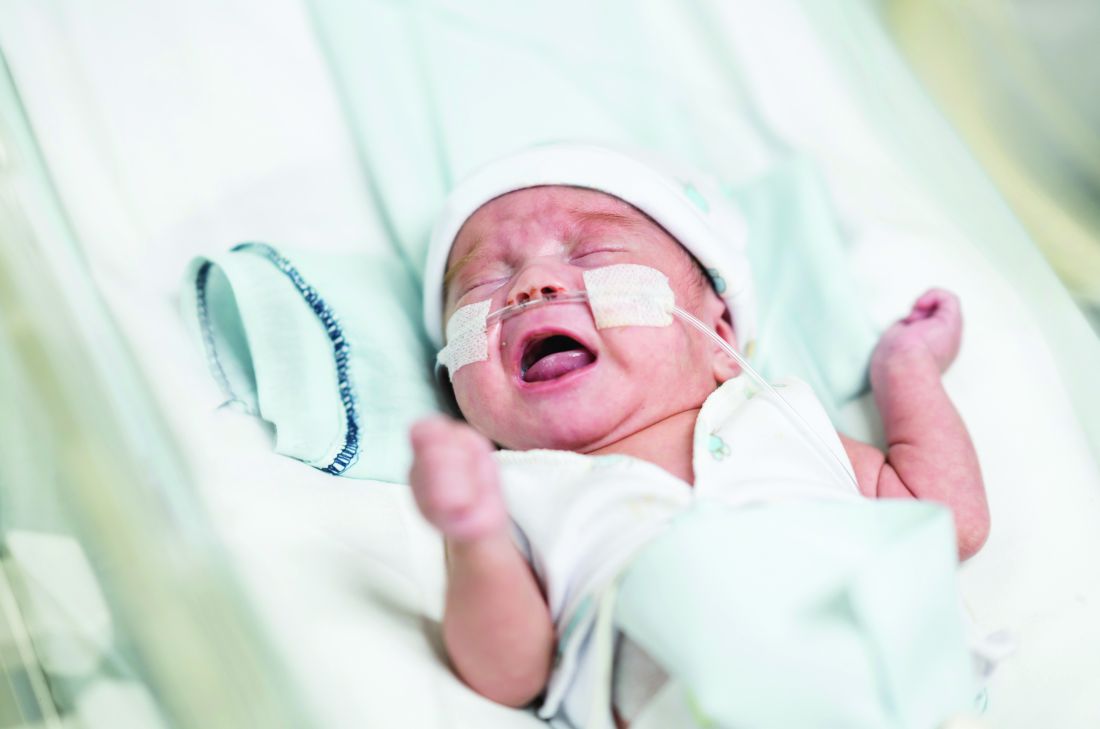
Preterm infant GER is a normal phenomenon
Treatment of gastroesophageal reflux (GER) in preterm infants with traditional treatments, such as body positioning, and newer treatments with pharmacologic agents appear to be ineffective, and pharmacologic agents in particular may cause significant harm, according to a clinical report by the American Academy of Pediatrics Committee on Fetus and Newborn.
“I think that probably the most important point for any physician, including neonatologists, is that the committee concluded on the basis of the evidence that Eric Eichenwald, MD, lead author of the committee’s clinical report and chief of neonatology at Children’s Hospital of Philadelphia, said in an interview. “So really the bottom line of the clinical report is watchful waiting, conservative management, and patience is the most important approach to a baby that you think is suffering from reflux.”
Pharmacologic management
The committee members focused on four categories of pharmacologic interventions in their report in Pediatrics.
Prokinetic (promotility) agents, such as metoclopramide, domperidone, and erythromycin, are widely used in treating symptoms of GER in older infants and appear to improve gastric emptying, reduce regurgitation, and enhance lower esophageal sphincter tone, but they do not appear to reduce GER symptoms in preterm infants. In addition to not being effective in these infants, there is also a potential for significant adverse events, including cardiac arrhythmia and neurologic side effects. Another common pharmacologic treatment is the use of sodium alginate in combination with sodium bicarbonate. In the presence of gastric acid, sodium alginate precipitates as a gel that forms a physical barrier that protects the gastric mucosa. When sodium bicarbonate is added, a carbon dioxide foam forms that is less harmful to the esophagus than GER-related fluids. While this combination treatment has reduced the number of acidic GER exposures and esophageal acid exposure in preterm infants in small studies, the long-term safety has not been evaluated in this populations.
Histamine2 (H2) blockers, like famotidine and ranitidine, also are commonly prescribed to treat preterm infant gastroesophageal reflux. H2 blockers compete with H2 for the histamine receptors of the parietal cells, which causes a decrease in hydrochloric acid and a subsequent increase in intragastric pH. These are often prescribed on the premise that GER symptoms are secondary to acid reflux in the lower esophagus, but there is no research on the efficacy of H2 blockers on the symptom profile of GER in preterm infants. This class of drugs also has been linked with an increased risk of necrotizing enterocolitis and a higher incidence of late-onset infections and death. This is thought to be caused by alteration of the intestinal microbiome, according to the clinical report.
Proton pump inhibitors (PPIs) are another treatment for reducing acid secretion by the parietal cells, but are largely ineffective in relieving clinical signs of GER in preterm infants. PPIs also have been associated with a higher risk of bacterial overgrowth, gastroenteritis, and community-acquired pneumonia in older children. It is theorized that, because of the acid mitigating effects of PPIs, they will have the potential for adverse effects similar to those seen with H2 blockers, although this has not been investigated.
Traditional treatments
Dr. Eichenwald also was quick to point out that even traditional methods of treating preterm infant GER are not particularly effective.
“Some of the conservative approaches that have been advocated include head-up position and different ways of side-lying to enhance emptying of the stomach after feeding. And none of those have been shown to reduce clinically appreciated signs of reflux in preterm infants. If anything – in term babies – some of those positions have been shown to increase the amount of reflux,” he said in an interview.
“I think that the other important point to make about this is that there are many signs that clinicians attribute to reflux in preterm babies, which include wakefulness, irritability, arching after a feeding. And none of those behaviors have been shown to be associated with reflux when it’s critically examined using either a pH Probe or multichannel impedance monitoring. And therefore the treatments to try to decrease reflux don’t really have an effect on those behaviors either.”
Parental concern
Treating a pediatric issue is not as simple as diagnosis and treatment. Often, parents are justifiably concerned about their children. Dr. Eichenwald sees educating parents as an important facet of treating GER in preterm infants.
“Quite honestly I think that there’s some projection on the part of adults who say, ‘I know how I feel when I have heartburn, which is the adult equivalent of reflux, and the baby must be experiencing the same thing, and that’s why they’re acting uncomfortable,’ ” suggested Dr. Eichenwald. “I think that it’s important for clinicians to educate families that a lot of the signs that we typically have attributed to gastroesophageal reflux are not really related to it.”
With both traditional and pharmacological interventions failing to treat preterm infant GER, Dr. Eichenwald believes that the most effective treatment could be patiently waiting. “I think that the important thing to stress is that reflux is a normal physiologic phenomenon. It rarely causes pathology in preterm infants, and therefore, in treating it, you’re not treating any pathology. You should just be patient and it will likely just go away on its own.”
Dr. Eichenwald has no potential conflicts of interest or external funding to report.
SOURCE: Eichenwald E et al. Pediatrics. 2018 June. doi: 10.1542/peds.2018-1061 .
Treatment of gastroesophageal reflux (GER) in preterm infants with traditional treatments, such as body positioning, and newer treatments with pharmacologic agents appear to be ineffective, and pharmacologic agents in particular may cause significant harm, according to a clinical report by the American Academy of Pediatrics Committee on Fetus and Newborn.
“I think that probably the most important point for any physician, including neonatologists, is that the committee concluded on the basis of the evidence that Eric Eichenwald, MD, lead author of the committee’s clinical report and chief of neonatology at Children’s Hospital of Philadelphia, said in an interview. “So really the bottom line of the clinical report is watchful waiting, conservative management, and patience is the most important approach to a baby that you think is suffering from reflux.”
Pharmacologic management
The committee members focused on four categories of pharmacologic interventions in their report in Pediatrics.
Prokinetic (promotility) agents, such as metoclopramide, domperidone, and erythromycin, are widely used in treating symptoms of GER in older infants and appear to improve gastric emptying, reduce regurgitation, and enhance lower esophageal sphincter tone, but they do not appear to reduce GER symptoms in preterm infants. In addition to not being effective in these infants, there is also a potential for significant adverse events, including cardiac arrhythmia and neurologic side effects. Another common pharmacologic treatment is the use of sodium alginate in combination with sodium bicarbonate. In the presence of gastric acid, sodium alginate precipitates as a gel that forms a physical barrier that protects the gastric mucosa. When sodium bicarbonate is added, a carbon dioxide foam forms that is less harmful to the esophagus than GER-related fluids. While this combination treatment has reduced the number of acidic GER exposures and esophageal acid exposure in preterm infants in small studies, the long-term safety has not been evaluated in this populations.
Histamine2 (H2) blockers, like famotidine and ranitidine, also are commonly prescribed to treat preterm infant gastroesophageal reflux. H2 blockers compete with H2 for the histamine receptors of the parietal cells, which causes a decrease in hydrochloric acid and a subsequent increase in intragastric pH. These are often prescribed on the premise that GER symptoms are secondary to acid reflux in the lower esophagus, but there is no research on the efficacy of H2 blockers on the symptom profile of GER in preterm infants. This class of drugs also has been linked with an increased risk of necrotizing enterocolitis and a higher incidence of late-onset infections and death. This is thought to be caused by alteration of the intestinal microbiome, according to the clinical report.
Proton pump inhibitors (PPIs) are another treatment for reducing acid secretion by the parietal cells, but are largely ineffective in relieving clinical signs of GER in preterm infants. PPIs also have been associated with a higher risk of bacterial overgrowth, gastroenteritis, and community-acquired pneumonia in older children. It is theorized that, because of the acid mitigating effects of PPIs, they will have the potential for adverse effects similar to those seen with H2 blockers, although this has not been investigated.
Traditional treatments
Dr. Eichenwald also was quick to point out that even traditional methods of treating preterm infant GER are not particularly effective.
“Some of the conservative approaches that have been advocated include head-up position and different ways of side-lying to enhance emptying of the stomach after feeding. And none of those have been shown to reduce clinically appreciated signs of reflux in preterm infants. If anything – in term babies – some of those positions have been shown to increase the amount of reflux,” he said in an interview.
“I think that the other important point to make about this is that there are many signs that clinicians attribute to reflux in preterm babies, which include wakefulness, irritability, arching after a feeding. And none of those behaviors have been shown to be associated with reflux when it’s critically examined using either a pH Probe or multichannel impedance monitoring. And therefore the treatments to try to decrease reflux don’t really have an effect on those behaviors either.”
Parental concern
Treating a pediatric issue is not as simple as diagnosis and treatment. Often, parents are justifiably concerned about their children. Dr. Eichenwald sees educating parents as an important facet of treating GER in preterm infants.
“Quite honestly I think that there’s some projection on the part of adults who say, ‘I know how I feel when I have heartburn, which is the adult equivalent of reflux, and the baby must be experiencing the same thing, and that’s why they’re acting uncomfortable,’ ” suggested Dr. Eichenwald. “I think that it’s important for clinicians to educate families that a lot of the signs that we typically have attributed to gastroesophageal reflux are not really related to it.”
With both traditional and pharmacological interventions failing to treat preterm infant GER, Dr. Eichenwald believes that the most effective treatment could be patiently waiting. “I think that the important thing to stress is that reflux is a normal physiologic phenomenon. It rarely causes pathology in preterm infants, and therefore, in treating it, you’re not treating any pathology. You should just be patient and it will likely just go away on its own.”
Dr. Eichenwald has no potential conflicts of interest or external funding to report.
SOURCE: Eichenwald E et al. Pediatrics. 2018 June. doi: 10.1542/peds.2018-1061 .
Treatment of gastroesophageal reflux (GER) in preterm infants with traditional treatments, such as body positioning, and newer treatments with pharmacologic agents appear to be ineffective, and pharmacologic agents in particular may cause significant harm, according to a clinical report by the American Academy of Pediatrics Committee on Fetus and Newborn.
“I think that probably the most important point for any physician, including neonatologists, is that the committee concluded on the basis of the evidence that Eric Eichenwald, MD, lead author of the committee’s clinical report and chief of neonatology at Children’s Hospital of Philadelphia, said in an interview. “So really the bottom line of the clinical report is watchful waiting, conservative management, and patience is the most important approach to a baby that you think is suffering from reflux.”
Pharmacologic management
The committee members focused on four categories of pharmacologic interventions in their report in Pediatrics.
Prokinetic (promotility) agents, such as metoclopramide, domperidone, and erythromycin, are widely used in treating symptoms of GER in older infants and appear to improve gastric emptying, reduce regurgitation, and enhance lower esophageal sphincter tone, but they do not appear to reduce GER symptoms in preterm infants. In addition to not being effective in these infants, there is also a potential for significant adverse events, including cardiac arrhythmia and neurologic side effects. Another common pharmacologic treatment is the use of sodium alginate in combination with sodium bicarbonate. In the presence of gastric acid, sodium alginate precipitates as a gel that forms a physical barrier that protects the gastric mucosa. When sodium bicarbonate is added, a carbon dioxide foam forms that is less harmful to the esophagus than GER-related fluids. While this combination treatment has reduced the number of acidic GER exposures and esophageal acid exposure in preterm infants in small studies, the long-term safety has not been evaluated in this populations.
Histamine2 (H2) blockers, like famotidine and ranitidine, also are commonly prescribed to treat preterm infant gastroesophageal reflux. H2 blockers compete with H2 for the histamine receptors of the parietal cells, which causes a decrease in hydrochloric acid and a subsequent increase in intragastric pH. These are often prescribed on the premise that GER symptoms are secondary to acid reflux in the lower esophagus, but there is no research on the efficacy of H2 blockers on the symptom profile of GER in preterm infants. This class of drugs also has been linked with an increased risk of necrotizing enterocolitis and a higher incidence of late-onset infections and death. This is thought to be caused by alteration of the intestinal microbiome, according to the clinical report.
Proton pump inhibitors (PPIs) are another treatment for reducing acid secretion by the parietal cells, but are largely ineffective in relieving clinical signs of GER in preterm infants. PPIs also have been associated with a higher risk of bacterial overgrowth, gastroenteritis, and community-acquired pneumonia in older children. It is theorized that, because of the acid mitigating effects of PPIs, they will have the potential for adverse effects similar to those seen with H2 blockers, although this has not been investigated.
Traditional treatments
Dr. Eichenwald also was quick to point out that even traditional methods of treating preterm infant GER are not particularly effective.
“Some of the conservative approaches that have been advocated include head-up position and different ways of side-lying to enhance emptying of the stomach after feeding. And none of those have been shown to reduce clinically appreciated signs of reflux in preterm infants. If anything – in term babies – some of those positions have been shown to increase the amount of reflux,” he said in an interview.
“I think that the other important point to make about this is that there are many signs that clinicians attribute to reflux in preterm babies, which include wakefulness, irritability, arching after a feeding. And none of those behaviors have been shown to be associated with reflux when it’s critically examined using either a pH Probe or multichannel impedance monitoring. And therefore the treatments to try to decrease reflux don’t really have an effect on those behaviors either.”
Parental concern
Treating a pediatric issue is not as simple as diagnosis and treatment. Often, parents are justifiably concerned about their children. Dr. Eichenwald sees educating parents as an important facet of treating GER in preterm infants.
“Quite honestly I think that there’s some projection on the part of adults who say, ‘I know how I feel when I have heartburn, which is the adult equivalent of reflux, and the baby must be experiencing the same thing, and that’s why they’re acting uncomfortable,’ ” suggested Dr. Eichenwald. “I think that it’s important for clinicians to educate families that a lot of the signs that we typically have attributed to gastroesophageal reflux are not really related to it.”
With both traditional and pharmacological interventions failing to treat preterm infant GER, Dr. Eichenwald believes that the most effective treatment could be patiently waiting. “I think that the important thing to stress is that reflux is a normal physiologic phenomenon. It rarely causes pathology in preterm infants, and therefore, in treating it, you’re not treating any pathology. You should just be patient and it will likely just go away on its own.”
Dr. Eichenwald has no potential conflicts of interest or external funding to report.
SOURCE: Eichenwald E et al. Pediatrics. 2018 June. doi: 10.1542/peds.2018-1061 .
FROM PEDIATRICS
Clinical trials to look for at ADA 2018
More than 2,000 abstracts will be presented at the annual scientific sessions of the American Diabetes Association in Orlando, from basic science studies to clinical trials. Maureen A. Gannon, PhD, who chairs the Scientific Sessions Meeting Planning Committee, highlighted several as being the most relevant to clinical practice.
TEDDY at 13
VADT at 15
Final follow-up data from Veterans Administration Diabetes Trial will be presented on Sunday, June 24, at 4:30 p.m. The trial randomized nearly 2,000 military veterans with poor glycemic control to a mean of 5.6 years of intensive glycemic therapy versus standard treatment, with a goal of lowering HbA1c below 8%.
RISE
Restoring Insulin Secretion (RISE) comprises three intervention trials, two in adults and one in adolescents. The trials are studying whether aggressive glucose lowering will lead to recovery of beta-cell function can be sustained after withdrawal of treatment. Initial results from the adolescent trial will be reported on Monday, June 25, at 2:15 p.m.
SGLT inhibition in type 1 diabetes
Presenters in this session, on Tuesday, June 26 at 10:15 a.m., will provide trial results an insights on a regulatory pathway for sodium-glucose cotransporter (SGLT)-1 and -2 inhibitors in type 1 diabetes patients. Julio Rosenstock, MD, who will present the latest data on empagliflozin from the EASE (Empagliflozin as Adjunctive to InSulin thErapy) trial program, said, “This symposium brings together the lead investigators from the three major competitors that are pursuing approval of a SGLT inhibitor for type 1 diabetes. They will report top-level data that will eventually be submitted to regulators.”
DIY technology
This symposium on Saturday at 1:45 pm, The Diabetes Do-It-Yourself Revolution, will explore the evolving, DIY revolution in diabetes, in which patients are upending traditional treatment pathways and closing their own insulin delivery loop.
“I’m excited about the variety we have in the program this year,” said Dr. Gannon, professor of medicine in the division of diabetes, endocrinology and metabolism; molecular physiology and biophysics; and cell and developmental biology at Vanderbilt University, Nashville, Tenn. “This is the place for cutting-edge information for anybody who is involved in diabetes research or patient care.”
More than 2,000 abstracts will be presented at the annual scientific sessions of the American Diabetes Association in Orlando, from basic science studies to clinical trials. Maureen A. Gannon, PhD, who chairs the Scientific Sessions Meeting Planning Committee, highlighted several as being the most relevant to clinical practice.
TEDDY at 13
VADT at 15
Final follow-up data from Veterans Administration Diabetes Trial will be presented on Sunday, June 24, at 4:30 p.m. The trial randomized nearly 2,000 military veterans with poor glycemic control to a mean of 5.6 years of intensive glycemic therapy versus standard treatment, with a goal of lowering HbA1c below 8%.
RISE
Restoring Insulin Secretion (RISE) comprises three intervention trials, two in adults and one in adolescents. The trials are studying whether aggressive glucose lowering will lead to recovery of beta-cell function can be sustained after withdrawal of treatment. Initial results from the adolescent trial will be reported on Monday, June 25, at 2:15 p.m.
SGLT inhibition in type 1 diabetes
Presenters in this session, on Tuesday, June 26 at 10:15 a.m., will provide trial results an insights on a regulatory pathway for sodium-glucose cotransporter (SGLT)-1 and -2 inhibitors in type 1 diabetes patients. Julio Rosenstock, MD, who will present the latest data on empagliflozin from the EASE (Empagliflozin as Adjunctive to InSulin thErapy) trial program, said, “This symposium brings together the lead investigators from the three major competitors that are pursuing approval of a SGLT inhibitor for type 1 diabetes. They will report top-level data that will eventually be submitted to regulators.”
DIY technology
This symposium on Saturday at 1:45 pm, The Diabetes Do-It-Yourself Revolution, will explore the evolving, DIY revolution in diabetes, in which patients are upending traditional treatment pathways and closing their own insulin delivery loop.
“I’m excited about the variety we have in the program this year,” said Dr. Gannon, professor of medicine in the division of diabetes, endocrinology and metabolism; molecular physiology and biophysics; and cell and developmental biology at Vanderbilt University, Nashville, Tenn. “This is the place for cutting-edge information for anybody who is involved in diabetes research or patient care.”
More than 2,000 abstracts will be presented at the annual scientific sessions of the American Diabetes Association in Orlando, from basic science studies to clinical trials. Maureen A. Gannon, PhD, who chairs the Scientific Sessions Meeting Planning Committee, highlighted several as being the most relevant to clinical practice.
TEDDY at 13
VADT at 15
Final follow-up data from Veterans Administration Diabetes Trial will be presented on Sunday, June 24, at 4:30 p.m. The trial randomized nearly 2,000 military veterans with poor glycemic control to a mean of 5.6 years of intensive glycemic therapy versus standard treatment, with a goal of lowering HbA1c below 8%.
RISE
Restoring Insulin Secretion (RISE) comprises three intervention trials, two in adults and one in adolescents. The trials are studying whether aggressive glucose lowering will lead to recovery of beta-cell function can be sustained after withdrawal of treatment. Initial results from the adolescent trial will be reported on Monday, June 25, at 2:15 p.m.
SGLT inhibition in type 1 diabetes
Presenters in this session, on Tuesday, June 26 at 10:15 a.m., will provide trial results an insights on a regulatory pathway for sodium-glucose cotransporter (SGLT)-1 and -2 inhibitors in type 1 diabetes patients. Julio Rosenstock, MD, who will present the latest data on empagliflozin from the EASE (Empagliflozin as Adjunctive to InSulin thErapy) trial program, said, “This symposium brings together the lead investigators from the three major competitors that are pursuing approval of a SGLT inhibitor for type 1 diabetes. They will report top-level data that will eventually be submitted to regulators.”
DIY technology
This symposium on Saturday at 1:45 pm, The Diabetes Do-It-Yourself Revolution, will explore the evolving, DIY revolution in diabetes, in which patients are upending traditional treatment pathways and closing their own insulin delivery loop.
“I’m excited about the variety we have in the program this year,” said Dr. Gannon, professor of medicine in the division of diabetes, endocrinology and metabolism; molecular physiology and biophysics; and cell and developmental biology at Vanderbilt University, Nashville, Tenn. “This is the place for cutting-edge information for anybody who is involved in diabetes research or patient care.”
ADA punts photography ban to presenters
Last year’s American Diabetes Association annual meeting gobbled up a lot of social media attention, most of it criticizing the organization’s ban on photography in sessions. This year, it’s the presenters who’ll be in the position of deciding whether to allow photography.
During ADA 2017, attendees tweeting photos of presentation slides were surprised to get tweets from the association saying, “Thanks for joining us! Photography isn’t allowed at #2017ADA presentations; we’d appreciate if you could delete this tweet.”
Social media responded with fury, news outlets wrote about it, and the uproar came to dominate public perception of the meeting. While the ADA claimed the policy was to protect intellectual property of unpublished data, critics countered that instant information is the norm and that medical innovation depends on it.
This year, it will be up to the presenters to decide whether their slides can be photographed and shared. The organizers state: “Each presenter/study author will announce, verbally and visually on a slide at the beginning of their presentation, whether or not he/she approves of photos being taken of their slides.”
So watch for the slides, and share your #2018ADA experiences and reactions with us at @ClinEndoNews.
Last year’s American Diabetes Association annual meeting gobbled up a lot of social media attention, most of it criticizing the organization’s ban on photography in sessions. This year, it’s the presenters who’ll be in the position of deciding whether to allow photography.
During ADA 2017, attendees tweeting photos of presentation slides were surprised to get tweets from the association saying, “Thanks for joining us! Photography isn’t allowed at #2017ADA presentations; we’d appreciate if you could delete this tweet.”

Social media responded with fury, news outlets wrote about it, and the uproar came to dominate public perception of the meeting. While the ADA claimed the policy was to protect intellectual property of unpublished data, critics countered that instant information is the norm and that medical innovation depends on it.
This year, it will be up to the presenters to decide whether their slides can be photographed and shared. The organizers state: “Each presenter/study author will announce, verbally and visually on a slide at the beginning of their presentation, whether or not he/she approves of photos being taken of their slides.”
So watch for the slides, and share your #2018ADA experiences and reactions with us at @ClinEndoNews.
Last year’s American Diabetes Association annual meeting gobbled up a lot of social media attention, most of it criticizing the organization’s ban on photography in sessions. This year, it’s the presenters who’ll be in the position of deciding whether to allow photography.
During ADA 2017, attendees tweeting photos of presentation slides were surprised to get tweets from the association saying, “Thanks for joining us! Photography isn’t allowed at #2017ADA presentations; we’d appreciate if you could delete this tweet.”

Social media responded with fury, news outlets wrote about it, and the uproar came to dominate public perception of the meeting. While the ADA claimed the policy was to protect intellectual property of unpublished data, critics countered that instant information is the norm and that medical innovation depends on it.
This year, it will be up to the presenters to decide whether their slides can be photographed and shared. The organizers state: “Each presenter/study author will announce, verbally and visually on a slide at the beginning of their presentation, whether or not he/she approves of photos being taken of their slides.”
So watch for the slides, and share your #2018ADA experiences and reactions with us at @ClinEndoNews.