User login
Has the time come for glucose monitors for people without diabetes?
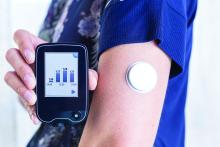
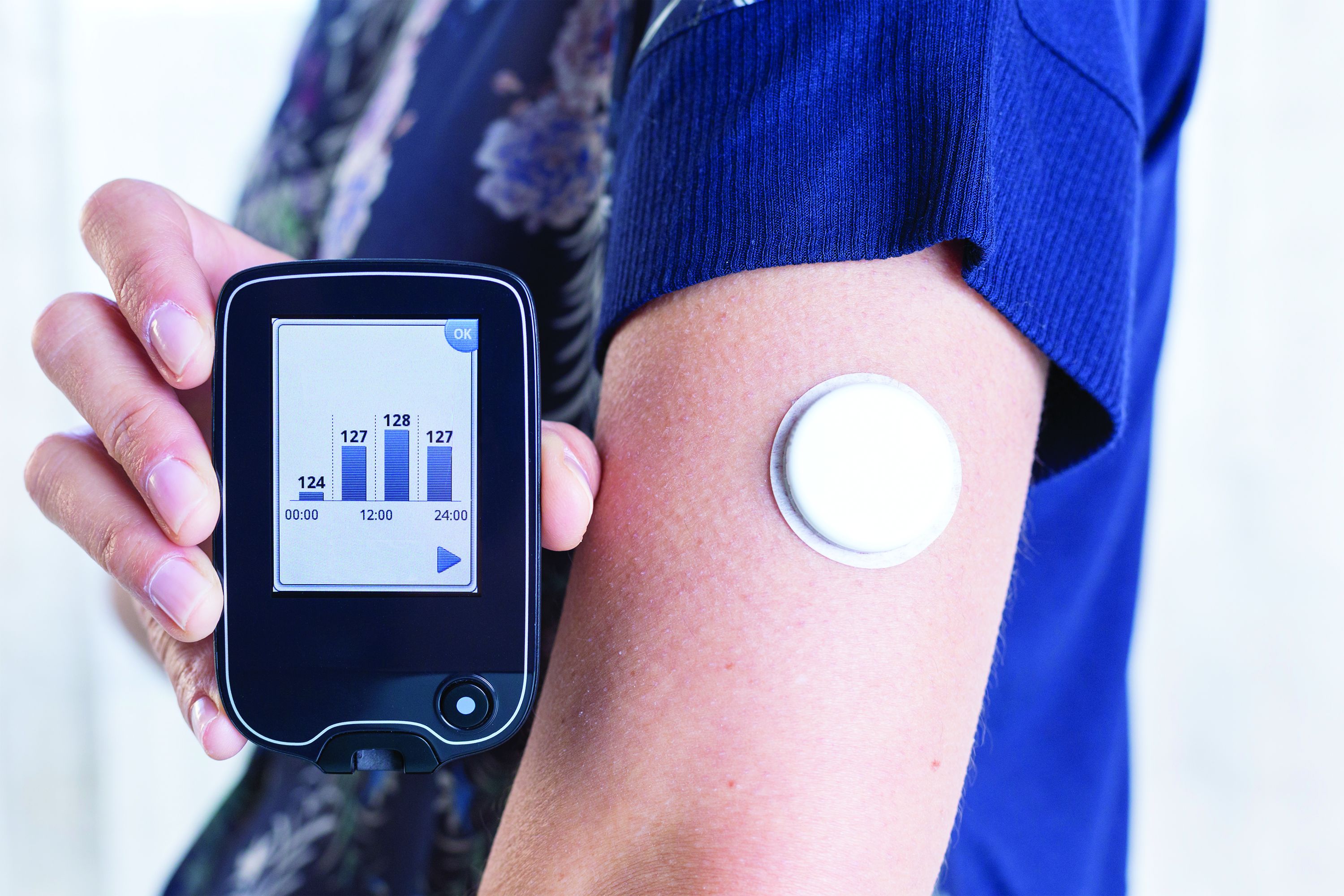
Use of continuous glucose monitoring (CGM) by people without diabetes is becoming increasingly popular despite little evidence of benefit thus far, prompting discussion in the diabetes technology community about best practices.
Emerging uses for CGM outside of diabetes include improving glucose patterns to avoid diabetes, improving mental or physical performance, and promoting motivation for healthy behavior change. Such uses are not approved by the Food and Drug Administration and not covered by health insurance, yet a growing number of people are paying digital health companies for the devices as part of wellness packages.
In a related issue that highlights a limitation in this area, new data suggest that the “glucose management indicator (GMI)” feature of CGMs used for diabetes management – a percentage derived from people with diabetes and elevated A1c – may overestimate the actual A1c level in people without diabetes or those with diabetes who maintain A1c less than 6.5%.
“This is an evolving space ... CGM in people with prediabetes may be beneficial, but we need more data and evidence to recommend it. CGM metrics such as time-in-range and GMI are designed for people with type 1 and type 2 diabetes, and therefore, they are not applicable for people without diabetes,” Viral Shah, MD, said in an interview.
During the recent virtual Diabetes Technology Society meeting, Dr. Shah presented results from a soon-to-be published study finding that on average, GMI was 0.59% higher in people with A1c less than 5.7% and 0.49% higher for A1c 5.7%-6.4%, both significant (P < .0001). Dr. Shah, of the Barbara Davis Center for Diabetes, Adult Clinic, Aurora, Colorado, also presented those data in June at the annual scientific sessions of the American Diabetes Association.
Juan Espinoza, MD, of Children’s Hospital Los Angeles, told this news organization that there are data showing that CGM can be a “powerful biofeedback tool” in people with obesity who don’t have diabetes. “Since they don’t have diabetes the time in range or GMI is meaningless. What’s useful for them is seeing the glucose changes in real time and then using that as a trigger for behavioral change.”
‘An idea whose time has come?’
Dr. Espinoza was a co-author on a review published online in the Journal of Diabetes Science and Technology, entitled, “Use of Continuous Glucose Monitors by People Without Diabetes: An Idea Whose Time Has Come?”
The review examines several aspects of the issue, beginning with studies that used CGM to investigate glucose concentrations in people with normal fasting glucose and glucose tolerance tests. Nearly all those individuals – from populations around the world – fell in the blood glucose range of 70-140 mg/dL.
Also reviewed are studies using CGM to study effects of diet, exercise, and stress on glucose levels in people without diabetes. Subsequent sections summarize the limited data that are available suggesting potential benefit for use of CGM in metabolic disease including prediabetes and obesity, non-metabolic conditions such as steroid treatment or parenteral nutrition, health and wellness, and among elite athletes. In that last group, glucose levels in both the hypoglycemic and hyperglycemic ranges during intensive activity have been documented.
Currently, there are four CGM devices that are FDA-approved for use in people with diabetes: FreeStyle Libre (Abbott), the implantable Eversense (Senseonics), and devices from Dexcom and Medtronic.
As Dr. Espinoza and colleagues explain in their review, most of the commercial health and wellness CGM programs, such as Nutrisense, Signos, and Supersapiens, actually use sensors made by those same manufacturers. Nutrisense and Supersapiens use the Libre, and Signos uses the Dexcom.
But, rather than the manufacturer’s apps meant for use by people with diabetes, the wellness companies pair the sensors with their own specially designed apps and typically offer additional services such as health coaching or nutrition counseling “to improve general health.”
Subscribers pay a monthly fee. Signos, for example, charges $399 for 1 month, $199/month for 3 months, or $159/month for 6 months. A prescription is required, but the company’s website says, “rest assured, an independent physician will handle the prescription for you, so you won’t need to arrange for a doctor visit. It is included in the cost of membership.”
Several consumer health product companies are now developing non-invasive glucose monitors, most often as a wristwatch, for people without diabetes to measure glucose optically from the skin in the wrist.
“It remains to be determined how accurate these new devices will be and how they will be regulated,” the researchers write.
What to do with the data?
The dedicated health and wellness apps typically provide average glucose and trend data but not the GMI. However, in theory users could access that metric by downloading the manufacturers’ viewing apps – for example, Clarity for Dexcom or LibreView for Libre.
Moreover, a person without diabetes could always obtain an off-label prescription from their physician for a FreeStyle Libre and purchase it at a pharmacy. At Walmart, for example, the cost for two boxes of two glucose meters with 14 days of wear each is $136.77. In that situation as well, users could download the viewing app that contains the summary data including the GMI that could potentially mislead in the setting of consistent normoglycemia.
Dr. Espinoza said: “I think there’s certainly value in glucose levels. We know the summary metrics are useful in type 1 diabetes. We don’t know which summary metrics are going to be useful in any other disease states. We may need brand new summary metrics for other disease states where it’s not about time in range. Maybe the thing that matters is the frequency or height of spikes. We don’t have a measure for that.”
He added that despite the availability of normative data, “even people without diabetes are a fairly heterogenous group. They can still have insulin resistance, so it’s tricky. From a science standpoint, we probably need studies with hundreds of patients with well-established A1c and [insulin resistance measures], weight, and body mass index. Then and only then will we be able to give an accurate glucose profile.”
In the meantime, “more data is always a good thing, but the hard thing is figuring out what do we do with it. Maybe it’s biofeedback for behavioral modification. We don’t know yet. But these are powerful tools and maybe we should learn how to use them better.”
Dr. Shah has reported receiving research grants and participating in advisory boards for Dexcom and Sanofi US. Dr. Espinoza has reported receiving research funding from the National Institutes of Health and FDA.
A version of this article first appeared on Medscape.com.
Use of continuous glucose monitoring (CGM) by people without diabetes is becoming increasingly popular despite little evidence of benefit thus far, prompting discussion in the diabetes technology community about best practices.
Emerging uses for CGM outside of diabetes include improving glucose patterns to avoid diabetes, improving mental or physical performance, and promoting motivation for healthy behavior change. Such uses are not approved by the Food and Drug Administration and not covered by health insurance, yet a growing number of people are paying digital health companies for the devices as part of wellness packages.
In a related issue that highlights a limitation in this area, new data suggest that the “glucose management indicator (GMI)” feature of CGMs used for diabetes management – a percentage derived from people with diabetes and elevated A1c – may overestimate the actual A1c level in people without diabetes or those with diabetes who maintain A1c less than 6.5%.
“This is an evolving space ... CGM in people with prediabetes may be beneficial, but we need more data and evidence to recommend it. CGM metrics such as time-in-range and GMI are designed for people with type 1 and type 2 diabetes, and therefore, they are not applicable for people without diabetes,” Viral Shah, MD, said in an interview.
During the recent virtual Diabetes Technology Society meeting, Dr. Shah presented results from a soon-to-be published study finding that on average, GMI was 0.59% higher in people with A1c less than 5.7% and 0.49% higher for A1c 5.7%-6.4%, both significant (P < .0001). Dr. Shah, of the Barbara Davis Center for Diabetes, Adult Clinic, Aurora, Colorado, also presented those data in June at the annual scientific sessions of the American Diabetes Association.
Juan Espinoza, MD, of Children’s Hospital Los Angeles, told this news organization that there are data showing that CGM can be a “powerful biofeedback tool” in people with obesity who don’t have diabetes. “Since they don’t have diabetes the time in range or GMI is meaningless. What’s useful for them is seeing the glucose changes in real time and then using that as a trigger for behavioral change.”
‘An idea whose time has come?’
Dr. Espinoza was a co-author on a review published online in the Journal of Diabetes Science and Technology, entitled, “Use of Continuous Glucose Monitors by People Without Diabetes: An Idea Whose Time Has Come?”
The review examines several aspects of the issue, beginning with studies that used CGM to investigate glucose concentrations in people with normal fasting glucose and glucose tolerance tests. Nearly all those individuals – from populations around the world – fell in the blood glucose range of 70-140 mg/dL.
Also reviewed are studies using CGM to study effects of diet, exercise, and stress on glucose levels in people without diabetes. Subsequent sections summarize the limited data that are available suggesting potential benefit for use of CGM in metabolic disease including prediabetes and obesity, non-metabolic conditions such as steroid treatment or parenteral nutrition, health and wellness, and among elite athletes. In that last group, glucose levels in both the hypoglycemic and hyperglycemic ranges during intensive activity have been documented.
Currently, there are four CGM devices that are FDA-approved for use in people with diabetes: FreeStyle Libre (Abbott), the implantable Eversense (Senseonics), and devices from Dexcom and Medtronic.
As Dr. Espinoza and colleagues explain in their review, most of the commercial health and wellness CGM programs, such as Nutrisense, Signos, and Supersapiens, actually use sensors made by those same manufacturers. Nutrisense and Supersapiens use the Libre, and Signos uses the Dexcom.
But, rather than the manufacturer’s apps meant for use by people with diabetes, the wellness companies pair the sensors with their own specially designed apps and typically offer additional services such as health coaching or nutrition counseling “to improve general health.”
Subscribers pay a monthly fee. Signos, for example, charges $399 for 1 month, $199/month for 3 months, or $159/month for 6 months. A prescription is required, but the company’s website says, “rest assured, an independent physician will handle the prescription for you, so you won’t need to arrange for a doctor visit. It is included in the cost of membership.”
Several consumer health product companies are now developing non-invasive glucose monitors, most often as a wristwatch, for people without diabetes to measure glucose optically from the skin in the wrist.
“It remains to be determined how accurate these new devices will be and how they will be regulated,” the researchers write.
What to do with the data?
The dedicated health and wellness apps typically provide average glucose and trend data but not the GMI. However, in theory users could access that metric by downloading the manufacturers’ viewing apps – for example, Clarity for Dexcom or LibreView for Libre.
Moreover, a person without diabetes could always obtain an off-label prescription from their physician for a FreeStyle Libre and purchase it at a pharmacy. At Walmart, for example, the cost for two boxes of two glucose meters with 14 days of wear each is $136.77. In that situation as well, users could download the viewing app that contains the summary data including the GMI that could potentially mislead in the setting of consistent normoglycemia.
Dr. Espinoza said: “I think there’s certainly value in glucose levels. We know the summary metrics are useful in type 1 diabetes. We don’t know which summary metrics are going to be useful in any other disease states. We may need brand new summary metrics for other disease states where it’s not about time in range. Maybe the thing that matters is the frequency or height of spikes. We don’t have a measure for that.”
He added that despite the availability of normative data, “even people without diabetes are a fairly heterogenous group. They can still have insulin resistance, so it’s tricky. From a science standpoint, we probably need studies with hundreds of patients with well-established A1c and [insulin resistance measures], weight, and body mass index. Then and only then will we be able to give an accurate glucose profile.”
In the meantime, “more data is always a good thing, but the hard thing is figuring out what do we do with it. Maybe it’s biofeedback for behavioral modification. We don’t know yet. But these are powerful tools and maybe we should learn how to use them better.”
Dr. Shah has reported receiving research grants and participating in advisory boards for Dexcom and Sanofi US. Dr. Espinoza has reported receiving research funding from the National Institutes of Health and FDA.
A version of this article first appeared on Medscape.com.
Use of continuous glucose monitoring (CGM) by people without diabetes is becoming increasingly popular despite little evidence of benefit thus far, prompting discussion in the diabetes technology community about best practices.
Emerging uses for CGM outside of diabetes include improving glucose patterns to avoid diabetes, improving mental or physical performance, and promoting motivation for healthy behavior change. Such uses are not approved by the Food and Drug Administration and not covered by health insurance, yet a growing number of people are paying digital health companies for the devices as part of wellness packages.
In a related issue that highlights a limitation in this area, new data suggest that the “glucose management indicator (GMI)” feature of CGMs used for diabetes management – a percentage derived from people with diabetes and elevated A1c – may overestimate the actual A1c level in people without diabetes or those with diabetes who maintain A1c less than 6.5%.
“This is an evolving space ... CGM in people with prediabetes may be beneficial, but we need more data and evidence to recommend it. CGM metrics such as time-in-range and GMI are designed for people with type 1 and type 2 diabetes, and therefore, they are not applicable for people without diabetes,” Viral Shah, MD, said in an interview.
During the recent virtual Diabetes Technology Society meeting, Dr. Shah presented results from a soon-to-be published study finding that on average, GMI was 0.59% higher in people with A1c less than 5.7% and 0.49% higher for A1c 5.7%-6.4%, both significant (P < .0001). Dr. Shah, of the Barbara Davis Center for Diabetes, Adult Clinic, Aurora, Colorado, also presented those data in June at the annual scientific sessions of the American Diabetes Association.
Juan Espinoza, MD, of Children’s Hospital Los Angeles, told this news organization that there are data showing that CGM can be a “powerful biofeedback tool” in people with obesity who don’t have diabetes. “Since they don’t have diabetes the time in range or GMI is meaningless. What’s useful for them is seeing the glucose changes in real time and then using that as a trigger for behavioral change.”
‘An idea whose time has come?’
Dr. Espinoza was a co-author on a review published online in the Journal of Diabetes Science and Technology, entitled, “Use of Continuous Glucose Monitors by People Without Diabetes: An Idea Whose Time Has Come?”
The review examines several aspects of the issue, beginning with studies that used CGM to investigate glucose concentrations in people with normal fasting glucose and glucose tolerance tests. Nearly all those individuals – from populations around the world – fell in the blood glucose range of 70-140 mg/dL.
Also reviewed are studies using CGM to study effects of diet, exercise, and stress on glucose levels in people without diabetes. Subsequent sections summarize the limited data that are available suggesting potential benefit for use of CGM in metabolic disease including prediabetes and obesity, non-metabolic conditions such as steroid treatment or parenteral nutrition, health and wellness, and among elite athletes. In that last group, glucose levels in both the hypoglycemic and hyperglycemic ranges during intensive activity have been documented.
Currently, there are four CGM devices that are FDA-approved for use in people with diabetes: FreeStyle Libre (Abbott), the implantable Eversense (Senseonics), and devices from Dexcom and Medtronic.
As Dr. Espinoza and colleagues explain in their review, most of the commercial health and wellness CGM programs, such as Nutrisense, Signos, and Supersapiens, actually use sensors made by those same manufacturers. Nutrisense and Supersapiens use the Libre, and Signos uses the Dexcom.
But, rather than the manufacturer’s apps meant for use by people with diabetes, the wellness companies pair the sensors with their own specially designed apps and typically offer additional services such as health coaching or nutrition counseling “to improve general health.”
Subscribers pay a monthly fee. Signos, for example, charges $399 for 1 month, $199/month for 3 months, or $159/month for 6 months. A prescription is required, but the company’s website says, “rest assured, an independent physician will handle the prescription for you, so you won’t need to arrange for a doctor visit. It is included in the cost of membership.”
Several consumer health product companies are now developing non-invasive glucose monitors, most often as a wristwatch, for people without diabetes to measure glucose optically from the skin in the wrist.
“It remains to be determined how accurate these new devices will be and how they will be regulated,” the researchers write.
What to do with the data?
The dedicated health and wellness apps typically provide average glucose and trend data but not the GMI. However, in theory users could access that metric by downloading the manufacturers’ viewing apps – for example, Clarity for Dexcom or LibreView for Libre.
Moreover, a person without diabetes could always obtain an off-label prescription from their physician for a FreeStyle Libre and purchase it at a pharmacy. At Walmart, for example, the cost for two boxes of two glucose meters with 14 days of wear each is $136.77. In that situation as well, users could download the viewing app that contains the summary data including the GMI that could potentially mislead in the setting of consistent normoglycemia.
Dr. Espinoza said: “I think there’s certainly value in glucose levels. We know the summary metrics are useful in type 1 diabetes. We don’t know which summary metrics are going to be useful in any other disease states. We may need brand new summary metrics for other disease states where it’s not about time in range. Maybe the thing that matters is the frequency or height of spikes. We don’t have a measure for that.”
He added that despite the availability of normative data, “even people without diabetes are a fairly heterogenous group. They can still have insulin resistance, so it’s tricky. From a science standpoint, we probably need studies with hundreds of patients with well-established A1c and [insulin resistance measures], weight, and body mass index. Then and only then will we be able to give an accurate glucose profile.”
In the meantime, “more data is always a good thing, but the hard thing is figuring out what do we do with it. Maybe it’s biofeedback for behavioral modification. We don’t know yet. But these are powerful tools and maybe we should learn how to use them better.”
Dr. Shah has reported receiving research grants and participating in advisory boards for Dexcom and Sanofi US. Dr. Espinoza has reported receiving research funding from the National Institutes of Health and FDA.
A version of this article first appeared on Medscape.com.
AT ADA 2022
‘Not their fault:’ Obesity warrants long-term management
This transcript has been edited for clarity.
It’s important to remember and to think about the first time when patients with obesity come to see us: What have they faced? What have been their struggles? What shame and blame and bias have they faced?
One of the first things that I do when a patient comes to see me is invite them to share their weight journey with me. I ask them to tell me about their struggles, about what’s worked and what hasn’t worked, what they would like, and what their health goals are.
As they share their stories, I look for the opportunity to share with them that obesity is not their fault, but that it’s biology driving their body to carry extra weight and their body is super smart. Neither their body nor their brain want them to starve.
Our bodies evolved during a time where there was food scarcity and the potential of famine. We have a complex system that was designed to make sure that we always held on to extra weight, specifically extra fat, because that’s how we store energy. In the current obesogenic environment, what happens is our bodies carry extra weight, or specifically, extra fat.
Again, I say to them, this is biology. Your body’s doing exactly what it was designed to do. Your body’s very smart, but now we have to figure out how to help your body want to carry less fat because it is impacting your health. This is not your fault. Having obesity is not your fault any more than having diabetes or hypertension is anyone’s fault. Now it’s time for all of us to use highly effective tools that target the pathophysiology of obesity.
When a patient comes to me for weight management or to help them treat their obesity, I listen to them, and I look for clues as to what might help that specific patient. Every patient deserves to have individualized treatment. One medicine may be right for one person, another medicine may be right for another, and surgery may be right for another patient. I really try to listen and hear what that patient is telling me.
What we as providers really need is tools – different options – to be able to provide for our patients and basically present them with different options, and then guide them toward the best therapy for them. Whether it’s semaglutide or tirzepatide potentially in the future, these types of medications are excellent options for our patients. They’re highly effective tools with safe profiles.
A question that I often get from providers or patients is, “Well, Doctor, I’ve lost the weight now. How long should I take this medicine? Can I stop it now?”
Then, we have a conversation, and we actually usually have this conversation even before we start the medicine. Basically, we talk about the fact that obesity is a chronic disease. There’s no cure for obesity. Because it’s a chronic disease, we need to treat it like we would treat any other chronic disease.
The example that I often use is, if you have a patient who has hypertension and you start them on an antihypertensive medication, what happens? Their blood pressure goes down. It improves. Now, if their blood pressure is improved with a specific antihypertensive, would you stop that medicine? What would happen if you stopped that antihypertensive? Well, their blood pressure would go up, and we wouldn’t be surprised.
In the same way, if you have a patient who has obesity and you start that patient on an antiobesity medication, and their weight decreases, and their body fat mass at that point decreases, what would happen if you stop that medicine? They lost the weight, but you stop the medicine. Well, their weight gain comes back. They regain the weight.
We should not be surprised that weight gain occurs when we stop the treatment. That really underscores the fact that treatment needs to be continued. If a patient is started on an antiobesity medication and they lose weight, that medication needs to be continued to maintain that weight loss.
Basically, we eat food and our body responds by releasing these hormones. The hormones are made in our gut and in our pancreas and these hormones inform our brain. Are we hungry? Are we full? Where are we with our homeostatic set point of fat mass? Based on that, our brain is like the sensor or the thermostat.
Obesity is a chronic, treatable disease. We should treat obesity as we treat any other chronic disease, with effective and safe approaches that target underlying disease mechanisms. These results in the SURMOUNT-1 trial underscore that tirzepatide may be doing just that. Remarkably, 9 in 10 individuals with obesity lost weight while taking tirzepatide. These results are impressive. They’re an important step forward in potentially expanding effective therapeutic options for people with obesity.
Dr. Jastreboff is an associate professor of medicine and pediatrics at Yale University, New Haven, Conn., and director of weight management and obesity prevention at Yale Stress Center. She reported conducting trials with Eli Lilly, Novo Nordisk, and Rhythm Pharmaceuticals; serving on scientific advisory boards for Ely Lilly, Intellihealth, Novo Nordisk, Pfizer, Rhythm Pharmaceuticals, and WW; and consulting for Boehringer Ingelheim and Scholar Rock.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
It’s important to remember and to think about the first time when patients with obesity come to see us: What have they faced? What have been their struggles? What shame and blame and bias have they faced?
One of the first things that I do when a patient comes to see me is invite them to share their weight journey with me. I ask them to tell me about their struggles, about what’s worked and what hasn’t worked, what they would like, and what their health goals are.
As they share their stories, I look for the opportunity to share with them that obesity is not their fault, but that it’s biology driving their body to carry extra weight and their body is super smart. Neither their body nor their brain want them to starve.
Our bodies evolved during a time where there was food scarcity and the potential of famine. We have a complex system that was designed to make sure that we always held on to extra weight, specifically extra fat, because that’s how we store energy. In the current obesogenic environment, what happens is our bodies carry extra weight, or specifically, extra fat.
Again, I say to them, this is biology. Your body’s doing exactly what it was designed to do. Your body’s very smart, but now we have to figure out how to help your body want to carry less fat because it is impacting your health. This is not your fault. Having obesity is not your fault any more than having diabetes or hypertension is anyone’s fault. Now it’s time for all of us to use highly effective tools that target the pathophysiology of obesity.
When a patient comes to me for weight management or to help them treat their obesity, I listen to them, and I look for clues as to what might help that specific patient. Every patient deserves to have individualized treatment. One medicine may be right for one person, another medicine may be right for another, and surgery may be right for another patient. I really try to listen and hear what that patient is telling me.
What we as providers really need is tools – different options – to be able to provide for our patients and basically present them with different options, and then guide them toward the best therapy for them. Whether it’s semaglutide or tirzepatide potentially in the future, these types of medications are excellent options for our patients. They’re highly effective tools with safe profiles.
A question that I often get from providers or patients is, “Well, Doctor, I’ve lost the weight now. How long should I take this medicine? Can I stop it now?”
Then, we have a conversation, and we actually usually have this conversation even before we start the medicine. Basically, we talk about the fact that obesity is a chronic disease. There’s no cure for obesity. Because it’s a chronic disease, we need to treat it like we would treat any other chronic disease.
The example that I often use is, if you have a patient who has hypertension and you start them on an antihypertensive medication, what happens? Their blood pressure goes down. It improves. Now, if their blood pressure is improved with a specific antihypertensive, would you stop that medicine? What would happen if you stopped that antihypertensive? Well, their blood pressure would go up, and we wouldn’t be surprised.
In the same way, if you have a patient who has obesity and you start that patient on an antiobesity medication, and their weight decreases, and their body fat mass at that point decreases, what would happen if you stop that medicine? They lost the weight, but you stop the medicine. Well, their weight gain comes back. They regain the weight.
We should not be surprised that weight gain occurs when we stop the treatment. That really underscores the fact that treatment needs to be continued. If a patient is started on an antiobesity medication and they lose weight, that medication needs to be continued to maintain that weight loss.
Basically, we eat food and our body responds by releasing these hormones. The hormones are made in our gut and in our pancreas and these hormones inform our brain. Are we hungry? Are we full? Where are we with our homeostatic set point of fat mass? Based on that, our brain is like the sensor or the thermostat.
Obesity is a chronic, treatable disease. We should treat obesity as we treat any other chronic disease, with effective and safe approaches that target underlying disease mechanisms. These results in the SURMOUNT-1 trial underscore that tirzepatide may be doing just that. Remarkably, 9 in 10 individuals with obesity lost weight while taking tirzepatide. These results are impressive. They’re an important step forward in potentially expanding effective therapeutic options for people with obesity.
Dr. Jastreboff is an associate professor of medicine and pediatrics at Yale University, New Haven, Conn., and director of weight management and obesity prevention at Yale Stress Center. She reported conducting trials with Eli Lilly, Novo Nordisk, and Rhythm Pharmaceuticals; serving on scientific advisory boards for Ely Lilly, Intellihealth, Novo Nordisk, Pfizer, Rhythm Pharmaceuticals, and WW; and consulting for Boehringer Ingelheim and Scholar Rock.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
It’s important to remember and to think about the first time when patients with obesity come to see us: What have they faced? What have been their struggles? What shame and blame and bias have they faced?
One of the first things that I do when a patient comes to see me is invite them to share their weight journey with me. I ask them to tell me about their struggles, about what’s worked and what hasn’t worked, what they would like, and what their health goals are.
As they share their stories, I look for the opportunity to share with them that obesity is not their fault, but that it’s biology driving their body to carry extra weight and their body is super smart. Neither their body nor their brain want them to starve.
Our bodies evolved during a time where there was food scarcity and the potential of famine. We have a complex system that was designed to make sure that we always held on to extra weight, specifically extra fat, because that’s how we store energy. In the current obesogenic environment, what happens is our bodies carry extra weight, or specifically, extra fat.
Again, I say to them, this is biology. Your body’s doing exactly what it was designed to do. Your body’s very smart, but now we have to figure out how to help your body want to carry less fat because it is impacting your health. This is not your fault. Having obesity is not your fault any more than having diabetes or hypertension is anyone’s fault. Now it’s time for all of us to use highly effective tools that target the pathophysiology of obesity.
When a patient comes to me for weight management or to help them treat their obesity, I listen to them, and I look for clues as to what might help that specific patient. Every patient deserves to have individualized treatment. One medicine may be right for one person, another medicine may be right for another, and surgery may be right for another patient. I really try to listen and hear what that patient is telling me.
What we as providers really need is tools – different options – to be able to provide for our patients and basically present them with different options, and then guide them toward the best therapy for them. Whether it’s semaglutide or tirzepatide potentially in the future, these types of medications are excellent options for our patients. They’re highly effective tools with safe profiles.
A question that I often get from providers or patients is, “Well, Doctor, I’ve lost the weight now. How long should I take this medicine? Can I stop it now?”
Then, we have a conversation, and we actually usually have this conversation even before we start the medicine. Basically, we talk about the fact that obesity is a chronic disease. There’s no cure for obesity. Because it’s a chronic disease, we need to treat it like we would treat any other chronic disease.
The example that I often use is, if you have a patient who has hypertension and you start them on an antihypertensive medication, what happens? Their blood pressure goes down. It improves. Now, if their blood pressure is improved with a specific antihypertensive, would you stop that medicine? What would happen if you stopped that antihypertensive? Well, their blood pressure would go up, and we wouldn’t be surprised.
In the same way, if you have a patient who has obesity and you start that patient on an antiobesity medication, and their weight decreases, and their body fat mass at that point decreases, what would happen if you stop that medicine? They lost the weight, but you stop the medicine. Well, their weight gain comes back. They regain the weight.
We should not be surprised that weight gain occurs when we stop the treatment. That really underscores the fact that treatment needs to be continued. If a patient is started on an antiobesity medication and they lose weight, that medication needs to be continued to maintain that weight loss.
Basically, we eat food and our body responds by releasing these hormones. The hormones are made in our gut and in our pancreas and these hormones inform our brain. Are we hungry? Are we full? Where are we with our homeostatic set point of fat mass? Based on that, our brain is like the sensor or the thermostat.
Obesity is a chronic, treatable disease. We should treat obesity as we treat any other chronic disease, with effective and safe approaches that target underlying disease mechanisms. These results in the SURMOUNT-1 trial underscore that tirzepatide may be doing just that. Remarkably, 9 in 10 individuals with obesity lost weight while taking tirzepatide. These results are impressive. They’re an important step forward in potentially expanding effective therapeutic options for people with obesity.
Dr. Jastreboff is an associate professor of medicine and pediatrics at Yale University, New Haven, Conn., and director of weight management and obesity prevention at Yale Stress Center. She reported conducting trials with Eli Lilly, Novo Nordisk, and Rhythm Pharmaceuticals; serving on scientific advisory boards for Ely Lilly, Intellihealth, Novo Nordisk, Pfizer, Rhythm Pharmaceuticals, and WW; and consulting for Boehringer Ingelheim and Scholar Rock.
A version of this article first appeared on Medscape.com.
Menstrual phase impacts exercise effects in type 1 diabetes
Women with type 1 diabetes may need additional glucose after exercise during the luteal phase of the menstrual cycle, compared with other times, according to a study in nine women.
“We know that exercise is very beneficial for people with type 1 diabetes; we also know that fear of hypoglycemia is a major barrier to exercise in this population,” said Jane E. Yardley, PhD, in a presentation at the annual scientific sessions of the American Diabetes Association, New Orleans. Women with type 1 diabetes (T1D) perceive more barriers, compared with men, she added.
The menstrual cycle could be an additional barrier to exercise for women with T1D because it increases glucose fluctuations that have not been well documented in the literature to date, said Dr. Yardley, of the University of Alberta, Augustana.
The follicular phase of the menstrual cycle lasts from menses to the midcycle, about 14 days later. This is followed by the luteal phase, which lasts until approximately day 28, Dr. Yardley explained. Data on insulin sensitivity have shown that the late luteal phase is associated with “a little less insulin sensitivity” in women with T1D, she noted.
To assess the relationship between menstrual cycle, glucose control, and exercise, Dr. Yardley and colleagues compared the effects of a moderate aerobic exercise on glycemic responses between the early follicular and late luteal phases of the menstrual cycle in nine female participants with T1D.
The exercise involved 45 minutes of aerobic cycling at 50% of predetermined peak oxygen uptake (VO2peak) for 45 min. The mean age of the participants was 30.2 years, the mean hemoglobin A1C was 7.4%, and the mean VO2peak was 32.5 mL/kg per min. The women reported regular menstrual cycles, and none were using oral contraceptives.
Blood samples were collected before and immediately after exercise and after an hour of recovery. Participants wore continuous glucose monitors for at least 1 hour before and after exercise.
Menstrual cycle was confirmed via estrogen, estradiol, and progesterone.
Insulin levels varied greatly among the study participants, but the differences were not significant, Dr. Yardley said. Glucose levels consistently decreased during exercise and increased after exercise, she noted.
No significant difference in glucose was observed between the follicular and luteal phases.
However, “this needs to be interpreted in the context of the safety profiles that are in place in our lab,” which include carbohydrate supplements for individuals whose blood glucose levels drop below 4.5 mmol/L, she said.
In the current study, 6 of 9 participants required additional carbohydrates during the luteal phase, but only 1 participant needed additional carbohydrates during the follicular phase, she noted. For this reason, no differences were noted. “We actually prevented changes,” she said.
No significant differences were noted in mean glucose levels or number of hypoglycemic episodes at any of the time points between the two phases.
“One place where we did see a difference was in hyperglycemia 24 hours after exercise,” Dr. Yardley said. Level 1 hyperglycemia 24 hours after exercise was significantly more frequent in the follicular phase, compared with the luteal phase (P = .028).
The study findings were limited by the small sample size and homogenous population, and more research is needed to interpret the data, said Dr. Yardley.
However, the need for more glucose supplementation to prevent hypoglycemia during the luteal phase suggests a higher hypoglycemic risk associated with aerobic exercise during this time, she said.
In addition, the results suggest that the menstrual cycle should be taken into consideration when female participants are involved in exercise studies, she noted.
Study supports personalized exercise plans
“It is important to evaluate effects of exercise in people with type 1 diabetes and evaluate whether there is a difference those effects in men and women,” said Helena W. Rodbard, MD, an endocrinologist in private practice in Rockville, Md., in an interview. “There is also a need to evaluate to what extent the changes in blood glucose patterns in women in response to exercise differ depending on the phase of the ovarian cycle,” said Dr. Rodbard, who was not involved in the study.
In the current study, “the researchers observed a decline in glucose during a 45-minute period of moderate aerobic exercise, cycling at 50% VO2peak followed by an increase during a 60-minute recovery period. There was a suggestive finding, in the nine subjects, that more carbohydrate supplementation was needed during the late luteal phase of the menstrual cycle than during the follicular phase,” Dr. Rodbard noted. “In contrast, the authors reported a significantly increased degree of hyperglycemia during the recovery phase for subjects during the follicular phase. These findings are consistent with and extend several recent studies from Dr. Yardley and coworkers, who have been focused on this area of research,” she said.
“This study provides provocative evidence that glucose responses to aerobic exercise in women may depend on the timing in relationship to their ovarian cycle,” said Dr. Rodbard. “These findings are based on a small group of subjects and were present in some but not all subjects. Clinicians should encourage women to evaluate and record their experiences during and after exercise in terms of need for carbohydrate supplementation for documented or symptomatic hypoglycemia and in terms of glucose changes as recorded using continuous glucose monitoring (CGM), both in relation to type of exercise and in relation to time in the menstrual cycle,” she said.
The findings also highlight the importance of individualized therapy that is “based on subjective inputs combined with analysis of CGM data during and following exercise,” said Dr. Rodbard. “It is likely that use of Automated Insulin Delivery (AID) will be helpful in achieving this level of individualization in view of the wide range of types, intensity, and duration of physical activity and exercise in which people with T1D engage and the myriad factors that can influence the glycemic response,” she said.
Looking ahead, “the authors and others should expand the present series of subjects using aerobic exercise and examine other types of exercise as well,” Dr. Rodbard noted. “It will be important to evaluate the consistency of these changes in glucose patterns within individuals on multiple occasions, and it would be helpful to repeat the studies in women using oral contraceptives.”
Dr. Yardley disclosed research support from Abbott, Dexcom, and LifeScan and disclosed serving on the speaker’s bureau for Abbott Diabetes. Dr. Rodbard had no financial conflicts to disclose. She serves on the Editorial Advisory Board of Clinical Endocrinology News.
Women with type 1 diabetes may need additional glucose after exercise during the luteal phase of the menstrual cycle, compared with other times, according to a study in nine women.
“We know that exercise is very beneficial for people with type 1 diabetes; we also know that fear of hypoglycemia is a major barrier to exercise in this population,” said Jane E. Yardley, PhD, in a presentation at the annual scientific sessions of the American Diabetes Association, New Orleans. Women with type 1 diabetes (T1D) perceive more barriers, compared with men, she added.
The menstrual cycle could be an additional barrier to exercise for women with T1D because it increases glucose fluctuations that have not been well documented in the literature to date, said Dr. Yardley, of the University of Alberta, Augustana.
The follicular phase of the menstrual cycle lasts from menses to the midcycle, about 14 days later. This is followed by the luteal phase, which lasts until approximately day 28, Dr. Yardley explained. Data on insulin sensitivity have shown that the late luteal phase is associated with “a little less insulin sensitivity” in women with T1D, she noted.
To assess the relationship between menstrual cycle, glucose control, and exercise, Dr. Yardley and colleagues compared the effects of a moderate aerobic exercise on glycemic responses between the early follicular and late luteal phases of the menstrual cycle in nine female participants with T1D.
The exercise involved 45 minutes of aerobic cycling at 50% of predetermined peak oxygen uptake (VO2peak) for 45 min. The mean age of the participants was 30.2 years, the mean hemoglobin A1C was 7.4%, and the mean VO2peak was 32.5 mL/kg per min. The women reported regular menstrual cycles, and none were using oral contraceptives.
Blood samples were collected before and immediately after exercise and after an hour of recovery. Participants wore continuous glucose monitors for at least 1 hour before and after exercise.
Menstrual cycle was confirmed via estrogen, estradiol, and progesterone.
Insulin levels varied greatly among the study participants, but the differences were not significant, Dr. Yardley said. Glucose levels consistently decreased during exercise and increased after exercise, she noted.
No significant difference in glucose was observed between the follicular and luteal phases.
However, “this needs to be interpreted in the context of the safety profiles that are in place in our lab,” which include carbohydrate supplements for individuals whose blood glucose levels drop below 4.5 mmol/L, she said.
In the current study, 6 of 9 participants required additional carbohydrates during the luteal phase, but only 1 participant needed additional carbohydrates during the follicular phase, she noted. For this reason, no differences were noted. “We actually prevented changes,” she said.
No significant differences were noted in mean glucose levels or number of hypoglycemic episodes at any of the time points between the two phases.
“One place where we did see a difference was in hyperglycemia 24 hours after exercise,” Dr. Yardley said. Level 1 hyperglycemia 24 hours after exercise was significantly more frequent in the follicular phase, compared with the luteal phase (P = .028).
The study findings were limited by the small sample size and homogenous population, and more research is needed to interpret the data, said Dr. Yardley.
However, the need for more glucose supplementation to prevent hypoglycemia during the luteal phase suggests a higher hypoglycemic risk associated with aerobic exercise during this time, she said.
In addition, the results suggest that the menstrual cycle should be taken into consideration when female participants are involved in exercise studies, she noted.
Study supports personalized exercise plans
“It is important to evaluate effects of exercise in people with type 1 diabetes and evaluate whether there is a difference those effects in men and women,” said Helena W. Rodbard, MD, an endocrinologist in private practice in Rockville, Md., in an interview. “There is also a need to evaluate to what extent the changes in blood glucose patterns in women in response to exercise differ depending on the phase of the ovarian cycle,” said Dr. Rodbard, who was not involved in the study.
In the current study, “the researchers observed a decline in glucose during a 45-minute period of moderate aerobic exercise, cycling at 50% VO2peak followed by an increase during a 60-minute recovery period. There was a suggestive finding, in the nine subjects, that more carbohydrate supplementation was needed during the late luteal phase of the menstrual cycle than during the follicular phase,” Dr. Rodbard noted. “In contrast, the authors reported a significantly increased degree of hyperglycemia during the recovery phase for subjects during the follicular phase. These findings are consistent with and extend several recent studies from Dr. Yardley and coworkers, who have been focused on this area of research,” she said.
“This study provides provocative evidence that glucose responses to aerobic exercise in women may depend on the timing in relationship to their ovarian cycle,” said Dr. Rodbard. “These findings are based on a small group of subjects and were present in some but not all subjects. Clinicians should encourage women to evaluate and record their experiences during and after exercise in terms of need for carbohydrate supplementation for documented or symptomatic hypoglycemia and in terms of glucose changes as recorded using continuous glucose monitoring (CGM), both in relation to type of exercise and in relation to time in the menstrual cycle,” she said.
The findings also highlight the importance of individualized therapy that is “based on subjective inputs combined with analysis of CGM data during and following exercise,” said Dr. Rodbard. “It is likely that use of Automated Insulin Delivery (AID) will be helpful in achieving this level of individualization in view of the wide range of types, intensity, and duration of physical activity and exercise in which people with T1D engage and the myriad factors that can influence the glycemic response,” she said.
Looking ahead, “the authors and others should expand the present series of subjects using aerobic exercise and examine other types of exercise as well,” Dr. Rodbard noted. “It will be important to evaluate the consistency of these changes in glucose patterns within individuals on multiple occasions, and it would be helpful to repeat the studies in women using oral contraceptives.”
Dr. Yardley disclosed research support from Abbott, Dexcom, and LifeScan and disclosed serving on the speaker’s bureau for Abbott Diabetes. Dr. Rodbard had no financial conflicts to disclose. She serves on the Editorial Advisory Board of Clinical Endocrinology News.
Women with type 1 diabetes may need additional glucose after exercise during the luteal phase of the menstrual cycle, compared with other times, according to a study in nine women.
“We know that exercise is very beneficial for people with type 1 diabetes; we also know that fear of hypoglycemia is a major barrier to exercise in this population,” said Jane E. Yardley, PhD, in a presentation at the annual scientific sessions of the American Diabetes Association, New Orleans. Women with type 1 diabetes (T1D) perceive more barriers, compared with men, she added.
The menstrual cycle could be an additional barrier to exercise for women with T1D because it increases glucose fluctuations that have not been well documented in the literature to date, said Dr. Yardley, of the University of Alberta, Augustana.
The follicular phase of the menstrual cycle lasts from menses to the midcycle, about 14 days later. This is followed by the luteal phase, which lasts until approximately day 28, Dr. Yardley explained. Data on insulin sensitivity have shown that the late luteal phase is associated with “a little less insulin sensitivity” in women with T1D, she noted.
To assess the relationship between menstrual cycle, glucose control, and exercise, Dr. Yardley and colleagues compared the effects of a moderate aerobic exercise on glycemic responses between the early follicular and late luteal phases of the menstrual cycle in nine female participants with T1D.
The exercise involved 45 minutes of aerobic cycling at 50% of predetermined peak oxygen uptake (VO2peak) for 45 min. The mean age of the participants was 30.2 years, the mean hemoglobin A1C was 7.4%, and the mean VO2peak was 32.5 mL/kg per min. The women reported regular menstrual cycles, and none were using oral contraceptives.
Blood samples were collected before and immediately after exercise and after an hour of recovery. Participants wore continuous glucose monitors for at least 1 hour before and after exercise.
Menstrual cycle was confirmed via estrogen, estradiol, and progesterone.
Insulin levels varied greatly among the study participants, but the differences were not significant, Dr. Yardley said. Glucose levels consistently decreased during exercise and increased after exercise, she noted.
No significant difference in glucose was observed between the follicular and luteal phases.
However, “this needs to be interpreted in the context of the safety profiles that are in place in our lab,” which include carbohydrate supplements for individuals whose blood glucose levels drop below 4.5 mmol/L, she said.
In the current study, 6 of 9 participants required additional carbohydrates during the luteal phase, but only 1 participant needed additional carbohydrates during the follicular phase, she noted. For this reason, no differences were noted. “We actually prevented changes,” she said.
No significant differences were noted in mean glucose levels or number of hypoglycemic episodes at any of the time points between the two phases.
“One place where we did see a difference was in hyperglycemia 24 hours after exercise,” Dr. Yardley said. Level 1 hyperglycemia 24 hours after exercise was significantly more frequent in the follicular phase, compared with the luteal phase (P = .028).
The study findings were limited by the small sample size and homogenous population, and more research is needed to interpret the data, said Dr. Yardley.
However, the need for more glucose supplementation to prevent hypoglycemia during the luteal phase suggests a higher hypoglycemic risk associated with aerobic exercise during this time, she said.
In addition, the results suggest that the menstrual cycle should be taken into consideration when female participants are involved in exercise studies, she noted.
Study supports personalized exercise plans
“It is important to evaluate effects of exercise in people with type 1 diabetes and evaluate whether there is a difference those effects in men and women,” said Helena W. Rodbard, MD, an endocrinologist in private practice in Rockville, Md., in an interview. “There is also a need to evaluate to what extent the changes in blood glucose patterns in women in response to exercise differ depending on the phase of the ovarian cycle,” said Dr. Rodbard, who was not involved in the study.
In the current study, “the researchers observed a decline in glucose during a 45-minute period of moderate aerobic exercise, cycling at 50% VO2peak followed by an increase during a 60-minute recovery period. There was a suggestive finding, in the nine subjects, that more carbohydrate supplementation was needed during the late luteal phase of the menstrual cycle than during the follicular phase,” Dr. Rodbard noted. “In contrast, the authors reported a significantly increased degree of hyperglycemia during the recovery phase for subjects during the follicular phase. These findings are consistent with and extend several recent studies from Dr. Yardley and coworkers, who have been focused on this area of research,” she said.
“This study provides provocative evidence that glucose responses to aerobic exercise in women may depend on the timing in relationship to their ovarian cycle,” said Dr. Rodbard. “These findings are based on a small group of subjects and were present in some but not all subjects. Clinicians should encourage women to evaluate and record their experiences during and after exercise in terms of need for carbohydrate supplementation for documented or symptomatic hypoglycemia and in terms of glucose changes as recorded using continuous glucose monitoring (CGM), both in relation to type of exercise and in relation to time in the menstrual cycle,” she said.
The findings also highlight the importance of individualized therapy that is “based on subjective inputs combined with analysis of CGM data during and following exercise,” said Dr. Rodbard. “It is likely that use of Automated Insulin Delivery (AID) will be helpful in achieving this level of individualization in view of the wide range of types, intensity, and duration of physical activity and exercise in which people with T1D engage and the myriad factors that can influence the glycemic response,” she said.
Looking ahead, “the authors and others should expand the present series of subjects using aerobic exercise and examine other types of exercise as well,” Dr. Rodbard noted. “It will be important to evaluate the consistency of these changes in glucose patterns within individuals on multiple occasions, and it would be helpful to repeat the studies in women using oral contraceptives.”
Dr. Yardley disclosed research support from Abbott, Dexcom, and LifeScan and disclosed serving on the speaker’s bureau for Abbott Diabetes. Dr. Rodbard had no financial conflicts to disclose. She serves on the Editorial Advisory Board of Clinical Endocrinology News.
FROM ADA 2022
Food insecurity drives poor glycemic control
People with diabetes who had a poor-quality diet and food insecurity were significantly more likely to have poor glycemic and cholesterol control than were those with a healthier diet and food security, based on data from a national study of more than 2,000 individuals.
The American Diabetes Association recommends a high-quality diet for people with diabetes (PWD) to achieve treatment goals; however, roughly 18% of PWD in the United States are food insecure and/or have a poor-quality diet, Sarah S. Casagrande, PhD, of DLH Corporation, Silver Spring, Md., and colleagues wrote in a poster presented at the annual scientific sessions of the ADA in New Orleans.
To examine the impact of food insecurity and diet quality on diabetes and lipid management, the researchers reviewed data from 2,075 adults with self-reported diabetes who completed the National Health and Nutrition Examination Surveys between 2013 and 2018.
Diet quality was divided into quartiles based on the 2015 Healthy Eating Index. Food insecurity was assessed using a standard 10-item questionnaire including questions about running out of food and not being able to afford more, reducing meal sizes, eating less or not at all, and going hungry because of lack of money for food.
The logistic regression analysis controlled for factors including sociodemographics, health care use, smoking, diabetes medications, blood pressure medication use, cholesterol medication use, and body mass index.
Overall, 17.6% of the participants were food insecure and had a low-quality diet, 14.2% were food insecure with a high-quality diet, 33.1% were food secure with a low-quality diet, and 35.2% were food secure with a high-quality diet.
PWD in the food insecure/low-quality diet group were significantly more likely to be younger, non-Hispanic black or Hispanic, and uninsured compared to those in the food secure/high-quality diet group (P < .001 for all).
When the researchers examined glycemic control, they found that PWD in the food insecurity/low-quality diet groups were significantly more likely than were those with food security/high-quality diets to have hemoglobin A1c of at least 7.0% (adjusted odds ratio, 1.85), A1c of at least 8.0% (aOR, 1.79), low HDL cholesterol (aOR, 1.69), and high triglycerides (aOR, 3.26).
PWD with food insecurity but a high-quality diet also were significantly more likely than were those with food security and a high quality diet to have A1c of at least 7.0% (aOR, 1.69), A1c of at least 8.0% (aOR, 1.83), and high triglycerides (aOR, 2.44). PWD with food security but a low-quality diet were significantly more likely than was the food security/high-quality diet group to have A1c of at least 7% (aOR, 1.55).
The study findings were limited by several factors including the cross-sectional design, reliance on self-reports, and inability to distinguish between type 1 and type 2 diabetes, the researchers wrote.
However, the results were strengthened by the large, nationally representative sample and the inclusion of multiple clinical outcomes in the patient assessment, they said.
The results suggest that food insecurity had a significant impact on both glycemic control and cholesterol management independent of diet quality, the researchers noted. Based on these findings, health care providers treating PWD may wish to assess their patients’ food security status, and “interventions could address disparities in food security,” they concluded.
Food insecurity a growing problem
“With more communities being pushed into state of war, drought, and famine globally, it is important to track impact of food insecurity and low quality food on common medical conditions like diabetes in our vulnerable communities,” Romesh K. Khardori, MD, professor of medicine: endocrinology, and metabolism at Eastern Virginia Medical School, Norfolk, said in an interview.
Dr. Khardori, who was not involved in the study, said he was not surprised by the current study findings.
“Type of food, amount of food, and quality of food have been stressed in diabetes management for more than 100 years,” he said. “Organizations charged with recommendations, such as the ADA and American Dietetic Association, have regularly updated their recommendations,” he noted. “It was not surprising, therefore, to find food insecurity and low quality tied to poor glycemic control.”
The take-home message for clinicians is to consider the availability and quality of food that their patients are exposed to when evaluating barriers to proper glycemic control, Dr. Khardori emphasized.
However, additional research is needed to explore whether the prescription of a sufficient amount of good quality food would alleviate the adverse impact seen in the current study, he said.
The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. The researchers and Dr. Khardori had no financial conflicts to disclose.
People with diabetes who had a poor-quality diet and food insecurity were significantly more likely to have poor glycemic and cholesterol control than were those with a healthier diet and food security, based on data from a national study of more than 2,000 individuals.
The American Diabetes Association recommends a high-quality diet for people with diabetes (PWD) to achieve treatment goals; however, roughly 18% of PWD in the United States are food insecure and/or have a poor-quality diet, Sarah S. Casagrande, PhD, of DLH Corporation, Silver Spring, Md., and colleagues wrote in a poster presented at the annual scientific sessions of the ADA in New Orleans.
To examine the impact of food insecurity and diet quality on diabetes and lipid management, the researchers reviewed data from 2,075 adults with self-reported diabetes who completed the National Health and Nutrition Examination Surveys between 2013 and 2018.
Diet quality was divided into quartiles based on the 2015 Healthy Eating Index. Food insecurity was assessed using a standard 10-item questionnaire including questions about running out of food and not being able to afford more, reducing meal sizes, eating less or not at all, and going hungry because of lack of money for food.
The logistic regression analysis controlled for factors including sociodemographics, health care use, smoking, diabetes medications, blood pressure medication use, cholesterol medication use, and body mass index.
Overall, 17.6% of the participants were food insecure and had a low-quality diet, 14.2% were food insecure with a high-quality diet, 33.1% were food secure with a low-quality diet, and 35.2% were food secure with a high-quality diet.
PWD in the food insecure/low-quality diet group were significantly more likely to be younger, non-Hispanic black or Hispanic, and uninsured compared to those in the food secure/high-quality diet group (P < .001 for all).
When the researchers examined glycemic control, they found that PWD in the food insecurity/low-quality diet groups were significantly more likely than were those with food security/high-quality diets to have hemoglobin A1c of at least 7.0% (adjusted odds ratio, 1.85), A1c of at least 8.0% (aOR, 1.79), low HDL cholesterol (aOR, 1.69), and high triglycerides (aOR, 3.26).
PWD with food insecurity but a high-quality diet also were significantly more likely than were those with food security and a high quality diet to have A1c of at least 7.0% (aOR, 1.69), A1c of at least 8.0% (aOR, 1.83), and high triglycerides (aOR, 2.44). PWD with food security but a low-quality diet were significantly more likely than was the food security/high-quality diet group to have A1c of at least 7% (aOR, 1.55).
The study findings were limited by several factors including the cross-sectional design, reliance on self-reports, and inability to distinguish between type 1 and type 2 diabetes, the researchers wrote.
However, the results were strengthened by the large, nationally representative sample and the inclusion of multiple clinical outcomes in the patient assessment, they said.
The results suggest that food insecurity had a significant impact on both glycemic control and cholesterol management independent of diet quality, the researchers noted. Based on these findings, health care providers treating PWD may wish to assess their patients’ food security status, and “interventions could address disparities in food security,” they concluded.
Food insecurity a growing problem
“With more communities being pushed into state of war, drought, and famine globally, it is important to track impact of food insecurity and low quality food on common medical conditions like diabetes in our vulnerable communities,” Romesh K. Khardori, MD, professor of medicine: endocrinology, and metabolism at Eastern Virginia Medical School, Norfolk, said in an interview.
Dr. Khardori, who was not involved in the study, said he was not surprised by the current study findings.
“Type of food, amount of food, and quality of food have been stressed in diabetes management for more than 100 years,” he said. “Organizations charged with recommendations, such as the ADA and American Dietetic Association, have regularly updated their recommendations,” he noted. “It was not surprising, therefore, to find food insecurity and low quality tied to poor glycemic control.”
The take-home message for clinicians is to consider the availability and quality of food that their patients are exposed to when evaluating barriers to proper glycemic control, Dr. Khardori emphasized.
However, additional research is needed to explore whether the prescription of a sufficient amount of good quality food would alleviate the adverse impact seen in the current study, he said.
The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. The researchers and Dr. Khardori had no financial conflicts to disclose.
People with diabetes who had a poor-quality diet and food insecurity were significantly more likely to have poor glycemic and cholesterol control than were those with a healthier diet and food security, based on data from a national study of more than 2,000 individuals.
The American Diabetes Association recommends a high-quality diet for people with diabetes (PWD) to achieve treatment goals; however, roughly 18% of PWD in the United States are food insecure and/or have a poor-quality diet, Sarah S. Casagrande, PhD, of DLH Corporation, Silver Spring, Md., and colleagues wrote in a poster presented at the annual scientific sessions of the ADA in New Orleans.
To examine the impact of food insecurity and diet quality on diabetes and lipid management, the researchers reviewed data from 2,075 adults with self-reported diabetes who completed the National Health and Nutrition Examination Surveys between 2013 and 2018.
Diet quality was divided into quartiles based on the 2015 Healthy Eating Index. Food insecurity was assessed using a standard 10-item questionnaire including questions about running out of food and not being able to afford more, reducing meal sizes, eating less or not at all, and going hungry because of lack of money for food.
The logistic regression analysis controlled for factors including sociodemographics, health care use, smoking, diabetes medications, blood pressure medication use, cholesterol medication use, and body mass index.
Overall, 17.6% of the participants were food insecure and had a low-quality diet, 14.2% were food insecure with a high-quality diet, 33.1% were food secure with a low-quality diet, and 35.2% were food secure with a high-quality diet.
PWD in the food insecure/low-quality diet group were significantly more likely to be younger, non-Hispanic black or Hispanic, and uninsured compared to those in the food secure/high-quality diet group (P < .001 for all).
When the researchers examined glycemic control, they found that PWD in the food insecurity/low-quality diet groups were significantly more likely than were those with food security/high-quality diets to have hemoglobin A1c of at least 7.0% (adjusted odds ratio, 1.85), A1c of at least 8.0% (aOR, 1.79), low HDL cholesterol (aOR, 1.69), and high triglycerides (aOR, 3.26).
PWD with food insecurity but a high-quality diet also were significantly more likely than were those with food security and a high quality diet to have A1c of at least 7.0% (aOR, 1.69), A1c of at least 8.0% (aOR, 1.83), and high triglycerides (aOR, 2.44). PWD with food security but a low-quality diet were significantly more likely than was the food security/high-quality diet group to have A1c of at least 7% (aOR, 1.55).
The study findings were limited by several factors including the cross-sectional design, reliance on self-reports, and inability to distinguish between type 1 and type 2 diabetes, the researchers wrote.
However, the results were strengthened by the large, nationally representative sample and the inclusion of multiple clinical outcomes in the patient assessment, they said.
The results suggest that food insecurity had a significant impact on both glycemic control and cholesterol management independent of diet quality, the researchers noted. Based on these findings, health care providers treating PWD may wish to assess their patients’ food security status, and “interventions could address disparities in food security,” they concluded.
Food insecurity a growing problem
“With more communities being pushed into state of war, drought, and famine globally, it is important to track impact of food insecurity and low quality food on common medical conditions like diabetes in our vulnerable communities,” Romesh K. Khardori, MD, professor of medicine: endocrinology, and metabolism at Eastern Virginia Medical School, Norfolk, said in an interview.
Dr. Khardori, who was not involved in the study, said he was not surprised by the current study findings.
“Type of food, amount of food, and quality of food have been stressed in diabetes management for more than 100 years,” he said. “Organizations charged with recommendations, such as the ADA and American Dietetic Association, have regularly updated their recommendations,” he noted. “It was not surprising, therefore, to find food insecurity and low quality tied to poor glycemic control.”
The take-home message for clinicians is to consider the availability and quality of food that their patients are exposed to when evaluating barriers to proper glycemic control, Dr. Khardori emphasized.
However, additional research is needed to explore whether the prescription of a sufficient amount of good quality food would alleviate the adverse impact seen in the current study, he said.
The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. The researchers and Dr. Khardori had no financial conflicts to disclose.
FROM ADA 2022
Type 1 diabetes control worse in racially segregated teens
Racial residential segregation was significantly associated with poor glycemic control in Black adolescents with type 1 diabetes, according to data from 144 individuals.
Racial residential segregation is considered a form of systemic racism that involves limited access to resources, including health care resources, Deborah A. Ellis, MD, of Wayne State University, Detroit, and colleagues wrote in a poster presented at the annual meeting of the American Diabetes Association.
In the study, the researchers recruited youth aged 10-15 years with type 1 diabetes from seven pediatric clinics in two large U.S. cities. The mean age of the participants was 13.3 years, and the mean hemoglobin A1c was 11.5%.
Diabetes management was based on self-reports using the Diabetes Management Scale (DMS). Racial residential segregation, which refers to the separation of groups within a geographic area, was determined using data from the U.S. Census using Location Quotient (LQ) at the block group level; this showed the ratio of the Black population to the total population, compared with the same ratio in the metropolitan area.
The mean family income was $34,163, and the mean LQ was 3.04, “indicating residence in highly segregated neighborhoods,” the researchers wrote.
Overall, racial residential segregation was significantly associated with A1c (P = .001) but not with DMS (P = .311). The researchers also conducted a stepwise multiple regression analysis including age, insulin delivery method, neighborhood adversity (a 9-item composite with variables including percentage of persons living in poverty, percentage of households with no vehicle), and family income. They found that only age, insulin delivery method, and racial residential segregation had significant impacts of A1c levels.
The study was limited by several factors, including the use of self-reports.
However, the results are consistent with previous studies showing the potential negative health effects of structural racism, the researchers wrote. The findings suggest that racial residential segregation has an independent effect on glycemic control in Black youth with type 1 diabetes, and consequently, “advocacy and policy making to address such inequities could improve diabetes population health.”
Location makes a difference
“Poor neighborhoods have been associated with high rates of obesity, hypertension, type 2 diabetes and high cholesterol,” Romesh K. Khardori, MD, professor of medicine at Eastern Virginia Medical School, Norfolk, said in an interview. However, “not much is known about impact of racial segregation on type 1 diabetes,” said Dr. Khardori, who was not involved in the study.
Dr. Khardori was not surprised by the current study findings. “In our practice, Black youth coming from racially segregated or low-income housing projects often tend have poor diabetes control, with repeated admissions to local hospitals for managing acute/chronic complications of type 1 diabetes,” he said.
The current findings reflect Dr. Khardori’s clinical experience and highlight the need for clinicians to recognize the increased risk for poor glycemic control and poor outcomes in this vulnerable population.
More research is needed to expand the observations of the current study, Dr. Khardori said. Future researchers also should “involve community leaders and politicians to educate and garner more support for mitigation efforts.”
The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Ellis and Dr. Khardori had no financial conflicts to disclose.
Racial residential segregation was significantly associated with poor glycemic control in Black adolescents with type 1 diabetes, according to data from 144 individuals.
Racial residential segregation is considered a form of systemic racism that involves limited access to resources, including health care resources, Deborah A. Ellis, MD, of Wayne State University, Detroit, and colleagues wrote in a poster presented at the annual meeting of the American Diabetes Association.
In the study, the researchers recruited youth aged 10-15 years with type 1 diabetes from seven pediatric clinics in two large U.S. cities. The mean age of the participants was 13.3 years, and the mean hemoglobin A1c was 11.5%.
Diabetes management was based on self-reports using the Diabetes Management Scale (DMS). Racial residential segregation, which refers to the separation of groups within a geographic area, was determined using data from the U.S. Census using Location Quotient (LQ) at the block group level; this showed the ratio of the Black population to the total population, compared with the same ratio in the metropolitan area.
The mean family income was $34,163, and the mean LQ was 3.04, “indicating residence in highly segregated neighborhoods,” the researchers wrote.
Overall, racial residential segregation was significantly associated with A1c (P = .001) but not with DMS (P = .311). The researchers also conducted a stepwise multiple regression analysis including age, insulin delivery method, neighborhood adversity (a 9-item composite with variables including percentage of persons living in poverty, percentage of households with no vehicle), and family income. They found that only age, insulin delivery method, and racial residential segregation had significant impacts of A1c levels.
The study was limited by several factors, including the use of self-reports.
However, the results are consistent with previous studies showing the potential negative health effects of structural racism, the researchers wrote. The findings suggest that racial residential segregation has an independent effect on glycemic control in Black youth with type 1 diabetes, and consequently, “advocacy and policy making to address such inequities could improve diabetes population health.”
Location makes a difference
“Poor neighborhoods have been associated with high rates of obesity, hypertension, type 2 diabetes and high cholesterol,” Romesh K. Khardori, MD, professor of medicine at Eastern Virginia Medical School, Norfolk, said in an interview. However, “not much is known about impact of racial segregation on type 1 diabetes,” said Dr. Khardori, who was not involved in the study.
Dr. Khardori was not surprised by the current study findings. “In our practice, Black youth coming from racially segregated or low-income housing projects often tend have poor diabetes control, with repeated admissions to local hospitals for managing acute/chronic complications of type 1 diabetes,” he said.
The current findings reflect Dr. Khardori’s clinical experience and highlight the need for clinicians to recognize the increased risk for poor glycemic control and poor outcomes in this vulnerable population.
More research is needed to expand the observations of the current study, Dr. Khardori said. Future researchers also should “involve community leaders and politicians to educate and garner more support for mitigation efforts.”
The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Ellis and Dr. Khardori had no financial conflicts to disclose.
Racial residential segregation was significantly associated with poor glycemic control in Black adolescents with type 1 diabetes, according to data from 144 individuals.
Racial residential segregation is considered a form of systemic racism that involves limited access to resources, including health care resources, Deborah A. Ellis, MD, of Wayne State University, Detroit, and colleagues wrote in a poster presented at the annual meeting of the American Diabetes Association.
In the study, the researchers recruited youth aged 10-15 years with type 1 diabetes from seven pediatric clinics in two large U.S. cities. The mean age of the participants was 13.3 years, and the mean hemoglobin A1c was 11.5%.
Diabetes management was based on self-reports using the Diabetes Management Scale (DMS). Racial residential segregation, which refers to the separation of groups within a geographic area, was determined using data from the U.S. Census using Location Quotient (LQ) at the block group level; this showed the ratio of the Black population to the total population, compared with the same ratio in the metropolitan area.
The mean family income was $34,163, and the mean LQ was 3.04, “indicating residence in highly segregated neighborhoods,” the researchers wrote.
Overall, racial residential segregation was significantly associated with A1c (P = .001) but not with DMS (P = .311). The researchers also conducted a stepwise multiple regression analysis including age, insulin delivery method, neighborhood adversity (a 9-item composite with variables including percentage of persons living in poverty, percentage of households with no vehicle), and family income. They found that only age, insulin delivery method, and racial residential segregation had significant impacts of A1c levels.
The study was limited by several factors, including the use of self-reports.
However, the results are consistent with previous studies showing the potential negative health effects of structural racism, the researchers wrote. The findings suggest that racial residential segregation has an independent effect on glycemic control in Black youth with type 1 diabetes, and consequently, “advocacy and policy making to address such inequities could improve diabetes population health.”
Location makes a difference
“Poor neighborhoods have been associated with high rates of obesity, hypertension, type 2 diabetes and high cholesterol,” Romesh K. Khardori, MD, professor of medicine at Eastern Virginia Medical School, Norfolk, said in an interview. However, “not much is known about impact of racial segregation on type 1 diabetes,” said Dr. Khardori, who was not involved in the study.
Dr. Khardori was not surprised by the current study findings. “In our practice, Black youth coming from racially segregated or low-income housing projects often tend have poor diabetes control, with repeated admissions to local hospitals for managing acute/chronic complications of type 1 diabetes,” he said.
The current findings reflect Dr. Khardori’s clinical experience and highlight the need for clinicians to recognize the increased risk for poor glycemic control and poor outcomes in this vulnerable population.
More research is needed to expand the observations of the current study, Dr. Khardori said. Future researchers also should “involve community leaders and politicians to educate and garner more support for mitigation efforts.”
The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Ellis and Dr. Khardori had no financial conflicts to disclose.
FROM ADA 2022
Experts elevate new drugs for diabetic kidney disease
ATLANTA – U.S. clinicians caring for people with diabetes should take a more aggressive approach to using combined medical treatments proven to slow the otherwise relentless progression of chronic kidney disease (CKD), according to a new joint statement by the American Diabetes Association and a major international nephrology organization presented during the annual scientific sessions of the American Diabetes Association (ADA).
The statement elevates treatment with an agent from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class to first-line for people with diabetes and laboratory-based evidence of advancing CKD. It also re-emphasizes the key role of concurrent first-line treatment with a renin-angiotensin system inhibitor (an ACE inhibitor or angiotensin-receptor blocker), metformin, and a statin.
The new statement also urges clinicians to rapidly add treatment with the new nonsteroidal mineralocorticoid receptor antagonist finerenone (Kerendia) for further renal protection in the many patients suitable for treatment with this agent, and it recommends the second-line addition of a glucagon-like peptide-1 (GLP-1) receptor agonist as the best add-on for any patient who needs additional glycemic control on top of metformin and an SGLT2 inhibitor.
The consensus joint statement with these updates came from a nine-member writing group assembled by the ADA and the Kidney Disease: Improving Global Outcomes (KDIGO) organization.
“We’re going to try to make this feasible. We have to; I don’t think we have a choice,” commented Amy K. Mottl, MD, a nephrologist at the University of North Carolina, Chapel Hill. Dr. Mottl was not involved with writing the consensus statement but has been active in the Diabetic Kidney Disease Collaborative of the American Society of Nephrology, another group promoting a more aggressive multidrug-class approach to treating CKD in people with diabetes.
Wider use of costly drugs
Adoption of this evidence-based approach by U.S. clinicians will both increase the number of agents that many patients receive and drive a significant uptick in the cost and complexity of patient care, a consequence acknowledged by the authors of the joint statement as well as outside experts.
But they view this as unavoidable given what’s now known about the high incidence of worsening CKD in patients with diabetes and the types of interventions proven to blunt this.
Much of the financial implication stems from the price of agents from the new drug classes now emphasized in the consensus recommendations – SGLT2 inhibitors, finerenone, and GLP-1 receptor agonists. All these drugs currently remain on-patent with relatively expensive retail prices in the range of about $600 to $1,000/month.
Commenting on the cost concerns, Dr. Mottl highlighted that she currently has several patients in her practice on agents from two or more of these newer classes, and she has generally found it possible for patients to get much of their expenses covered by insurers and through drug-company assistance programs.
“The major gap is patients on Medicare,” she noted in an interview, because the Federal health insurance program does not allow beneficiaries to receive rebates for their drug costs. “The Diabetic Kidney Disease Collaborative is currently lobbying members of Congress to lift that barrier,” she emphasized.
Improved alignment
Details of the KDIGO recommendations feature in a guideline from that organization that appeared as a draft document online in March 2022. The ADA’s version recently appeared as an update to its Standards of Medical Care in Diabetes – 2022, as reported by this news organization. A panel of five KDIGO representatives and four members appointed by the ADA produced the harmonization statement.
Recommendations from both organizations were largely in agreement at the outset, but following the panel’s review, the two groups are now “very well-aligned,” said Peter Rossing, MD, DMSc, a diabetologist and professor at the Steno Diabetes Center, Copenhagen, and a KDIGO representative to the writing committee, who presented the joint statement at the ADA meeting.
“These are very important drugs that are vastly underused,” commented Josef Coresh, MD, PhD, an epidemiologist and professor at Johns Hopkins Bloomberg School of Public Health, Baltimore, who specializes in CKD and was not involved with the new statement.
“Coherence and simplicity are what we need so that there are no excuses about moving forward” with the recommended combination treatment, he stressed.
Moving too slow
“No one is resisting using these new medications, but they are just moving too slowly, and data now show that it’s moving more slowly in the United States than elsewhere. That may be partly because U.S. patients are charged much more for these drugs, and partly because U.S. health care is so much more fragmented,” Dr. Coresh said in an interview.
The new joint consensus statement may help, “but the fragmentation of the United States system and COVID-19 are big enemies” for any short-term increased use of the highlighted agents, he added.
Evidence for low U.S. use of SGLT2 inhibitors, finerenone, and GLP-1 receptor agonists is becoming well known.
Dr. Rossing cited a 2019 report from the CURE-CKD registry of more than 600,000 U.S. patients with CKD showing that less than 1% received an SGLT2 inhibitor and less than 1% a GLP-1 receptor agonist. Not all these patients had diabetes, but a subgroup analysis of those with diabetes, prediabetes, or hypertension showed that usage of each of these two classes remained at less than 1% even in this group.
A separate report at the ADA meeting documented that of more than 1.3 million people with type 2 diabetes in the U.S. Veterans Affairs Healthcare System during 2019 and 2020, just 10% received an SGLT2 inhibitor and 7% a GLP-1 receptor agonist. And this is in a setting where drug cost is not a limiting factor.
In addition to focusing on the updated scheme for drug intervention in the consensus statement, Dr. Rossing highlighted several other important points that the writing committee emphasized.
Lifestyle optimization is a core first-line element of managing patients with diabetes and CKD, including a healthy diet, exercise, smoking cessation, and weight control. Other key steps for management include optimization of blood pressure, glucose, and lipids. The statement also calls out a potentially helpful role for continuous glucose monitoring in patients with type 1 or type 2 diabetes and CKD.
The statement notes that patients who also have atherosclerotic cardiovascular disease usually qualify for and could potentially benefit from more intensified lipid management with ezetimibe or a PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor, as well as a potential role for treatment with antiplatelet agents.
‘If you don’t screen, you won’t find it’
Dr. Rossing also stressed the importance of regular screening for the onset of advanced CKD in patients. Patients whose estimated glomerular filtration rate (eGFR) drops below 60 mL/min/1.73m2, as well as those who develop microalbuminuria with a urinary albumin-to-creatinine ratio of at least 30 mg/g (30 mg/mmol), have a stage of CKD that warrants the drug interventions he outlined.
Guidelines from both the ADA and KDIGO were already in place, recommending annual screening of patients with diabetes for both these parameters starting at diagnosis of type 2 diabetes or 5 years following initial diagnosis of type 1 diabetes.
“If you don’t screen, you won’t find it, and you won’t be able to treat,” Dr. Rossing warned. He also highlighted the panel’s recommendation to treat these patients with an SGLT2 inhibitor as long as their eGFR is at least 20 mL/min/1.73m2. Treatment can then continue even when their eGFR drops lower.
Starting treatment with finerenone requires that patients have a normal level of serum potassium, he emphasized.
One reason for developing the new ADA and KDIGO statement is that “discrepancies in clinical practice guideline recommendations from various professional organizations add to confusion that impedes understanding of best practices,” write Katherine R. Tuttle, MD, and associates in a recent commentary.
The goal of the new statement is to harmonize and promote the shared recommendations of the two organizations, added Dr. Tuttle, who is executive director for research at Providence Healthcare, Spokane, Washington, and a KDIGO representative on the statement writing panel.
Dr. Mottl has reported being a consultant to Bayer. Dr. Rossing has reported being a consultant to or speaker on behalf of Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Gilead, MSD, Mundipharma, Novo Nordisk, Sanofi Aventis, and Vifor, as well as receiving research grants from AstraZeneca and Novo Nordisk. Dr. Coresh has reported no relevant financial relationships. Dr. Tuttle has reported being a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Goldfinch Bio, Janssen, Novo Nordisk, and Travere; receiving honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, Goldfinch Bio, Novo Nordisk, and Travere; and receiving research funding from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Gilead, Goldfinch Bio, Novo Nordisk, and Travere.
A version of this article first appeared on Medscape.com.
ATLANTA – U.S. clinicians caring for people with diabetes should take a more aggressive approach to using combined medical treatments proven to slow the otherwise relentless progression of chronic kidney disease (CKD), according to a new joint statement by the American Diabetes Association and a major international nephrology organization presented during the annual scientific sessions of the American Diabetes Association (ADA).
The statement elevates treatment with an agent from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class to first-line for people with diabetes and laboratory-based evidence of advancing CKD. It also re-emphasizes the key role of concurrent first-line treatment with a renin-angiotensin system inhibitor (an ACE inhibitor or angiotensin-receptor blocker), metformin, and a statin.
The new statement also urges clinicians to rapidly add treatment with the new nonsteroidal mineralocorticoid receptor antagonist finerenone (Kerendia) for further renal protection in the many patients suitable for treatment with this agent, and it recommends the second-line addition of a glucagon-like peptide-1 (GLP-1) receptor agonist as the best add-on for any patient who needs additional glycemic control on top of metformin and an SGLT2 inhibitor.
The consensus joint statement with these updates came from a nine-member writing group assembled by the ADA and the Kidney Disease: Improving Global Outcomes (KDIGO) organization.
“We’re going to try to make this feasible. We have to; I don’t think we have a choice,” commented Amy K. Mottl, MD, a nephrologist at the University of North Carolina, Chapel Hill. Dr. Mottl was not involved with writing the consensus statement but has been active in the Diabetic Kidney Disease Collaborative of the American Society of Nephrology, another group promoting a more aggressive multidrug-class approach to treating CKD in people with diabetes.
Wider use of costly drugs
Adoption of this evidence-based approach by U.S. clinicians will both increase the number of agents that many patients receive and drive a significant uptick in the cost and complexity of patient care, a consequence acknowledged by the authors of the joint statement as well as outside experts.
But they view this as unavoidable given what’s now known about the high incidence of worsening CKD in patients with diabetes and the types of interventions proven to blunt this.
Much of the financial implication stems from the price of agents from the new drug classes now emphasized in the consensus recommendations – SGLT2 inhibitors, finerenone, and GLP-1 receptor agonists. All these drugs currently remain on-patent with relatively expensive retail prices in the range of about $600 to $1,000/month.
Commenting on the cost concerns, Dr. Mottl highlighted that she currently has several patients in her practice on agents from two or more of these newer classes, and she has generally found it possible for patients to get much of their expenses covered by insurers and through drug-company assistance programs.
“The major gap is patients on Medicare,” she noted in an interview, because the Federal health insurance program does not allow beneficiaries to receive rebates for their drug costs. “The Diabetic Kidney Disease Collaborative is currently lobbying members of Congress to lift that barrier,” she emphasized.
Improved alignment
Details of the KDIGO recommendations feature in a guideline from that organization that appeared as a draft document online in March 2022. The ADA’s version recently appeared as an update to its Standards of Medical Care in Diabetes – 2022, as reported by this news organization. A panel of five KDIGO representatives and four members appointed by the ADA produced the harmonization statement.
Recommendations from both organizations were largely in agreement at the outset, but following the panel’s review, the two groups are now “very well-aligned,” said Peter Rossing, MD, DMSc, a diabetologist and professor at the Steno Diabetes Center, Copenhagen, and a KDIGO representative to the writing committee, who presented the joint statement at the ADA meeting.
“These are very important drugs that are vastly underused,” commented Josef Coresh, MD, PhD, an epidemiologist and professor at Johns Hopkins Bloomberg School of Public Health, Baltimore, who specializes in CKD and was not involved with the new statement.
“Coherence and simplicity are what we need so that there are no excuses about moving forward” with the recommended combination treatment, he stressed.
Moving too slow
“No one is resisting using these new medications, but they are just moving too slowly, and data now show that it’s moving more slowly in the United States than elsewhere. That may be partly because U.S. patients are charged much more for these drugs, and partly because U.S. health care is so much more fragmented,” Dr. Coresh said in an interview.
The new joint consensus statement may help, “but the fragmentation of the United States system and COVID-19 are big enemies” for any short-term increased use of the highlighted agents, he added.
Evidence for low U.S. use of SGLT2 inhibitors, finerenone, and GLP-1 receptor agonists is becoming well known.
Dr. Rossing cited a 2019 report from the CURE-CKD registry of more than 600,000 U.S. patients with CKD showing that less than 1% received an SGLT2 inhibitor and less than 1% a GLP-1 receptor agonist. Not all these patients had diabetes, but a subgroup analysis of those with diabetes, prediabetes, or hypertension showed that usage of each of these two classes remained at less than 1% even in this group.
A separate report at the ADA meeting documented that of more than 1.3 million people with type 2 diabetes in the U.S. Veterans Affairs Healthcare System during 2019 and 2020, just 10% received an SGLT2 inhibitor and 7% a GLP-1 receptor agonist. And this is in a setting where drug cost is not a limiting factor.
In addition to focusing on the updated scheme for drug intervention in the consensus statement, Dr. Rossing highlighted several other important points that the writing committee emphasized.
Lifestyle optimization is a core first-line element of managing patients with diabetes and CKD, including a healthy diet, exercise, smoking cessation, and weight control. Other key steps for management include optimization of blood pressure, glucose, and lipids. The statement also calls out a potentially helpful role for continuous glucose monitoring in patients with type 1 or type 2 diabetes and CKD.
The statement notes that patients who also have atherosclerotic cardiovascular disease usually qualify for and could potentially benefit from more intensified lipid management with ezetimibe or a PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor, as well as a potential role for treatment with antiplatelet agents.
‘If you don’t screen, you won’t find it’
Dr. Rossing also stressed the importance of regular screening for the onset of advanced CKD in patients. Patients whose estimated glomerular filtration rate (eGFR) drops below 60 mL/min/1.73m2, as well as those who develop microalbuminuria with a urinary albumin-to-creatinine ratio of at least 30 mg/g (30 mg/mmol), have a stage of CKD that warrants the drug interventions he outlined.
Guidelines from both the ADA and KDIGO were already in place, recommending annual screening of patients with diabetes for both these parameters starting at diagnosis of type 2 diabetes or 5 years following initial diagnosis of type 1 diabetes.
“If you don’t screen, you won’t find it, and you won’t be able to treat,” Dr. Rossing warned. He also highlighted the panel’s recommendation to treat these patients with an SGLT2 inhibitor as long as their eGFR is at least 20 mL/min/1.73m2. Treatment can then continue even when their eGFR drops lower.
Starting treatment with finerenone requires that patients have a normal level of serum potassium, he emphasized.
One reason for developing the new ADA and KDIGO statement is that “discrepancies in clinical practice guideline recommendations from various professional organizations add to confusion that impedes understanding of best practices,” write Katherine R. Tuttle, MD, and associates in a recent commentary.
The goal of the new statement is to harmonize and promote the shared recommendations of the two organizations, added Dr. Tuttle, who is executive director for research at Providence Healthcare, Spokane, Washington, and a KDIGO representative on the statement writing panel.
Dr. Mottl has reported being a consultant to Bayer. Dr. Rossing has reported being a consultant to or speaker on behalf of Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Gilead, MSD, Mundipharma, Novo Nordisk, Sanofi Aventis, and Vifor, as well as receiving research grants from AstraZeneca and Novo Nordisk. Dr. Coresh has reported no relevant financial relationships. Dr. Tuttle has reported being a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Goldfinch Bio, Janssen, Novo Nordisk, and Travere; receiving honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, Goldfinch Bio, Novo Nordisk, and Travere; and receiving research funding from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Gilead, Goldfinch Bio, Novo Nordisk, and Travere.
A version of this article first appeared on Medscape.com.
ATLANTA – U.S. clinicians caring for people with diabetes should take a more aggressive approach to using combined medical treatments proven to slow the otherwise relentless progression of chronic kidney disease (CKD), according to a new joint statement by the American Diabetes Association and a major international nephrology organization presented during the annual scientific sessions of the American Diabetes Association (ADA).
The statement elevates treatment with an agent from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class to first-line for people with diabetes and laboratory-based evidence of advancing CKD. It also re-emphasizes the key role of concurrent first-line treatment with a renin-angiotensin system inhibitor (an ACE inhibitor or angiotensin-receptor blocker), metformin, and a statin.
The new statement also urges clinicians to rapidly add treatment with the new nonsteroidal mineralocorticoid receptor antagonist finerenone (Kerendia) for further renal protection in the many patients suitable for treatment with this agent, and it recommends the second-line addition of a glucagon-like peptide-1 (GLP-1) receptor agonist as the best add-on for any patient who needs additional glycemic control on top of metformin and an SGLT2 inhibitor.
The consensus joint statement with these updates came from a nine-member writing group assembled by the ADA and the Kidney Disease: Improving Global Outcomes (KDIGO) organization.
“We’re going to try to make this feasible. We have to; I don’t think we have a choice,” commented Amy K. Mottl, MD, a nephrologist at the University of North Carolina, Chapel Hill. Dr. Mottl was not involved with writing the consensus statement but has been active in the Diabetic Kidney Disease Collaborative of the American Society of Nephrology, another group promoting a more aggressive multidrug-class approach to treating CKD in people with diabetes.
Wider use of costly drugs
Adoption of this evidence-based approach by U.S. clinicians will both increase the number of agents that many patients receive and drive a significant uptick in the cost and complexity of patient care, a consequence acknowledged by the authors of the joint statement as well as outside experts.
But they view this as unavoidable given what’s now known about the high incidence of worsening CKD in patients with diabetes and the types of interventions proven to blunt this.
Much of the financial implication stems from the price of agents from the new drug classes now emphasized in the consensus recommendations – SGLT2 inhibitors, finerenone, and GLP-1 receptor agonists. All these drugs currently remain on-patent with relatively expensive retail prices in the range of about $600 to $1,000/month.
Commenting on the cost concerns, Dr. Mottl highlighted that she currently has several patients in her practice on agents from two or more of these newer classes, and she has generally found it possible for patients to get much of their expenses covered by insurers and through drug-company assistance programs.
“The major gap is patients on Medicare,” she noted in an interview, because the Federal health insurance program does not allow beneficiaries to receive rebates for their drug costs. “The Diabetic Kidney Disease Collaborative is currently lobbying members of Congress to lift that barrier,” she emphasized.
Improved alignment
Details of the KDIGO recommendations feature in a guideline from that organization that appeared as a draft document online in March 2022. The ADA’s version recently appeared as an update to its Standards of Medical Care in Diabetes – 2022, as reported by this news organization. A panel of five KDIGO representatives and four members appointed by the ADA produced the harmonization statement.
Recommendations from both organizations were largely in agreement at the outset, but following the panel’s review, the two groups are now “very well-aligned,” said Peter Rossing, MD, DMSc, a diabetologist and professor at the Steno Diabetes Center, Copenhagen, and a KDIGO representative to the writing committee, who presented the joint statement at the ADA meeting.
“These are very important drugs that are vastly underused,” commented Josef Coresh, MD, PhD, an epidemiologist and professor at Johns Hopkins Bloomberg School of Public Health, Baltimore, who specializes in CKD and was not involved with the new statement.
“Coherence and simplicity are what we need so that there are no excuses about moving forward” with the recommended combination treatment, he stressed.
Moving too slow
“No one is resisting using these new medications, but they are just moving too slowly, and data now show that it’s moving more slowly in the United States than elsewhere. That may be partly because U.S. patients are charged much more for these drugs, and partly because U.S. health care is so much more fragmented,” Dr. Coresh said in an interview.
The new joint consensus statement may help, “but the fragmentation of the United States system and COVID-19 are big enemies” for any short-term increased use of the highlighted agents, he added.
Evidence for low U.S. use of SGLT2 inhibitors, finerenone, and GLP-1 receptor agonists is becoming well known.
Dr. Rossing cited a 2019 report from the CURE-CKD registry of more than 600,000 U.S. patients with CKD showing that less than 1% received an SGLT2 inhibitor and less than 1% a GLP-1 receptor agonist. Not all these patients had diabetes, but a subgroup analysis of those with diabetes, prediabetes, or hypertension showed that usage of each of these two classes remained at less than 1% even in this group.
A separate report at the ADA meeting documented that of more than 1.3 million people with type 2 diabetes in the U.S. Veterans Affairs Healthcare System during 2019 and 2020, just 10% received an SGLT2 inhibitor and 7% a GLP-1 receptor agonist. And this is in a setting where drug cost is not a limiting factor.
In addition to focusing on the updated scheme for drug intervention in the consensus statement, Dr. Rossing highlighted several other important points that the writing committee emphasized.
Lifestyle optimization is a core first-line element of managing patients with diabetes and CKD, including a healthy diet, exercise, smoking cessation, and weight control. Other key steps for management include optimization of blood pressure, glucose, and lipids. The statement also calls out a potentially helpful role for continuous glucose monitoring in patients with type 1 or type 2 diabetes and CKD.
The statement notes that patients who also have atherosclerotic cardiovascular disease usually qualify for and could potentially benefit from more intensified lipid management with ezetimibe or a PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor, as well as a potential role for treatment with antiplatelet agents.
‘If you don’t screen, you won’t find it’
Dr. Rossing also stressed the importance of regular screening for the onset of advanced CKD in patients. Patients whose estimated glomerular filtration rate (eGFR) drops below 60 mL/min/1.73m2, as well as those who develop microalbuminuria with a urinary albumin-to-creatinine ratio of at least 30 mg/g (30 mg/mmol), have a stage of CKD that warrants the drug interventions he outlined.
Guidelines from both the ADA and KDIGO were already in place, recommending annual screening of patients with diabetes for both these parameters starting at diagnosis of type 2 diabetes or 5 years following initial diagnosis of type 1 diabetes.
“If you don’t screen, you won’t find it, and you won’t be able to treat,” Dr. Rossing warned. He also highlighted the panel’s recommendation to treat these patients with an SGLT2 inhibitor as long as their eGFR is at least 20 mL/min/1.73m2. Treatment can then continue even when their eGFR drops lower.
Starting treatment with finerenone requires that patients have a normal level of serum potassium, he emphasized.
One reason for developing the new ADA and KDIGO statement is that “discrepancies in clinical practice guideline recommendations from various professional organizations add to confusion that impedes understanding of best practices,” write Katherine R. Tuttle, MD, and associates in a recent commentary.
The goal of the new statement is to harmonize and promote the shared recommendations of the two organizations, added Dr. Tuttle, who is executive director for research at Providence Healthcare, Spokane, Washington, and a KDIGO representative on the statement writing panel.
Dr. Mottl has reported being a consultant to Bayer. Dr. Rossing has reported being a consultant to or speaker on behalf of Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Gilead, MSD, Mundipharma, Novo Nordisk, Sanofi Aventis, and Vifor, as well as receiving research grants from AstraZeneca and Novo Nordisk. Dr. Coresh has reported no relevant financial relationships. Dr. Tuttle has reported being a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Goldfinch Bio, Janssen, Novo Nordisk, and Travere; receiving honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, Goldfinch Bio, Novo Nordisk, and Travere; and receiving research funding from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Gilead, Goldfinch Bio, Novo Nordisk, and Travere.
A version of this article first appeared on Medscape.com.
AT ADA 2022
Diabetes tied to risk of long COVID, too
Individuals with diabetes who experience COVID-19 are at increased risk for long COVID compared to individuals without diabetes, according to data from a literature review of seven studies.
Diabetes remains a risk factor for severe COVID-19, but whether it is a risk factor for postacute sequelae of COVID-19 (PASC), also known as long COVID, remains unclear, Jessica L. Harding, PhD, of Emory University, said in a late-breaking poster session at the annual scientific sessions of the American Diabetes Association.
Long COVID is generally defined as “sequelae that extend beyond the 4 weeks after initial infection” and may include a range of symptoms that affect multiple organs, Dr. Harding said. A study conducted in January of 2022 suggested that type 2 diabetes was one of several strong risk factors for long COVID, she noted.
Dr. Harding and colleagues reviewed data from seven studies published from Jan. 1, 2020, to Jan. 27, 2022, on the risk of PASC in people with and without diabetes. The studies included patients with a minimum of 4 weeks’ follow-up after COVID-19 diagnosis. All seven studies had a longitudinal cohort design, and included adults from high-income countries, with study populations ranging from 104 to 4,182.
Across the studies, long COVID definitions varied, but included ongoing symptoms of fatigue, cough, and dyspnea, with follow-up periods of 4 weeks to 7 months.
Overall, three of the seven studies indicated that diabetes was a risk factor for long COVID (odds ratio [OR] greater than 4 for all) and four studies indicated that diabetes was not a risk factor for long COVID (OR, 0.5-2.2).
One of the three studies showing increased risk included 2,334 individuals hospitalized with COVID-19; of these about 5% had diabetes. The odds ratio for PASC for individuals with diabetes was 4.18. In another study of 209 persons with COVID-19, of whom 22% had diabetes, diabetes was significantly correlated with respiratory viral disease (meaning at least two respiratory symptoms). The third study showing an increased risk of long COVID in diabetes patients included 104 kidney transplant patients, of whom 20% had diabetes; the odds ratio for PASC was 4.42.
The findings were limited by several factors, including the relatively small number of studies and the heterogeneity of studies regarding definitions of long COVID, specific populations at risk, follow-up times, and risk adjustment, Dr. Harding noted.
More high-quality studies across multiple populations and settings are needed to determine if diabetes is indeed a risk factor for long COVID, she said.
In the meantime, “careful monitoring of people with diabetes for development of PASC may be advised,” Dr. Harding concluded.
Findings support need for screening
“Given the devastating impact of COVID on people with diabetes, it’s important to know what data has been accumulated on long COVID for future research and discoveries in this area,” Robert A. Gabbay, MD, chief science and medical officer for the American Diabetes Association, said in an interview. “The more information we have, the better we can understand the implications.”
Dr. Gabbay said he was surprised by the current study findings. “We know very little on this subject, so yes, I am surprised to see just how significant the risk of long COVID for people with diabetes seems to be, but clearly, more research needs to be done to understand long COVID,” he emphasized.
The take-home message for clinicians is the importance of screening patients for PASC; also “ask your patients if they had COVID, to better understand any symptoms they might have that could be related to PACS,” he noted.
“It is crucial that we confirm these results and then look at risk factors in people with diabetes that might explain who is at highest risk and ultimately understand the causes and potential cure,” Dr. Gabbay added.
The study was supported by the National Heart, Lung, and Blood Institute. Dr. Harding and Dr. Gabbay had no financial conflicts to disclose.
Individuals with diabetes who experience COVID-19 are at increased risk for long COVID compared to individuals without diabetes, according to data from a literature review of seven studies.
Diabetes remains a risk factor for severe COVID-19, but whether it is a risk factor for postacute sequelae of COVID-19 (PASC), also known as long COVID, remains unclear, Jessica L. Harding, PhD, of Emory University, said in a late-breaking poster session at the annual scientific sessions of the American Diabetes Association.
Long COVID is generally defined as “sequelae that extend beyond the 4 weeks after initial infection” and may include a range of symptoms that affect multiple organs, Dr. Harding said. A study conducted in January of 2022 suggested that type 2 diabetes was one of several strong risk factors for long COVID, she noted.
Dr. Harding and colleagues reviewed data from seven studies published from Jan. 1, 2020, to Jan. 27, 2022, on the risk of PASC in people with and without diabetes. The studies included patients with a minimum of 4 weeks’ follow-up after COVID-19 diagnosis. All seven studies had a longitudinal cohort design, and included adults from high-income countries, with study populations ranging from 104 to 4,182.
Across the studies, long COVID definitions varied, but included ongoing symptoms of fatigue, cough, and dyspnea, with follow-up periods of 4 weeks to 7 months.
Overall, three of the seven studies indicated that diabetes was a risk factor for long COVID (odds ratio [OR] greater than 4 for all) and four studies indicated that diabetes was not a risk factor for long COVID (OR, 0.5-2.2).
One of the three studies showing increased risk included 2,334 individuals hospitalized with COVID-19; of these about 5% had diabetes. The odds ratio for PASC for individuals with diabetes was 4.18. In another study of 209 persons with COVID-19, of whom 22% had diabetes, diabetes was significantly correlated with respiratory viral disease (meaning at least two respiratory symptoms). The third study showing an increased risk of long COVID in diabetes patients included 104 kidney transplant patients, of whom 20% had diabetes; the odds ratio for PASC was 4.42.
The findings were limited by several factors, including the relatively small number of studies and the heterogeneity of studies regarding definitions of long COVID, specific populations at risk, follow-up times, and risk adjustment, Dr. Harding noted.
More high-quality studies across multiple populations and settings are needed to determine if diabetes is indeed a risk factor for long COVID, she said.
In the meantime, “careful monitoring of people with diabetes for development of PASC may be advised,” Dr. Harding concluded.
Findings support need for screening
“Given the devastating impact of COVID on people with diabetes, it’s important to know what data has been accumulated on long COVID for future research and discoveries in this area,” Robert A. Gabbay, MD, chief science and medical officer for the American Diabetes Association, said in an interview. “The more information we have, the better we can understand the implications.”
Dr. Gabbay said he was surprised by the current study findings. “We know very little on this subject, so yes, I am surprised to see just how significant the risk of long COVID for people with diabetes seems to be, but clearly, more research needs to be done to understand long COVID,” he emphasized.
The take-home message for clinicians is the importance of screening patients for PASC; also “ask your patients if they had COVID, to better understand any symptoms they might have that could be related to PACS,” he noted.
“It is crucial that we confirm these results and then look at risk factors in people with diabetes that might explain who is at highest risk and ultimately understand the causes and potential cure,” Dr. Gabbay added.
The study was supported by the National Heart, Lung, and Blood Institute. Dr. Harding and Dr. Gabbay had no financial conflicts to disclose.
Individuals with diabetes who experience COVID-19 are at increased risk for long COVID compared to individuals without diabetes, according to data from a literature review of seven studies.
Diabetes remains a risk factor for severe COVID-19, but whether it is a risk factor for postacute sequelae of COVID-19 (PASC), also known as long COVID, remains unclear, Jessica L. Harding, PhD, of Emory University, said in a late-breaking poster session at the annual scientific sessions of the American Diabetes Association.
Long COVID is generally defined as “sequelae that extend beyond the 4 weeks after initial infection” and may include a range of symptoms that affect multiple organs, Dr. Harding said. A study conducted in January of 2022 suggested that type 2 diabetes was one of several strong risk factors for long COVID, she noted.
Dr. Harding and colleagues reviewed data from seven studies published from Jan. 1, 2020, to Jan. 27, 2022, on the risk of PASC in people with and without diabetes. The studies included patients with a minimum of 4 weeks’ follow-up after COVID-19 diagnosis. All seven studies had a longitudinal cohort design, and included adults from high-income countries, with study populations ranging from 104 to 4,182.
Across the studies, long COVID definitions varied, but included ongoing symptoms of fatigue, cough, and dyspnea, with follow-up periods of 4 weeks to 7 months.
Overall, three of the seven studies indicated that diabetes was a risk factor for long COVID (odds ratio [OR] greater than 4 for all) and four studies indicated that diabetes was not a risk factor for long COVID (OR, 0.5-2.2).
One of the three studies showing increased risk included 2,334 individuals hospitalized with COVID-19; of these about 5% had diabetes. The odds ratio for PASC for individuals with diabetes was 4.18. In another study of 209 persons with COVID-19, of whom 22% had diabetes, diabetes was significantly correlated with respiratory viral disease (meaning at least two respiratory symptoms). The third study showing an increased risk of long COVID in diabetes patients included 104 kidney transplant patients, of whom 20% had diabetes; the odds ratio for PASC was 4.42.
The findings were limited by several factors, including the relatively small number of studies and the heterogeneity of studies regarding definitions of long COVID, specific populations at risk, follow-up times, and risk adjustment, Dr. Harding noted.
More high-quality studies across multiple populations and settings are needed to determine if diabetes is indeed a risk factor for long COVID, she said.
In the meantime, “careful monitoring of people with diabetes for development of PASC may be advised,” Dr. Harding concluded.
Findings support need for screening
“Given the devastating impact of COVID on people with diabetes, it’s important to know what data has been accumulated on long COVID for future research and discoveries in this area,” Robert A. Gabbay, MD, chief science and medical officer for the American Diabetes Association, said in an interview. “The more information we have, the better we can understand the implications.”
Dr. Gabbay said he was surprised by the current study findings. “We know very little on this subject, so yes, I am surprised to see just how significant the risk of long COVID for people with diabetes seems to be, but clearly, more research needs to be done to understand long COVID,” he emphasized.
The take-home message for clinicians is the importance of screening patients for PASC; also “ask your patients if they had COVID, to better understand any symptoms they might have that could be related to PACS,” he noted.
“It is crucial that we confirm these results and then look at risk factors in people with diabetes that might explain who is at highest risk and ultimately understand the causes and potential cure,” Dr. Gabbay added.
The study was supported by the National Heart, Lung, and Blood Institute. Dr. Harding and Dr. Gabbay had no financial conflicts to disclose.
FROM ADA 2022
Exercise of any type boosts type 1 diabetes time in range
Adults with type 1 diabetes had significantly better glycemic control on days they exercised, regardless of exercise type, compared to days when they were inactive, according to a prospective study in nearly 500 individuals.
Different types of exercise, such as aerobic workouts, interval training, or resistance training, may have different immediate glycemic effects in adults with type 1 diabetes (T1D), but the impact of exercise type on the percentage of time diabetes patients maintain glucose in the 70-180 mg/dL range on days when they are active vs. inactive has not been well studied, Zoey Li said in a presentation at the annual scientific sessions of the American Diabetes Association.
In the Type 1 Diabetes Exercise Initiative (T1DEXI) study, Ms. Li and colleagues examined continuous glucose monitoring (CGM) data from 497 adults with T1D. The observational study included self-referred adults aged 18 years and older who had been living with T1D for at least 2 years. Participants were assigned to programs of aerobic exercise (defined as a target heart rate of 70%-80% of age-predicted maximum), interval exercise (defined as an interval heart rate of 80%-90% of age-predicted maximum), or resistance exercise (defined as muscle group fatigue after three sets of eight repetitions).
Participants completed the workouts at home via 30-minute videos at least six times over the 4-week study period. The study design involved an activity goal of at least 150 minutes per week, including the videos and self-reported usual activity, such as walking. The data were collected through an app designed for the study, a heart rate monitor, and a CGM.
The researchers compared glucose levels on days when the participants reported being active compared to days when they were sedentary. The goal of the study was to assess the effect of exercise type on time spent with glucose in the range of 70-180 mg/dL, defined as time in range (TIR).
The mean age of the participants was 37 years; 89% were White. The mean duration of diabetes was 18 years, and the mean hemoglobin A1c was 6.6%. “An astounding 95% were current continuous glucose monitoring [CGM] users,” said Ms. Li, a statistician at the Jaeb Center for Health Research in Tampa, Fla.
A total of 398 participants reported at least one exercise day and one sedentary day, for a total of 1,302 exercise days and 2,470 sedentary days.
Overall, the mean TIR was significantly higher on exercise days compared to sedentary days (75% vs. 70%, P < .001). The median time above 180 mg/dL also was significantly lower on exercise days compared to sedentary days (17% vs. 23%, P < .001), and mean glucose levels were 10 mg/dL lower on exercise days (145 mg/dL vs. 155 mg/dL)
“This all came with a slight hit to their time below range,” Ms. Li noted. The median time below 70 mg/dL was 1.1% on exercise days compared to 0.4% on sedentary days (P < .001). The percentage of days with hypoglycemic events was higher on exercise days compared to sedentary days (47% vs. 40%, P < .001), as they are related to time below 70 mg/dL, she added.
The differences for mean glucose level and TIR between exercise days and sedentary days were significant for each of the three exercise types, Ms. Li said.
“After establishing these glycemic trends, we looked at whether there were any factors that influenced the glycemic differences on exercise vs. sedentary days,” Ms. Li said.
Regardless of exercise type, age, sex, baseline A1c, diabetes duration, body mass index, insulin modality, CGM use, and percentage of time below range in the past 24 hours, there was higher TIR and higher hypoglycemia on exercise days compared to sedentary days.
Although the study was limited in part by the observational design, “with these data, we can better understand the glycemic benefits and disadvantages of exercise in adults with type 1 diabetes,” Ms. Li said.
Don’t forget the negative effects of exercise
“It is well known that the three types of exercise can modulate glucose levels. This can be very useful when attempting to reduce excessively high glucose levels, and when encouraging people to engage in frequent, regular, and consistent physical activity and exercise for general cardiovascular pulmonary and musculoskeletal health,” Helena W. Rodbard, MD, an endocrinologist in private practice in Rockville, Md., said in an interview.
“However, it was not known what effects various types of exercise would have on time in range (70-180 mg/dL) and time below range (< 70 mg/dL) measured over a full 24-hour period in people with type 1 diabetes,” said Dr. Rodbard, who was not involved with the study.
“I was surprised to see that the effect of the three different types of exercise were so similar,” Dr. Rodbard noted. “There had been previous reports suggesting that the time course of glucose could be different for these three types of exercise.”
The current study confirms prior knowledge that exercise can help reduce blood glucose, and increase TIR, said Dr. Rodbard. The study shows that TIR increases by roughly 5-7 percentage points (about 1 hour per day) and reduces mean glucose by 9-13 mg/dL irrespective of the three types of exercise,” she said. “There was a suggestion that the risk of increasing hypoglycemia below 70 mg/dL was less likely for resistance exercise than for the interval or aerobic types of exercise,” she noted.
As for additional research, “This study did not address the various ways in which one can mitigate the potentially deleterious effects of exercise, specifically with reference to rates of hypoglycemia, even mild symptomatic biochemical hypoglycemia,” said Dr. Rodbard. “Since the actual amount of time below 70 mg/dL is usually so small (0.3%-0.7% of the 1,440 minutes in the day, or about 5-10 minutes per day on average), it is difficult to measure and there is considerable variability between different people,” she emphasized. “Finding optimal and robust ways to achieve consistency in the reduction of glucose, between days within subjects, and between subjects, will need further examination of various types of protocols for diet, exercise and insulin administration, and of various methods for education of the patient,” she said.
The study was supported in part by the Leona M. and Harry B. Helmsley Charitable Trust. Ms. Li and Dr. Rodbard had no financial conflicts to disclose. Dr. Rodbard serves on the editorial advisory board of Clinical Endocrinology News.
Adults with type 1 diabetes had significantly better glycemic control on days they exercised, regardless of exercise type, compared to days when they were inactive, according to a prospective study in nearly 500 individuals.
Different types of exercise, such as aerobic workouts, interval training, or resistance training, may have different immediate glycemic effects in adults with type 1 diabetes (T1D), but the impact of exercise type on the percentage of time diabetes patients maintain glucose in the 70-180 mg/dL range on days when they are active vs. inactive has not been well studied, Zoey Li said in a presentation at the annual scientific sessions of the American Diabetes Association.
In the Type 1 Diabetes Exercise Initiative (T1DEXI) study, Ms. Li and colleagues examined continuous glucose monitoring (CGM) data from 497 adults with T1D. The observational study included self-referred adults aged 18 years and older who had been living with T1D for at least 2 years. Participants were assigned to programs of aerobic exercise (defined as a target heart rate of 70%-80% of age-predicted maximum), interval exercise (defined as an interval heart rate of 80%-90% of age-predicted maximum), or resistance exercise (defined as muscle group fatigue after three sets of eight repetitions).
Participants completed the workouts at home via 30-minute videos at least six times over the 4-week study period. The study design involved an activity goal of at least 150 minutes per week, including the videos and self-reported usual activity, such as walking. The data were collected through an app designed for the study, a heart rate monitor, and a CGM.
The researchers compared glucose levels on days when the participants reported being active compared to days when they were sedentary. The goal of the study was to assess the effect of exercise type on time spent with glucose in the range of 70-180 mg/dL, defined as time in range (TIR).
The mean age of the participants was 37 years; 89% were White. The mean duration of diabetes was 18 years, and the mean hemoglobin A1c was 6.6%. “An astounding 95% were current continuous glucose monitoring [CGM] users,” said Ms. Li, a statistician at the Jaeb Center for Health Research in Tampa, Fla.
A total of 398 participants reported at least one exercise day and one sedentary day, for a total of 1,302 exercise days and 2,470 sedentary days.
Overall, the mean TIR was significantly higher on exercise days compared to sedentary days (75% vs. 70%, P < .001). The median time above 180 mg/dL also was significantly lower on exercise days compared to sedentary days (17% vs. 23%, P < .001), and mean glucose levels were 10 mg/dL lower on exercise days (145 mg/dL vs. 155 mg/dL)
“This all came with a slight hit to their time below range,” Ms. Li noted. The median time below 70 mg/dL was 1.1% on exercise days compared to 0.4% on sedentary days (P < .001). The percentage of days with hypoglycemic events was higher on exercise days compared to sedentary days (47% vs. 40%, P < .001), as they are related to time below 70 mg/dL, she added.
The differences for mean glucose level and TIR between exercise days and sedentary days were significant for each of the three exercise types, Ms. Li said.
“After establishing these glycemic trends, we looked at whether there were any factors that influenced the glycemic differences on exercise vs. sedentary days,” Ms. Li said.
Regardless of exercise type, age, sex, baseline A1c, diabetes duration, body mass index, insulin modality, CGM use, and percentage of time below range in the past 24 hours, there was higher TIR and higher hypoglycemia on exercise days compared to sedentary days.
Although the study was limited in part by the observational design, “with these data, we can better understand the glycemic benefits and disadvantages of exercise in adults with type 1 diabetes,” Ms. Li said.
Don’t forget the negative effects of exercise
“It is well known that the three types of exercise can modulate glucose levels. This can be very useful when attempting to reduce excessively high glucose levels, and when encouraging people to engage in frequent, regular, and consistent physical activity and exercise for general cardiovascular pulmonary and musculoskeletal health,” Helena W. Rodbard, MD, an endocrinologist in private practice in Rockville, Md., said in an interview.
“However, it was not known what effects various types of exercise would have on time in range (70-180 mg/dL) and time below range (< 70 mg/dL) measured over a full 24-hour period in people with type 1 diabetes,” said Dr. Rodbard, who was not involved with the study.
“I was surprised to see that the effect of the three different types of exercise were so similar,” Dr. Rodbard noted. “There had been previous reports suggesting that the time course of glucose could be different for these three types of exercise.”
The current study confirms prior knowledge that exercise can help reduce blood glucose, and increase TIR, said Dr. Rodbard. The study shows that TIR increases by roughly 5-7 percentage points (about 1 hour per day) and reduces mean glucose by 9-13 mg/dL irrespective of the three types of exercise,” she said. “There was a suggestion that the risk of increasing hypoglycemia below 70 mg/dL was less likely for resistance exercise than for the interval or aerobic types of exercise,” she noted.
As for additional research, “This study did not address the various ways in which one can mitigate the potentially deleterious effects of exercise, specifically with reference to rates of hypoglycemia, even mild symptomatic biochemical hypoglycemia,” said Dr. Rodbard. “Since the actual amount of time below 70 mg/dL is usually so small (0.3%-0.7% of the 1,440 minutes in the day, or about 5-10 minutes per day on average), it is difficult to measure and there is considerable variability between different people,” she emphasized. “Finding optimal and robust ways to achieve consistency in the reduction of glucose, between days within subjects, and between subjects, will need further examination of various types of protocols for diet, exercise and insulin administration, and of various methods for education of the patient,” she said.
The study was supported in part by the Leona M. and Harry B. Helmsley Charitable Trust. Ms. Li and Dr. Rodbard had no financial conflicts to disclose. Dr. Rodbard serves on the editorial advisory board of Clinical Endocrinology News.
Adults with type 1 diabetes had significantly better glycemic control on days they exercised, regardless of exercise type, compared to days when they were inactive, according to a prospective study in nearly 500 individuals.
Different types of exercise, such as aerobic workouts, interval training, or resistance training, may have different immediate glycemic effects in adults with type 1 diabetes (T1D), but the impact of exercise type on the percentage of time diabetes patients maintain glucose in the 70-180 mg/dL range on days when they are active vs. inactive has not been well studied, Zoey Li said in a presentation at the annual scientific sessions of the American Diabetes Association.
In the Type 1 Diabetes Exercise Initiative (T1DEXI) study, Ms. Li and colleagues examined continuous glucose monitoring (CGM) data from 497 adults with T1D. The observational study included self-referred adults aged 18 years and older who had been living with T1D for at least 2 years. Participants were assigned to programs of aerobic exercise (defined as a target heart rate of 70%-80% of age-predicted maximum), interval exercise (defined as an interval heart rate of 80%-90% of age-predicted maximum), or resistance exercise (defined as muscle group fatigue after three sets of eight repetitions).
Participants completed the workouts at home via 30-minute videos at least six times over the 4-week study period. The study design involved an activity goal of at least 150 minutes per week, including the videos and self-reported usual activity, such as walking. The data were collected through an app designed for the study, a heart rate monitor, and a CGM.
The researchers compared glucose levels on days when the participants reported being active compared to days when they were sedentary. The goal of the study was to assess the effect of exercise type on time spent with glucose in the range of 70-180 mg/dL, defined as time in range (TIR).
The mean age of the participants was 37 years; 89% were White. The mean duration of diabetes was 18 years, and the mean hemoglobin A1c was 6.6%. “An astounding 95% were current continuous glucose monitoring [CGM] users,” said Ms. Li, a statistician at the Jaeb Center for Health Research in Tampa, Fla.
A total of 398 participants reported at least one exercise day and one sedentary day, for a total of 1,302 exercise days and 2,470 sedentary days.
Overall, the mean TIR was significantly higher on exercise days compared to sedentary days (75% vs. 70%, P < .001). The median time above 180 mg/dL also was significantly lower on exercise days compared to sedentary days (17% vs. 23%, P < .001), and mean glucose levels were 10 mg/dL lower on exercise days (145 mg/dL vs. 155 mg/dL)
“This all came with a slight hit to their time below range,” Ms. Li noted. The median time below 70 mg/dL was 1.1% on exercise days compared to 0.4% on sedentary days (P < .001). The percentage of days with hypoglycemic events was higher on exercise days compared to sedentary days (47% vs. 40%, P < .001), as they are related to time below 70 mg/dL, she added.
The differences for mean glucose level and TIR between exercise days and sedentary days were significant for each of the three exercise types, Ms. Li said.
“After establishing these glycemic trends, we looked at whether there were any factors that influenced the glycemic differences on exercise vs. sedentary days,” Ms. Li said.
Regardless of exercise type, age, sex, baseline A1c, diabetes duration, body mass index, insulin modality, CGM use, and percentage of time below range in the past 24 hours, there was higher TIR and higher hypoglycemia on exercise days compared to sedentary days.
Although the study was limited in part by the observational design, “with these data, we can better understand the glycemic benefits and disadvantages of exercise in adults with type 1 diabetes,” Ms. Li said.
Don’t forget the negative effects of exercise
“It is well known that the three types of exercise can modulate glucose levels. This can be very useful when attempting to reduce excessively high glucose levels, and when encouraging people to engage in frequent, regular, and consistent physical activity and exercise for general cardiovascular pulmonary and musculoskeletal health,” Helena W. Rodbard, MD, an endocrinologist in private practice in Rockville, Md., said in an interview.
“However, it was not known what effects various types of exercise would have on time in range (70-180 mg/dL) and time below range (< 70 mg/dL) measured over a full 24-hour period in people with type 1 diabetes,” said Dr. Rodbard, who was not involved with the study.
“I was surprised to see that the effect of the three different types of exercise were so similar,” Dr. Rodbard noted. “There had been previous reports suggesting that the time course of glucose could be different for these three types of exercise.”
The current study confirms prior knowledge that exercise can help reduce blood glucose, and increase TIR, said Dr. Rodbard. The study shows that TIR increases by roughly 5-7 percentage points (about 1 hour per day) and reduces mean glucose by 9-13 mg/dL irrespective of the three types of exercise,” she said. “There was a suggestion that the risk of increasing hypoglycemia below 70 mg/dL was less likely for resistance exercise than for the interval or aerobic types of exercise,” she noted.
As for additional research, “This study did not address the various ways in which one can mitigate the potentially deleterious effects of exercise, specifically with reference to rates of hypoglycemia, even mild symptomatic biochemical hypoglycemia,” said Dr. Rodbard. “Since the actual amount of time below 70 mg/dL is usually so small (0.3%-0.7% of the 1,440 minutes in the day, or about 5-10 minutes per day on average), it is difficult to measure and there is considerable variability between different people,” she emphasized. “Finding optimal and robust ways to achieve consistency in the reduction of glucose, between days within subjects, and between subjects, will need further examination of various types of protocols for diet, exercise and insulin administration, and of various methods for education of the patient,” she said.
The study was supported in part by the Leona M. and Harry B. Helmsley Charitable Trust. Ms. Li and Dr. Rodbard had no financial conflicts to disclose. Dr. Rodbard serves on the editorial advisory board of Clinical Endocrinology News.
FROM ADA 2022
‘DIY’ artificial pancreas systems found to be safe, effective: CREATE trial
NEW ORLEANS – Open-source automated insulin delivery systems appear to be both effective and safe in adults and children, new research finds.
Automated insulin delivery (AID) system, also known as closed-loop systems or an artificial pancreas, link an insulin pump and a continuous glucose monitor (CGM) with an algorithm that automatically adjusts insulin delivery to optimize glycemic control.
Prior to the availability of commercial AID systems, Dana Lewis, a patient with type 1 diabetes, and her partner codeveloped an algorithm that could link older versions of an insulin pump and CGM.
In 2015, they made the code and all related materials open-source, so that anyone who wanted to create their own AID system could do so. Today thousands of people worldwide with type 1 diabetes are using the systems, which are sometimes called “do-it-yourself (DIY)” AID systems although the approach has been community based.
AID systems are not approved by any regulatory body, and despite several nonrandomized studies demonstrating their effectiveness and safety, there is still concern among some health professionals about their safety. In 2019, the U.S. Food and Drug Administration warned against the use of any nonapproved devices or algorithms. (Now, though, at least one open-source AID system algorithm is under FDA review.)
Aimed at addressing those concerns, CREATE (Community Derived Automated Insulin Delivery) is the first randomized controlled clinical trial to compare an open-source AID system to insulin pump therapy and CGM (without any communication between the two) in patients with type 1 diabetes, most of whom were naive to AID systems.
Doctors uncomfortable with open source; study provides reassurance
The findings were presented at the American Diabetes Association scientific sessions by Martin I. de Bock, PhD, a pediatric endocrinologist and senior lecturer at the University of Otago, Christchurch, New Zealand.
The study compared the most commonly used open-source AID system (using the OpenAPS algorithm from a version of AndroidAPS implemented in a smartphone with the DANA-i insulin pump and Dexcom G6 CGM) to any insulin pump plus CGM as a comparator group.
The open-source AID system led to a significant reduction in hemoglobin A1c with no major safety issues.
“The acceptance [among clinicians] of open-source systems is diverse and complicated, [with varying] personal comfort levels of seeing someone using an AID system that has no regulatory approval,” Dr. de Bock told this news organization.
“This is one of the reasons that it was so important to conduct the CREATE trial for the many thousands of open-source AID users. Given that the trial demonstrated safety and efficacy using the most robust scientific methodology available – a long-term randomized controlled trial – it may go some way to provide assurance for providers when they are seeing people using an open-source automated system,” he said.
Asked for comment, session moderator Diana Isaacs, PharmD, CDCES, an endocrine clinical pharmacist at the Cleveland Clinic, told this news organization: “There has been concern that these systems aren’t safe, so showing the safety is important. I think people deserve choice. As long as they’re safe, patients should be able to use what they want to use, and we should support them.”
Dr. Isaacs pointed out that an advantage of open-source systems over current commercial AIDs for patients is the ability to customize glucose targets, but in CREATE, those targets were established in the protocol by the investigators.
“I think it’s nice having the data, although in the trial they had specific requirements. They had a target range and active insulin time that they were recommending. So it’s a little different than true DIY where you don’t really have those guidelines you have to follow. It is exciting, it’s very interesting, but I wouldn’t say it’s a true mirror of the real world.”
Open-source systems improved time-in-range, no safety issues
For the CREATE study, 100 participants were enrolled, including 50 children aged 7-15 years and 50 adults aged 16-70 years. All participants had been using insulin pumps for at least 6 months. Most of the children and about two-thirds of the adults were also using CGMs, but just 6% of the children and 18% of the adults had prior experience with AID systems.
Baseline A1c in children was 7.5% and in adults was 7.7%.
After a 4-week run-in, all patients were randomized to the open-source AID or insulin pump plus CGM for 6 months.
The final group analyzed consisted of 42 patients in the open-source AID group and 53 patients in the comparator group.
The primary outcome, the adjusted mean difference in percent time-in-range (glucose of 70-180 mg/dL) during the final 2 weeks of the 6-month trial, showed a significant difference of 14% (P < .001) with open-source AID compared with pump plus CGM only.
Time-in-range in the open-source AID group rose from 61.2% to 71.2%, while it actually dropped slightly in the comparator group, from 57.7% to 54.5%.
The proportion of patients achieving time-in-range greater than 70% with open-source AID was 60% versus just 15% with pump plus CGM.
Glycemic improvements with open-source AID were significant for adults and children and were greater for those with higher baseline A1c levels. The effect was immediate and sustained throughout the study period, “which is super-pleasing, because there was a worry that the technical burden of open source might be [leading to] dropout, but we didn’t see that. It was sustained right through to the end of the trial,” Dr. de Bock commented.
Hypoglycemic rates didn’t differ between groups, and there were no episodes of severe hypoglycemia or diabetic ketoacidosis.
No more waiting: What is the future of open-source AID?
When the open-source APS was first developed, users coined the motto: “We are not waiting.” But now that the “wait” is over and several commercial AIDs have been approved by regulatory bodies, with others still in the pipeline, will people still use open-source systems?
There are no current data on people moving from DIY to commercial systems. However, Dr. de Bock said, “For most who undertook an open-source option, the precision of the settings that they can use and enjoy would mean that most would likely stick to their open source.”
Dr. Isaacs agrees: “I actually don’t think it’s going to go away in the near future, because the FDA has very specific criteria for where these [formally approved] devices can be in terms of their target ranges and requirements versus with open source you can really customize. So I still think there’s going to be a subset of people who want that customization, who want the lower targets.”
Dana Lewis, the originator of the DIY system and a CREATE coauthor, told this news organization: “I don’t believe there has been a fall-off, and in fact, I think open-source AID has continued to have ongoing uptake as awareness increases about options and as more pumps and CGMs become interoperable with various open-source AID choices.”
“I think uptake increasing is also influenced by the fact that in places like Europe, Asia, and Australia there are in-warranty on-the-market pumps that are compatible and interoperable with open-source AID. I think awareness of AID overall increases uptake of commercial and open source alike,” she said.
“Clinicians, as emphasized in recent position statements, must maintain support of the person with diabetes, irrespective of the mode of treatment they are on. ... Health care providers should be encouraged to learn from the experiences of the people who have stuck with open-source AID or switched, so that they can inform themselves of the relative strengths and benefits of each system,” Dr. de Bock advised.
Ms. Lewis noted: “We are seeing increasing awareness and comfort in endocrinologists from the community perspective, and we do hope that this study helps increase conversation and awareness of the safety and efficacy of open-source AID systems as an option for people with diabetes.”
In fact, the team published an article specifically about clinicians’ experience in CREATE. “The learning curve is similar across AID technology,” she observed.
Findings of a 6-month continuation phase of CREATE, in which all participants used the open-source AID, are scheduled to be presented in September at the European Association for the Study of Diabetes annual meeting.
The study was funded by the Health Research Council of New Zealand, with hardware support from SOOIL Developments, South Korea; Dexcom; and Vodafone New Zealand. Dr. de Bock has reported receiving honoraria and/or research funding from Novo Nordisk, Sanofi, Pfizer, Medtronic, Lilly, Ypsomed, and Dexcom. Dr. Isaacs has reported serving as a consultant for LifeScan, Lilly, and Insulet, and as a speaker for Dexcom, Medtronic, Abbott, and Novo Nordisk. Ms. Lewis has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – Open-source automated insulin delivery systems appear to be both effective and safe in adults and children, new research finds.
Automated insulin delivery (AID) system, also known as closed-loop systems or an artificial pancreas, link an insulin pump and a continuous glucose monitor (CGM) with an algorithm that automatically adjusts insulin delivery to optimize glycemic control.
Prior to the availability of commercial AID systems, Dana Lewis, a patient with type 1 diabetes, and her partner codeveloped an algorithm that could link older versions of an insulin pump and CGM.
In 2015, they made the code and all related materials open-source, so that anyone who wanted to create their own AID system could do so. Today thousands of people worldwide with type 1 diabetes are using the systems, which are sometimes called “do-it-yourself (DIY)” AID systems although the approach has been community based.
AID systems are not approved by any regulatory body, and despite several nonrandomized studies demonstrating their effectiveness and safety, there is still concern among some health professionals about their safety. In 2019, the U.S. Food and Drug Administration warned against the use of any nonapproved devices or algorithms. (Now, though, at least one open-source AID system algorithm is under FDA review.)
Aimed at addressing those concerns, CREATE (Community Derived Automated Insulin Delivery) is the first randomized controlled clinical trial to compare an open-source AID system to insulin pump therapy and CGM (without any communication between the two) in patients with type 1 diabetes, most of whom were naive to AID systems.
Doctors uncomfortable with open source; study provides reassurance
The findings were presented at the American Diabetes Association scientific sessions by Martin I. de Bock, PhD, a pediatric endocrinologist and senior lecturer at the University of Otago, Christchurch, New Zealand.
The study compared the most commonly used open-source AID system (using the OpenAPS algorithm from a version of AndroidAPS implemented in a smartphone with the DANA-i insulin pump and Dexcom G6 CGM) to any insulin pump plus CGM as a comparator group.
The open-source AID system led to a significant reduction in hemoglobin A1c with no major safety issues.
“The acceptance [among clinicians] of open-source systems is diverse and complicated, [with varying] personal comfort levels of seeing someone using an AID system that has no regulatory approval,” Dr. de Bock told this news organization.
“This is one of the reasons that it was so important to conduct the CREATE trial for the many thousands of open-source AID users. Given that the trial demonstrated safety and efficacy using the most robust scientific methodology available – a long-term randomized controlled trial – it may go some way to provide assurance for providers when they are seeing people using an open-source automated system,” he said.
Asked for comment, session moderator Diana Isaacs, PharmD, CDCES, an endocrine clinical pharmacist at the Cleveland Clinic, told this news organization: “There has been concern that these systems aren’t safe, so showing the safety is important. I think people deserve choice. As long as they’re safe, patients should be able to use what they want to use, and we should support them.”
Dr. Isaacs pointed out that an advantage of open-source systems over current commercial AIDs for patients is the ability to customize glucose targets, but in CREATE, those targets were established in the protocol by the investigators.
“I think it’s nice having the data, although in the trial they had specific requirements. They had a target range and active insulin time that they were recommending. So it’s a little different than true DIY where you don’t really have those guidelines you have to follow. It is exciting, it’s very interesting, but I wouldn’t say it’s a true mirror of the real world.”
Open-source systems improved time-in-range, no safety issues
For the CREATE study, 100 participants were enrolled, including 50 children aged 7-15 years and 50 adults aged 16-70 years. All participants had been using insulin pumps for at least 6 months. Most of the children and about two-thirds of the adults were also using CGMs, but just 6% of the children and 18% of the adults had prior experience with AID systems.
Baseline A1c in children was 7.5% and in adults was 7.7%.
After a 4-week run-in, all patients were randomized to the open-source AID or insulin pump plus CGM for 6 months.
The final group analyzed consisted of 42 patients in the open-source AID group and 53 patients in the comparator group.
The primary outcome, the adjusted mean difference in percent time-in-range (glucose of 70-180 mg/dL) during the final 2 weeks of the 6-month trial, showed a significant difference of 14% (P < .001) with open-source AID compared with pump plus CGM only.
Time-in-range in the open-source AID group rose from 61.2% to 71.2%, while it actually dropped slightly in the comparator group, from 57.7% to 54.5%.
The proportion of patients achieving time-in-range greater than 70% with open-source AID was 60% versus just 15% with pump plus CGM.
Glycemic improvements with open-source AID were significant for adults and children and were greater for those with higher baseline A1c levels. The effect was immediate and sustained throughout the study period, “which is super-pleasing, because there was a worry that the technical burden of open source might be [leading to] dropout, but we didn’t see that. It was sustained right through to the end of the trial,” Dr. de Bock commented.
Hypoglycemic rates didn’t differ between groups, and there were no episodes of severe hypoglycemia or diabetic ketoacidosis.
No more waiting: What is the future of open-source AID?
When the open-source APS was first developed, users coined the motto: “We are not waiting.” But now that the “wait” is over and several commercial AIDs have been approved by regulatory bodies, with others still in the pipeline, will people still use open-source systems?
There are no current data on people moving from DIY to commercial systems. However, Dr. de Bock said, “For most who undertook an open-source option, the precision of the settings that they can use and enjoy would mean that most would likely stick to their open source.”
Dr. Isaacs agrees: “I actually don’t think it’s going to go away in the near future, because the FDA has very specific criteria for where these [formally approved] devices can be in terms of their target ranges and requirements versus with open source you can really customize. So I still think there’s going to be a subset of people who want that customization, who want the lower targets.”
Dana Lewis, the originator of the DIY system and a CREATE coauthor, told this news organization: “I don’t believe there has been a fall-off, and in fact, I think open-source AID has continued to have ongoing uptake as awareness increases about options and as more pumps and CGMs become interoperable with various open-source AID choices.”
“I think uptake increasing is also influenced by the fact that in places like Europe, Asia, and Australia there are in-warranty on-the-market pumps that are compatible and interoperable with open-source AID. I think awareness of AID overall increases uptake of commercial and open source alike,” she said.
“Clinicians, as emphasized in recent position statements, must maintain support of the person with diabetes, irrespective of the mode of treatment they are on. ... Health care providers should be encouraged to learn from the experiences of the people who have stuck with open-source AID or switched, so that they can inform themselves of the relative strengths and benefits of each system,” Dr. de Bock advised.
Ms. Lewis noted: “We are seeing increasing awareness and comfort in endocrinologists from the community perspective, and we do hope that this study helps increase conversation and awareness of the safety and efficacy of open-source AID systems as an option for people with diabetes.”
In fact, the team published an article specifically about clinicians’ experience in CREATE. “The learning curve is similar across AID technology,” she observed.
Findings of a 6-month continuation phase of CREATE, in which all participants used the open-source AID, are scheduled to be presented in September at the European Association for the Study of Diabetes annual meeting.
The study was funded by the Health Research Council of New Zealand, with hardware support from SOOIL Developments, South Korea; Dexcom; and Vodafone New Zealand. Dr. de Bock has reported receiving honoraria and/or research funding from Novo Nordisk, Sanofi, Pfizer, Medtronic, Lilly, Ypsomed, and Dexcom. Dr. Isaacs has reported serving as a consultant for LifeScan, Lilly, and Insulet, and as a speaker for Dexcom, Medtronic, Abbott, and Novo Nordisk. Ms. Lewis has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – Open-source automated insulin delivery systems appear to be both effective and safe in adults and children, new research finds.
Automated insulin delivery (AID) system, also known as closed-loop systems or an artificial pancreas, link an insulin pump and a continuous glucose monitor (CGM) with an algorithm that automatically adjusts insulin delivery to optimize glycemic control.
Prior to the availability of commercial AID systems, Dana Lewis, a patient with type 1 diabetes, and her partner codeveloped an algorithm that could link older versions of an insulin pump and CGM.
In 2015, they made the code and all related materials open-source, so that anyone who wanted to create their own AID system could do so. Today thousands of people worldwide with type 1 diabetes are using the systems, which are sometimes called “do-it-yourself (DIY)” AID systems although the approach has been community based.
AID systems are not approved by any regulatory body, and despite several nonrandomized studies demonstrating their effectiveness and safety, there is still concern among some health professionals about their safety. In 2019, the U.S. Food and Drug Administration warned against the use of any nonapproved devices or algorithms. (Now, though, at least one open-source AID system algorithm is under FDA review.)
Aimed at addressing those concerns, CREATE (Community Derived Automated Insulin Delivery) is the first randomized controlled clinical trial to compare an open-source AID system to insulin pump therapy and CGM (without any communication between the two) in patients with type 1 diabetes, most of whom were naive to AID systems.
Doctors uncomfortable with open source; study provides reassurance
The findings were presented at the American Diabetes Association scientific sessions by Martin I. de Bock, PhD, a pediatric endocrinologist and senior lecturer at the University of Otago, Christchurch, New Zealand.
The study compared the most commonly used open-source AID system (using the OpenAPS algorithm from a version of AndroidAPS implemented in a smartphone with the DANA-i insulin pump and Dexcom G6 CGM) to any insulin pump plus CGM as a comparator group.
The open-source AID system led to a significant reduction in hemoglobin A1c with no major safety issues.
“The acceptance [among clinicians] of open-source systems is diverse and complicated, [with varying] personal comfort levels of seeing someone using an AID system that has no regulatory approval,” Dr. de Bock told this news organization.
“This is one of the reasons that it was so important to conduct the CREATE trial for the many thousands of open-source AID users. Given that the trial demonstrated safety and efficacy using the most robust scientific methodology available – a long-term randomized controlled trial – it may go some way to provide assurance for providers when they are seeing people using an open-source automated system,” he said.
Asked for comment, session moderator Diana Isaacs, PharmD, CDCES, an endocrine clinical pharmacist at the Cleveland Clinic, told this news organization: “There has been concern that these systems aren’t safe, so showing the safety is important. I think people deserve choice. As long as they’re safe, patients should be able to use what they want to use, and we should support them.”
Dr. Isaacs pointed out that an advantage of open-source systems over current commercial AIDs for patients is the ability to customize glucose targets, but in CREATE, those targets were established in the protocol by the investigators.
“I think it’s nice having the data, although in the trial they had specific requirements. They had a target range and active insulin time that they were recommending. So it’s a little different than true DIY where you don’t really have those guidelines you have to follow. It is exciting, it’s very interesting, but I wouldn’t say it’s a true mirror of the real world.”
Open-source systems improved time-in-range, no safety issues
For the CREATE study, 100 participants were enrolled, including 50 children aged 7-15 years and 50 adults aged 16-70 years. All participants had been using insulin pumps for at least 6 months. Most of the children and about two-thirds of the adults were also using CGMs, but just 6% of the children and 18% of the adults had prior experience with AID systems.
Baseline A1c in children was 7.5% and in adults was 7.7%.
After a 4-week run-in, all patients were randomized to the open-source AID or insulin pump plus CGM for 6 months.
The final group analyzed consisted of 42 patients in the open-source AID group and 53 patients in the comparator group.
The primary outcome, the adjusted mean difference in percent time-in-range (glucose of 70-180 mg/dL) during the final 2 weeks of the 6-month trial, showed a significant difference of 14% (P < .001) with open-source AID compared with pump plus CGM only.
Time-in-range in the open-source AID group rose from 61.2% to 71.2%, while it actually dropped slightly in the comparator group, from 57.7% to 54.5%.
The proportion of patients achieving time-in-range greater than 70% with open-source AID was 60% versus just 15% with pump plus CGM.
Glycemic improvements with open-source AID were significant for adults and children and were greater for those with higher baseline A1c levels. The effect was immediate and sustained throughout the study period, “which is super-pleasing, because there was a worry that the technical burden of open source might be [leading to] dropout, but we didn’t see that. It was sustained right through to the end of the trial,” Dr. de Bock commented.
Hypoglycemic rates didn’t differ between groups, and there were no episodes of severe hypoglycemia or diabetic ketoacidosis.
No more waiting: What is the future of open-source AID?
When the open-source APS was first developed, users coined the motto: “We are not waiting.” But now that the “wait” is over and several commercial AIDs have been approved by regulatory bodies, with others still in the pipeline, will people still use open-source systems?
There are no current data on people moving from DIY to commercial systems. However, Dr. de Bock said, “For most who undertook an open-source option, the precision of the settings that they can use and enjoy would mean that most would likely stick to their open source.”
Dr. Isaacs agrees: “I actually don’t think it’s going to go away in the near future, because the FDA has very specific criteria for where these [formally approved] devices can be in terms of their target ranges and requirements versus with open source you can really customize. So I still think there’s going to be a subset of people who want that customization, who want the lower targets.”
Dana Lewis, the originator of the DIY system and a CREATE coauthor, told this news organization: “I don’t believe there has been a fall-off, and in fact, I think open-source AID has continued to have ongoing uptake as awareness increases about options and as more pumps and CGMs become interoperable with various open-source AID choices.”
“I think uptake increasing is also influenced by the fact that in places like Europe, Asia, and Australia there are in-warranty on-the-market pumps that are compatible and interoperable with open-source AID. I think awareness of AID overall increases uptake of commercial and open source alike,” she said.
“Clinicians, as emphasized in recent position statements, must maintain support of the person with diabetes, irrespective of the mode of treatment they are on. ... Health care providers should be encouraged to learn from the experiences of the people who have stuck with open-source AID or switched, so that they can inform themselves of the relative strengths and benefits of each system,” Dr. de Bock advised.
Ms. Lewis noted: “We are seeing increasing awareness and comfort in endocrinologists from the community perspective, and we do hope that this study helps increase conversation and awareness of the safety and efficacy of open-source AID systems as an option for people with diabetes.”
In fact, the team published an article specifically about clinicians’ experience in CREATE. “The learning curve is similar across AID technology,” she observed.
Findings of a 6-month continuation phase of CREATE, in which all participants used the open-source AID, are scheduled to be presented in September at the European Association for the Study of Diabetes annual meeting.
The study was funded by the Health Research Council of New Zealand, with hardware support from SOOIL Developments, South Korea; Dexcom; and Vodafone New Zealand. Dr. de Bock has reported receiving honoraria and/or research funding from Novo Nordisk, Sanofi, Pfizer, Medtronic, Lilly, Ypsomed, and Dexcom. Dr. Isaacs has reported serving as a consultant for LifeScan, Lilly, and Insulet, and as a speaker for Dexcom, Medtronic, Abbott, and Novo Nordisk. Ms. Lewis has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ADA 2022
SGLT2 inhibitors cut AFib risk in real-word analysis
NEW ORLEANS – The case continues to grow for prioritizing a sodium-glucose transporter 2 (SGLT2) inhibitor in patients with type 2 diabetes, as real-world evidence of benefit and safety accumulates on top of the data from randomized trials that first established this class as a management pillar.
Another important effect of these agents gaining increasing currency, on top of their well-established benefits in patients with type 2 diabetes for preventing acute heart failure exacerbations and slowing progression of diabetic kidney disease, is that they cut the incidence of new-onset atrial fibrillation (AFib). That effect was confirmed in an analysis of data from about 300,000 U.S. patients included in recent Medicare records, Elisabetta Patorno, MD, reported at the annual scientific sessions of the American Diabetes Association.
But despite documentation like this, real-world evidence also continues to show limited uptake of SGLT2 inhibitors in U.S. patients with type 2 diabetes. Records from more than 1.3 million patients with type 2 diabetes managed in the Veterans Affairs Healthcare System during 2019 or 2022 documented that just 10% of these patients received an agent from this class, even though all were eligible to receive it, according to findings in a separate report at the meeting.
The AFib analysis analyzed two sets of propensity score–matched Medicare patients during 2013-2018 aged 65 years or older with type 2 diabetes and no history of AFib. One analysis focused on 80,475 matched patients who started on treatment with either an SGLT2 inhibitor or a glucagonlike peptide–1 (GLP-1) receptor agonist, and a second on 74,868 matched patients who began either an SGTL2 inhibitor or a dipeptidyl peptidase–4 (DPP4) inhibitor. In both analyses, matching involved more than 130 variables. In both pair sets, patients at baseline averaged about 72 years old, nearly two-thirds were women, about 8%-9% had heart failure, 77%-80% were on metformin, and 20%-25% were using insulin.
The study’s primary endpoint was the incidence of hospitalization for AFib, which occurred a significant 18% less often in the patients who started on an SGLT2, compared with those who started a DPP4 inhibitor during median follow-up of 6.7 months, and a significant 10% less often, compared with those starting a GLP-1 receptor agonist during a median follow-up of 6.0 months, Elisabetta Patorno, MD, DrPH, reported at the meeting. This worked out to 3.7 fewer hospitalizations for AFib per 1,000 patient-years of follow-up among the people who received an SGLT2 inhibitor, compared with a DPP4 inhibitor, and a decrease of 1.8 hospitalizations/1,000 patient-years when compared against patients in a GLP-1 receptor agonist.
Two secondary outcomes showed significantly fewer episodes of newly diagnosed AFib, and significantly fewer patients initiating AFib treatment among those who received an SGLT2 inhibitor relative to the comparator groups. In addition, these associations were consistent across subgroup analyses that divided patients by their age, sex, history of heart failure, and history of atherosclerotic cardiovascular disease.
AFib effects add to benefits
The findings “suggest that initiation of an SGLT2 inhibitor may be beneficial in older adults with type 2 diabetes who are at risk for AFib,” said Dr. Patorno, a researcher in the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital, Boston. “These new findings on AFib may be helpful when weighing the potential risks and benefits of various glucose-lowering drugs in older patients with type 2 diabetes.”
This new evidence follows several prior reports from other research groups of data supporting an AFib benefit from SGLT2 inhibitors. The earlier reports include a post hoc analysis of more than 17,000 patients enrolled in the DECLARE-TIMI 58 cardiovascular outcome trial of dapagliflozin (Farxiga), which showed a 19% relative decrease in the rate of incident AFib or atrial flutter events during a median 4.2 year follow-up.
Other prior reports that found a reduced incidence of AFib events linked with SGLT2 inhibitor treatment include a 2020 meta-analysis based on data from more than 38,000 patients with type 2 diabetes enrolled in any of 16 randomized, controlled trials, which found a 24% relative risk reduction. And an as-yet unpublished report from researchers at the University of Rochester (N.Y.) and their associates presented in November 2021 at the annual scientific sessions of the American Heart Association that documented a significant 24% relative risk reduction in incident AFib events linked to SGLT2 inhibitor treatment in a prospective study of 13,890 patients at several hospitals in Israel or the United States.
Evidence ‘convincing’ in totality
The accumulated evidence for a reduced incidence of AFib when patients were on treatment with an SGLT2 inhibitor are “convincing because it’s real world data that complements what we know from clinical trials,” commented Silvio E. Inzucchi, MD, professor of medicine at Yale University and director of the Yale Medicine Diabetes Center in New Haven, Conn., who was not involved with the study.
“If these drugs reduce heart failure, they may also reduce AFib. Heart failure patients easily slip into AFib,” he noted in an interview, but added that “I don’t think this explains all cases” of the reduced AFib incidence.
Dr. Patorno offered a few other possible mechanisms for the observed effect. The class may work by reducing blood pressure, weight, inflammation, and oxidative stress, mitochondrial dysfunction, atrial remodeling, and AFib susceptibility. These agents are also known to cause natriuresis and diuresis, which could reduce atrial dilation, a mechanism that again relates the AFib effect to the better documented reduction in acute heart failure exacerbations.
“With the diuretic effect, we’d expect less overload at the atrium and less dilation, and the same mechanism would reduce heart failure,” she said in an interview.
“If you reduce preload and afterload you may reduce stress on the ventricle and reduce atrial stretch, and that might have a significant effect on atrial arrhythmia,” agreed Dr. Inzucchi.
EMPRISE produces more real-world evidence
A pair of additional reports at the meeting that Dr. Patorno coauthored provided real-world evidence supporting the dramatic heart failure benefit of the SGLT2 inhibitor empagliflozin (Jardiance) in U.S. patients with type 2 diabetes, compared with alternative drug classes. The EMPRISE study used data from the Medicare, Optum Clinformatics, and MarketScan databases during the period from August 2014, when empagliflozin became available, to September 2019. The study used more than 140 variables to match patients treated with either empagliflozin or a comparator agent.
The results showed that, in an analysis of more than 130,000 matched pairs, treatment with empagliflozin was linked to a significant 30% reduction in the incidence of hospitalization for heart failure, compared with patients treated with a GLP-1 receptor agonist. Analysis of more than 116,000 matched pairs of patients showed that treatment with empagliflozin linked with a significant 29%-50% reduced rate of hospitalization for heart failure, compared with matched patients treated with a DPP4 inhibitor.
These findings “add to the pool of information” on the efficacy of agents from the SGLT2 inhibitor class, Dr. Patorno said in an interview. “We wanted to look at the full range of patients with type 2 diabetes who we see in practice,” rather than the more selected group of patients enrolled in randomized trials.
SGLT2 inhibitor use lags even when cost isn’t an issue
Despite all the accumulated evidence for efficacy and safety of the class, usage remains low, Julio A. Lamprea-Montealegre, MD, PhD, a cardiologist at the University of California, San Francisco, reported in a separate talk at the meeting. The study he presented examined records for 1,319,500 adults with type 2 diabetes managed in the VA Healthcare System during 2019 and 2020. Despite being in a system that “removes the influence of cost,” just 10% of these patients received treatment with an SGLT2 inhibitor, and 7% received treatment with a GLP-1 receptor agonist.
Notably, his analysis further showed that treatment with an SGLT2 inhibitor was especially depressed among patients with an estimated glomerular filtration rate (eGFR) of 30-44 mL/min per 1.73m2. In this subgroup, usage of a drug from this class was at two-thirds of the rate, compared with patients with an eGFR of at least 90 mL/min per 1.73m2. His findings also documented lower rates of use in patients with higher risk for atherosclerotic cardiovascular disease. Dr. Lamprea-Montealegre called this a “treatment paradox,” in which patients likely to get the most benefit from an SGLT2 inhibitor were also less likely to actually receive it.
While his findings from the VA System suggest that drug cost is not the only factor driving underuse, the high price set for the SGLT2 inhibitor drugs that all currently remain on U.S. patents is widely considered an important factor.
“There is a big problem of affordability,” said Dr. Patorno.
“SGLT2 inhibitors should probably be first-line therapy” for many patients with type 2 diabetes, said Dr. Inzucchi. “The only thing holding it back is cost,” a situation that he hopes will dramatically shift once agents from this class become generic and have substantially lower price tags.
The EMPRISE study received funding from Boehringer Ingelheim, the company that markets empagliflozin (Jardiance). Dr. Patorno had no relevant commercial disclosures. Dr. Inzucchi is an adviser to Abbott Diagnostics, Esperion Therapeutics, and vTv Therapeutics, a consultant to Merck and Pfizer, and has other relationships with AstraZeneca, Boehringer Ingelheim, Lexicon, and Novo Nordisk. Dr. Lamprea-Montealegre had received research funding from Bayer.
NEW ORLEANS – The case continues to grow for prioritizing a sodium-glucose transporter 2 (SGLT2) inhibitor in patients with type 2 diabetes, as real-world evidence of benefit and safety accumulates on top of the data from randomized trials that first established this class as a management pillar.
Another important effect of these agents gaining increasing currency, on top of their well-established benefits in patients with type 2 diabetes for preventing acute heart failure exacerbations and slowing progression of diabetic kidney disease, is that they cut the incidence of new-onset atrial fibrillation (AFib). That effect was confirmed in an analysis of data from about 300,000 U.S. patients included in recent Medicare records, Elisabetta Patorno, MD, reported at the annual scientific sessions of the American Diabetes Association.
But despite documentation like this, real-world evidence also continues to show limited uptake of SGLT2 inhibitors in U.S. patients with type 2 diabetes. Records from more than 1.3 million patients with type 2 diabetes managed in the Veterans Affairs Healthcare System during 2019 or 2022 documented that just 10% of these patients received an agent from this class, even though all were eligible to receive it, according to findings in a separate report at the meeting.
The AFib analysis analyzed two sets of propensity score–matched Medicare patients during 2013-2018 aged 65 years or older with type 2 diabetes and no history of AFib. One analysis focused on 80,475 matched patients who started on treatment with either an SGLT2 inhibitor or a glucagonlike peptide–1 (GLP-1) receptor agonist, and a second on 74,868 matched patients who began either an SGTL2 inhibitor or a dipeptidyl peptidase–4 (DPP4) inhibitor. In both analyses, matching involved more than 130 variables. In both pair sets, patients at baseline averaged about 72 years old, nearly two-thirds were women, about 8%-9% had heart failure, 77%-80% were on metformin, and 20%-25% were using insulin.
The study’s primary endpoint was the incidence of hospitalization for AFib, which occurred a significant 18% less often in the patients who started on an SGLT2, compared with those who started a DPP4 inhibitor during median follow-up of 6.7 months, and a significant 10% less often, compared with those starting a GLP-1 receptor agonist during a median follow-up of 6.0 months, Elisabetta Patorno, MD, DrPH, reported at the meeting. This worked out to 3.7 fewer hospitalizations for AFib per 1,000 patient-years of follow-up among the people who received an SGLT2 inhibitor, compared with a DPP4 inhibitor, and a decrease of 1.8 hospitalizations/1,000 patient-years when compared against patients in a GLP-1 receptor agonist.
Two secondary outcomes showed significantly fewer episodes of newly diagnosed AFib, and significantly fewer patients initiating AFib treatment among those who received an SGLT2 inhibitor relative to the comparator groups. In addition, these associations were consistent across subgroup analyses that divided patients by their age, sex, history of heart failure, and history of atherosclerotic cardiovascular disease.
AFib effects add to benefits
The findings “suggest that initiation of an SGLT2 inhibitor may be beneficial in older adults with type 2 diabetes who are at risk for AFib,” said Dr. Patorno, a researcher in the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital, Boston. “These new findings on AFib may be helpful when weighing the potential risks and benefits of various glucose-lowering drugs in older patients with type 2 diabetes.”
This new evidence follows several prior reports from other research groups of data supporting an AFib benefit from SGLT2 inhibitors. The earlier reports include a post hoc analysis of more than 17,000 patients enrolled in the DECLARE-TIMI 58 cardiovascular outcome trial of dapagliflozin (Farxiga), which showed a 19% relative decrease in the rate of incident AFib or atrial flutter events during a median 4.2 year follow-up.
Other prior reports that found a reduced incidence of AFib events linked with SGLT2 inhibitor treatment include a 2020 meta-analysis based on data from more than 38,000 patients with type 2 diabetes enrolled in any of 16 randomized, controlled trials, which found a 24% relative risk reduction. And an as-yet unpublished report from researchers at the University of Rochester (N.Y.) and their associates presented in November 2021 at the annual scientific sessions of the American Heart Association that documented a significant 24% relative risk reduction in incident AFib events linked to SGLT2 inhibitor treatment in a prospective study of 13,890 patients at several hospitals in Israel or the United States.
Evidence ‘convincing’ in totality
The accumulated evidence for a reduced incidence of AFib when patients were on treatment with an SGLT2 inhibitor are “convincing because it’s real world data that complements what we know from clinical trials,” commented Silvio E. Inzucchi, MD, professor of medicine at Yale University and director of the Yale Medicine Diabetes Center in New Haven, Conn., who was not involved with the study.
“If these drugs reduce heart failure, they may also reduce AFib. Heart failure patients easily slip into AFib,” he noted in an interview, but added that “I don’t think this explains all cases” of the reduced AFib incidence.
Dr. Patorno offered a few other possible mechanisms for the observed effect. The class may work by reducing blood pressure, weight, inflammation, and oxidative stress, mitochondrial dysfunction, atrial remodeling, and AFib susceptibility. These agents are also known to cause natriuresis and diuresis, which could reduce atrial dilation, a mechanism that again relates the AFib effect to the better documented reduction in acute heart failure exacerbations.
“With the diuretic effect, we’d expect less overload at the atrium and less dilation, and the same mechanism would reduce heart failure,” she said in an interview.
“If you reduce preload and afterload you may reduce stress on the ventricle and reduce atrial stretch, and that might have a significant effect on atrial arrhythmia,” agreed Dr. Inzucchi.
EMPRISE produces more real-world evidence
A pair of additional reports at the meeting that Dr. Patorno coauthored provided real-world evidence supporting the dramatic heart failure benefit of the SGLT2 inhibitor empagliflozin (Jardiance) in U.S. patients with type 2 diabetes, compared with alternative drug classes. The EMPRISE study used data from the Medicare, Optum Clinformatics, and MarketScan databases during the period from August 2014, when empagliflozin became available, to September 2019. The study used more than 140 variables to match patients treated with either empagliflozin or a comparator agent.
The results showed that, in an analysis of more than 130,000 matched pairs, treatment with empagliflozin was linked to a significant 30% reduction in the incidence of hospitalization for heart failure, compared with patients treated with a GLP-1 receptor agonist. Analysis of more than 116,000 matched pairs of patients showed that treatment with empagliflozin linked with a significant 29%-50% reduced rate of hospitalization for heart failure, compared with matched patients treated with a DPP4 inhibitor.
These findings “add to the pool of information” on the efficacy of agents from the SGLT2 inhibitor class, Dr. Patorno said in an interview. “We wanted to look at the full range of patients with type 2 diabetes who we see in practice,” rather than the more selected group of patients enrolled in randomized trials.
SGLT2 inhibitor use lags even when cost isn’t an issue
Despite all the accumulated evidence for efficacy and safety of the class, usage remains low, Julio A. Lamprea-Montealegre, MD, PhD, a cardiologist at the University of California, San Francisco, reported in a separate talk at the meeting. The study he presented examined records for 1,319,500 adults with type 2 diabetes managed in the VA Healthcare System during 2019 and 2020. Despite being in a system that “removes the influence of cost,” just 10% of these patients received treatment with an SGLT2 inhibitor, and 7% received treatment with a GLP-1 receptor agonist.
Notably, his analysis further showed that treatment with an SGLT2 inhibitor was especially depressed among patients with an estimated glomerular filtration rate (eGFR) of 30-44 mL/min per 1.73m2. In this subgroup, usage of a drug from this class was at two-thirds of the rate, compared with patients with an eGFR of at least 90 mL/min per 1.73m2. His findings also documented lower rates of use in patients with higher risk for atherosclerotic cardiovascular disease. Dr. Lamprea-Montealegre called this a “treatment paradox,” in which patients likely to get the most benefit from an SGLT2 inhibitor were also less likely to actually receive it.
While his findings from the VA System suggest that drug cost is not the only factor driving underuse, the high price set for the SGLT2 inhibitor drugs that all currently remain on U.S. patents is widely considered an important factor.
“There is a big problem of affordability,” said Dr. Patorno.
“SGLT2 inhibitors should probably be first-line therapy” for many patients with type 2 diabetes, said Dr. Inzucchi. “The only thing holding it back is cost,” a situation that he hopes will dramatically shift once agents from this class become generic and have substantially lower price tags.
The EMPRISE study received funding from Boehringer Ingelheim, the company that markets empagliflozin (Jardiance). Dr. Patorno had no relevant commercial disclosures. Dr. Inzucchi is an adviser to Abbott Diagnostics, Esperion Therapeutics, and vTv Therapeutics, a consultant to Merck and Pfizer, and has other relationships with AstraZeneca, Boehringer Ingelheim, Lexicon, and Novo Nordisk. Dr. Lamprea-Montealegre had received research funding from Bayer.
NEW ORLEANS – The case continues to grow for prioritizing a sodium-glucose transporter 2 (SGLT2) inhibitor in patients with type 2 diabetes, as real-world evidence of benefit and safety accumulates on top of the data from randomized trials that first established this class as a management pillar.
Another important effect of these agents gaining increasing currency, on top of their well-established benefits in patients with type 2 diabetes for preventing acute heart failure exacerbations and slowing progression of diabetic kidney disease, is that they cut the incidence of new-onset atrial fibrillation (AFib). That effect was confirmed in an analysis of data from about 300,000 U.S. patients included in recent Medicare records, Elisabetta Patorno, MD, reported at the annual scientific sessions of the American Diabetes Association.
But despite documentation like this, real-world evidence also continues to show limited uptake of SGLT2 inhibitors in U.S. patients with type 2 diabetes. Records from more than 1.3 million patients with type 2 diabetes managed in the Veterans Affairs Healthcare System during 2019 or 2022 documented that just 10% of these patients received an agent from this class, even though all were eligible to receive it, according to findings in a separate report at the meeting.
The AFib analysis analyzed two sets of propensity score–matched Medicare patients during 2013-2018 aged 65 years or older with type 2 diabetes and no history of AFib. One analysis focused on 80,475 matched patients who started on treatment with either an SGLT2 inhibitor or a glucagonlike peptide–1 (GLP-1) receptor agonist, and a second on 74,868 matched patients who began either an SGTL2 inhibitor or a dipeptidyl peptidase–4 (DPP4) inhibitor. In both analyses, matching involved more than 130 variables. In both pair sets, patients at baseline averaged about 72 years old, nearly two-thirds were women, about 8%-9% had heart failure, 77%-80% were on metformin, and 20%-25% were using insulin.
The study’s primary endpoint was the incidence of hospitalization for AFib, which occurred a significant 18% less often in the patients who started on an SGLT2, compared with those who started a DPP4 inhibitor during median follow-up of 6.7 months, and a significant 10% less often, compared with those starting a GLP-1 receptor agonist during a median follow-up of 6.0 months, Elisabetta Patorno, MD, DrPH, reported at the meeting. This worked out to 3.7 fewer hospitalizations for AFib per 1,000 patient-years of follow-up among the people who received an SGLT2 inhibitor, compared with a DPP4 inhibitor, and a decrease of 1.8 hospitalizations/1,000 patient-years when compared against patients in a GLP-1 receptor agonist.
Two secondary outcomes showed significantly fewer episodes of newly diagnosed AFib, and significantly fewer patients initiating AFib treatment among those who received an SGLT2 inhibitor relative to the comparator groups. In addition, these associations were consistent across subgroup analyses that divided patients by their age, sex, history of heart failure, and history of atherosclerotic cardiovascular disease.
AFib effects add to benefits
The findings “suggest that initiation of an SGLT2 inhibitor may be beneficial in older adults with type 2 diabetes who are at risk for AFib,” said Dr. Patorno, a researcher in the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital, Boston. “These new findings on AFib may be helpful when weighing the potential risks and benefits of various glucose-lowering drugs in older patients with type 2 diabetes.”
This new evidence follows several prior reports from other research groups of data supporting an AFib benefit from SGLT2 inhibitors. The earlier reports include a post hoc analysis of more than 17,000 patients enrolled in the DECLARE-TIMI 58 cardiovascular outcome trial of dapagliflozin (Farxiga), which showed a 19% relative decrease in the rate of incident AFib or atrial flutter events during a median 4.2 year follow-up.
Other prior reports that found a reduced incidence of AFib events linked with SGLT2 inhibitor treatment include a 2020 meta-analysis based on data from more than 38,000 patients with type 2 diabetes enrolled in any of 16 randomized, controlled trials, which found a 24% relative risk reduction. And an as-yet unpublished report from researchers at the University of Rochester (N.Y.) and their associates presented in November 2021 at the annual scientific sessions of the American Heart Association that documented a significant 24% relative risk reduction in incident AFib events linked to SGLT2 inhibitor treatment in a prospective study of 13,890 patients at several hospitals in Israel or the United States.
Evidence ‘convincing’ in totality
The accumulated evidence for a reduced incidence of AFib when patients were on treatment with an SGLT2 inhibitor are “convincing because it’s real world data that complements what we know from clinical trials,” commented Silvio E. Inzucchi, MD, professor of medicine at Yale University and director of the Yale Medicine Diabetes Center in New Haven, Conn., who was not involved with the study.
“If these drugs reduce heart failure, they may also reduce AFib. Heart failure patients easily slip into AFib,” he noted in an interview, but added that “I don’t think this explains all cases” of the reduced AFib incidence.
Dr. Patorno offered a few other possible mechanisms for the observed effect. The class may work by reducing blood pressure, weight, inflammation, and oxidative stress, mitochondrial dysfunction, atrial remodeling, and AFib susceptibility. These agents are also known to cause natriuresis and diuresis, which could reduce atrial dilation, a mechanism that again relates the AFib effect to the better documented reduction in acute heart failure exacerbations.
“With the diuretic effect, we’d expect less overload at the atrium and less dilation, and the same mechanism would reduce heart failure,” she said in an interview.
“If you reduce preload and afterload you may reduce stress on the ventricle and reduce atrial stretch, and that might have a significant effect on atrial arrhythmia,” agreed Dr. Inzucchi.
EMPRISE produces more real-world evidence
A pair of additional reports at the meeting that Dr. Patorno coauthored provided real-world evidence supporting the dramatic heart failure benefit of the SGLT2 inhibitor empagliflozin (Jardiance) in U.S. patients with type 2 diabetes, compared with alternative drug classes. The EMPRISE study used data from the Medicare, Optum Clinformatics, and MarketScan databases during the period from August 2014, when empagliflozin became available, to September 2019. The study used more than 140 variables to match patients treated with either empagliflozin or a comparator agent.
The results showed that, in an analysis of more than 130,000 matched pairs, treatment with empagliflozin was linked to a significant 30% reduction in the incidence of hospitalization for heart failure, compared with patients treated with a GLP-1 receptor agonist. Analysis of more than 116,000 matched pairs of patients showed that treatment with empagliflozin linked with a significant 29%-50% reduced rate of hospitalization for heart failure, compared with matched patients treated with a DPP4 inhibitor.
These findings “add to the pool of information” on the efficacy of agents from the SGLT2 inhibitor class, Dr. Patorno said in an interview. “We wanted to look at the full range of patients with type 2 diabetes who we see in practice,” rather than the more selected group of patients enrolled in randomized trials.
SGLT2 inhibitor use lags even when cost isn’t an issue
Despite all the accumulated evidence for efficacy and safety of the class, usage remains low, Julio A. Lamprea-Montealegre, MD, PhD, a cardiologist at the University of California, San Francisco, reported in a separate talk at the meeting. The study he presented examined records for 1,319,500 adults with type 2 diabetes managed in the VA Healthcare System during 2019 and 2020. Despite being in a system that “removes the influence of cost,” just 10% of these patients received treatment with an SGLT2 inhibitor, and 7% received treatment with a GLP-1 receptor agonist.
Notably, his analysis further showed that treatment with an SGLT2 inhibitor was especially depressed among patients with an estimated glomerular filtration rate (eGFR) of 30-44 mL/min per 1.73m2. In this subgroup, usage of a drug from this class was at two-thirds of the rate, compared with patients with an eGFR of at least 90 mL/min per 1.73m2. His findings also documented lower rates of use in patients with higher risk for atherosclerotic cardiovascular disease. Dr. Lamprea-Montealegre called this a “treatment paradox,” in which patients likely to get the most benefit from an SGLT2 inhibitor were also less likely to actually receive it.
While his findings from the VA System suggest that drug cost is not the only factor driving underuse, the high price set for the SGLT2 inhibitor drugs that all currently remain on U.S. patents is widely considered an important factor.
“There is a big problem of affordability,” said Dr. Patorno.
“SGLT2 inhibitors should probably be first-line therapy” for many patients with type 2 diabetes, said Dr. Inzucchi. “The only thing holding it back is cost,” a situation that he hopes will dramatically shift once agents from this class become generic and have substantially lower price tags.
The EMPRISE study received funding from Boehringer Ingelheim, the company that markets empagliflozin (Jardiance). Dr. Patorno had no relevant commercial disclosures. Dr. Inzucchi is an adviser to Abbott Diagnostics, Esperion Therapeutics, and vTv Therapeutics, a consultant to Merck and Pfizer, and has other relationships with AstraZeneca, Boehringer Ingelheim, Lexicon, and Novo Nordisk. Dr. Lamprea-Montealegre had received research funding from Bayer.
AT ADA 2022