User login
Cannabis crimps teen cognitive development
BARCELONA – What would you predict has a greater detrimental effect on adolescent cognitive development: alcohol or cannabis use?
The evidence-based answer may come as a surprise. It certainly did for Patricia Conrod, PhD, who led the large population-based study that addressed the question.
“Generally, we found no effect of alcohol on cognitive development, which was a huge surprise to us. It might be related to the fact that the quantity of alcohol consumption in this young sample just wasn’t high enough to produce significant effects on cognitive development. But, to our surprise, we found rather significant effects of cannabis use on cognitive development,” she said at the annual congress of the European College of Neuropsychopharmacology.
Indeed, cannabis use proved to have detrimental effects on all four cognitive domains assessed in the study: working memory, perceptual reasoning, delayed recall, and inhibitory control, reported Dr. Conrod, professor of psychiatry at the University of Montreal.
Her recently study, published in the American Journal of Psychiatry, included 3,826 seventh-grade students at 31 Montreal-area schools. They constituted 5% of all students entering that grade in the greater Montreal area. Participants were prospectively assessed annually for 4 years regarding their use or nonuse of alcohol or cannabis and also underwent neurocognitive testing on the four domains of interest. The assessments were done on school computers with preservation of student confidentiality. Investigators used a Big Data approach to model the relationship between the extent of substance use and neurocognitive function variables over time.
Abstinent students were the best performers on the neurocognitive testing. Cannabis use, but not alcohol, in a given year was associated with concurrent adverse effects on all four cognitive domains. In addition, cannabis use showed evidence of having a neurotoxic lag effect on inhibitory control and working memory. This took the form of a lasting effect: A student who reported using cannabis 1 year but not the next showed impairment of inhibitory control and working memory during both years. And a student who used cannabis both years was even more impaired in those domains.
Dr. Conrod found the evidence of a neurotoxic effect of cannabis use on inhibitory control to be of particular concern because in earlier studies she established that impaired inhibitory control is a strong independent risk factor for subsequent substance use disorders.
”So what we’re seeing is indeed that early onset substance use is interfering with cognitive development, which now sets us up to be able to answer the question of whether evidence-based prevention protects cognitive development by delaying early onset of substance use. And over the longer term, does that protect young people against addiction?”
Dr. Conrod and her coworkers are now in the process of obtaining answers to those questions in the large ongoing Canadian Institutes of Health Research-funded Co-Venture Trial. This randomized trial involving thousands of adolescent students used the investigators’ Preventure Program, a school-based, personality-targeted intervention for prevention of substance use and abuse.
The Preventure Program involves two 90-minute group sessions of manual-based cognitive-behavioral therapy. Students are invited to participate if they score at least one standard deviation above the school mean on one of four personality traits that have been shown to increase the risk of substance misuse and psychiatric disorders. The four personality traits are sensation seeking, impulsivity, anxiety sensitivity, and hopelessness. Typically, about 45% of students met that threshold, and 85% of those invited to participate in the program volunteered to do so. Students of similar personality type are grouped together for the targeted therapy sessions.
This brief coping skills intervention has been shown in multiple randomized trials around the world to reduce the likelihood of substance use in at-risk adolescents. For example, in an early trial involving 732 high school students in London, participation in the Preventure Program was associated with a 30% reduction in the likelihood of taking up the use of cannabis within the next 2 years, an 80% reduction in the likelihood of taking up cocaine, and a 50% reduction in the use of other drugs (Arch Gen Psychiatry. 2010 Jan;67[1]:85-93).
bjancin@mdedge.com
SOURCE: Conrod P. Am J Psychiatry. 2018 Oct 3. doi: 10.1176/appi.ajp.2018.18020202.
BARCELONA – What would you predict has a greater detrimental effect on adolescent cognitive development: alcohol or cannabis use?
The evidence-based answer may come as a surprise. It certainly did for Patricia Conrod, PhD, who led the large population-based study that addressed the question.
“Generally, we found no effect of alcohol on cognitive development, which was a huge surprise to us. It might be related to the fact that the quantity of alcohol consumption in this young sample just wasn’t high enough to produce significant effects on cognitive development. But, to our surprise, we found rather significant effects of cannabis use on cognitive development,” she said at the annual congress of the European College of Neuropsychopharmacology.
Indeed, cannabis use proved to have detrimental effects on all four cognitive domains assessed in the study: working memory, perceptual reasoning, delayed recall, and inhibitory control, reported Dr. Conrod, professor of psychiatry at the University of Montreal.
Her recently study, published in the American Journal of Psychiatry, included 3,826 seventh-grade students at 31 Montreal-area schools. They constituted 5% of all students entering that grade in the greater Montreal area. Participants were prospectively assessed annually for 4 years regarding their use or nonuse of alcohol or cannabis and also underwent neurocognitive testing on the four domains of interest. The assessments were done on school computers with preservation of student confidentiality. Investigators used a Big Data approach to model the relationship between the extent of substance use and neurocognitive function variables over time.
Abstinent students were the best performers on the neurocognitive testing. Cannabis use, but not alcohol, in a given year was associated with concurrent adverse effects on all four cognitive domains. In addition, cannabis use showed evidence of having a neurotoxic lag effect on inhibitory control and working memory. This took the form of a lasting effect: A student who reported using cannabis 1 year but not the next showed impairment of inhibitory control and working memory during both years. And a student who used cannabis both years was even more impaired in those domains.
Dr. Conrod found the evidence of a neurotoxic effect of cannabis use on inhibitory control to be of particular concern because in earlier studies she established that impaired inhibitory control is a strong independent risk factor for subsequent substance use disorders.
”So what we’re seeing is indeed that early onset substance use is interfering with cognitive development, which now sets us up to be able to answer the question of whether evidence-based prevention protects cognitive development by delaying early onset of substance use. And over the longer term, does that protect young people against addiction?”
Dr. Conrod and her coworkers are now in the process of obtaining answers to those questions in the large ongoing Canadian Institutes of Health Research-funded Co-Venture Trial. This randomized trial involving thousands of adolescent students used the investigators’ Preventure Program, a school-based, personality-targeted intervention for prevention of substance use and abuse.
The Preventure Program involves two 90-minute group sessions of manual-based cognitive-behavioral therapy. Students are invited to participate if they score at least one standard deviation above the school mean on one of four personality traits that have been shown to increase the risk of substance misuse and psychiatric disorders. The four personality traits are sensation seeking, impulsivity, anxiety sensitivity, and hopelessness. Typically, about 45% of students met that threshold, and 85% of those invited to participate in the program volunteered to do so. Students of similar personality type are grouped together for the targeted therapy sessions.
This brief coping skills intervention has been shown in multiple randomized trials around the world to reduce the likelihood of substance use in at-risk adolescents. For example, in an early trial involving 732 high school students in London, participation in the Preventure Program was associated with a 30% reduction in the likelihood of taking up the use of cannabis within the next 2 years, an 80% reduction in the likelihood of taking up cocaine, and a 50% reduction in the use of other drugs (Arch Gen Psychiatry. 2010 Jan;67[1]:85-93).
bjancin@mdedge.com
SOURCE: Conrod P. Am J Psychiatry. 2018 Oct 3. doi: 10.1176/appi.ajp.2018.18020202.
BARCELONA – What would you predict has a greater detrimental effect on adolescent cognitive development: alcohol or cannabis use?
The evidence-based answer may come as a surprise. It certainly did for Patricia Conrod, PhD, who led the large population-based study that addressed the question.
“Generally, we found no effect of alcohol on cognitive development, which was a huge surprise to us. It might be related to the fact that the quantity of alcohol consumption in this young sample just wasn’t high enough to produce significant effects on cognitive development. But, to our surprise, we found rather significant effects of cannabis use on cognitive development,” she said at the annual congress of the European College of Neuropsychopharmacology.
Indeed, cannabis use proved to have detrimental effects on all four cognitive domains assessed in the study: working memory, perceptual reasoning, delayed recall, and inhibitory control, reported Dr. Conrod, professor of psychiatry at the University of Montreal.
Her recently study, published in the American Journal of Psychiatry, included 3,826 seventh-grade students at 31 Montreal-area schools. They constituted 5% of all students entering that grade in the greater Montreal area. Participants were prospectively assessed annually for 4 years regarding their use or nonuse of alcohol or cannabis and also underwent neurocognitive testing on the four domains of interest. The assessments were done on school computers with preservation of student confidentiality. Investigators used a Big Data approach to model the relationship between the extent of substance use and neurocognitive function variables over time.
Abstinent students were the best performers on the neurocognitive testing. Cannabis use, but not alcohol, in a given year was associated with concurrent adverse effects on all four cognitive domains. In addition, cannabis use showed evidence of having a neurotoxic lag effect on inhibitory control and working memory. This took the form of a lasting effect: A student who reported using cannabis 1 year but not the next showed impairment of inhibitory control and working memory during both years. And a student who used cannabis both years was even more impaired in those domains.
Dr. Conrod found the evidence of a neurotoxic effect of cannabis use on inhibitory control to be of particular concern because in earlier studies she established that impaired inhibitory control is a strong independent risk factor for subsequent substance use disorders.
”So what we’re seeing is indeed that early onset substance use is interfering with cognitive development, which now sets us up to be able to answer the question of whether evidence-based prevention protects cognitive development by delaying early onset of substance use. And over the longer term, does that protect young people against addiction?”
Dr. Conrod and her coworkers are now in the process of obtaining answers to those questions in the large ongoing Canadian Institutes of Health Research-funded Co-Venture Trial. This randomized trial involving thousands of adolescent students used the investigators’ Preventure Program, a school-based, personality-targeted intervention for prevention of substance use and abuse.
The Preventure Program involves two 90-minute group sessions of manual-based cognitive-behavioral therapy. Students are invited to participate if they score at least one standard deviation above the school mean on one of four personality traits that have been shown to increase the risk of substance misuse and psychiatric disorders. The four personality traits are sensation seeking, impulsivity, anxiety sensitivity, and hopelessness. Typically, about 45% of students met that threshold, and 85% of those invited to participate in the program volunteered to do so. Students of similar personality type are grouped together for the targeted therapy sessions.
This brief coping skills intervention has been shown in multiple randomized trials around the world to reduce the likelihood of substance use in at-risk adolescents. For example, in an early trial involving 732 high school students in London, participation in the Preventure Program was associated with a 30% reduction in the likelihood of taking up the use of cannabis within the next 2 years, an 80% reduction in the likelihood of taking up cocaine, and a 50% reduction in the use of other drugs (Arch Gen Psychiatry. 2010 Jan;67[1]:85-93).
bjancin@mdedge.com
SOURCE: Conrod P. Am J Psychiatry. 2018 Oct 3. doi: 10.1176/appi.ajp.2018.18020202.
REPORTING FROM THE ECNP CONGRESS
Key clinical point:
Major finding: The observed neurotoxic effect on impulse control may spell future trouble.
Study details: This population-based study included 3,826 Montreal-area seventh graders who were prospectively assessed annually for 4 years regarding their cannabis and alcohol use and also underwent neurocognitive testing.
Disclosures: The study was funded by the Canadian Institutes of Health Research.
Source: Conrod P. Am J Psychiatry. 2018 Oct 3. doi: 10.1176/appi.ajp.2018.18020202.
Bipolar patients’ relatives face increased cardiovascular risk
BARCELONA – Young patients recently diagnosed with bipolar disorder are at double the 30-year risk of cardiovascular disease, compared with the general population, and their unaffected first-degree relatives are nearly as high risk, Klara Coello, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
The clinical implication of this finding is that unaffected first-degree relatives of patients with bipolar disorder – an affective disorder typically diagnosed at age 15-24 – should be targeted for intensified primary cardiovascular prevention, with a focus on smoking and dyslipidemia, both of which were more prevalent in these patients and their unaffected relatives than in the general population in her study, noted Dr. Coello, a doctoral candidate with the Copenhagen Affective Disorders Research Center at the University of Copenhagen.
She and her coinvestigators presented a cross-sectional study in which they calculated the 30-year Framingham Risk Scores for 221 patients recently diagnosed bipolar disorder – 95% of whom had been diagnosed within the past 2 years – along with 50 unaffected first-degree relatives and 119 age- and sex-matched controls. The investigators used the Framingham Risk Score because the widely used American Heart Association/American College of Cardiology Atherosclerotic Cardiovascular Disease Risk Estimator applies only to individuals aged 40 and up.
The key findings: The 30-year risk of cardiovascular disease for patients with bipolar was 98.5% greater than that of controls, and the calculated risk of the unaffected first-degree relatives was increased by 85.4%, compared with that of controls.
The Framingham Risk Score is determined on the basis of old-school cardiovascular risk factors, including age, gender, lipids, systolic blood pressure, diabetes, and smoking. 45% of the bipolar patients were smokers, as were 20% of their first-degree relatives and 13% of controls.
The Danish finding of increased cardiovascular risk in young adults with bipolar disorder recapitulates an American Heart Association Scientific Statement, which was published in Circulation (2015 Sep 8;132[10]:965-86). The statement was intended to alert clinicians that these affective disorders constitute “moderate-risk” conditions for arterial dysfunction prior to age 30 and for premature cardiovascular disease (CVD). The statement declared that this risk is likely mediated not only by the classic cardiovascular risk factors but also by disease-related inflammation, oxidative stress, sleep disruption, and the adverse metabolic effects of many psychotropic medications.
“The magnitude of increased risk for CVD in adulthood is substantial,” according to the AHA expert panel’s scientific statement.
Dr. Coello’s study only took into account levels of the traditional cardiovascular risk factors. Where the study broke new ground that hadn’t been explored in the AHA scientific statement, however, was in identifying unaffected first-degree relatives as an additional at-risk group.
She reported having no financial conflicts regarding her study, which constitutes her PhD thesis.
BARCELONA – Young patients recently diagnosed with bipolar disorder are at double the 30-year risk of cardiovascular disease, compared with the general population, and their unaffected first-degree relatives are nearly as high risk, Klara Coello, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
The clinical implication of this finding is that unaffected first-degree relatives of patients with bipolar disorder – an affective disorder typically diagnosed at age 15-24 – should be targeted for intensified primary cardiovascular prevention, with a focus on smoking and dyslipidemia, both of which were more prevalent in these patients and their unaffected relatives than in the general population in her study, noted Dr. Coello, a doctoral candidate with the Copenhagen Affective Disorders Research Center at the University of Copenhagen.
She and her coinvestigators presented a cross-sectional study in which they calculated the 30-year Framingham Risk Scores for 221 patients recently diagnosed bipolar disorder – 95% of whom had been diagnosed within the past 2 years – along with 50 unaffected first-degree relatives and 119 age- and sex-matched controls. The investigators used the Framingham Risk Score because the widely used American Heart Association/American College of Cardiology Atherosclerotic Cardiovascular Disease Risk Estimator applies only to individuals aged 40 and up.
The key findings: The 30-year risk of cardiovascular disease for patients with bipolar was 98.5% greater than that of controls, and the calculated risk of the unaffected first-degree relatives was increased by 85.4%, compared with that of controls.
The Framingham Risk Score is determined on the basis of old-school cardiovascular risk factors, including age, gender, lipids, systolic blood pressure, diabetes, and smoking. 45% of the bipolar patients were smokers, as were 20% of their first-degree relatives and 13% of controls.
The Danish finding of increased cardiovascular risk in young adults with bipolar disorder recapitulates an American Heart Association Scientific Statement, which was published in Circulation (2015 Sep 8;132[10]:965-86). The statement was intended to alert clinicians that these affective disorders constitute “moderate-risk” conditions for arterial dysfunction prior to age 30 and for premature cardiovascular disease (CVD). The statement declared that this risk is likely mediated not only by the classic cardiovascular risk factors but also by disease-related inflammation, oxidative stress, sleep disruption, and the adverse metabolic effects of many psychotropic medications.
“The magnitude of increased risk for CVD in adulthood is substantial,” according to the AHA expert panel’s scientific statement.
Dr. Coello’s study only took into account levels of the traditional cardiovascular risk factors. Where the study broke new ground that hadn’t been explored in the AHA scientific statement, however, was in identifying unaffected first-degree relatives as an additional at-risk group.
She reported having no financial conflicts regarding her study, which constitutes her PhD thesis.
BARCELONA – Young patients recently diagnosed with bipolar disorder are at double the 30-year risk of cardiovascular disease, compared with the general population, and their unaffected first-degree relatives are nearly as high risk, Klara Coello, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
The clinical implication of this finding is that unaffected first-degree relatives of patients with bipolar disorder – an affective disorder typically diagnosed at age 15-24 – should be targeted for intensified primary cardiovascular prevention, with a focus on smoking and dyslipidemia, both of which were more prevalent in these patients and their unaffected relatives than in the general population in her study, noted Dr. Coello, a doctoral candidate with the Copenhagen Affective Disorders Research Center at the University of Copenhagen.
She and her coinvestigators presented a cross-sectional study in which they calculated the 30-year Framingham Risk Scores for 221 patients recently diagnosed bipolar disorder – 95% of whom had been diagnosed within the past 2 years – along with 50 unaffected first-degree relatives and 119 age- and sex-matched controls. The investigators used the Framingham Risk Score because the widely used American Heart Association/American College of Cardiology Atherosclerotic Cardiovascular Disease Risk Estimator applies only to individuals aged 40 and up.
The key findings: The 30-year risk of cardiovascular disease for patients with bipolar was 98.5% greater than that of controls, and the calculated risk of the unaffected first-degree relatives was increased by 85.4%, compared with that of controls.
The Framingham Risk Score is determined on the basis of old-school cardiovascular risk factors, including age, gender, lipids, systolic blood pressure, diabetes, and smoking. 45% of the bipolar patients were smokers, as were 20% of their first-degree relatives and 13% of controls.
The Danish finding of increased cardiovascular risk in young adults with bipolar disorder recapitulates an American Heart Association Scientific Statement, which was published in Circulation (2015 Sep 8;132[10]:965-86). The statement was intended to alert clinicians that these affective disorders constitute “moderate-risk” conditions for arterial dysfunction prior to age 30 and for premature cardiovascular disease (CVD). The statement declared that this risk is likely mediated not only by the classic cardiovascular risk factors but also by disease-related inflammation, oxidative stress, sleep disruption, and the adverse metabolic effects of many psychotropic medications.
“The magnitude of increased risk for CVD in adulthood is substantial,” according to the AHA expert panel’s scientific statement.
Dr. Coello’s study only took into account levels of the traditional cardiovascular risk factors. Where the study broke new ground that hadn’t been explored in the AHA scientific statement, however, was in identifying unaffected first-degree relatives as an additional at-risk group.
She reported having no financial conflicts regarding her study, which constitutes her PhD thesis.
REPORTING FROM THE ECNP CONGRESS
Key clinical point: The first-degree relatives of patients with bipolar disorder should be targeted for intensified primary cardiovascular prevention.
Major finding: Thirty-year cardiovascular risk was increased by 98.5% in recently diagnosed bipolar patients and by 85.4% in their unaffected first-degree relatives, compared with the general population.
Study details: This cross-sectional study involved calculation of 30-year Framingham Risk Scores for 221 patients recently diagnosed with bipolar disorder, 50 unaffected first-degree relatives, and 119 age- and sex-matched controls.
Disclosures: The study presenter reported having no financial conflicts of interest.
Big drinkers face newly appreciated massive health burden
BARCELONA – Individuals who regularly consume alcohol in quantities defined by the World Health Organization as “very high risk” face a daunting and yet widely underappreciated health burden, Rainer Spanagel, MD, observed at the annual congress of the European College of Neuropsychopharmacology.
He cited a recent study led by Jürgen Rehm, PhD, of the Centre for Addiction and Mental Health in Toronto in which the investigators estimated the prevalence of what the WHO has defined as a “very-high-risk drinking level” among people aged 15-65 years in 13 E.U. countries. The researchers then went on to determine the associated annual risk of disease and injury, as well as the effects on life expectancy.
“The numbers are so shocking that you have to take it seriously,” said Dr. Spanagel, chair of the department of psychopharmacology at the Central Institute of Mental Health in Mannheim, Germany.
Nearly 2 decades ago, the WHO defined very-high-risk level of alcohol consumption as more than 100 g/day of ethanol for men and more than 60 g/day for women. That translates to a threshold of 7.1 and 4.3 standard drinks – a 12-ounce beer, 5-ounce glass of wine, or 1.5-ounce serving of liquor – on a daily basis.
“This WHO categorization of drinking risk levels has been pretty much ignored in clinical trials and epidemiologic studies until 3 or 4 years ago,” according to Dr. Spanagel.
The study by Dr. Rehm and his colleagues suggests this has been a serious mistake. By using data from the WHO’s Global Information System on Alcohol and Health, as well as from clinical trials, the investigators determined that the prevalence of this level of alcohol consumption was less than 1% overall across 13 European countries. However, rates varied markedly: in excess of 4% in Ireland and more than 3.5% in the United Kingdom, compared with less than 0.5% in Germany, Sweden, Denmark, Finland, Hungary, and the Netherlands. The Czech Republic came in at about 3%, while Italy, Spain, France, and Austria had rates of more than 0.5% but less than 1%.
The investigators estimated that the risk of disease or injury associated with this very-high-risk drinking level was 13.5% per year. Based on data from nine E.U. countries, Dr. Rehm and his colleagues found that a very-high-risk level of alcohol consumption caused nearly 54% of all cases of hepatic cirrhosis in those countries, 41% of esophageal and oral cancers, and 44% of pancreatitis.
Life expectancy in the European Union stands at 80.6 years. compared with the general population. By comparison, all cancers considered together resulted in 10 years of life lost.
Dr. Spanagel is editor in chief of the journal Addiction Biology, in which this study appeared (Addict Biol. 2018 Jul;23[4]:961-8).
bjancin@mdedge.com
BARCELONA – Individuals who regularly consume alcohol in quantities defined by the World Health Organization as “very high risk” face a daunting and yet widely underappreciated health burden, Rainer Spanagel, MD, observed at the annual congress of the European College of Neuropsychopharmacology.
He cited a recent study led by Jürgen Rehm, PhD, of the Centre for Addiction and Mental Health in Toronto in which the investigators estimated the prevalence of what the WHO has defined as a “very-high-risk drinking level” among people aged 15-65 years in 13 E.U. countries. The researchers then went on to determine the associated annual risk of disease and injury, as well as the effects on life expectancy.
“The numbers are so shocking that you have to take it seriously,” said Dr. Spanagel, chair of the department of psychopharmacology at the Central Institute of Mental Health in Mannheim, Germany.
Nearly 2 decades ago, the WHO defined very-high-risk level of alcohol consumption as more than 100 g/day of ethanol for men and more than 60 g/day for women. That translates to a threshold of 7.1 and 4.3 standard drinks – a 12-ounce beer, 5-ounce glass of wine, or 1.5-ounce serving of liquor – on a daily basis.
“This WHO categorization of drinking risk levels has been pretty much ignored in clinical trials and epidemiologic studies until 3 or 4 years ago,” according to Dr. Spanagel.
The study by Dr. Rehm and his colleagues suggests this has been a serious mistake. By using data from the WHO’s Global Information System on Alcohol and Health, as well as from clinical trials, the investigators determined that the prevalence of this level of alcohol consumption was less than 1% overall across 13 European countries. However, rates varied markedly: in excess of 4% in Ireland and more than 3.5% in the United Kingdom, compared with less than 0.5% in Germany, Sweden, Denmark, Finland, Hungary, and the Netherlands. The Czech Republic came in at about 3%, while Italy, Spain, France, and Austria had rates of more than 0.5% but less than 1%.
The investigators estimated that the risk of disease or injury associated with this very-high-risk drinking level was 13.5% per year. Based on data from nine E.U. countries, Dr. Rehm and his colleagues found that a very-high-risk level of alcohol consumption caused nearly 54% of all cases of hepatic cirrhosis in those countries, 41% of esophageal and oral cancers, and 44% of pancreatitis.
Life expectancy in the European Union stands at 80.6 years. compared with the general population. By comparison, all cancers considered together resulted in 10 years of life lost.
Dr. Spanagel is editor in chief of the journal Addiction Biology, in which this study appeared (Addict Biol. 2018 Jul;23[4]:961-8).
bjancin@mdedge.com
BARCELONA – Individuals who regularly consume alcohol in quantities defined by the World Health Organization as “very high risk” face a daunting and yet widely underappreciated health burden, Rainer Spanagel, MD, observed at the annual congress of the European College of Neuropsychopharmacology.
He cited a recent study led by Jürgen Rehm, PhD, of the Centre for Addiction and Mental Health in Toronto in which the investigators estimated the prevalence of what the WHO has defined as a “very-high-risk drinking level” among people aged 15-65 years in 13 E.U. countries. The researchers then went on to determine the associated annual risk of disease and injury, as well as the effects on life expectancy.
“The numbers are so shocking that you have to take it seriously,” said Dr. Spanagel, chair of the department of psychopharmacology at the Central Institute of Mental Health in Mannheim, Germany.
Nearly 2 decades ago, the WHO defined very-high-risk level of alcohol consumption as more than 100 g/day of ethanol for men and more than 60 g/day for women. That translates to a threshold of 7.1 and 4.3 standard drinks – a 12-ounce beer, 5-ounce glass of wine, or 1.5-ounce serving of liquor – on a daily basis.
“This WHO categorization of drinking risk levels has been pretty much ignored in clinical trials and epidemiologic studies until 3 or 4 years ago,” according to Dr. Spanagel.
The study by Dr. Rehm and his colleagues suggests this has been a serious mistake. By using data from the WHO’s Global Information System on Alcohol and Health, as well as from clinical trials, the investigators determined that the prevalence of this level of alcohol consumption was less than 1% overall across 13 European countries. However, rates varied markedly: in excess of 4% in Ireland and more than 3.5% in the United Kingdom, compared with less than 0.5% in Germany, Sweden, Denmark, Finland, Hungary, and the Netherlands. The Czech Republic came in at about 3%, while Italy, Spain, France, and Austria had rates of more than 0.5% but less than 1%.
The investigators estimated that the risk of disease or injury associated with this very-high-risk drinking level was 13.5% per year. Based on data from nine E.U. countries, Dr. Rehm and his colleagues found that a very-high-risk level of alcohol consumption caused nearly 54% of all cases of hepatic cirrhosis in those countries, 41% of esophageal and oral cancers, and 44% of pancreatitis.
Life expectancy in the European Union stands at 80.6 years. compared with the general population. By comparison, all cancers considered together resulted in 10 years of life lost.
Dr. Spanagel is editor in chief of the journal Addiction Biology, in which this study appeared (Addict Biol. 2018 Jul;23[4]:961-8).
bjancin@mdedge.com
REPORTING FROM THE ECNP CONGRESS
This year’s top papers on mood disorders
BARCELONA – Among the handful of top publications on mood disorders during the first three-quarters of 2018 was a landmark comparison of the efficacy and acceptability of 21 antidepressants for acute treatment of major depressive disorder, Íria Grande, MD, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
Dr. Grande, a psychiatrist at the bipolar disorders clinic of the University of Barcelona, shared her personal top picks.
‘Antidepressants work’
This epic systematic review and network meta-analysis (Lancet. 2018 Apr 7;391[10128]:1357-66) encompassed 522 randomized double-blind trials with 116,477 participants with major depressive disorder assigned to 21 antidepressants or placebo, in some instances with an additional active comparator antidepressant arm. The report is a major extension of previous work by the same multinational group of investigators (Lancet. 2009 Feb 28;373[9665]:746-58), who initially scrutinized 12 older antidepressants in a total population only one-quarter the size of the updated analysis.
Based upon this vast randomized trial evidence, some of which came from unpublished studies tracked down by the investigators, the 21 antidepressants were rank-ordered in terms of effectiveness and acceptability. But in Dr. Grande’s view, the most important study finding wasn’t which antidepressant donned the crown of most effective or patient acceptable, it was the fact that all 21 drugs proved significantly more effective than placebo, with odds ratios ranging from 2.13 at the top end to 1.37 for reboxetine.
“The results showed antidepressants work. All of the antipsychiatry system is trying to show us that antidepressants do not work in major depression. Well, in this study, it has been proven that all antidepressants are more effective than placebo in major depressive disorder. I think social media should be made aware of that. (Lead investigator) Dr. Andrea Cipriani talked on the BBC about this article, and it had a high impact,” according to Dr. Grande.
All but three of the 21 antidepressants were deemed to be as acceptable as placebo, based upon study dropout rates. The exceptions were agomelatine and fluoxetine, which were 12%-14% more acceptable than placebo. “That’s strange, I think, but that’s what the clinical trial results showed,” she noted. The findings on clomipramine, which was 30% less acceptable than placebo, make sense, Dr. Grande said, “due to its muscarinic effects.”
She took issue with some of the specific study findings. For example, the two top-rated antidepressants in terms of efficacy were amitriptyline and mirtazapine, with odds ratios of 2.13 and 1.89, respectively.
“As a clinician, I don’t consider mirtazapine to be one of the best antidepressants, especially in major depression,” she said. “But these are the results, and as always, we have to adapt the evidence-based medicine and consider it from our clinical point of view.”
The investigators conducted a subanalysis restricted to placebo-controlled head-to-head studies with a comparator antidepressant which Dr. Grande found more interesting and informative than the overall analysis. In the head-to-head analysis, vortioxetine emerged as the top-rated antidepressant, both in efficacy, with an odds ratio of 2.0, as well as in acceptability.
Lithium vs. quetiapine
Finnish investigators used prospective national databases to examine the rates of psychiatric and all-cause hospitalization during a mean 7.2 years of follow-up in all 18,018 Finns hospitalized for bipolar disorder. The purpose was to assess the impact of various mood stabilizers on overall health outcomes in a real-world setting.
The big winner was lithium. In an analysis adjusted for concomitant psychotropic medications, duration of bipolar illness, and intervals of drug exposure and nonexposure, lithium was associated with the lowest risks of psychiatric rehospitalization and all-cause hospitalization, with relative risk reductions of 33% and 29%, respectively. In contrast, quetiapine, the most widely used antipsychotic agent, paled by comparison, achieving only an 8% reduction in the risk of psychiatric rehospitalization and a 7% decrease in all-cause hospitalization (JAMA Psychiatry. 2018 Apr 1;75[4]:347-55).
In addition, long-acting injectable antipsychotics were significantly more effective for prevention of hospitalization than oral antipsychotics.
“That is kind of shocking, because in some countries, long-acting injectables are not authorized and cannot be used. But I think after this article some regulatory changes are going to take place as a result,” Dr. Grande predicted.
“Another issue I thought was interesting, although it was not the main aim of the study, involved benzodiazepines. They increased the risk of hospitalizations, both for psychiatric illness and all other causes. So apart from giving lithium and long-acting injectable antipsychotics to our bipolar patients, we should also be really careful about the use of benzodiazepines,” she commented.
Intranasal esketamine for suicidality?
Esketamine nasal spray, a fast-acting N-methyl-D-aspartate antagonist whose application for marketing approval in combination with a standard oral antidepressant in treatment-resistant depression is now under Food and Drug Administration review, also is being developed for another indication: reduction of suicidality in patients at imminent suicide risk. In a proof-of-concept study, intranasal esketamine resulted in a significant reduction in suicidal thoughts 4 hours after administration, compared with usual care – but not at 24 hours (Am J Psychiatry. 2018 Jul 1;175[7]:620-30).
New and effective medications for this indication are sorely needed. The only drug approved for the indication of suicide prevention is clozapine.
‘Latest thinking’ on bipolar disorders
Dr. Grande coauthored a comprehensive review article on bipolar disorders that she recommended as worthwhile reading (Nat Rev Dis Primers. 2018 Mar 8;4:18008. doi: 10.1038/nrdp.2018.8).
“It covers all the latest thinking. It focuses on the early stages of the disorder, how epigenetic factors are essential, and many other topics, including the bipolarity index being developed at the University of Barcelona to classify drugs in terms of their capacity to prevent episodes of mania or depression in terms of number needed to treat and number needed to harm. It emphasizes the importance of intervening early and focusing on cognitive dysfunction,” Dr Grande said.
Psychedelics making a comeback
German and Swiss investigators used a facial expression discrimination task to demonstrate that psilocybin, a 5-hydroxytryptamine2A–receptor agonist, decreases connectivity between the amygdala and regions of the brain important in emotion processing, including the striatum and frontal pole. The investigators theorized that this might be the mechanism for the psychedelic’s apparent antidepressant effects (Eur Neuropsychopharmacol. 2018 Jun;28[6]:691-700).
Dr. Grande included this study in her top publications list because it reflects the rapidly growing rebirth of interest in psychedelics research among European psychiatrists.
Indeed, elsewhere at the ECNP congress David J. Nutt, DM, declared, “We now have the beginnings of some swinging of the pendulum back in a modern direction. Over the last 10 years there have been a small number of open studies, all done with psilocybin, which is somewhat easier to use than LSD. There are studies in OCD [obsessive-compulsive disorder], tobacco dependence, alcoholism, resistant depression, end-of-life mood changes with cancer and other terminal diseases, and at least two ongoing randomized trials in resistant depression.”
Dr. Nutt, professor of neuropsychopharmacology at Imperial College London, was senior author of the first proof-of-concept study of psilocybin accompanied by psychologic support as a novel therapy for moderate to severe treatment-resistant major depression (Lancet Psychiatry. 2016 Jul;3[7]:619-27).
Methylphenidate ineffective for treatment of acute mania
The MEMAP study was a randomized, double-blind, placebo-controlled multicenter clinical trial testing what has been called the vigilance regulation model of mania. This model hypothesized that unstable regulation of wakefulness figures prominently in the pathogenesis of both mania and attention-deficit/hyperactivity disorder. If true, investigators reasoned, then 2.5 days of methylphenidate at 20-40 mg/day should have a rapid antimanic effect similar to the drug’s benefits in ADHD. Dr. Grande had been a skeptic, and indeed, the trial was halted early for futility (Eur Neuropsychopharmacol. 2018 Jan;28[1]:185-94).
She reported serving as a paid speaker for Lunbeck, Ferrer, GlaxoSmithKline, and Janssen. Her own research is funded by the Spanish Ministry of Economy and Competitiveness.
BARCELONA – Among the handful of top publications on mood disorders during the first three-quarters of 2018 was a landmark comparison of the efficacy and acceptability of 21 antidepressants for acute treatment of major depressive disorder, Íria Grande, MD, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
Dr. Grande, a psychiatrist at the bipolar disorders clinic of the University of Barcelona, shared her personal top picks.
‘Antidepressants work’
This epic systematic review and network meta-analysis (Lancet. 2018 Apr 7;391[10128]:1357-66) encompassed 522 randomized double-blind trials with 116,477 participants with major depressive disorder assigned to 21 antidepressants or placebo, in some instances with an additional active comparator antidepressant arm. The report is a major extension of previous work by the same multinational group of investigators (Lancet. 2009 Feb 28;373[9665]:746-58), who initially scrutinized 12 older antidepressants in a total population only one-quarter the size of the updated analysis.
Based upon this vast randomized trial evidence, some of which came from unpublished studies tracked down by the investigators, the 21 antidepressants were rank-ordered in terms of effectiveness and acceptability. But in Dr. Grande’s view, the most important study finding wasn’t which antidepressant donned the crown of most effective or patient acceptable, it was the fact that all 21 drugs proved significantly more effective than placebo, with odds ratios ranging from 2.13 at the top end to 1.37 for reboxetine.
“The results showed antidepressants work. All of the antipsychiatry system is trying to show us that antidepressants do not work in major depression. Well, in this study, it has been proven that all antidepressants are more effective than placebo in major depressive disorder. I think social media should be made aware of that. (Lead investigator) Dr. Andrea Cipriani talked on the BBC about this article, and it had a high impact,” according to Dr. Grande.
All but three of the 21 antidepressants were deemed to be as acceptable as placebo, based upon study dropout rates. The exceptions were agomelatine and fluoxetine, which were 12%-14% more acceptable than placebo. “That’s strange, I think, but that’s what the clinical trial results showed,” she noted. The findings on clomipramine, which was 30% less acceptable than placebo, make sense, Dr. Grande said, “due to its muscarinic effects.”
She took issue with some of the specific study findings. For example, the two top-rated antidepressants in terms of efficacy were amitriptyline and mirtazapine, with odds ratios of 2.13 and 1.89, respectively.
“As a clinician, I don’t consider mirtazapine to be one of the best antidepressants, especially in major depression,” she said. “But these are the results, and as always, we have to adapt the evidence-based medicine and consider it from our clinical point of view.”
The investigators conducted a subanalysis restricted to placebo-controlled head-to-head studies with a comparator antidepressant which Dr. Grande found more interesting and informative than the overall analysis. In the head-to-head analysis, vortioxetine emerged as the top-rated antidepressant, both in efficacy, with an odds ratio of 2.0, as well as in acceptability.
Lithium vs. quetiapine
Finnish investigators used prospective national databases to examine the rates of psychiatric and all-cause hospitalization during a mean 7.2 years of follow-up in all 18,018 Finns hospitalized for bipolar disorder. The purpose was to assess the impact of various mood stabilizers on overall health outcomes in a real-world setting.
The big winner was lithium. In an analysis adjusted for concomitant psychotropic medications, duration of bipolar illness, and intervals of drug exposure and nonexposure, lithium was associated with the lowest risks of psychiatric rehospitalization and all-cause hospitalization, with relative risk reductions of 33% and 29%, respectively. In contrast, quetiapine, the most widely used antipsychotic agent, paled by comparison, achieving only an 8% reduction in the risk of psychiatric rehospitalization and a 7% decrease in all-cause hospitalization (JAMA Psychiatry. 2018 Apr 1;75[4]:347-55).
In addition, long-acting injectable antipsychotics were significantly more effective for prevention of hospitalization than oral antipsychotics.
“That is kind of shocking, because in some countries, long-acting injectables are not authorized and cannot be used. But I think after this article some regulatory changes are going to take place as a result,” Dr. Grande predicted.
“Another issue I thought was interesting, although it was not the main aim of the study, involved benzodiazepines. They increased the risk of hospitalizations, both for psychiatric illness and all other causes. So apart from giving lithium and long-acting injectable antipsychotics to our bipolar patients, we should also be really careful about the use of benzodiazepines,” she commented.
Intranasal esketamine for suicidality?
Esketamine nasal spray, a fast-acting N-methyl-D-aspartate antagonist whose application for marketing approval in combination with a standard oral antidepressant in treatment-resistant depression is now under Food and Drug Administration review, also is being developed for another indication: reduction of suicidality in patients at imminent suicide risk. In a proof-of-concept study, intranasal esketamine resulted in a significant reduction in suicidal thoughts 4 hours after administration, compared with usual care – but not at 24 hours (Am J Psychiatry. 2018 Jul 1;175[7]:620-30).
New and effective medications for this indication are sorely needed. The only drug approved for the indication of suicide prevention is clozapine.
‘Latest thinking’ on bipolar disorders
Dr. Grande coauthored a comprehensive review article on bipolar disorders that she recommended as worthwhile reading (Nat Rev Dis Primers. 2018 Mar 8;4:18008. doi: 10.1038/nrdp.2018.8).
“It covers all the latest thinking. It focuses on the early stages of the disorder, how epigenetic factors are essential, and many other topics, including the bipolarity index being developed at the University of Barcelona to classify drugs in terms of their capacity to prevent episodes of mania or depression in terms of number needed to treat and number needed to harm. It emphasizes the importance of intervening early and focusing on cognitive dysfunction,” Dr Grande said.
Psychedelics making a comeback
German and Swiss investigators used a facial expression discrimination task to demonstrate that psilocybin, a 5-hydroxytryptamine2A–receptor agonist, decreases connectivity between the amygdala and regions of the brain important in emotion processing, including the striatum and frontal pole. The investigators theorized that this might be the mechanism for the psychedelic’s apparent antidepressant effects (Eur Neuropsychopharmacol. 2018 Jun;28[6]:691-700).
Dr. Grande included this study in her top publications list because it reflects the rapidly growing rebirth of interest in psychedelics research among European psychiatrists.
Indeed, elsewhere at the ECNP congress David J. Nutt, DM, declared, “We now have the beginnings of some swinging of the pendulum back in a modern direction. Over the last 10 years there have been a small number of open studies, all done with psilocybin, which is somewhat easier to use than LSD. There are studies in OCD [obsessive-compulsive disorder], tobacco dependence, alcoholism, resistant depression, end-of-life mood changes with cancer and other terminal diseases, and at least two ongoing randomized trials in resistant depression.”
Dr. Nutt, professor of neuropsychopharmacology at Imperial College London, was senior author of the first proof-of-concept study of psilocybin accompanied by psychologic support as a novel therapy for moderate to severe treatment-resistant major depression (Lancet Psychiatry. 2016 Jul;3[7]:619-27).
Methylphenidate ineffective for treatment of acute mania
The MEMAP study was a randomized, double-blind, placebo-controlled multicenter clinical trial testing what has been called the vigilance regulation model of mania. This model hypothesized that unstable regulation of wakefulness figures prominently in the pathogenesis of both mania and attention-deficit/hyperactivity disorder. If true, investigators reasoned, then 2.5 days of methylphenidate at 20-40 mg/day should have a rapid antimanic effect similar to the drug’s benefits in ADHD. Dr. Grande had been a skeptic, and indeed, the trial was halted early for futility (Eur Neuropsychopharmacol. 2018 Jan;28[1]:185-94).
She reported serving as a paid speaker for Lunbeck, Ferrer, GlaxoSmithKline, and Janssen. Her own research is funded by the Spanish Ministry of Economy and Competitiveness.
BARCELONA – Among the handful of top publications on mood disorders during the first three-quarters of 2018 was a landmark comparison of the efficacy and acceptability of 21 antidepressants for acute treatment of major depressive disorder, Íria Grande, MD, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
Dr. Grande, a psychiatrist at the bipolar disorders clinic of the University of Barcelona, shared her personal top picks.
‘Antidepressants work’
This epic systematic review and network meta-analysis (Lancet. 2018 Apr 7;391[10128]:1357-66) encompassed 522 randomized double-blind trials with 116,477 participants with major depressive disorder assigned to 21 antidepressants or placebo, in some instances with an additional active comparator antidepressant arm. The report is a major extension of previous work by the same multinational group of investigators (Lancet. 2009 Feb 28;373[9665]:746-58), who initially scrutinized 12 older antidepressants in a total population only one-quarter the size of the updated analysis.
Based upon this vast randomized trial evidence, some of which came from unpublished studies tracked down by the investigators, the 21 antidepressants were rank-ordered in terms of effectiveness and acceptability. But in Dr. Grande’s view, the most important study finding wasn’t which antidepressant donned the crown of most effective or patient acceptable, it was the fact that all 21 drugs proved significantly more effective than placebo, with odds ratios ranging from 2.13 at the top end to 1.37 for reboxetine.
“The results showed antidepressants work. All of the antipsychiatry system is trying to show us that antidepressants do not work in major depression. Well, in this study, it has been proven that all antidepressants are more effective than placebo in major depressive disorder. I think social media should be made aware of that. (Lead investigator) Dr. Andrea Cipriani talked on the BBC about this article, and it had a high impact,” according to Dr. Grande.
All but three of the 21 antidepressants were deemed to be as acceptable as placebo, based upon study dropout rates. The exceptions were agomelatine and fluoxetine, which were 12%-14% more acceptable than placebo. “That’s strange, I think, but that’s what the clinical trial results showed,” she noted. The findings on clomipramine, which was 30% less acceptable than placebo, make sense, Dr. Grande said, “due to its muscarinic effects.”
She took issue with some of the specific study findings. For example, the two top-rated antidepressants in terms of efficacy were amitriptyline and mirtazapine, with odds ratios of 2.13 and 1.89, respectively.
“As a clinician, I don’t consider mirtazapine to be one of the best antidepressants, especially in major depression,” she said. “But these are the results, and as always, we have to adapt the evidence-based medicine and consider it from our clinical point of view.”
The investigators conducted a subanalysis restricted to placebo-controlled head-to-head studies with a comparator antidepressant which Dr. Grande found more interesting and informative than the overall analysis. In the head-to-head analysis, vortioxetine emerged as the top-rated antidepressant, both in efficacy, with an odds ratio of 2.0, as well as in acceptability.
Lithium vs. quetiapine
Finnish investigators used prospective national databases to examine the rates of psychiatric and all-cause hospitalization during a mean 7.2 years of follow-up in all 18,018 Finns hospitalized for bipolar disorder. The purpose was to assess the impact of various mood stabilizers on overall health outcomes in a real-world setting.
The big winner was lithium. In an analysis adjusted for concomitant psychotropic medications, duration of bipolar illness, and intervals of drug exposure and nonexposure, lithium was associated with the lowest risks of psychiatric rehospitalization and all-cause hospitalization, with relative risk reductions of 33% and 29%, respectively. In contrast, quetiapine, the most widely used antipsychotic agent, paled by comparison, achieving only an 8% reduction in the risk of psychiatric rehospitalization and a 7% decrease in all-cause hospitalization (JAMA Psychiatry. 2018 Apr 1;75[4]:347-55).
In addition, long-acting injectable antipsychotics were significantly more effective for prevention of hospitalization than oral antipsychotics.
“That is kind of shocking, because in some countries, long-acting injectables are not authorized and cannot be used. But I think after this article some regulatory changes are going to take place as a result,” Dr. Grande predicted.
“Another issue I thought was interesting, although it was not the main aim of the study, involved benzodiazepines. They increased the risk of hospitalizations, both for psychiatric illness and all other causes. So apart from giving lithium and long-acting injectable antipsychotics to our bipolar patients, we should also be really careful about the use of benzodiazepines,” she commented.
Intranasal esketamine for suicidality?
Esketamine nasal spray, a fast-acting N-methyl-D-aspartate antagonist whose application for marketing approval in combination with a standard oral antidepressant in treatment-resistant depression is now under Food and Drug Administration review, also is being developed for another indication: reduction of suicidality in patients at imminent suicide risk. In a proof-of-concept study, intranasal esketamine resulted in a significant reduction in suicidal thoughts 4 hours after administration, compared with usual care – but not at 24 hours (Am J Psychiatry. 2018 Jul 1;175[7]:620-30).
New and effective medications for this indication are sorely needed. The only drug approved for the indication of suicide prevention is clozapine.
‘Latest thinking’ on bipolar disorders
Dr. Grande coauthored a comprehensive review article on bipolar disorders that she recommended as worthwhile reading (Nat Rev Dis Primers. 2018 Mar 8;4:18008. doi: 10.1038/nrdp.2018.8).
“It covers all the latest thinking. It focuses on the early stages of the disorder, how epigenetic factors are essential, and many other topics, including the bipolarity index being developed at the University of Barcelona to classify drugs in terms of their capacity to prevent episodes of mania or depression in terms of number needed to treat and number needed to harm. It emphasizes the importance of intervening early and focusing on cognitive dysfunction,” Dr Grande said.
Psychedelics making a comeback
German and Swiss investigators used a facial expression discrimination task to demonstrate that psilocybin, a 5-hydroxytryptamine2A–receptor agonist, decreases connectivity between the amygdala and regions of the brain important in emotion processing, including the striatum and frontal pole. The investigators theorized that this might be the mechanism for the psychedelic’s apparent antidepressant effects (Eur Neuropsychopharmacol. 2018 Jun;28[6]:691-700).
Dr. Grande included this study in her top publications list because it reflects the rapidly growing rebirth of interest in psychedelics research among European psychiatrists.
Indeed, elsewhere at the ECNP congress David J. Nutt, DM, declared, “We now have the beginnings of some swinging of the pendulum back in a modern direction. Over the last 10 years there have been a small number of open studies, all done with psilocybin, which is somewhat easier to use than LSD. There are studies in OCD [obsessive-compulsive disorder], tobacco dependence, alcoholism, resistant depression, end-of-life mood changes with cancer and other terminal diseases, and at least two ongoing randomized trials in resistant depression.”
Dr. Nutt, professor of neuropsychopharmacology at Imperial College London, was senior author of the first proof-of-concept study of psilocybin accompanied by psychologic support as a novel therapy for moderate to severe treatment-resistant major depression (Lancet Psychiatry. 2016 Jul;3[7]:619-27).
Methylphenidate ineffective for treatment of acute mania
The MEMAP study was a randomized, double-blind, placebo-controlled multicenter clinical trial testing what has been called the vigilance regulation model of mania. This model hypothesized that unstable regulation of wakefulness figures prominently in the pathogenesis of both mania and attention-deficit/hyperactivity disorder. If true, investigators reasoned, then 2.5 days of methylphenidate at 20-40 mg/day should have a rapid antimanic effect similar to the drug’s benefits in ADHD. Dr. Grande had been a skeptic, and indeed, the trial was halted early for futility (Eur Neuropsychopharmacol. 2018 Jan;28[1]:185-94).
She reported serving as a paid speaker for Lunbeck, Ferrer, GlaxoSmithKline, and Janssen. Her own research is funded by the Spanish Ministry of Economy and Competitiveness.
REPORTING FROM THE ECNP CONGRESS
Improve cognitive symptoms of depression to boost work productivity
BARCELONA – (Assessment in Work Productivity and the Relationship with Cognitive Symptoms).
“We found that as patients rated themselves as improved in terms of cognition – ‘I can think better,’ ‘I can focus,’ ‘I’m concentrating better’ – there was a strong correlation at 12 weeks and later extended to 1 year with improved work productivity by as much as 75%. It’s pretty dramatic,” lead investigator Pratap Chokka, MD, said in an interview at the annual congress of the European College of Neuropsychopharmacology.
AtWoRK was a multicenter, open-label, naturalistic intervention study in which 219 gainfully employed Canadian adults with major depressive disorder (MDD) who had presented to primary care physicians or psychiatrists were placed on vortioxetine (Trintellex) flexibly dosed at 10-20 mg/day and scheduled for routine follow-up visits every 4 weeks for 52 weeks.
This was a patient population with severe depression, severe cognitive dysfunction, severe anxiety, and substantial functional impairment as reflected in their baseline scores on a variety of validated measures (see graphic). The study was designed to emulate real-world clinical practice.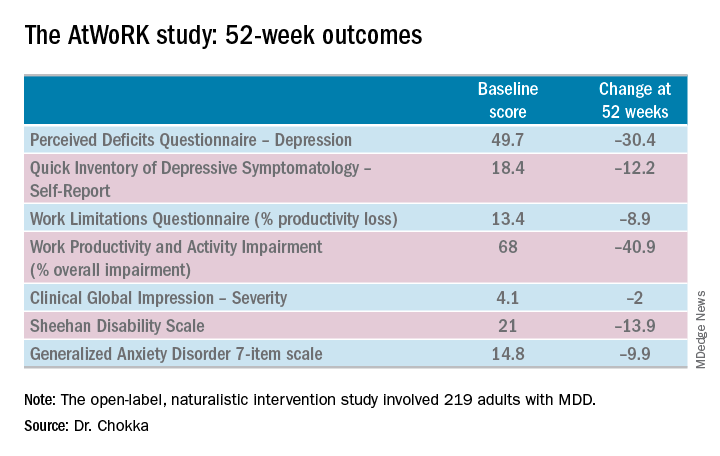
“We know that patients with depression are very impaired in terms of work productivity. Depressed patients really suffer from absenteeism and presenteeism [reduced productivity at work caused by depression]. And very few naturalistic studies have been done in working patients with depression,” according to Dr. Chokka. “The randomized trials are really important. They show us that a drug is working. But in terms of the real world that I work in, I need to have effectiveness: Does the drug work in patients with comorbid conditions, problems in their home lives, who are maybe drinking alcohol? Those are cases we’d rule out from participation in the RCTs.”
“The patients in our study walked into our clinics saying, ‘You know what, doctor, my mind isn’t working very good. I’m depressed, I can’t think, I can’t focus, I’m missing work, my boss is on my case, I’m making errors. I need help.’ These are the kinds of practicalities we wanted to address,” explained Dr. Chokka, a psychiatrist at Grey Nuns Community Hospital in Edmonton, Can.
The primary endpoint in AtWoRK was the correlation between changes in patients’ self-reported cognitive symptoms on the 20-item Perceived Deficits Questionnaire–Depression (PDQ-D-20) and changes in work productivity loss measured on the Work Limitations Questionnaire (WLQ) at week 12. Those 12-week results were recently published (CNS Spectr. 2018 May 24:1-10). At the ECNP congress, Dr. Chokka presented the expanded 52-week outcomes.
The correlation between change from baseline to week 12 in PDQ-D-20 and change in WLQ was strong (r = 0.606), and it remained strong at week 52 (r = 0.731; P less than .001).
At 52 weeks on vortioxetine, 77% of patients fulfilled criteria for MDD response, which required at least a 50% reduction in Quick Inventory of Depressive Symptomatology – Self-Report (QIDS-SR) score from baseline, and 56% for disease remission, which meant the QIDS-SR score was 5 or less. The response and remission rates were 71% and 45%, respectively, in the 107 subjects for whom the drug was the first treatment for their current MDD episode and 83% and 67% for the 112 switched to vortioxetine at study outset because the antidepressant they’d been on was ineffective.
Subjects also displayed significant improvement at 12 and 52 weeks in mood as assessed using QIDS-SR and global functioning as measured using the Sheehan Disability Scale (SDS). Of note, however, improvement in cognitive symptoms was independent of and not predictive of improvement in overall depressive symptoms on the QIDS-SR. Nor did improvement in depressive symptoms predict functional outcomes as assessed by the WLQ or SDS.
In Dr. Chokka’s view, these findings have clear implications for clinical practice: “In the past we thought that, if we can get the mood better, things will all get better. We now we know that treating depression is about more than just getting the mood better.”
Vortioxetine is an antidepressant with multiple agonist and antagonist effects on various 5-HT serotonin receptors.
The AtWoRK study was supported by Lundbeck Canada. Dr. Chokka reported receiving research grants from and serving on advisory boards and as a speaker for that company and others.
SOURCE: Chokka P. ECNP, P.022.
BARCELONA – (Assessment in Work Productivity and the Relationship with Cognitive Symptoms).
“We found that as patients rated themselves as improved in terms of cognition – ‘I can think better,’ ‘I can focus,’ ‘I’m concentrating better’ – there was a strong correlation at 12 weeks and later extended to 1 year with improved work productivity by as much as 75%. It’s pretty dramatic,” lead investigator Pratap Chokka, MD, said in an interview at the annual congress of the European College of Neuropsychopharmacology.
AtWoRK was a multicenter, open-label, naturalistic intervention study in which 219 gainfully employed Canadian adults with major depressive disorder (MDD) who had presented to primary care physicians or psychiatrists were placed on vortioxetine (Trintellex) flexibly dosed at 10-20 mg/day and scheduled for routine follow-up visits every 4 weeks for 52 weeks.
This was a patient population with severe depression, severe cognitive dysfunction, severe anxiety, and substantial functional impairment as reflected in their baseline scores on a variety of validated measures (see graphic). The study was designed to emulate real-world clinical practice.
“We know that patients with depression are very impaired in terms of work productivity. Depressed patients really suffer from absenteeism and presenteeism [reduced productivity at work caused by depression]. And very few naturalistic studies have been done in working patients with depression,” according to Dr. Chokka. “The randomized trials are really important. They show us that a drug is working. But in terms of the real world that I work in, I need to have effectiveness: Does the drug work in patients with comorbid conditions, problems in their home lives, who are maybe drinking alcohol? Those are cases we’d rule out from participation in the RCTs.”
“The patients in our study walked into our clinics saying, ‘You know what, doctor, my mind isn’t working very good. I’m depressed, I can’t think, I can’t focus, I’m missing work, my boss is on my case, I’m making errors. I need help.’ These are the kinds of practicalities we wanted to address,” explained Dr. Chokka, a psychiatrist at Grey Nuns Community Hospital in Edmonton, Can.
The primary endpoint in AtWoRK was the correlation between changes in patients’ self-reported cognitive symptoms on the 20-item Perceived Deficits Questionnaire–Depression (PDQ-D-20) and changes in work productivity loss measured on the Work Limitations Questionnaire (WLQ) at week 12. Those 12-week results were recently published (CNS Spectr. 2018 May 24:1-10). At the ECNP congress, Dr. Chokka presented the expanded 52-week outcomes.
The correlation between change from baseline to week 12 in PDQ-D-20 and change in WLQ was strong (r = 0.606), and it remained strong at week 52 (r = 0.731; P less than .001).
At 52 weeks on vortioxetine, 77% of patients fulfilled criteria for MDD response, which required at least a 50% reduction in Quick Inventory of Depressive Symptomatology – Self-Report (QIDS-SR) score from baseline, and 56% for disease remission, which meant the QIDS-SR score was 5 or less. The response and remission rates were 71% and 45%, respectively, in the 107 subjects for whom the drug was the first treatment for their current MDD episode and 83% and 67% for the 112 switched to vortioxetine at study outset because the antidepressant they’d been on was ineffective.
Subjects also displayed significant improvement at 12 and 52 weeks in mood as assessed using QIDS-SR and global functioning as measured using the Sheehan Disability Scale (SDS). Of note, however, improvement in cognitive symptoms was independent of and not predictive of improvement in overall depressive symptoms on the QIDS-SR. Nor did improvement in depressive symptoms predict functional outcomes as assessed by the WLQ or SDS.
In Dr. Chokka’s view, these findings have clear implications for clinical practice: “In the past we thought that, if we can get the mood better, things will all get better. We now we know that treating depression is about more than just getting the mood better.”
Vortioxetine is an antidepressant with multiple agonist and antagonist effects on various 5-HT serotonin receptors.
The AtWoRK study was supported by Lundbeck Canada. Dr. Chokka reported receiving research grants from and serving on advisory boards and as a speaker for that company and others.
SOURCE: Chokka P. ECNP, P.022.
BARCELONA – (Assessment in Work Productivity and the Relationship with Cognitive Symptoms).
“We found that as patients rated themselves as improved in terms of cognition – ‘I can think better,’ ‘I can focus,’ ‘I’m concentrating better’ – there was a strong correlation at 12 weeks and later extended to 1 year with improved work productivity by as much as 75%. It’s pretty dramatic,” lead investigator Pratap Chokka, MD, said in an interview at the annual congress of the European College of Neuropsychopharmacology.
AtWoRK was a multicenter, open-label, naturalistic intervention study in which 219 gainfully employed Canadian adults with major depressive disorder (MDD) who had presented to primary care physicians or psychiatrists were placed on vortioxetine (Trintellex) flexibly dosed at 10-20 mg/day and scheduled for routine follow-up visits every 4 weeks for 52 weeks.
This was a patient population with severe depression, severe cognitive dysfunction, severe anxiety, and substantial functional impairment as reflected in their baseline scores on a variety of validated measures (see graphic). The study was designed to emulate real-world clinical practice.
“We know that patients with depression are very impaired in terms of work productivity. Depressed patients really suffer from absenteeism and presenteeism [reduced productivity at work caused by depression]. And very few naturalistic studies have been done in working patients with depression,” according to Dr. Chokka. “The randomized trials are really important. They show us that a drug is working. But in terms of the real world that I work in, I need to have effectiveness: Does the drug work in patients with comorbid conditions, problems in their home lives, who are maybe drinking alcohol? Those are cases we’d rule out from participation in the RCTs.”
“The patients in our study walked into our clinics saying, ‘You know what, doctor, my mind isn’t working very good. I’m depressed, I can’t think, I can’t focus, I’m missing work, my boss is on my case, I’m making errors. I need help.’ These are the kinds of practicalities we wanted to address,” explained Dr. Chokka, a psychiatrist at Grey Nuns Community Hospital in Edmonton, Can.
The primary endpoint in AtWoRK was the correlation between changes in patients’ self-reported cognitive symptoms on the 20-item Perceived Deficits Questionnaire–Depression (PDQ-D-20) and changes in work productivity loss measured on the Work Limitations Questionnaire (WLQ) at week 12. Those 12-week results were recently published (CNS Spectr. 2018 May 24:1-10). At the ECNP congress, Dr. Chokka presented the expanded 52-week outcomes.
The correlation between change from baseline to week 12 in PDQ-D-20 and change in WLQ was strong (r = 0.606), and it remained strong at week 52 (r = 0.731; P less than .001).
At 52 weeks on vortioxetine, 77% of patients fulfilled criteria for MDD response, which required at least a 50% reduction in Quick Inventory of Depressive Symptomatology – Self-Report (QIDS-SR) score from baseline, and 56% for disease remission, which meant the QIDS-SR score was 5 or less. The response and remission rates were 71% and 45%, respectively, in the 107 subjects for whom the drug was the first treatment for their current MDD episode and 83% and 67% for the 112 switched to vortioxetine at study outset because the antidepressant they’d been on was ineffective.
Subjects also displayed significant improvement at 12 and 52 weeks in mood as assessed using QIDS-SR and global functioning as measured using the Sheehan Disability Scale (SDS). Of note, however, improvement in cognitive symptoms was independent of and not predictive of improvement in overall depressive symptoms on the QIDS-SR. Nor did improvement in depressive symptoms predict functional outcomes as assessed by the WLQ or SDS.
In Dr. Chokka’s view, these findings have clear implications for clinical practice: “In the past we thought that, if we can get the mood better, things will all get better. We now we know that treating depression is about more than just getting the mood better.”
Vortioxetine is an antidepressant with multiple agonist and antagonist effects on various 5-HT serotonin receptors.
The AtWoRK study was supported by Lundbeck Canada. Dr. Chokka reported receiving research grants from and serving on advisory boards and as a speaker for that company and others.
SOURCE: Chokka P. ECNP, P.022.
REPORTING FROM THE ECNP CONGRESS
Key clinical point: Treat cognitive symptoms of depression to improve impaired work productivity.
Major finding: Impaired work productivity in depressed patients improved greatly in response to reduction in cognitive symptoms, but not with enhanced mood.
Study details: A 52-week, multicenter, open-label study in which 219 employed adults with major depression were placed on vortioxetine and serially assessed for changes in cognitive dysfunction, mood, and work productivity.
Disclosures: The AtWoRK study was supported by Lundbeck Canada. The presenter reported receiving research grants from and serving on advisory boards and as a speaker for that company and others.
Source: Chokka P. ECNP, P.022.
Brexanolone injection quickly improves postpartum depression
BARCELONA – Brexanolone injection provided rapid and durable improvement in postpartum depression in an integrated analysis of three pivotal randomized trials collectively known as the Hummingbird trials, Christine Clemson, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
This was accomplished with a favorable safety experience. The most common treatment-emergent adverse events – dizziness and sleepiness – were roughly twice as common as with placebo in the 247-patient Hummingbird safety analysis.
On Dec. 19, 2018, the agency is expected to respond to SAGE Therapeutics’s application for marketing approval of intravenous brexanolone given as a continuous 60-hour infusion at 90 mcg/kg per hour, according to Dr. Clemson, senior medical director at Cambridge, Mass.–based SAGE Therapeutics, which is developing the therapy.
Brexanolone is a proprietary IV formulation of allopregnanolone, a metabolite of progesterone. The drug’s mechanism of action involves modulation of the neurotransmitter gamma-aminobutyric acid (GABA). The drug binds to both synaptic and extra-synaptic GABA A receptors, thereby increasing receptor functionality.
The decision to target GABA as a novel therapeutic strategy in PPD was based upon translational studies demonstrating that GABA is the chief neuroinhibitory mechanism in the brain, and its actions are mediated mainly by GABA A receptors. Brexanolone’s efficacy is consistent with a theory that the pathogenesis of PPD involves triggers such as inflammation, hormonal fluctuations, or chronic stress, which in some women cause GABA hypofunction, both at the receptors and in terms of tissue GABA levels. This, in turn, leads to an overactive HPA axis and dysregulated neural networks, with resultant PPD, Dr. Clemson explained.
The three Hummingbird clinical trials were double blind, randomized, and placebo controlled. Two were restricted to women with severe PPD. The third and largest focused on moderately affected patients as defined by a baseline Hamilton Depression Scale for Depression (HAM-D) score of 20-25.
The efficacy analysis included 207 patients who received brexanolone at 90 mcg/kg per hour or placebo for 60 hours in an inpatient setting and were followed for 30 days. The primary endpoint was the change in HAM-D total score from baseline to 60 hours. The mean 17-point reduction in the active treatment arm was significantly better than the 12.8-point decrease with placebo. The between-group difference was significant within the first 24 hours and remained so at all time points out to the study’s end at day 30. There was no individual item on the HAM-D in which the drug performed worse than placebo, and there were many in which brexanolone performed significantly better, including depressed mood, anxiety, insomnia, and feelings of guilt.
In terms of the rigorous secondary endpoint of HAM-D remission as defined by a total score of 7 or less, the brexanolone injection significantly outperformed placebo at every time point except for day 30.
There was a 2% rate of serious adverse events in both study arms. These included suicidal ideation, an intentional overdose attempt post discharge, altered state of consciousness, and syncope.
A vastly more convenient once-daily oral formulation of brexanolone is now in phase 3 clinical trials for PPD and major depressive disorder, and in phase 2 for insomnia and bipolar depression.
Elsewhere at the ECNP congress, other investigators from Sage Therapeutics presented for the first time the outcomes of an 89-patient, randomized, double-blind, multicenter, placebo-controlled, phase 2 clinical trial of SAGE-217 for treatment of major depressive disorder.
Participants received a nightly 30-mg dose of the drug or placebo for 2 weeks, with a primary study endpoint being the change in HAM-D total score from baseline to day 15. Patients on the oral GABA A receptor positive modulator averaged a 17.4-point improvement, significantly better than the 10.3-point spread in placebo-treated controls. A statistically significant between-group difference was noted on days 2 through 28. HAM-D remission at day 15 was documented in 64% of the oral brexanolone group, compared with 26% of controls.
Those improvements in depression were accompanied by significant gains in numerous domains of health-related quality of life as assessed via the 36-Item Short Form Health Survey. Indeed, day 15 health-related quality of life scores in the oral brexanolone group approached normative values for the general population.
The studies were funded by SAGE Therapeutics.
BARCELONA – Brexanolone injection provided rapid and durable improvement in postpartum depression in an integrated analysis of three pivotal randomized trials collectively known as the Hummingbird trials, Christine Clemson, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
This was accomplished with a favorable safety experience. The most common treatment-emergent adverse events – dizziness and sleepiness – were roughly twice as common as with placebo in the 247-patient Hummingbird safety analysis.
On Dec. 19, 2018, the agency is expected to respond to SAGE Therapeutics’s application for marketing approval of intravenous brexanolone given as a continuous 60-hour infusion at 90 mcg/kg per hour, according to Dr. Clemson, senior medical director at Cambridge, Mass.–based SAGE Therapeutics, which is developing the therapy.
Brexanolone is a proprietary IV formulation of allopregnanolone, a metabolite of progesterone. The drug’s mechanism of action involves modulation of the neurotransmitter gamma-aminobutyric acid (GABA). The drug binds to both synaptic and extra-synaptic GABA A receptors, thereby increasing receptor functionality.
The decision to target GABA as a novel therapeutic strategy in PPD was based upon translational studies demonstrating that GABA is the chief neuroinhibitory mechanism in the brain, and its actions are mediated mainly by GABA A receptors. Brexanolone’s efficacy is consistent with a theory that the pathogenesis of PPD involves triggers such as inflammation, hormonal fluctuations, or chronic stress, which in some women cause GABA hypofunction, both at the receptors and in terms of tissue GABA levels. This, in turn, leads to an overactive HPA axis and dysregulated neural networks, with resultant PPD, Dr. Clemson explained.
The three Hummingbird clinical trials were double blind, randomized, and placebo controlled. Two were restricted to women with severe PPD. The third and largest focused on moderately affected patients as defined by a baseline Hamilton Depression Scale for Depression (HAM-D) score of 20-25.
The efficacy analysis included 207 patients who received brexanolone at 90 mcg/kg per hour or placebo for 60 hours in an inpatient setting and were followed for 30 days. The primary endpoint was the change in HAM-D total score from baseline to 60 hours. The mean 17-point reduction in the active treatment arm was significantly better than the 12.8-point decrease with placebo. The between-group difference was significant within the first 24 hours and remained so at all time points out to the study’s end at day 30. There was no individual item on the HAM-D in which the drug performed worse than placebo, and there were many in which brexanolone performed significantly better, including depressed mood, anxiety, insomnia, and feelings of guilt.
In terms of the rigorous secondary endpoint of HAM-D remission as defined by a total score of 7 or less, the brexanolone injection significantly outperformed placebo at every time point except for day 30.
There was a 2% rate of serious adverse events in both study arms. These included suicidal ideation, an intentional overdose attempt post discharge, altered state of consciousness, and syncope.
A vastly more convenient once-daily oral formulation of brexanolone is now in phase 3 clinical trials for PPD and major depressive disorder, and in phase 2 for insomnia and bipolar depression.
Elsewhere at the ECNP congress, other investigators from Sage Therapeutics presented for the first time the outcomes of an 89-patient, randomized, double-blind, multicenter, placebo-controlled, phase 2 clinical trial of SAGE-217 for treatment of major depressive disorder.
Participants received a nightly 30-mg dose of the drug or placebo for 2 weeks, with a primary study endpoint being the change in HAM-D total score from baseline to day 15. Patients on the oral GABA A receptor positive modulator averaged a 17.4-point improvement, significantly better than the 10.3-point spread in placebo-treated controls. A statistically significant between-group difference was noted on days 2 through 28. HAM-D remission at day 15 was documented in 64% of the oral brexanolone group, compared with 26% of controls.
Those improvements in depression were accompanied by significant gains in numerous domains of health-related quality of life as assessed via the 36-Item Short Form Health Survey. Indeed, day 15 health-related quality of life scores in the oral brexanolone group approached normative values for the general population.
The studies were funded by SAGE Therapeutics.
BARCELONA – Brexanolone injection provided rapid and durable improvement in postpartum depression in an integrated analysis of three pivotal randomized trials collectively known as the Hummingbird trials, Christine Clemson, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
This was accomplished with a favorable safety experience. The most common treatment-emergent adverse events – dizziness and sleepiness – were roughly twice as common as with placebo in the 247-patient Hummingbird safety analysis.
On Dec. 19, 2018, the agency is expected to respond to SAGE Therapeutics’s application for marketing approval of intravenous brexanolone given as a continuous 60-hour infusion at 90 mcg/kg per hour, according to Dr. Clemson, senior medical director at Cambridge, Mass.–based SAGE Therapeutics, which is developing the therapy.
Brexanolone is a proprietary IV formulation of allopregnanolone, a metabolite of progesterone. The drug’s mechanism of action involves modulation of the neurotransmitter gamma-aminobutyric acid (GABA). The drug binds to both synaptic and extra-synaptic GABA A receptors, thereby increasing receptor functionality.
The decision to target GABA as a novel therapeutic strategy in PPD was based upon translational studies demonstrating that GABA is the chief neuroinhibitory mechanism in the brain, and its actions are mediated mainly by GABA A receptors. Brexanolone’s efficacy is consistent with a theory that the pathogenesis of PPD involves triggers such as inflammation, hormonal fluctuations, or chronic stress, which in some women cause GABA hypofunction, both at the receptors and in terms of tissue GABA levels. This, in turn, leads to an overactive HPA axis and dysregulated neural networks, with resultant PPD, Dr. Clemson explained.
The three Hummingbird clinical trials were double blind, randomized, and placebo controlled. Two were restricted to women with severe PPD. The third and largest focused on moderately affected patients as defined by a baseline Hamilton Depression Scale for Depression (HAM-D) score of 20-25.
The efficacy analysis included 207 patients who received brexanolone at 90 mcg/kg per hour or placebo for 60 hours in an inpatient setting and were followed for 30 days. The primary endpoint was the change in HAM-D total score from baseline to 60 hours. The mean 17-point reduction in the active treatment arm was significantly better than the 12.8-point decrease with placebo. The between-group difference was significant within the first 24 hours and remained so at all time points out to the study’s end at day 30. There was no individual item on the HAM-D in which the drug performed worse than placebo, and there were many in which brexanolone performed significantly better, including depressed mood, anxiety, insomnia, and feelings of guilt.
In terms of the rigorous secondary endpoint of HAM-D remission as defined by a total score of 7 or less, the brexanolone injection significantly outperformed placebo at every time point except for day 30.
There was a 2% rate of serious adverse events in both study arms. These included suicidal ideation, an intentional overdose attempt post discharge, altered state of consciousness, and syncope.
A vastly more convenient once-daily oral formulation of brexanolone is now in phase 3 clinical trials for PPD and major depressive disorder, and in phase 2 for insomnia and bipolar depression.
Elsewhere at the ECNP congress, other investigators from Sage Therapeutics presented for the first time the outcomes of an 89-patient, randomized, double-blind, multicenter, placebo-controlled, phase 2 clinical trial of SAGE-217 for treatment of major depressive disorder.
Participants received a nightly 30-mg dose of the drug or placebo for 2 weeks, with a primary study endpoint being the change in HAM-D total score from baseline to day 15. Patients on the oral GABA A receptor positive modulator averaged a 17.4-point improvement, significantly better than the 10.3-point spread in placebo-treated controls. A statistically significant between-group difference was noted on days 2 through 28. HAM-D remission at day 15 was documented in 64% of the oral brexanolone group, compared with 26% of controls.
Those improvements in depression were accompanied by significant gains in numerous domains of health-related quality of life as assessed via the 36-Item Short Form Health Survey. Indeed, day 15 health-related quality of life scores in the oral brexanolone group approached normative values for the general population.
The studies were funded by SAGE Therapeutics.
REPORTING FROM THE ECNP CONGRESS
Key clinical point: A novel investigational GABA modulator is turning heads for treatment of postpartum depression.
Major finding: At 60 hours, mean HAM-D total scores had dropped by 17 points with brexanolone, versus 12.8 with placebo.
Study details: A prespecified integrated safety and efficacy analysis incorporating three pivotal clinical trials.
Disclosures: The studies were funded by SAGE Therapeutics and presented by a company executive.
Lithium/cancer link debunked
BARCELONA – A large Swedish national registry study has found no hint of increased cancer risk in bipolar patients on long-term lithium therapy.
“This is a very important null result. There is no increased risk for cancer for bipolar patients on lithium. It’s a bad rumor. It’s important to tell patients we’re very confident this is true. We studied every single type of cancer. We would have seen something here if there was something to see,” Lina Martinsson, MD, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
Using comprehensive registry data on nearly 2.6 million Swedes aged 50-84 years with 4 years of follow-up, including 2,393 patients with bipolar disorder on long-term lithium and 3,049 patients not on lithium, the overall cancer incidence rate was 5.9% in the group on lithium and 6.0% in those not taking the drug. Those rates were not different from the general Swedish population, reported Dr. Martinsson, a senior psychiatrist at the Karolinska Institute in Stockholm.
Such patients had a 72% greater risk of lung cancer and other cancers of the respiratory system than the general population, a 47% increased risk of GI cancers, and a 150% greater risk of endocrine organ cancers.
“The increase in respiratory and digestive organ cancers might depend upon bipolar patients’ tendency for smoking and other types of hard living. We can’t explain the increase in endocrine cancers,” she said.
In contrast, the rates of these types of cancer were no different from the general population in bipolar patients taking lithium, hinting at a possible protective effect of the drug, although this remains speculative, the psychiatrist added.
The question of whether lithium is associated with increased cancer risk has been controversial. In particular, several groups have reported a possible increased risk of renal cancer on the basis of what Dr. Martinsson considers weak evidence. She felt a responsibility to undertake this definitive Swedish national study examining the issue because the cancer speculation arose following her earlier study demonstrating that bipolar patients on lithium had much longer telomeres than those not on the drug, and that the ones who responded well to lithium had longer telomeres than those who did not (Transl Psychiatry. 2013 May 21. doi: 10.1038/tp.2013.37).
“If longer telomere length gives longer life to the wrong cells, it might enhance the risk of cancer,” she noted.
But this theoretical concern did not hold up under close Swedish scrutiny. “Warnings for cancer in patients with long-term lithium treatment are unnecessary and ought to be omitted from the current policies,” Dr. Martinsson said.
She reported no financial conflicts regarding her study, which was funded by the Swedish Research Council.
BARCELONA – A large Swedish national registry study has found no hint of increased cancer risk in bipolar patients on long-term lithium therapy.
“This is a very important null result. There is no increased risk for cancer for bipolar patients on lithium. It’s a bad rumor. It’s important to tell patients we’re very confident this is true. We studied every single type of cancer. We would have seen something here if there was something to see,” Lina Martinsson, MD, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
Using comprehensive registry data on nearly 2.6 million Swedes aged 50-84 years with 4 years of follow-up, including 2,393 patients with bipolar disorder on long-term lithium and 3,049 patients not on lithium, the overall cancer incidence rate was 5.9% in the group on lithium and 6.0% in those not taking the drug. Those rates were not different from the general Swedish population, reported Dr. Martinsson, a senior psychiatrist at the Karolinska Institute in Stockholm.
Such patients had a 72% greater risk of lung cancer and other cancers of the respiratory system than the general population, a 47% increased risk of GI cancers, and a 150% greater risk of endocrine organ cancers.
“The increase in respiratory and digestive organ cancers might depend upon bipolar patients’ tendency for smoking and other types of hard living. We can’t explain the increase in endocrine cancers,” she said.
In contrast, the rates of these types of cancer were no different from the general population in bipolar patients taking lithium, hinting at a possible protective effect of the drug, although this remains speculative, the psychiatrist added.
The question of whether lithium is associated with increased cancer risk has been controversial. In particular, several groups have reported a possible increased risk of renal cancer on the basis of what Dr. Martinsson considers weak evidence. She felt a responsibility to undertake this definitive Swedish national study examining the issue because the cancer speculation arose following her earlier study demonstrating that bipolar patients on lithium had much longer telomeres than those not on the drug, and that the ones who responded well to lithium had longer telomeres than those who did not (Transl Psychiatry. 2013 May 21. doi: 10.1038/tp.2013.37).
“If longer telomere length gives longer life to the wrong cells, it might enhance the risk of cancer,” she noted.
But this theoretical concern did not hold up under close Swedish scrutiny. “Warnings for cancer in patients with long-term lithium treatment are unnecessary and ought to be omitted from the current policies,” Dr. Martinsson said.
She reported no financial conflicts regarding her study, which was funded by the Swedish Research Council.
BARCELONA – A large Swedish national registry study has found no hint of increased cancer risk in bipolar patients on long-term lithium therapy.
“This is a very important null result. There is no increased risk for cancer for bipolar patients on lithium. It’s a bad rumor. It’s important to tell patients we’re very confident this is true. We studied every single type of cancer. We would have seen something here if there was something to see,” Lina Martinsson, MD, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
Using comprehensive registry data on nearly 2.6 million Swedes aged 50-84 years with 4 years of follow-up, including 2,393 patients with bipolar disorder on long-term lithium and 3,049 patients not on lithium, the overall cancer incidence rate was 5.9% in the group on lithium and 6.0% in those not taking the drug. Those rates were not different from the general Swedish population, reported Dr. Martinsson, a senior psychiatrist at the Karolinska Institute in Stockholm.
Such patients had a 72% greater risk of lung cancer and other cancers of the respiratory system than the general population, a 47% increased risk of GI cancers, and a 150% greater risk of endocrine organ cancers.
“The increase in respiratory and digestive organ cancers might depend upon bipolar patients’ tendency for smoking and other types of hard living. We can’t explain the increase in endocrine cancers,” she said.
In contrast, the rates of these types of cancer were no different from the general population in bipolar patients taking lithium, hinting at a possible protective effect of the drug, although this remains speculative, the psychiatrist added.
The question of whether lithium is associated with increased cancer risk has been controversial. In particular, several groups have reported a possible increased risk of renal cancer on the basis of what Dr. Martinsson considers weak evidence. She felt a responsibility to undertake this definitive Swedish national study examining the issue because the cancer speculation arose following her earlier study demonstrating that bipolar patients on lithium had much longer telomeres than those not on the drug, and that the ones who responded well to lithium had longer telomeres than those who did not (Transl Psychiatry. 2013 May 21. doi: 10.1038/tp.2013.37).
“If longer telomere length gives longer life to the wrong cells, it might enhance the risk of cancer,” she noted.
But this theoretical concern did not hold up under close Swedish scrutiny. “Warnings for cancer in patients with long-term lithium treatment are unnecessary and ought to be omitted from the current policies,” Dr. Martinsson said.
She reported no financial conflicts regarding her study, which was funded by the Swedish Research Council.
REPORTING FROM THE ECNP CONGRESS
Key clinical point: Long-term lithium therapy does not increase cancer risk.
Major finding: The overall incidence of cancer during 4 years of follow-up was 5.9% in bipolar patients on long-term lithium and 6.0% in those who were not.
Study details: This Swedish national registry study compared cancer incidence rates in more than 5,400 patients with bipolar disorder and nearly 2.6 million controls.
Disclosures: The presenter reported no financial conflicts regarding the study, which was supported by the Swedish Research Council.
Promising novel antidepressant cruising in pipeline
BARCELONA – An investigational antidepressant known for now simply as MIN-117 shows the potential – at least, in phase 2 development – of offering significant advantages over currently available antidepressants in patients with major depressive disorder, Michael Davidson, MD, said at the annual congress of the European College of Neuropsychopharmacology.
“This is a compound with a very, very rich pharmacology – so rich that we don’t know exactly which of the pharmacologic effects are really making the difference. This rich pharmacology is related to pathways that may confer to MIN-117 a unique positioning in the field of antidepressants and address unmet medical needs not well covered by existing therapies. For example, faster onset of action, complete restoration of euthymia, and beneficial effects on cognition and sexual functioning,” according to Michael Davidson, MD, chief medical officer at Minerva Neurosciences, which is developing the drug..
In the completed phase 2 study, the drug also displayed a strong anxiolytic effect and no prolongation of REM sleep latency in polysomnographic testing.
“So it may be that this is the first antidepressant which does not affect sleep architecture,” observed Dr. Davidson, professor of psychiatry at the Sackler School of Medicine in Tel Aviv.
Preclinical studies established that MIN-117 has high affinity for the 5HT serotonin transporter, serotonin 1A and 2A receptors, the dopamine transporter, and the alpha-1A and -1B adrenergic receptors. In animal models of depression, the drug results in sustained release of dopamine and serotonin. In human studies, the drug has a long half-life of roughly 60 hours.
“One possibility is that MIN-117 will be administered not once a day, but once or twice a week. It may be that here we have an antidepressant that doesn’t have to be administered every day,” the psychiatrist said.
In the completed phase 2 study, however, the drug was given once daily in what he described as a “classic design” for an antidepressant clinical trial: a 4-week washout period, then 6 weeks of double-blind treatment with MIN-117 at 2.5 or 0.5 mg/day, paroxetine at 20 mg/day, or placebo, then a 2-week posttreatment follow-up phase.
In describing the results of the study of 84 patients with major depressive disorder, Dr. Davidson painted a picture of MIN-117’s safety and efficacy with broad strokes because the trial wasn’t powered to demonstrate statistically significant differences. But the treatment effect sizes for the novel drug were impressive. A much more complete picture of the safety and efficacy of MIN-117 will be provided by an ongoing 324-patient phase 2b multicenter U.S. and European trial of the drug given at 2.5 or 5 mg/day or placebo.
The primary endpoint in the completed trial was change from baseline to 6 weeks in mean Montgomery-Åsberg Rating Depression Scale (MADRS) score. From a baseline score of about 33, MADRS scores improved by 9 points with placebo, 11 with MIN-117 at 0.5 mg, and by 12 points with 2.5 mg of the drug. MIN-117 was superior to placebo in this regard from the time of the earliest assessment, at 2 weeks.
One-quarter of patients on MIN-117 at 2.5 mg/day achieved remission, prospectively defined as a MADRS score below 12. Remission was 2.1-fold more likely in this group than with placebo at week 4 and 3.1-fold more likely at 6 weeks. The remission rate with MIN-117 also was better than with paroxetine.
“So , and that we’ll be able to produce full remission of the depressive symptoms,” Dr. Davidson said.
MIN-117 also showed a solid anxiolytic effect, with mean scores on the Hamilton Anxiety Rating Scale – a secondary endpoint – improving by 10 points in both the 0.5- and 2.5-mg groups from a baseline of 26 points. As a result of this impressive showing, the large ongoing phase 2b study is recruiting patients with an ongoing episode of major depressive disorder and prominent secondary anxiety.
Both doses of MIN-117 were well tolerated. Scores on the Arizona Sexual Experiences Scale showed that the drug did not result in any impairment of sexual function. Nor was MIN-117 associated with evidence of cognitive impairment; in fact, scores on the Digit-Symbol Substitution Test and Digit Span Backwards tool were better than in the placebo arm.
The study was sponsored by Minerva Neurosciences and presented by the company’s chief medical officer.
BARCELONA – An investigational antidepressant known for now simply as MIN-117 shows the potential – at least, in phase 2 development – of offering significant advantages over currently available antidepressants in patients with major depressive disorder, Michael Davidson, MD, said at the annual congress of the European College of Neuropsychopharmacology.
“This is a compound with a very, very rich pharmacology – so rich that we don’t know exactly which of the pharmacologic effects are really making the difference. This rich pharmacology is related to pathways that may confer to MIN-117 a unique positioning in the field of antidepressants and address unmet medical needs not well covered by existing therapies. For example, faster onset of action, complete restoration of euthymia, and beneficial effects on cognition and sexual functioning,” according to Michael Davidson, MD, chief medical officer at Minerva Neurosciences, which is developing the drug..
In the completed phase 2 study, the drug also displayed a strong anxiolytic effect and no prolongation of REM sleep latency in polysomnographic testing.
“So it may be that this is the first antidepressant which does not affect sleep architecture,” observed Dr. Davidson, professor of psychiatry at the Sackler School of Medicine in Tel Aviv.
Preclinical studies established that MIN-117 has high affinity for the 5HT serotonin transporter, serotonin 1A and 2A receptors, the dopamine transporter, and the alpha-1A and -1B adrenergic receptors. In animal models of depression, the drug results in sustained release of dopamine and serotonin. In human studies, the drug has a long half-life of roughly 60 hours.
“One possibility is that MIN-117 will be administered not once a day, but once or twice a week. It may be that here we have an antidepressant that doesn’t have to be administered every day,” the psychiatrist said.
In the completed phase 2 study, however, the drug was given once daily in what he described as a “classic design” for an antidepressant clinical trial: a 4-week washout period, then 6 weeks of double-blind treatment with MIN-117 at 2.5 or 0.5 mg/day, paroxetine at 20 mg/day, or placebo, then a 2-week posttreatment follow-up phase.
In describing the results of the study of 84 patients with major depressive disorder, Dr. Davidson painted a picture of MIN-117’s safety and efficacy with broad strokes because the trial wasn’t powered to demonstrate statistically significant differences. But the treatment effect sizes for the novel drug were impressive. A much more complete picture of the safety and efficacy of MIN-117 will be provided by an ongoing 324-patient phase 2b multicenter U.S. and European trial of the drug given at 2.5 or 5 mg/day or placebo.
The primary endpoint in the completed trial was change from baseline to 6 weeks in mean Montgomery-Åsberg Rating Depression Scale (MADRS) score. From a baseline score of about 33, MADRS scores improved by 9 points with placebo, 11 with MIN-117 at 0.5 mg, and by 12 points with 2.5 mg of the drug. MIN-117 was superior to placebo in this regard from the time of the earliest assessment, at 2 weeks.
One-quarter of patients on MIN-117 at 2.5 mg/day achieved remission, prospectively defined as a MADRS score below 12. Remission was 2.1-fold more likely in this group than with placebo at week 4 and 3.1-fold more likely at 6 weeks. The remission rate with MIN-117 also was better than with paroxetine.
“So , and that we’ll be able to produce full remission of the depressive symptoms,” Dr. Davidson said.
MIN-117 also showed a solid anxiolytic effect, with mean scores on the Hamilton Anxiety Rating Scale – a secondary endpoint – improving by 10 points in both the 0.5- and 2.5-mg groups from a baseline of 26 points. As a result of this impressive showing, the large ongoing phase 2b study is recruiting patients with an ongoing episode of major depressive disorder and prominent secondary anxiety.
Both doses of MIN-117 were well tolerated. Scores on the Arizona Sexual Experiences Scale showed that the drug did not result in any impairment of sexual function. Nor was MIN-117 associated with evidence of cognitive impairment; in fact, scores on the Digit-Symbol Substitution Test and Digit Span Backwards tool were better than in the placebo arm.
The study was sponsored by Minerva Neurosciences and presented by the company’s chief medical officer.
BARCELONA – An investigational antidepressant known for now simply as MIN-117 shows the potential – at least, in phase 2 development – of offering significant advantages over currently available antidepressants in patients with major depressive disorder, Michael Davidson, MD, said at the annual congress of the European College of Neuropsychopharmacology.
“This is a compound with a very, very rich pharmacology – so rich that we don’t know exactly which of the pharmacologic effects are really making the difference. This rich pharmacology is related to pathways that may confer to MIN-117 a unique positioning in the field of antidepressants and address unmet medical needs not well covered by existing therapies. For example, faster onset of action, complete restoration of euthymia, and beneficial effects on cognition and sexual functioning,” according to Michael Davidson, MD, chief medical officer at Minerva Neurosciences, which is developing the drug..
In the completed phase 2 study, the drug also displayed a strong anxiolytic effect and no prolongation of REM sleep latency in polysomnographic testing.
“So it may be that this is the first antidepressant which does not affect sleep architecture,” observed Dr. Davidson, professor of psychiatry at the Sackler School of Medicine in Tel Aviv.
Preclinical studies established that MIN-117 has high affinity for the 5HT serotonin transporter, serotonin 1A and 2A receptors, the dopamine transporter, and the alpha-1A and -1B adrenergic receptors. In animal models of depression, the drug results in sustained release of dopamine and serotonin. In human studies, the drug has a long half-life of roughly 60 hours.
“One possibility is that MIN-117 will be administered not once a day, but once or twice a week. It may be that here we have an antidepressant that doesn’t have to be administered every day,” the psychiatrist said.
In the completed phase 2 study, however, the drug was given once daily in what he described as a “classic design” for an antidepressant clinical trial: a 4-week washout period, then 6 weeks of double-blind treatment with MIN-117 at 2.5 or 0.5 mg/day, paroxetine at 20 mg/day, or placebo, then a 2-week posttreatment follow-up phase.
In describing the results of the study of 84 patients with major depressive disorder, Dr. Davidson painted a picture of MIN-117’s safety and efficacy with broad strokes because the trial wasn’t powered to demonstrate statistically significant differences. But the treatment effect sizes for the novel drug were impressive. A much more complete picture of the safety and efficacy of MIN-117 will be provided by an ongoing 324-patient phase 2b multicenter U.S. and European trial of the drug given at 2.5 or 5 mg/day or placebo.
The primary endpoint in the completed trial was change from baseline to 6 weeks in mean Montgomery-Åsberg Rating Depression Scale (MADRS) score. From a baseline score of about 33, MADRS scores improved by 9 points with placebo, 11 with MIN-117 at 0.5 mg, and by 12 points with 2.5 mg of the drug. MIN-117 was superior to placebo in this regard from the time of the earliest assessment, at 2 weeks.
One-quarter of patients on MIN-117 at 2.5 mg/day achieved remission, prospectively defined as a MADRS score below 12. Remission was 2.1-fold more likely in this group than with placebo at week 4 and 3.1-fold more likely at 6 weeks. The remission rate with MIN-117 also was better than with paroxetine.
“So , and that we’ll be able to produce full remission of the depressive symptoms,” Dr. Davidson said.
MIN-117 also showed a solid anxiolytic effect, with mean scores on the Hamilton Anxiety Rating Scale – a secondary endpoint – improving by 10 points in both the 0.5- and 2.5-mg groups from a baseline of 26 points. As a result of this impressive showing, the large ongoing phase 2b study is recruiting patients with an ongoing episode of major depressive disorder and prominent secondary anxiety.
Both doses of MIN-117 were well tolerated. Scores on the Arizona Sexual Experiences Scale showed that the drug did not result in any impairment of sexual function. Nor was MIN-117 associated with evidence of cognitive impairment; in fact, scores on the Digit-Symbol Substitution Test and Digit Span Backwards tool were better than in the placebo arm.
The study was sponsored by Minerva Neurosciences and presented by the company’s chief medical officer.
REPORTING FROM THE ECNP CONGRESS
Key clinical point: An investigational antidepressant might address important unmet needs in the treatment of major depression.
Major finding: Patients with major depressive disorder were 3.1-fold more likely to be in remission after 6 weeks on MIN-117 at 2.5 mg/day than with placebo.
Study details: This prospective, double-blind, randomized, double-blind, placebo- and active-controlled study included 84 patients with major depressive disorder.
Disclosures: The study was sponsored by Minerva Neurosciences and presented by the company’s chief medical officer.













