User login
Know the red flags for synaptic autoimmune psychosis
BARCELONA – Consider the possibility of an autoantibody-related etiology in all cases of first-onset psychosis, Josep Dalmau, MD, PhD, urged at the annual congress of the European College of Neuropsychopharmacology.
“There are patients in our clinics all of us – neurologists and psychiatrists – are missing. These patients are believed to have psychiatric presentations, but they do not. They are autoimmune,” said Dr. Dalmau, professor of neurology at the University of Barcelona.
Dr. Dalmau urged psychiatrists to become familiar with the red flags suggestive of synaptic autoimmunity as the underlying cause of first-episode, out-of-the-blue psychosis.
“If you have a patient with a classical presentation of schizophrenia or bipolar disorder, you probably won’t find antibodies,” according to the neurologist.
It’s important to have a high index of suspicion, because anti–NMDA receptor encephalitis is treatable with immunotherapy. And firm evidence shows that earlier recognition and treatment lead to improved outcomes. Also, the disorder is refractory to antipsychotics; indeed, 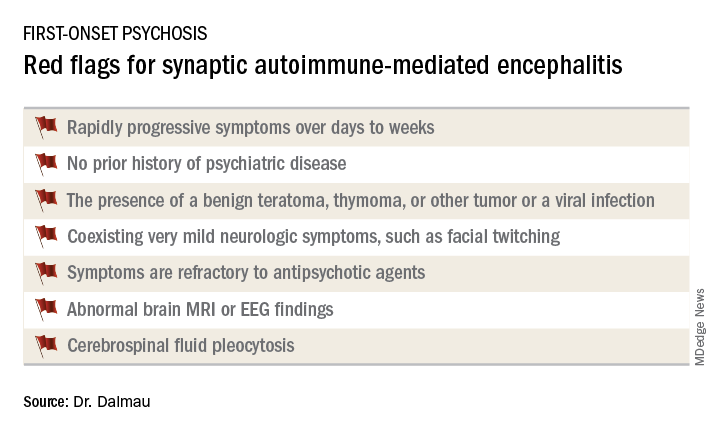
Manifestations of anti–NMDA receptor encephalitis follow a characteristic pattern, beginning with a prodromal flulike phase lasting several days to a week. This is followed by acute-onset bizarre behavioral changes, irritability, and psychosis with delusions and/or hallucinations, often progressing to catatonia. After 1-4 weeks of this, florid neurologic symptoms usually appear, including seizures, abnormal movements, autonomic dysregulation, and hypoventilation requiring prolonged ICU support for weeks to months. This is followed by a prolonged recovery phase lasting 5-24 months, and a period marked by deficits in executive function and working memory, impulsivity, and disinhibition. Impressively, the patient has no memory of the illness.
In one large series of patients with confirmed anti–NMDA receptor encephalitis reported by Dr. Dalmau and coinvestigators, psychiatric symptoms occurred in isolation without subsequent neurologic involvement in just 4% of cases (JAMA Neurol. 2013 Sep 1;70[9]:1133-9).
Dr. Dalmau was senior author of an international cohort study including 577 patients with anti-NMDA receptor encephalitis with serial follow-up for 24 months. The study provided an unprecedented picture of the epidemiology and clinical features of the disorder.
“It’s a disease predominantly of women and young people,” he observed.
Indeed, the median age of the study population was 21 years, and 37% of subjects were less than 18 years of age. Roughly 80% of patients were female and most of them had a benign ovarian teratoma, which played a key role in their neuropsychiatric disease (Lancet Neurol. 2013 Feb;12[2]:157-65). These benign tumors express the NMDA receptor in ectopic nerve tissue, triggering a systemic immune response.
One or more relapses – again treatable via immunotherapy – occurred in 12% of patients during 24 months of follow-up.
When a red flag suggestive of synaptic autoimmunity is present, it’s important to obtain a cerebrospinal fluid (CSF) sample for analysis, along with an EEG and/or brain MRI.
“I don’t know if you as psychiatrists are set up to do spinal taps in all persons with first presentation of psychosis, but this would be my suggestion. It’s extremely useful in this situation,” Dr. Dalmau said.
The vast majority of patients with anti–NMDA receptor encephalitis have CSF pleocytosis with a mild lymphocytic predominance. The MRI is abnormal in about 35% of cases. EEG abnormalities are common but nonspecific. The diagnosis is confirmed by identification of anti–NMDA receptor antibodies in the CSF.
First-line therapy is corticosteroids, intravenous immunoglobulin, and/or plasma exchange to remove the pathogenic antibodies, along with resection of the tumor if present. These treatments are effective in almost half of affected patients. When they’re not, the second-line options are rituximab (Rituxan) and cyclophosphamide, alone or combined.
Antibodies to the NMDA receptor are far and away the most common cause of synaptic autoimmunity-induced psychosis, but other targets of autoimmunity have been documented as well, including the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, contactin-associated protein-like 2 (CASPR2), and neurexin-3-alpha.
Dr. Dalmau and various collaborators continue to advance the understanding of this novel category of neuropsychiatric disease. They have developed a simple 5-point score, known as the NEOS score, that predicts 1-year functional status in patients with anti–NMDA receptor encephalitis (Neurology. 2018 Dec 21. doi: 10.1212/WNL.0000000000006783). He and his colleagues have also recently shown in a prospective study that herpes simplex encephalitis can result in an autoimmune encephalitis, with NMDA receptor antibodies present in most cases (Lancet Neurol. 2018 Sep;17[9]:760-72).
Dr. Dalmau’s research is supported by the U.S. National Institute of Neurological Disorders and Stroke, the Spanish Ministry of Health, and Spanish research foundations. He reported receiving royalties from the use of several neuronal antibody tests.
BARCELONA – Consider the possibility of an autoantibody-related etiology in all cases of first-onset psychosis, Josep Dalmau, MD, PhD, urged at the annual congress of the European College of Neuropsychopharmacology.
“There are patients in our clinics all of us – neurologists and psychiatrists – are missing. These patients are believed to have psychiatric presentations, but they do not. They are autoimmune,” said Dr. Dalmau, professor of neurology at the University of Barcelona.
Dr. Dalmau urged psychiatrists to become familiar with the red flags suggestive of synaptic autoimmunity as the underlying cause of first-episode, out-of-the-blue psychosis.
“If you have a patient with a classical presentation of schizophrenia or bipolar disorder, you probably won’t find antibodies,” according to the neurologist.
It’s important to have a high index of suspicion, because anti–NMDA receptor encephalitis is treatable with immunotherapy. And firm evidence shows that earlier recognition and treatment lead to improved outcomes. Also, the disorder is refractory to antipsychotics; indeed, 
Manifestations of anti–NMDA receptor encephalitis follow a characteristic pattern, beginning with a prodromal flulike phase lasting several days to a week. This is followed by acute-onset bizarre behavioral changes, irritability, and psychosis with delusions and/or hallucinations, often progressing to catatonia. After 1-4 weeks of this, florid neurologic symptoms usually appear, including seizures, abnormal movements, autonomic dysregulation, and hypoventilation requiring prolonged ICU support for weeks to months. This is followed by a prolonged recovery phase lasting 5-24 months, and a period marked by deficits in executive function and working memory, impulsivity, and disinhibition. Impressively, the patient has no memory of the illness.
In one large series of patients with confirmed anti–NMDA receptor encephalitis reported by Dr. Dalmau and coinvestigators, psychiatric symptoms occurred in isolation without subsequent neurologic involvement in just 4% of cases (JAMA Neurol. 2013 Sep 1;70[9]:1133-9).
Dr. Dalmau was senior author of an international cohort study including 577 patients with anti-NMDA receptor encephalitis with serial follow-up for 24 months. The study provided an unprecedented picture of the epidemiology and clinical features of the disorder.
“It’s a disease predominantly of women and young people,” he observed.
Indeed, the median age of the study population was 21 years, and 37% of subjects were less than 18 years of age. Roughly 80% of patients were female and most of them had a benign ovarian teratoma, which played a key role in their neuropsychiatric disease (Lancet Neurol. 2013 Feb;12[2]:157-65). These benign tumors express the NMDA receptor in ectopic nerve tissue, triggering a systemic immune response.
One or more relapses – again treatable via immunotherapy – occurred in 12% of patients during 24 months of follow-up.
When a red flag suggestive of synaptic autoimmunity is present, it’s important to obtain a cerebrospinal fluid (CSF) sample for analysis, along with an EEG and/or brain MRI.
“I don’t know if you as psychiatrists are set up to do spinal taps in all persons with first presentation of psychosis, but this would be my suggestion. It’s extremely useful in this situation,” Dr. Dalmau said.
The vast majority of patients with anti–NMDA receptor encephalitis have CSF pleocytosis with a mild lymphocytic predominance. The MRI is abnormal in about 35% of cases. EEG abnormalities are common but nonspecific. The diagnosis is confirmed by identification of anti–NMDA receptor antibodies in the CSF.
First-line therapy is corticosteroids, intravenous immunoglobulin, and/or plasma exchange to remove the pathogenic antibodies, along with resection of the tumor if present. These treatments are effective in almost half of affected patients. When they’re not, the second-line options are rituximab (Rituxan) and cyclophosphamide, alone or combined.
Antibodies to the NMDA receptor are far and away the most common cause of synaptic autoimmunity-induced psychosis, but other targets of autoimmunity have been documented as well, including the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, contactin-associated protein-like 2 (CASPR2), and neurexin-3-alpha.
Dr. Dalmau and various collaborators continue to advance the understanding of this novel category of neuropsychiatric disease. They have developed a simple 5-point score, known as the NEOS score, that predicts 1-year functional status in patients with anti–NMDA receptor encephalitis (Neurology. 2018 Dec 21. doi: 10.1212/WNL.0000000000006783). He and his colleagues have also recently shown in a prospective study that herpes simplex encephalitis can result in an autoimmune encephalitis, with NMDA receptor antibodies present in most cases (Lancet Neurol. 2018 Sep;17[9]:760-72).
Dr. Dalmau’s research is supported by the U.S. National Institute of Neurological Disorders and Stroke, the Spanish Ministry of Health, and Spanish research foundations. He reported receiving royalties from the use of several neuronal antibody tests.
BARCELONA – Consider the possibility of an autoantibody-related etiology in all cases of first-onset psychosis, Josep Dalmau, MD, PhD, urged at the annual congress of the European College of Neuropsychopharmacology.
“There are patients in our clinics all of us – neurologists and psychiatrists – are missing. These patients are believed to have psychiatric presentations, but they do not. They are autoimmune,” said Dr. Dalmau, professor of neurology at the University of Barcelona.
Dr. Dalmau urged psychiatrists to become familiar with the red flags suggestive of synaptic autoimmunity as the underlying cause of first-episode, out-of-the-blue psychosis.
“If you have a patient with a classical presentation of schizophrenia or bipolar disorder, you probably won’t find antibodies,” according to the neurologist.
It’s important to have a high index of suspicion, because anti–NMDA receptor encephalitis is treatable with immunotherapy. And firm evidence shows that earlier recognition and treatment lead to improved outcomes. Also, the disorder is refractory to antipsychotics; indeed, 
Manifestations of anti–NMDA receptor encephalitis follow a characteristic pattern, beginning with a prodromal flulike phase lasting several days to a week. This is followed by acute-onset bizarre behavioral changes, irritability, and psychosis with delusions and/or hallucinations, often progressing to catatonia. After 1-4 weeks of this, florid neurologic symptoms usually appear, including seizures, abnormal movements, autonomic dysregulation, and hypoventilation requiring prolonged ICU support for weeks to months. This is followed by a prolonged recovery phase lasting 5-24 months, and a period marked by deficits in executive function and working memory, impulsivity, and disinhibition. Impressively, the patient has no memory of the illness.
In one large series of patients with confirmed anti–NMDA receptor encephalitis reported by Dr. Dalmau and coinvestigators, psychiatric symptoms occurred in isolation without subsequent neurologic involvement in just 4% of cases (JAMA Neurol. 2013 Sep 1;70[9]:1133-9).
Dr. Dalmau was senior author of an international cohort study including 577 patients with anti-NMDA receptor encephalitis with serial follow-up for 24 months. The study provided an unprecedented picture of the epidemiology and clinical features of the disorder.
“It’s a disease predominantly of women and young people,” he observed.
Indeed, the median age of the study population was 21 years, and 37% of subjects were less than 18 years of age. Roughly 80% of patients were female and most of them had a benign ovarian teratoma, which played a key role in their neuropsychiatric disease (Lancet Neurol. 2013 Feb;12[2]:157-65). These benign tumors express the NMDA receptor in ectopic nerve tissue, triggering a systemic immune response.
One or more relapses – again treatable via immunotherapy – occurred in 12% of patients during 24 months of follow-up.
When a red flag suggestive of synaptic autoimmunity is present, it’s important to obtain a cerebrospinal fluid (CSF) sample for analysis, along with an EEG and/or brain MRI.
“I don’t know if you as psychiatrists are set up to do spinal taps in all persons with first presentation of psychosis, but this would be my suggestion. It’s extremely useful in this situation,” Dr. Dalmau said.
The vast majority of patients with anti–NMDA receptor encephalitis have CSF pleocytosis with a mild lymphocytic predominance. The MRI is abnormal in about 35% of cases. EEG abnormalities are common but nonspecific. The diagnosis is confirmed by identification of anti–NMDA receptor antibodies in the CSF.
First-line therapy is corticosteroids, intravenous immunoglobulin, and/or plasma exchange to remove the pathogenic antibodies, along with resection of the tumor if present. These treatments are effective in almost half of affected patients. When they’re not, the second-line options are rituximab (Rituxan) and cyclophosphamide, alone or combined.
Antibodies to the NMDA receptor are far and away the most common cause of synaptic autoimmunity-induced psychosis, but other targets of autoimmunity have been documented as well, including the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, contactin-associated protein-like 2 (CASPR2), and neurexin-3-alpha.
Dr. Dalmau and various collaborators continue to advance the understanding of this novel category of neuropsychiatric disease. They have developed a simple 5-point score, known as the NEOS score, that predicts 1-year functional status in patients with anti–NMDA receptor encephalitis (Neurology. 2018 Dec 21. doi: 10.1212/WNL.0000000000006783). He and his colleagues have also recently shown in a prospective study that herpes simplex encephalitis can result in an autoimmune encephalitis, with NMDA receptor antibodies present in most cases (Lancet Neurol. 2018 Sep;17[9]:760-72).
Dr. Dalmau’s research is supported by the U.S. National Institute of Neurological Disorders and Stroke, the Spanish Ministry of Health, and Spanish research foundations. He reported receiving royalties from the use of several neuronal antibody tests.
REPORTING FROM THE ECNP CONGRESS
Single-dose propranolol tied to ‘selective erasure’ of anxiety disorders
BARCELONA – A single 40-mg dose of oral propranolol, judiciously timed, constitutes an outside-the-box yet highly promising treatment for anxiety disorders, and perhaps for posttraumatic stress disorder as well, Marieke Soeter, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
The concept here is that the beta-blocker, when given with a brief therapist-led reactivation of a fear memory, blocks beta-adrenergic receptors in the brain so as to interfere with the specific proteins required for reconsolidation of that memory, thereby disrupting the reconsolidation process and neutralizing subsequent expression of that memory in its toxic form. In effect, timely administration of one dose of propranolol, a drug that readily crosses the blood/brain barrier, achieves pharmacologically induced amnesia regarding the learned fear, explained Dr. Soeter, a clinical psychologist at TNO, the Netherlands Organization for Scientific Research, an independent nonprofit translational research organization.
“It looks like permanent fear erasure. You can never say that something is erased, but we have not been able to get it back,” she said. “Propranolol achieves selective erasure: It targets the emotional component, but knowledge is intact. They know what happened, but they aren’t scared anymore. The fear association is affected, but not the innate fear response to a threat stimulus, so it doesn’t alter reactions to potentially dangerous situations, which is important. If there is a bomb, they still know to run away from it.”
This single-session therapy addressing what psychologists call fear memory reconsolidation is totally outside the box relative to contemporary psychotherapy for anxiety disorders, which typically entails gradual fear extinction learning requiring multiple treatment sessions. But contemporary psychotherapy for anxiety disorders leaves much room for improvement, given that up to 60% of patients experience relapse. That’s probably because the original fear memory remains intact and resurfaces at some point despite initial treatment success, according to Dr. Soeter.
Nearly 2 decades ago, other investigators showed in animal studies that fear memories are not necessarily permanent. Rather, they are modifiable, and even erasable, during the vulnerable period that occurs when the memories are reactivated and become labile.
Later, Dr. Soeter – then at the University of Amsterdam – and her colleagues demonstrated the same phenomenon using Pavlovian fear-conditioning techniques involving pictures and electric shocks in healthy human volunteers. They showed that a dose of propranolol given before memory reactivation blocked the fear response, while nadolol, a beta-blocker that does not cross the blood/brain barrier, did not.
However, since the fear memories they could ethically induce in the psychology laboratory are far less intense than those experienced by patients with anxiety disorders, the researchers next conducted a randomized, double-blind clinical trial in 45 individuals with arachnophobia. Fifteen received 40 mg of propranolol after spending 2 minutes in proximity to a large tarantula, 15 got placebo, and another 15 received propranolol without exposure to a tarantula. One week later, all patients who received propranolol with spider exposure were able to approach and actually pet the tarantula. Pharmacologic disruption of reconsolidation and storage of their fear memory had turned avoidance behavior into approach behavior. This benefit was maintained for at least a year after the brief treatment session (Biol Psychiatry. 2015 Dec 15;78[12]:880-6).
“Interestingly, there was no direct effect of propranolol on spider beliefs. Therefore, do we need treatment that targets the cognitive level? These findings challenge one of the fundamental tenets of cognitive-behavioral therapy that emphasizes changes in cognition as central to behavioral modification,” Dr. Soeter said.
Most recently, she and a coinvestigator have been working to pin down the precise conditions under which memory reconsolidation can be targeted to extinguish fear memories. They have shown in a 30-subject study that the process is both time- and sleep-dependent. The propranolol must be given within roughly an hour before to 1 hour after therapeutic reactivation of the fear memory to be effective. And sleep is an absolute necessity: When subjects were rechallenged 12 hours after memory reactivation and administration of propranolol earlier on the same day, with no opportunity for sleep, there was no therapeutic effect: The disturbing fear memory was elicited. However, when subjects were rechallenged 12 hours after taking propranolol the previous day – that is, after a night’s sleep – the fear memory was gone (Nat Commun. 2018 Apr 3;9[1]:1316. doi: 10.1038/s41467-018-03659-1).
“Postretrieval amnesia requires sleep to happen. ,” Dr. Soeter said. It’s still unclear, however, how much sleep is required. Perhaps a nap will turn out to be sufficient, she said.
Colleagues at the University of Amsterdam are now using single-dose propranolol-based therapy in patients with a wide range of phobias.
“The effects are pretty amazing,” Dr. Soeter said. “Everything is treatable. It’s almost too good to be true, but these are our findings.”
Based upon her favorable anecdotal experience in treating a Dutch military veteran with severe combat-related PTSD of 10 years’ duration which had proved resistant to multiple conventional and unconventional interventions, a pilot study of single-dose propranolol with traumatic memory reactivation is now being planned in patients with war-related PTSD.
“After one pill and a 20-minute session, this veteran with severe chronic PTSD has no more nightmares, insomnia, or alcohol problems, and he now travels the world,” she said.
Her research met with an enthusiastic reception from other speakers at the ECNP session on PTSD. Eric Vermetten, MD, PhD, welcomed the concept that pharmacologic therapy upon reexposure to fearful cues can impede the molecular and cellular cascade required to reestablish fearful memories. This also is the basis for the extremely encouraging, albeit preliminary, clinical data on ketamine, an N-methyl-D-aspartate receptor antagonist, as well as 3,4-Methylenedioxymethamphetamine (MDMA) for therapeutic manipulation of trauma memories.
“Targeting reconsolidation of existing fear memories is worthy of looking into further,” declared Dr. Vermetten, professor of psychiatry at Leiden (the Netherlands) University and a military mental health researcher for the Dutch Ministry of Defense.
New thinking regarding pharmacotherapy for PTSD is sorely needed, he added. He endorsed a consensus statement by the PTSD Psychopharmacology Working Group that decried what was termed a crisis in pharmacotherapy of PTSD (Biol Psychiatry. 2017 Oct 1;82[7]:e51-e59. doi: 10.1016/j.biopsych.2017.03.007. Epub 2017 Mar 14).
“We only have two [Food and Drug Administration]-approved medications for PTSD – sertraline and paroxetine – and they were approved back in 2001,” Dr. Vermetten noted. “Research has stalled, and there is a void in new drug development.”
Dr. Soeter’s study of the time- and sleep-dependent nature of propranolol-induced amnesia was supported by the Netherlands Organization for Scientific Research, where she is employed.
BARCELONA – A single 40-mg dose of oral propranolol, judiciously timed, constitutes an outside-the-box yet highly promising treatment for anxiety disorders, and perhaps for posttraumatic stress disorder as well, Marieke Soeter, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
The concept here is that the beta-blocker, when given with a brief therapist-led reactivation of a fear memory, blocks beta-adrenergic receptors in the brain so as to interfere with the specific proteins required for reconsolidation of that memory, thereby disrupting the reconsolidation process and neutralizing subsequent expression of that memory in its toxic form. In effect, timely administration of one dose of propranolol, a drug that readily crosses the blood/brain barrier, achieves pharmacologically induced amnesia regarding the learned fear, explained Dr. Soeter, a clinical psychologist at TNO, the Netherlands Organization for Scientific Research, an independent nonprofit translational research organization.
“It looks like permanent fear erasure. You can never say that something is erased, but we have not been able to get it back,” she said. “Propranolol achieves selective erasure: It targets the emotional component, but knowledge is intact. They know what happened, but they aren’t scared anymore. The fear association is affected, but not the innate fear response to a threat stimulus, so it doesn’t alter reactions to potentially dangerous situations, which is important. If there is a bomb, they still know to run away from it.”
This single-session therapy addressing what psychologists call fear memory reconsolidation is totally outside the box relative to contemporary psychotherapy for anxiety disorders, which typically entails gradual fear extinction learning requiring multiple treatment sessions. But contemporary psychotherapy for anxiety disorders leaves much room for improvement, given that up to 60% of patients experience relapse. That’s probably because the original fear memory remains intact and resurfaces at some point despite initial treatment success, according to Dr. Soeter.
Nearly 2 decades ago, other investigators showed in animal studies that fear memories are not necessarily permanent. Rather, they are modifiable, and even erasable, during the vulnerable period that occurs when the memories are reactivated and become labile.
Later, Dr. Soeter – then at the University of Amsterdam – and her colleagues demonstrated the same phenomenon using Pavlovian fear-conditioning techniques involving pictures and electric shocks in healthy human volunteers. They showed that a dose of propranolol given before memory reactivation blocked the fear response, while nadolol, a beta-blocker that does not cross the blood/brain barrier, did not.
However, since the fear memories they could ethically induce in the psychology laboratory are far less intense than those experienced by patients with anxiety disorders, the researchers next conducted a randomized, double-blind clinical trial in 45 individuals with arachnophobia. Fifteen received 40 mg of propranolol after spending 2 minutes in proximity to a large tarantula, 15 got placebo, and another 15 received propranolol without exposure to a tarantula. One week later, all patients who received propranolol with spider exposure were able to approach and actually pet the tarantula. Pharmacologic disruption of reconsolidation and storage of their fear memory had turned avoidance behavior into approach behavior. This benefit was maintained for at least a year after the brief treatment session (Biol Psychiatry. 2015 Dec 15;78[12]:880-6).
“Interestingly, there was no direct effect of propranolol on spider beliefs. Therefore, do we need treatment that targets the cognitive level? These findings challenge one of the fundamental tenets of cognitive-behavioral therapy that emphasizes changes in cognition as central to behavioral modification,” Dr. Soeter said.
Most recently, she and a coinvestigator have been working to pin down the precise conditions under which memory reconsolidation can be targeted to extinguish fear memories. They have shown in a 30-subject study that the process is both time- and sleep-dependent. The propranolol must be given within roughly an hour before to 1 hour after therapeutic reactivation of the fear memory to be effective. And sleep is an absolute necessity: When subjects were rechallenged 12 hours after memory reactivation and administration of propranolol earlier on the same day, with no opportunity for sleep, there was no therapeutic effect: The disturbing fear memory was elicited. However, when subjects were rechallenged 12 hours after taking propranolol the previous day – that is, after a night’s sleep – the fear memory was gone (Nat Commun. 2018 Apr 3;9[1]:1316. doi: 10.1038/s41467-018-03659-1).
“Postretrieval amnesia requires sleep to happen. ,” Dr. Soeter said. It’s still unclear, however, how much sleep is required. Perhaps a nap will turn out to be sufficient, she said.
Colleagues at the University of Amsterdam are now using single-dose propranolol-based therapy in patients with a wide range of phobias.
“The effects are pretty amazing,” Dr. Soeter said. “Everything is treatable. It’s almost too good to be true, but these are our findings.”
Based upon her favorable anecdotal experience in treating a Dutch military veteran with severe combat-related PTSD of 10 years’ duration which had proved resistant to multiple conventional and unconventional interventions, a pilot study of single-dose propranolol with traumatic memory reactivation is now being planned in patients with war-related PTSD.
“After one pill and a 20-minute session, this veteran with severe chronic PTSD has no more nightmares, insomnia, or alcohol problems, and he now travels the world,” she said.
Her research met with an enthusiastic reception from other speakers at the ECNP session on PTSD. Eric Vermetten, MD, PhD, welcomed the concept that pharmacologic therapy upon reexposure to fearful cues can impede the molecular and cellular cascade required to reestablish fearful memories. This also is the basis for the extremely encouraging, albeit preliminary, clinical data on ketamine, an N-methyl-D-aspartate receptor antagonist, as well as 3,4-Methylenedioxymethamphetamine (MDMA) for therapeutic manipulation of trauma memories.
“Targeting reconsolidation of existing fear memories is worthy of looking into further,” declared Dr. Vermetten, professor of psychiatry at Leiden (the Netherlands) University and a military mental health researcher for the Dutch Ministry of Defense.
New thinking regarding pharmacotherapy for PTSD is sorely needed, he added. He endorsed a consensus statement by the PTSD Psychopharmacology Working Group that decried what was termed a crisis in pharmacotherapy of PTSD (Biol Psychiatry. 2017 Oct 1;82[7]:e51-e59. doi: 10.1016/j.biopsych.2017.03.007. Epub 2017 Mar 14).
“We only have two [Food and Drug Administration]-approved medications for PTSD – sertraline and paroxetine – and they were approved back in 2001,” Dr. Vermetten noted. “Research has stalled, and there is a void in new drug development.”
Dr. Soeter’s study of the time- and sleep-dependent nature of propranolol-induced amnesia was supported by the Netherlands Organization for Scientific Research, where she is employed.
BARCELONA – A single 40-mg dose of oral propranolol, judiciously timed, constitutes an outside-the-box yet highly promising treatment for anxiety disorders, and perhaps for posttraumatic stress disorder as well, Marieke Soeter, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
The concept here is that the beta-blocker, when given with a brief therapist-led reactivation of a fear memory, blocks beta-adrenergic receptors in the brain so as to interfere with the specific proteins required for reconsolidation of that memory, thereby disrupting the reconsolidation process and neutralizing subsequent expression of that memory in its toxic form. In effect, timely administration of one dose of propranolol, a drug that readily crosses the blood/brain barrier, achieves pharmacologically induced amnesia regarding the learned fear, explained Dr. Soeter, a clinical psychologist at TNO, the Netherlands Organization for Scientific Research, an independent nonprofit translational research organization.
“It looks like permanent fear erasure. You can never say that something is erased, but we have not been able to get it back,” she said. “Propranolol achieves selective erasure: It targets the emotional component, but knowledge is intact. They know what happened, but they aren’t scared anymore. The fear association is affected, but not the innate fear response to a threat stimulus, so it doesn’t alter reactions to potentially dangerous situations, which is important. If there is a bomb, they still know to run away from it.”
This single-session therapy addressing what psychologists call fear memory reconsolidation is totally outside the box relative to contemporary psychotherapy for anxiety disorders, which typically entails gradual fear extinction learning requiring multiple treatment sessions. But contemporary psychotherapy for anxiety disorders leaves much room for improvement, given that up to 60% of patients experience relapse. That’s probably because the original fear memory remains intact and resurfaces at some point despite initial treatment success, according to Dr. Soeter.
Nearly 2 decades ago, other investigators showed in animal studies that fear memories are not necessarily permanent. Rather, they are modifiable, and even erasable, during the vulnerable period that occurs when the memories are reactivated and become labile.
Later, Dr. Soeter – then at the University of Amsterdam – and her colleagues demonstrated the same phenomenon using Pavlovian fear-conditioning techniques involving pictures and electric shocks in healthy human volunteers. They showed that a dose of propranolol given before memory reactivation blocked the fear response, while nadolol, a beta-blocker that does not cross the blood/brain barrier, did not.
However, since the fear memories they could ethically induce in the psychology laboratory are far less intense than those experienced by patients with anxiety disorders, the researchers next conducted a randomized, double-blind clinical trial in 45 individuals with arachnophobia. Fifteen received 40 mg of propranolol after spending 2 minutes in proximity to a large tarantula, 15 got placebo, and another 15 received propranolol without exposure to a tarantula. One week later, all patients who received propranolol with spider exposure were able to approach and actually pet the tarantula. Pharmacologic disruption of reconsolidation and storage of their fear memory had turned avoidance behavior into approach behavior. This benefit was maintained for at least a year after the brief treatment session (Biol Psychiatry. 2015 Dec 15;78[12]:880-6).
“Interestingly, there was no direct effect of propranolol on spider beliefs. Therefore, do we need treatment that targets the cognitive level? These findings challenge one of the fundamental tenets of cognitive-behavioral therapy that emphasizes changes in cognition as central to behavioral modification,” Dr. Soeter said.
Most recently, she and a coinvestigator have been working to pin down the precise conditions under which memory reconsolidation can be targeted to extinguish fear memories. They have shown in a 30-subject study that the process is both time- and sleep-dependent. The propranolol must be given within roughly an hour before to 1 hour after therapeutic reactivation of the fear memory to be effective. And sleep is an absolute necessity: When subjects were rechallenged 12 hours after memory reactivation and administration of propranolol earlier on the same day, with no opportunity for sleep, there was no therapeutic effect: The disturbing fear memory was elicited. However, when subjects were rechallenged 12 hours after taking propranolol the previous day – that is, after a night’s sleep – the fear memory was gone (Nat Commun. 2018 Apr 3;9[1]:1316. doi: 10.1038/s41467-018-03659-1).
“Postretrieval amnesia requires sleep to happen. ,” Dr. Soeter said. It’s still unclear, however, how much sleep is required. Perhaps a nap will turn out to be sufficient, she said.
Colleagues at the University of Amsterdam are now using single-dose propranolol-based therapy in patients with a wide range of phobias.
“The effects are pretty amazing,” Dr. Soeter said. “Everything is treatable. It’s almost too good to be true, but these are our findings.”
Based upon her favorable anecdotal experience in treating a Dutch military veteran with severe combat-related PTSD of 10 years’ duration which had proved resistant to multiple conventional and unconventional interventions, a pilot study of single-dose propranolol with traumatic memory reactivation is now being planned in patients with war-related PTSD.
“After one pill and a 20-minute session, this veteran with severe chronic PTSD has no more nightmares, insomnia, or alcohol problems, and he now travels the world,” she said.
Her research met with an enthusiastic reception from other speakers at the ECNP session on PTSD. Eric Vermetten, MD, PhD, welcomed the concept that pharmacologic therapy upon reexposure to fearful cues can impede the molecular and cellular cascade required to reestablish fearful memories. This also is the basis for the extremely encouraging, albeit preliminary, clinical data on ketamine, an N-methyl-D-aspartate receptor antagonist, as well as 3,4-Methylenedioxymethamphetamine (MDMA) for therapeutic manipulation of trauma memories.
“Targeting reconsolidation of existing fear memories is worthy of looking into further,” declared Dr. Vermetten, professor of psychiatry at Leiden (the Netherlands) University and a military mental health researcher for the Dutch Ministry of Defense.
New thinking regarding pharmacotherapy for PTSD is sorely needed, he added. He endorsed a consensus statement by the PTSD Psychopharmacology Working Group that decried what was termed a crisis in pharmacotherapy of PTSD (Biol Psychiatry. 2017 Oct 1;82[7]:e51-e59. doi: 10.1016/j.biopsych.2017.03.007. Epub 2017 Mar 14).
“We only have two [Food and Drug Administration]-approved medications for PTSD – sertraline and paroxetine – and they were approved back in 2001,” Dr. Vermetten noted. “Research has stalled, and there is a void in new drug development.”
Dr. Soeter’s study of the time- and sleep-dependent nature of propranolol-induced amnesia was supported by the Netherlands Organization for Scientific Research, where she is employed.
REPORTING FROM THE ECNP CONGRESS
Key clinical point: A single 40-mg dose of oral propranolol, judiciously timed, is a highly promising novel treatment for anxiety disorders.
Major finding: The beta-blocker must be given within an hour before to an hour after therapist-facilitated reactivation of the fear memory.
Study details: This study included 30 healthy volunteers who underwent a cued Pavlovian fear-conditioning program.
Disclosures: Dr. Soeter’s study of the time- and sleep-dependent nature of propranolol-induced amnesia was supported by the Netherlands Organization for Scientific Research, where she is employed.
‘Walk and talk’ 3MDR psychotherapy for PTSD
BARCELONA – The therapeutic setting for individual psychotherapy has shifted over the years from the analytic couch, with the therapist discretely tucked out of sight, to facing chairs, a similarly sedentary format. The next evolutionary development might be to plop a patient with posttraumatic stress disorder on an exercise treadmill and don a virtual reality helmet to engage in an interactive motion-assisted form of psychotherapy in which the therapist stands alongside the walking patient while providing guidance on processing traumatic memories, Eric Vermetten, MD, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
He and his colleagues have developed an innovative approach to delivering trauma-focused psychotherapy. They call it Multimodular Motion-Assisted Memory Desensitization and Reconsolidation (3MDR), or more informally, “walk and talk therapy,” explained Dr. Vermetten, professor of psychiatry at Leiden (the Netherlands) University and a military mental health researcher for the Dutch Ministry of Defense.
3MDR is a combination of personalized virtual reality using a headset, multisensory input using self-selected trauma-related pictures, and a dual-attention task borrowed from eye movement desensitization and reprocessing therapy, with treadmill walking throughout the treatment session.
3MDR is designed to boost this process of memory retrieval and reconsolidation by creating a more totally immersive patient experience intended to enhance treatment engagement and overcome behavioral avoidance. Through virtual reality, the PTSD patient literally walks toward his personal fear-related images.
Dr. Vermetten and his coinvestigators came up with 3MDR as a treatment designed for military veterans with chronic, combat-related, treatment-resistant PTSD. The impetus was the evident need for new and better forms of psychotherapy for such patients. Even though an array of evidence-based psychotherapies are available as guideline-recommended first-line treatments for PTSD, individuals with combat-related PTSD have a notoriously low response rate to these interventions, presumably because of the intensity and repetitive nature of their traumatic experiences. Indeed, up to two-thirds of veterans with PTSD experience substantial residual symptoms post treatment such that they still meet diagnostic criteria for the disorder.
3MDR is an amped up form of exposure-based therapy in which patients walk through a personalized virtual reality installation toward self-chosen trauma-related pictures of their deployment. The investigators developed this intensely immersive type of psychotherapy because they believe avoidance and lack of emotional engagement figure prominently in the low success rate of established forms of psychotherapy in combat-related PTSD. The treadmill walking aspect is considered key because of the large body of research showing that walking entails cognitive-motor interactions that facilitate problem solving, the psychiatrist explained.
The investigators recently published a detailed description of the therapeutic rationale for 3MDR and the nuts and bolts of the novel therapy (Front Psychiatry. 2018 May 4;9:176. doi: 10.3389/fpsyt.2018.00176). Early anecdotal experience has been positive. However, as cochair of the ECNP Traumatic Stress Network, Dr. Vermetten is acutely aware of the need to demonstrate efficacy in rigorous randomized controlled trials.
“This is a way psychotherapy can be shaped in the future. We’re collaborating with various centers across the globe now to see whether this is effective for treatment-resistant PTSD patients,” Dr. Vermetten said.
If those studies prove positive, it will be worthwhile to determine whether 3MDR also has a role as a first-line treatment for earlier-stage PTSD and for forms of the disorder unrelated to military combat, he added.
Funding for the project has been provided by the Dutch Ministry of Defense.
BARCELONA – The therapeutic setting for individual psychotherapy has shifted over the years from the analytic couch, with the therapist discretely tucked out of sight, to facing chairs, a similarly sedentary format. The next evolutionary development might be to plop a patient with posttraumatic stress disorder on an exercise treadmill and don a virtual reality helmet to engage in an interactive motion-assisted form of psychotherapy in which the therapist stands alongside the walking patient while providing guidance on processing traumatic memories, Eric Vermetten, MD, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
He and his colleagues have developed an innovative approach to delivering trauma-focused psychotherapy. They call it Multimodular Motion-Assisted Memory Desensitization and Reconsolidation (3MDR), or more informally, “walk and talk therapy,” explained Dr. Vermetten, professor of psychiatry at Leiden (the Netherlands) University and a military mental health researcher for the Dutch Ministry of Defense.
3MDR is a combination of personalized virtual reality using a headset, multisensory input using self-selected trauma-related pictures, and a dual-attention task borrowed from eye movement desensitization and reprocessing therapy, with treadmill walking throughout the treatment session.
3MDR is designed to boost this process of memory retrieval and reconsolidation by creating a more totally immersive patient experience intended to enhance treatment engagement and overcome behavioral avoidance. Through virtual reality, the PTSD patient literally walks toward his personal fear-related images.
Dr. Vermetten and his coinvestigators came up with 3MDR as a treatment designed for military veterans with chronic, combat-related, treatment-resistant PTSD. The impetus was the evident need for new and better forms of psychotherapy for such patients. Even though an array of evidence-based psychotherapies are available as guideline-recommended first-line treatments for PTSD, individuals with combat-related PTSD have a notoriously low response rate to these interventions, presumably because of the intensity and repetitive nature of their traumatic experiences. Indeed, up to two-thirds of veterans with PTSD experience substantial residual symptoms post treatment such that they still meet diagnostic criteria for the disorder.
3MDR is an amped up form of exposure-based therapy in which patients walk through a personalized virtual reality installation toward self-chosen trauma-related pictures of their deployment. The investigators developed this intensely immersive type of psychotherapy because they believe avoidance and lack of emotional engagement figure prominently in the low success rate of established forms of psychotherapy in combat-related PTSD. The treadmill walking aspect is considered key because of the large body of research showing that walking entails cognitive-motor interactions that facilitate problem solving, the psychiatrist explained.
The investigators recently published a detailed description of the therapeutic rationale for 3MDR and the nuts and bolts of the novel therapy (Front Psychiatry. 2018 May 4;9:176. doi: 10.3389/fpsyt.2018.00176). Early anecdotal experience has been positive. However, as cochair of the ECNP Traumatic Stress Network, Dr. Vermetten is acutely aware of the need to demonstrate efficacy in rigorous randomized controlled trials.
“This is a way psychotherapy can be shaped in the future. We’re collaborating with various centers across the globe now to see whether this is effective for treatment-resistant PTSD patients,” Dr. Vermetten said.
If those studies prove positive, it will be worthwhile to determine whether 3MDR also has a role as a first-line treatment for earlier-stage PTSD and for forms of the disorder unrelated to military combat, he added.
Funding for the project has been provided by the Dutch Ministry of Defense.
BARCELONA – The therapeutic setting for individual psychotherapy has shifted over the years from the analytic couch, with the therapist discretely tucked out of sight, to facing chairs, a similarly sedentary format. The next evolutionary development might be to plop a patient with posttraumatic stress disorder on an exercise treadmill and don a virtual reality helmet to engage in an interactive motion-assisted form of psychotherapy in which the therapist stands alongside the walking patient while providing guidance on processing traumatic memories, Eric Vermetten, MD, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
He and his colleagues have developed an innovative approach to delivering trauma-focused psychotherapy. They call it Multimodular Motion-Assisted Memory Desensitization and Reconsolidation (3MDR), or more informally, “walk and talk therapy,” explained Dr. Vermetten, professor of psychiatry at Leiden (the Netherlands) University and a military mental health researcher for the Dutch Ministry of Defense.
3MDR is a combination of personalized virtual reality using a headset, multisensory input using self-selected trauma-related pictures, and a dual-attention task borrowed from eye movement desensitization and reprocessing therapy, with treadmill walking throughout the treatment session.
3MDR is designed to boost this process of memory retrieval and reconsolidation by creating a more totally immersive patient experience intended to enhance treatment engagement and overcome behavioral avoidance. Through virtual reality, the PTSD patient literally walks toward his personal fear-related images.
Dr. Vermetten and his coinvestigators came up with 3MDR as a treatment designed for military veterans with chronic, combat-related, treatment-resistant PTSD. The impetus was the evident need for new and better forms of psychotherapy for such patients. Even though an array of evidence-based psychotherapies are available as guideline-recommended first-line treatments for PTSD, individuals with combat-related PTSD have a notoriously low response rate to these interventions, presumably because of the intensity and repetitive nature of their traumatic experiences. Indeed, up to two-thirds of veterans with PTSD experience substantial residual symptoms post treatment such that they still meet diagnostic criteria for the disorder.
3MDR is an amped up form of exposure-based therapy in which patients walk through a personalized virtual reality installation toward self-chosen trauma-related pictures of their deployment. The investigators developed this intensely immersive type of psychotherapy because they believe avoidance and lack of emotional engagement figure prominently in the low success rate of established forms of psychotherapy in combat-related PTSD. The treadmill walking aspect is considered key because of the large body of research showing that walking entails cognitive-motor interactions that facilitate problem solving, the psychiatrist explained.
The investigators recently published a detailed description of the therapeutic rationale for 3MDR and the nuts and bolts of the novel therapy (Front Psychiatry. 2018 May 4;9:176. doi: 10.3389/fpsyt.2018.00176). Early anecdotal experience has been positive. However, as cochair of the ECNP Traumatic Stress Network, Dr. Vermetten is acutely aware of the need to demonstrate efficacy in rigorous randomized controlled trials.
“This is a way psychotherapy can be shaped in the future. We’re collaborating with various centers across the globe now to see whether this is effective for treatment-resistant PTSD patients,” Dr. Vermetten said.
If those studies prove positive, it will be worthwhile to determine whether 3MDR also has a role as a first-line treatment for earlier-stage PTSD and for forms of the disorder unrelated to military combat, he added.
Funding for the project has been provided by the Dutch Ministry of Defense.
REPORTING FROM THE ECNP CONGRESS
New PTSD prevention guidelines released
Hydrocortisone is only drug rated as an ‘intervention with emerging evidence of efficacy’
Barcelona – New evidence-based guidelines on posttraumatic stress disorder prevention and treatment from the International Society for Traumatic Stress Studies (ISTSS) highlight an uncomfortable truth: Namely, the basis for early formal intervention of any sort is sorely lacking.
“I’m acutely aware that a lot of people in the mental health field are not aware of the evidence base as it stands at the moment,” Jonathan I. Bisson, MD, said at the annual congress of the European College of Neuropsychopharmacology. “There’s something very human about trying to do something. I think we find it very hard to do nothing following a traumatic event.”
Dr. Bisson, a professor of psychiatry at Cardiff (Wales) University and the chair of the ISTSS guidelines committee, provided an advance look at the ISTSS guidelines, which have since been released.
Secondary prevention of PTSD can entail either blocking development of symptoms after exposure to trauma or treating early emergent PTSD symptoms. Dr. Bisson emphasized that, although multiple exciting prospects are on the horizon for secondary prevention, those interventions need further work before implementation. The ISTSS guidelines, based on the group’s meta-analyses of 361 randomized controlled trials, rated most of the diverse psychosocial, psychological, and pharmacologic interventions that have been proposed or are now actually being used in clinical practice as either “low effect,” “interventions with emerging evidence,” or “insufficient evidence to recommend.” Those interventions are not backed by sufficient evidence of efficacy to be ready for prime time use in clinical practice.
Morever, the potential for iatrogenic harm is very real.
to a trauma,” the psychiatrist observed. “It’s normal to cry after a bereavement, for example. But should we be pathologizing that, or is that the body’s way of actually bringing itself to terms with something that’s very extreme?
“So we’ve got to be careful in our efforts to shape emotional processing, which might do absolutely nothing – which I’d argue is a problem when we’ve got limited resources because we should be focusing those resources on things that make a difference. Or it could minimize or prevent prolonged distress or pathology, which is what we’re after. Or it could interfere with the adaptive acute stress response – and that’s a real problem and one we’ve got to be very careful about,” Dr. Bisson said. “So ‘primum non nocere’ – first do no harm – should be a principle we adhere to.”
Neurobiology of PTSD
The accepted view of the neurobiology of PTSD is that it represents a failure of the medial prefrontal/anterior cingulate network to regulate activity in the amygdala, with resultant hyperreactivity to threat. Enhanced negative feedback of cortisol occurs. The brain’s response to low cortisol is to increase levels of corticotropin-releasing factor, which has the unwanted consequence of increased locus coeruleus activity and noradrenaline release. The resultant adrenergic surge facilitates the laying down and consolidation of traumatic memories.
Also, low cortisol levels disinhibit retrieval of traumatic memories, so the affected individual thinks more about the trauma. All of this elicits an uncontrolled sympathetic response, so the patient remains in a constant state of hyperarousal characteristic of PTSD.
“In theory we should have some really simple ways to prevent PTSD from occurring if we get in there soon enough: reducing noradrenergic overactivity via alpha2-adrenergic receptor agonism with an agent such as clonidine; postsynaptic beta-adrenergic blocking with a drug such as propranolol; or alpha1-adrenergic receptor blocking, as with prazosin. All of these approaches reduce noradrenergic tone and therefore should be effective, in theory, to prevent PTSD.
“We should also be able to use indirect strategies to reduce noradrenergic overactivity: GABA agents like benzodiazepines, alcohol, and gabapentin oppose noradrenaline action in the amygdala. I’m not suggesting drinking all the time to prevent PTSD, but there’s a strong association in several studies, with about a 50% reduction in rates of PTSD in those who are intoxicated at the time of the trauma,” according to Dr. Bisson.
Unfortunately, to date, none of those pharmacologic approaches have been effective when studied in randomized trials.
One pharmacologic intervention
Only one drug, hydrocortisone, was rated an “intervention with emerging evidence of efficacy” for prevention of PTSD symptoms in adults when given within the first 3 months after a traumatic event. Three placebo-controlled, randomized trials have shown a positive effect.
“It should be said that most of the studies of hydrocortisone have been done in individuals following extreme physical illness, such as septic shock sufferers, so the generalizability is a bit of a question. Nevertheless, it’s the one agent that has meta-analytic evidence of being effective at preventing PTSD, although more research is needed,” Dr. Bisson said.
Results of randomized trials featuring those agents have been “really disappointing” in light of what seems a sound theoretic rationale, he continued.
“We’re really struggling from a pharmacologic perspective to know what to do. I would say we are still at the experimental stage, and there’s no real good evidence that we should give any medication to prevent PTSD,” Dr. Bisson said.
Early psychosocial interventions
The ISTSS guidelines rate only two single-session interventions for prevention as rising to the promising level of “emerging evidence” of clinically important benefit: single-session eye movement desensitization and reprocessing (EMDR), which in its multisession format is a well-established treatment with strong evidence of efficacy in established PTSD, and a program known as Group 512 PM, which combines group debriefing with group cohesion–building exercises.
“Group 512 PM was done in groups of Chinese army personnel helping in recovery efforts following a 2008 earthquake in China that killed 80,000 people. It resulted in nearly a 50% reduction in PTSD versus no debriefing. This cohesion training might be a clue to us as something to work on in the future,” Dr. Bisson said.
The ISTSS guidelines deem there is insufficient evidence to recommend single-session group debriefing, group stress management, heart stress management, group education, trauma-focused counselling, computerized visuospatial task, individual psychoeducation, or individual debriefing.
“In six randomized controlled trials over nearly the last 20 years, we see a strong signal that individual psychological debriefing isn’t effective. So, certainly, going into a room with an individual or a couple and talking about what they’ve been through in great detail and getting them to express their emotions and advising them that’s a normal reaction doesn’t seem to be enough. And rather worryingly, the people who tend to do worse with that sort of intervention are the people who’ve got the most symptoms when they started, so they’re the ones at highest risk of developing PTSD,” Dr. Bisson said.
Multisession prevention interventions such as brief dyadic therapy and self-guided Internet interventions are supported by emerging evidence. Less promising, and with insufficient evidence to recommend, according to the ISTSS, are brief interpersonal therapy, brief individual trauma processing therapy, telephone-based cognitive-behavioral therapy (CBT), and nurse-led intensive care recovery programs.
For multisession early treatment interventions for patients with emerging traumatic stress symptoms within the first 3 months, the new ISTSS guidelines recommend as standard therapy CBT with a trauma focus, EMDR, or cognitive therapy. Stepped or collaborative care is rated as having “low effect.” There is emerging evidence for structured writing interventions and Internet-based guided self-help. And there is insufficient evidence to recommend behavioral activation, Internet virtual reality therapy, telephone-based CBT with a trauma focus, computerized neurobehavioral training, or supportive counseling.
Treating adults with established PTSD
Pharmacotherapy, including fluoxetine, sertraline, paroxetine, and venlafaxine is rated in the guidelines as a low-effect treatment. Quetiapine has emerging evidence of efficacy. Everything else has insufficient evidence.
Psychological therapies such as EMDR, CBT with a trauma focus, prolonged exposure, cognitive therapy, and cognitive processing therapy received strong recommendations. In fact, those are the only interventions in the entire ISTSS guidelines that received a “strong recommendation” rating. A weaker “standard recommendation” is given to CBT without a trauma focus, narrative exposure therapy, present-centered therapy, group CBT with a trauma focus, and guided Internet-based therapy with a trauma focus. Interventions with emerging evidence of efficacy include virtual reality therapy, reconsolidation of traumatic memories, and couples CBT with a trauma focus.
Best-practice approach to prevention
“In my view, and what I tell people, is that after a traumatic event I think practical pragmatic support in an empathic manner is the best first step,” Dr. Bisson said. “And it doesn’t have to be provided by a mental health professional. In fact, your family and friends are the best people to provide that. And then, we watchfully wait to see if traumatic stress symptoms emerge. If they do, and particularly if their trajectory is going up, then at about 1 month, I would get in there and deliver a therapy, either CBT with a trauma focus, EMDR, or cognitive therapy with a trauma focus. All of those have a significant positive effect for this group.”
Although he restricted his talk to secondary prevention of PTSD in adults, the ISTSS guidelines also address early intervention in children and adolescents.
Dr. Bisson reported having no financial conflicts of interest regarding his presentation.
Hydrocortisone is only drug rated as an ‘intervention with emerging evidence of efficacy’
Hydrocortisone is only drug rated as an ‘intervention with emerging evidence of efficacy’
Barcelona – New evidence-based guidelines on posttraumatic stress disorder prevention and treatment from the International Society for Traumatic Stress Studies (ISTSS) highlight an uncomfortable truth: Namely, the basis for early formal intervention of any sort is sorely lacking.
“I’m acutely aware that a lot of people in the mental health field are not aware of the evidence base as it stands at the moment,” Jonathan I. Bisson, MD, said at the annual congress of the European College of Neuropsychopharmacology. “There’s something very human about trying to do something. I think we find it very hard to do nothing following a traumatic event.”
Dr. Bisson, a professor of psychiatry at Cardiff (Wales) University and the chair of the ISTSS guidelines committee, provided an advance look at the ISTSS guidelines, which have since been released.
Secondary prevention of PTSD can entail either blocking development of symptoms after exposure to trauma or treating early emergent PTSD symptoms. Dr. Bisson emphasized that, although multiple exciting prospects are on the horizon for secondary prevention, those interventions need further work before implementation. The ISTSS guidelines, based on the group’s meta-analyses of 361 randomized controlled trials, rated most of the diverse psychosocial, psychological, and pharmacologic interventions that have been proposed or are now actually being used in clinical practice as either “low effect,” “interventions with emerging evidence,” or “insufficient evidence to recommend.” Those interventions are not backed by sufficient evidence of efficacy to be ready for prime time use in clinical practice.
Morever, the potential for iatrogenic harm is very real.
to a trauma,” the psychiatrist observed. “It’s normal to cry after a bereavement, for example. But should we be pathologizing that, or is that the body’s way of actually bringing itself to terms with something that’s very extreme?
“So we’ve got to be careful in our efforts to shape emotional processing, which might do absolutely nothing – which I’d argue is a problem when we’ve got limited resources because we should be focusing those resources on things that make a difference. Or it could minimize or prevent prolonged distress or pathology, which is what we’re after. Or it could interfere with the adaptive acute stress response – and that’s a real problem and one we’ve got to be very careful about,” Dr. Bisson said. “So ‘primum non nocere’ – first do no harm – should be a principle we adhere to.”
Neurobiology of PTSD
The accepted view of the neurobiology of PTSD is that it represents a failure of the medial prefrontal/anterior cingulate network to regulate activity in the amygdala, with resultant hyperreactivity to threat. Enhanced negative feedback of cortisol occurs. The brain’s response to low cortisol is to increase levels of corticotropin-releasing factor, which has the unwanted consequence of increased locus coeruleus activity and noradrenaline release. The resultant adrenergic surge facilitates the laying down and consolidation of traumatic memories.
Also, low cortisol levels disinhibit retrieval of traumatic memories, so the affected individual thinks more about the trauma. All of this elicits an uncontrolled sympathetic response, so the patient remains in a constant state of hyperarousal characteristic of PTSD.
“In theory we should have some really simple ways to prevent PTSD from occurring if we get in there soon enough: reducing noradrenergic overactivity via alpha2-adrenergic receptor agonism with an agent such as clonidine; postsynaptic beta-adrenergic blocking with a drug such as propranolol; or alpha1-adrenergic receptor blocking, as with prazosin. All of these approaches reduce noradrenergic tone and therefore should be effective, in theory, to prevent PTSD.
“We should also be able to use indirect strategies to reduce noradrenergic overactivity: GABA agents like benzodiazepines, alcohol, and gabapentin oppose noradrenaline action in the amygdala. I’m not suggesting drinking all the time to prevent PTSD, but there’s a strong association in several studies, with about a 50% reduction in rates of PTSD in those who are intoxicated at the time of the trauma,” according to Dr. Bisson.
Unfortunately, to date, none of those pharmacologic approaches have been effective when studied in randomized trials.
One pharmacologic intervention
Only one drug, hydrocortisone, was rated an “intervention with emerging evidence of efficacy” for prevention of PTSD symptoms in adults when given within the first 3 months after a traumatic event. Three placebo-controlled, randomized trials have shown a positive effect.
“It should be said that most of the studies of hydrocortisone have been done in individuals following extreme physical illness, such as septic shock sufferers, so the generalizability is a bit of a question. Nevertheless, it’s the one agent that has meta-analytic evidence of being effective at preventing PTSD, although more research is needed,” Dr. Bisson said.
Results of randomized trials featuring those agents have been “really disappointing” in light of what seems a sound theoretic rationale, he continued.
“We’re really struggling from a pharmacologic perspective to know what to do. I would say we are still at the experimental stage, and there’s no real good evidence that we should give any medication to prevent PTSD,” Dr. Bisson said.
Early psychosocial interventions
The ISTSS guidelines rate only two single-session interventions for prevention as rising to the promising level of “emerging evidence” of clinically important benefit: single-session eye movement desensitization and reprocessing (EMDR), which in its multisession format is a well-established treatment with strong evidence of efficacy in established PTSD, and a program known as Group 512 PM, which combines group debriefing with group cohesion–building exercises.
“Group 512 PM was done in groups of Chinese army personnel helping in recovery efforts following a 2008 earthquake in China that killed 80,000 people. It resulted in nearly a 50% reduction in PTSD versus no debriefing. This cohesion training might be a clue to us as something to work on in the future,” Dr. Bisson said.
The ISTSS guidelines deem there is insufficient evidence to recommend single-session group debriefing, group stress management, heart stress management, group education, trauma-focused counselling, computerized visuospatial task, individual psychoeducation, or individual debriefing.
“In six randomized controlled trials over nearly the last 20 years, we see a strong signal that individual psychological debriefing isn’t effective. So, certainly, going into a room with an individual or a couple and talking about what they’ve been through in great detail and getting them to express their emotions and advising them that’s a normal reaction doesn’t seem to be enough. And rather worryingly, the people who tend to do worse with that sort of intervention are the people who’ve got the most symptoms when they started, so they’re the ones at highest risk of developing PTSD,” Dr. Bisson said.
Multisession prevention interventions such as brief dyadic therapy and self-guided Internet interventions are supported by emerging evidence. Less promising, and with insufficient evidence to recommend, according to the ISTSS, are brief interpersonal therapy, brief individual trauma processing therapy, telephone-based cognitive-behavioral therapy (CBT), and nurse-led intensive care recovery programs.
For multisession early treatment interventions for patients with emerging traumatic stress symptoms within the first 3 months, the new ISTSS guidelines recommend as standard therapy CBT with a trauma focus, EMDR, or cognitive therapy. Stepped or collaborative care is rated as having “low effect.” There is emerging evidence for structured writing interventions and Internet-based guided self-help. And there is insufficient evidence to recommend behavioral activation, Internet virtual reality therapy, telephone-based CBT with a trauma focus, computerized neurobehavioral training, or supportive counseling.
Treating adults with established PTSD
Pharmacotherapy, including fluoxetine, sertraline, paroxetine, and venlafaxine is rated in the guidelines as a low-effect treatment. Quetiapine has emerging evidence of efficacy. Everything else has insufficient evidence.
Psychological therapies such as EMDR, CBT with a trauma focus, prolonged exposure, cognitive therapy, and cognitive processing therapy received strong recommendations. In fact, those are the only interventions in the entire ISTSS guidelines that received a “strong recommendation” rating. A weaker “standard recommendation” is given to CBT without a trauma focus, narrative exposure therapy, present-centered therapy, group CBT with a trauma focus, and guided Internet-based therapy with a trauma focus. Interventions with emerging evidence of efficacy include virtual reality therapy, reconsolidation of traumatic memories, and couples CBT with a trauma focus.
Best-practice approach to prevention
“In my view, and what I tell people, is that after a traumatic event I think practical pragmatic support in an empathic manner is the best first step,” Dr. Bisson said. “And it doesn’t have to be provided by a mental health professional. In fact, your family and friends are the best people to provide that. And then, we watchfully wait to see if traumatic stress symptoms emerge. If they do, and particularly if their trajectory is going up, then at about 1 month, I would get in there and deliver a therapy, either CBT with a trauma focus, EMDR, or cognitive therapy with a trauma focus. All of those have a significant positive effect for this group.”
Although he restricted his talk to secondary prevention of PTSD in adults, the ISTSS guidelines also address early intervention in children and adolescents.
Dr. Bisson reported having no financial conflicts of interest regarding his presentation.
Barcelona – New evidence-based guidelines on posttraumatic stress disorder prevention and treatment from the International Society for Traumatic Stress Studies (ISTSS) highlight an uncomfortable truth: Namely, the basis for early formal intervention of any sort is sorely lacking.
“I’m acutely aware that a lot of people in the mental health field are not aware of the evidence base as it stands at the moment,” Jonathan I. Bisson, MD, said at the annual congress of the European College of Neuropsychopharmacology. “There’s something very human about trying to do something. I think we find it very hard to do nothing following a traumatic event.”
Dr. Bisson, a professor of psychiatry at Cardiff (Wales) University and the chair of the ISTSS guidelines committee, provided an advance look at the ISTSS guidelines, which have since been released.
Secondary prevention of PTSD can entail either blocking development of symptoms after exposure to trauma or treating early emergent PTSD symptoms. Dr. Bisson emphasized that, although multiple exciting prospects are on the horizon for secondary prevention, those interventions need further work before implementation. The ISTSS guidelines, based on the group’s meta-analyses of 361 randomized controlled trials, rated most of the diverse psychosocial, psychological, and pharmacologic interventions that have been proposed or are now actually being used in clinical practice as either “low effect,” “interventions with emerging evidence,” or “insufficient evidence to recommend.” Those interventions are not backed by sufficient evidence of efficacy to be ready for prime time use in clinical practice.
Morever, the potential for iatrogenic harm is very real.
to a trauma,” the psychiatrist observed. “It’s normal to cry after a bereavement, for example. But should we be pathologizing that, or is that the body’s way of actually bringing itself to terms with something that’s very extreme?
“So we’ve got to be careful in our efforts to shape emotional processing, which might do absolutely nothing – which I’d argue is a problem when we’ve got limited resources because we should be focusing those resources on things that make a difference. Or it could minimize or prevent prolonged distress or pathology, which is what we’re after. Or it could interfere with the adaptive acute stress response – and that’s a real problem and one we’ve got to be very careful about,” Dr. Bisson said. “So ‘primum non nocere’ – first do no harm – should be a principle we adhere to.”
Neurobiology of PTSD
The accepted view of the neurobiology of PTSD is that it represents a failure of the medial prefrontal/anterior cingulate network to regulate activity in the amygdala, with resultant hyperreactivity to threat. Enhanced negative feedback of cortisol occurs. The brain’s response to low cortisol is to increase levels of corticotropin-releasing factor, which has the unwanted consequence of increased locus coeruleus activity and noradrenaline release. The resultant adrenergic surge facilitates the laying down and consolidation of traumatic memories.
Also, low cortisol levels disinhibit retrieval of traumatic memories, so the affected individual thinks more about the trauma. All of this elicits an uncontrolled sympathetic response, so the patient remains in a constant state of hyperarousal characteristic of PTSD.
“In theory we should have some really simple ways to prevent PTSD from occurring if we get in there soon enough: reducing noradrenergic overactivity via alpha2-adrenergic receptor agonism with an agent such as clonidine; postsynaptic beta-adrenergic blocking with a drug such as propranolol; or alpha1-adrenergic receptor blocking, as with prazosin. All of these approaches reduce noradrenergic tone and therefore should be effective, in theory, to prevent PTSD.
“We should also be able to use indirect strategies to reduce noradrenergic overactivity: GABA agents like benzodiazepines, alcohol, and gabapentin oppose noradrenaline action in the amygdala. I’m not suggesting drinking all the time to prevent PTSD, but there’s a strong association in several studies, with about a 50% reduction in rates of PTSD in those who are intoxicated at the time of the trauma,” according to Dr. Bisson.
Unfortunately, to date, none of those pharmacologic approaches have been effective when studied in randomized trials.
One pharmacologic intervention
Only one drug, hydrocortisone, was rated an “intervention with emerging evidence of efficacy” for prevention of PTSD symptoms in adults when given within the first 3 months after a traumatic event. Three placebo-controlled, randomized trials have shown a positive effect.
“It should be said that most of the studies of hydrocortisone have been done in individuals following extreme physical illness, such as septic shock sufferers, so the generalizability is a bit of a question. Nevertheless, it’s the one agent that has meta-analytic evidence of being effective at preventing PTSD, although more research is needed,” Dr. Bisson said.
Results of randomized trials featuring those agents have been “really disappointing” in light of what seems a sound theoretic rationale, he continued.
“We’re really struggling from a pharmacologic perspective to know what to do. I would say we are still at the experimental stage, and there’s no real good evidence that we should give any medication to prevent PTSD,” Dr. Bisson said.
Early psychosocial interventions
The ISTSS guidelines rate only two single-session interventions for prevention as rising to the promising level of “emerging evidence” of clinically important benefit: single-session eye movement desensitization and reprocessing (EMDR), which in its multisession format is a well-established treatment with strong evidence of efficacy in established PTSD, and a program known as Group 512 PM, which combines group debriefing with group cohesion–building exercises.
“Group 512 PM was done in groups of Chinese army personnel helping in recovery efforts following a 2008 earthquake in China that killed 80,000 people. It resulted in nearly a 50% reduction in PTSD versus no debriefing. This cohesion training might be a clue to us as something to work on in the future,” Dr. Bisson said.
The ISTSS guidelines deem there is insufficient evidence to recommend single-session group debriefing, group stress management, heart stress management, group education, trauma-focused counselling, computerized visuospatial task, individual psychoeducation, or individual debriefing.
“In six randomized controlled trials over nearly the last 20 years, we see a strong signal that individual psychological debriefing isn’t effective. So, certainly, going into a room with an individual or a couple and talking about what they’ve been through in great detail and getting them to express their emotions and advising them that’s a normal reaction doesn’t seem to be enough. And rather worryingly, the people who tend to do worse with that sort of intervention are the people who’ve got the most symptoms when they started, so they’re the ones at highest risk of developing PTSD,” Dr. Bisson said.
Multisession prevention interventions such as brief dyadic therapy and self-guided Internet interventions are supported by emerging evidence. Less promising, and with insufficient evidence to recommend, according to the ISTSS, are brief interpersonal therapy, brief individual trauma processing therapy, telephone-based cognitive-behavioral therapy (CBT), and nurse-led intensive care recovery programs.
For multisession early treatment interventions for patients with emerging traumatic stress symptoms within the first 3 months, the new ISTSS guidelines recommend as standard therapy CBT with a trauma focus, EMDR, or cognitive therapy. Stepped or collaborative care is rated as having “low effect.” There is emerging evidence for structured writing interventions and Internet-based guided self-help. And there is insufficient evidence to recommend behavioral activation, Internet virtual reality therapy, telephone-based CBT with a trauma focus, computerized neurobehavioral training, or supportive counseling.
Treating adults with established PTSD
Pharmacotherapy, including fluoxetine, sertraline, paroxetine, and venlafaxine is rated in the guidelines as a low-effect treatment. Quetiapine has emerging evidence of efficacy. Everything else has insufficient evidence.
Psychological therapies such as EMDR, CBT with a trauma focus, prolonged exposure, cognitive therapy, and cognitive processing therapy received strong recommendations. In fact, those are the only interventions in the entire ISTSS guidelines that received a “strong recommendation” rating. A weaker “standard recommendation” is given to CBT without a trauma focus, narrative exposure therapy, present-centered therapy, group CBT with a trauma focus, and guided Internet-based therapy with a trauma focus. Interventions with emerging evidence of efficacy include virtual reality therapy, reconsolidation of traumatic memories, and couples CBT with a trauma focus.
Best-practice approach to prevention
“In my view, and what I tell people, is that after a traumatic event I think practical pragmatic support in an empathic manner is the best first step,” Dr. Bisson said. “And it doesn’t have to be provided by a mental health professional. In fact, your family and friends are the best people to provide that. And then, we watchfully wait to see if traumatic stress symptoms emerge. If they do, and particularly if their trajectory is going up, then at about 1 month, I would get in there and deliver a therapy, either CBT with a trauma focus, EMDR, or cognitive therapy with a trauma focus. All of those have a significant positive effect for this group.”
Although he restricted his talk to secondary prevention of PTSD in adults, the ISTSS guidelines also address early intervention in children and adolescents.
Dr. Bisson reported having no financial conflicts of interest regarding his presentation.
EXPERT ANALYSIS FROM THE ECNP CONGRESS
Ask depressed patients about hypersomnia to screen for mixicity
BARCELONA – Hypersomnia is a novel, previously unappreciated factor useful in tipping the balance in favor of an underlying bipolar predisposition in patients with an acute major depressive episode, Andrea Murru, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
“This may help us in clinical practice. It’s an effective, costless, and highly objective clinical measure. It’s one question, and it takes a second. It’s simply asking the patient: ‘Are you sleeping more hours at night than usual?’ It’s a very simple clinical question that could really change the focus of treatment for a patient,” said Dr. Murru, a clinical psychiatrist in the bipolar disorders program of the University of Barcelona.
He presented a post hoc analysis of 2,514 acutely depressed individuals who participated in the BRIDGE-II-MIX (Bipolar Disorders: Improving Diagnosis, Guidance and Education) study, an international, multicenter, cross-sectional, observational study aimed at better characterizing clinically valid mixed features of depression indicative of concurrent manic symptoms.
“This is one of a whole series of hypothesis-generating studies from BRIDGE-II-MIX that are trying to deal with the struggle of understanding whether all the elements that favor mixicity and an underpinning bipolar diathesis are fairly represented in the diagnostic criteria in DSM-5. And what we are basically finding is the DSM-5 criteria are leaving out important symptoms that really do play a role,” the psychiatrist said in an interview.
One of those missing factors, he continued, is hypersomnia. It was present in 16.8% of the study population, and he and his coinvestigators compared them in terms of clinical variables, current and past psychiatric symptoms, and sociodemographics with the 83.2% of patients with insomnia. That is, patients who got fewer hours of sleep than normal and felt fatigued during the next day were compared with those who felt a reduced need to sleep.
The two groups differed in important ways. Hypersomnia showed a powerful correlation with a physician diagnosis of major depressive episode with atypical features, being present in 32.2% of such patients, while a mere 1.8% had insomnia. Moreover, among patients diagnosed with bipolar disorder I or II, 20.6% reported hypersomnia, a significantly higher proportion than the 16% who had insomnia.
The finding that only 5% of BRIDGE-II-MIX participants with hypersomnia met DSM-5 criteria for a mixed-state specifier, a rate not significantly different from the 8.3% figure in those with insomnia, underscores the drawbacks of the DSM-5 criteria, according to Dr. Murru. He noted that, in contrast to the DSM-5 criteria, 32.9% of the hypersomniac patients with a major depressive episode met Research Diagnostic Criteria (RDC) for a mixed-state specifier, a rate significantly higher than the 27.6% figure in patients with insomnia.
Specifically, the individual RDC mixed-state specifiers that stood out as significantly more frequent among depressed patients with hypersomnia than insomnia were racing thoughts, by a margin of 15.1% to 10.6%; impulsivity, 16.8% versus 13.2%; distractibility, 29.6% versus 23.4%; hypersexuality, which was present in 4% of patients with hypersomnia but only 2.3% with insomnia; irritable mood, 33.1% versus 24.8%; and a history of insufficient response to previous antidepressant therapy, 34.3%, compared with 27.1% in insomniacs.
When Dr. Murru and his coinvestigators performed a stepwise linear regression analysis to identify significant predictors of hypersomnia in patients with a major depressive episode, they found that the sleep abnormality keeps some interesting company. Patients with current bulimia were 4.21-fold more likely to have hypersomnia than those without the eating disorder. Current social phobia was associated with a 1.77-fold increased risk of hypersomnia; mood lability on prior antidepressant therapy carried a 1.37-fold risk, as did current mood lability; prior attempted suicide was associated with a 1.31-fold increased risk; being overweight or obese was associated with a 1.42-fold risk; currently being on a mood stabilizer carried a 1.33-fold increased risk of hypersomnia; and currently being on an atypical antipsychotic agent had a 1.36-fold greater risk.
Dr. Murru concluded that the take-home message of this study – “Of course, conceding it’s highly exploratory nature intrinsic to a post hoc analysis,” he noted – is that hypersomnia should be included among the symptoms that trigger the “with mixed features” specifier in patients with a major depressive episode.
The BRIDGE-II-MIX study was sponsored by Sanofi-Aventis. Dr. Murru reported having no financial conflicts of interest regarding the study.
BARCELONA – Hypersomnia is a novel, previously unappreciated factor useful in tipping the balance in favor of an underlying bipolar predisposition in patients with an acute major depressive episode, Andrea Murru, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
“This may help us in clinical practice. It’s an effective, costless, and highly objective clinical measure. It’s one question, and it takes a second. It’s simply asking the patient: ‘Are you sleeping more hours at night than usual?’ It’s a very simple clinical question that could really change the focus of treatment for a patient,” said Dr. Murru, a clinical psychiatrist in the bipolar disorders program of the University of Barcelona.
He presented a post hoc analysis of 2,514 acutely depressed individuals who participated in the BRIDGE-II-MIX (Bipolar Disorders: Improving Diagnosis, Guidance and Education) study, an international, multicenter, cross-sectional, observational study aimed at better characterizing clinically valid mixed features of depression indicative of concurrent manic symptoms.
“This is one of a whole series of hypothesis-generating studies from BRIDGE-II-MIX that are trying to deal with the struggle of understanding whether all the elements that favor mixicity and an underpinning bipolar diathesis are fairly represented in the diagnostic criteria in DSM-5. And what we are basically finding is the DSM-5 criteria are leaving out important symptoms that really do play a role,” the psychiatrist said in an interview.
One of those missing factors, he continued, is hypersomnia. It was present in 16.8% of the study population, and he and his coinvestigators compared them in terms of clinical variables, current and past psychiatric symptoms, and sociodemographics with the 83.2% of patients with insomnia. That is, patients who got fewer hours of sleep than normal and felt fatigued during the next day were compared with those who felt a reduced need to sleep.
The two groups differed in important ways. Hypersomnia showed a powerful correlation with a physician diagnosis of major depressive episode with atypical features, being present in 32.2% of such patients, while a mere 1.8% had insomnia. Moreover, among patients diagnosed with bipolar disorder I or II, 20.6% reported hypersomnia, a significantly higher proportion than the 16% who had insomnia.
The finding that only 5% of BRIDGE-II-MIX participants with hypersomnia met DSM-5 criteria for a mixed-state specifier, a rate not significantly different from the 8.3% figure in those with insomnia, underscores the drawbacks of the DSM-5 criteria, according to Dr. Murru. He noted that, in contrast to the DSM-5 criteria, 32.9% of the hypersomniac patients with a major depressive episode met Research Diagnostic Criteria (RDC) for a mixed-state specifier, a rate significantly higher than the 27.6% figure in patients with insomnia.
Specifically, the individual RDC mixed-state specifiers that stood out as significantly more frequent among depressed patients with hypersomnia than insomnia were racing thoughts, by a margin of 15.1% to 10.6%; impulsivity, 16.8% versus 13.2%; distractibility, 29.6% versus 23.4%; hypersexuality, which was present in 4% of patients with hypersomnia but only 2.3% with insomnia; irritable mood, 33.1% versus 24.8%; and a history of insufficient response to previous antidepressant therapy, 34.3%, compared with 27.1% in insomniacs.
When Dr. Murru and his coinvestigators performed a stepwise linear regression analysis to identify significant predictors of hypersomnia in patients with a major depressive episode, they found that the sleep abnormality keeps some interesting company. Patients with current bulimia were 4.21-fold more likely to have hypersomnia than those without the eating disorder. Current social phobia was associated with a 1.77-fold increased risk of hypersomnia; mood lability on prior antidepressant therapy carried a 1.37-fold risk, as did current mood lability; prior attempted suicide was associated with a 1.31-fold increased risk; being overweight or obese was associated with a 1.42-fold risk; currently being on a mood stabilizer carried a 1.33-fold increased risk of hypersomnia; and currently being on an atypical antipsychotic agent had a 1.36-fold greater risk.
Dr. Murru concluded that the take-home message of this study – “Of course, conceding it’s highly exploratory nature intrinsic to a post hoc analysis,” he noted – is that hypersomnia should be included among the symptoms that trigger the “with mixed features” specifier in patients with a major depressive episode.
The BRIDGE-II-MIX study was sponsored by Sanofi-Aventis. Dr. Murru reported having no financial conflicts of interest regarding the study.
BARCELONA – Hypersomnia is a novel, previously unappreciated factor useful in tipping the balance in favor of an underlying bipolar predisposition in patients with an acute major depressive episode, Andrea Murru, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
“This may help us in clinical practice. It’s an effective, costless, and highly objective clinical measure. It’s one question, and it takes a second. It’s simply asking the patient: ‘Are you sleeping more hours at night than usual?’ It’s a very simple clinical question that could really change the focus of treatment for a patient,” said Dr. Murru, a clinical psychiatrist in the bipolar disorders program of the University of Barcelona.
He presented a post hoc analysis of 2,514 acutely depressed individuals who participated in the BRIDGE-II-MIX (Bipolar Disorders: Improving Diagnosis, Guidance and Education) study, an international, multicenter, cross-sectional, observational study aimed at better characterizing clinically valid mixed features of depression indicative of concurrent manic symptoms.
“This is one of a whole series of hypothesis-generating studies from BRIDGE-II-MIX that are trying to deal with the struggle of understanding whether all the elements that favor mixicity and an underpinning bipolar diathesis are fairly represented in the diagnostic criteria in DSM-5. And what we are basically finding is the DSM-5 criteria are leaving out important symptoms that really do play a role,” the psychiatrist said in an interview.
One of those missing factors, he continued, is hypersomnia. It was present in 16.8% of the study population, and he and his coinvestigators compared them in terms of clinical variables, current and past psychiatric symptoms, and sociodemographics with the 83.2% of patients with insomnia. That is, patients who got fewer hours of sleep than normal and felt fatigued during the next day were compared with those who felt a reduced need to sleep.
The two groups differed in important ways. Hypersomnia showed a powerful correlation with a physician diagnosis of major depressive episode with atypical features, being present in 32.2% of such patients, while a mere 1.8% had insomnia. Moreover, among patients diagnosed with bipolar disorder I or II, 20.6% reported hypersomnia, a significantly higher proportion than the 16% who had insomnia.
The finding that only 5% of BRIDGE-II-MIX participants with hypersomnia met DSM-5 criteria for a mixed-state specifier, a rate not significantly different from the 8.3% figure in those with insomnia, underscores the drawbacks of the DSM-5 criteria, according to Dr. Murru. He noted that, in contrast to the DSM-5 criteria, 32.9% of the hypersomniac patients with a major depressive episode met Research Diagnostic Criteria (RDC) for a mixed-state specifier, a rate significantly higher than the 27.6% figure in patients with insomnia.
Specifically, the individual RDC mixed-state specifiers that stood out as significantly more frequent among depressed patients with hypersomnia than insomnia were racing thoughts, by a margin of 15.1% to 10.6%; impulsivity, 16.8% versus 13.2%; distractibility, 29.6% versus 23.4%; hypersexuality, which was present in 4% of patients with hypersomnia but only 2.3% with insomnia; irritable mood, 33.1% versus 24.8%; and a history of insufficient response to previous antidepressant therapy, 34.3%, compared with 27.1% in insomniacs.
When Dr. Murru and his coinvestigators performed a stepwise linear regression analysis to identify significant predictors of hypersomnia in patients with a major depressive episode, they found that the sleep abnormality keeps some interesting company. Patients with current bulimia were 4.21-fold more likely to have hypersomnia than those without the eating disorder. Current social phobia was associated with a 1.77-fold increased risk of hypersomnia; mood lability on prior antidepressant therapy carried a 1.37-fold risk, as did current mood lability; prior attempted suicide was associated with a 1.31-fold increased risk; being overweight or obese was associated with a 1.42-fold risk; currently being on a mood stabilizer carried a 1.33-fold increased risk of hypersomnia; and currently being on an atypical antipsychotic agent had a 1.36-fold greater risk.
Dr. Murru concluded that the take-home message of this study – “Of course, conceding it’s highly exploratory nature intrinsic to a post hoc analysis,” he noted – is that hypersomnia should be included among the symptoms that trigger the “with mixed features” specifier in patients with a major depressive episode.
The BRIDGE-II-MIX study was sponsored by Sanofi-Aventis. Dr. Murru reported having no financial conflicts of interest regarding the study.
REPORTING FROM THE ECNP CONGRESS
Key clinical point: Ask patients with a major depressive episode about hypersomnia.
Major finding: Hypersomnia in patients with an acute major depressive episode clusters with numerous elements of a bipolar diathesis.
Study details: This was a post hoc analysis of 2,514 acutely depressed individuals who participated in an international, multicenter, cross-sectional, observational study.
Disclosures: The BRIDGE-II-MIX study was sponsored by Sanofi-Aventis. The presenter reported having no financial conflicts of interest regarding the study.
Novel theory explains SSRIs’ variable efficacy
BARCELONA – New evidence from the landmark 12-year-old STAR*D clinical trial provides fresh support for a novel Italian hypothesis that the efficacy of selective serotonin reuptake inhibitors in major depressive disorder results from their capacity to amplify the influence of living conditions upon mood.
“This hypothesis claims that SSRIs do not affect mood by themselves, but by increasing brain plasticity to render an individual more susceptible to the influence of living conditions. So in a positive environment, treatment leads to a beneficial outcome, and in a stressful environment it may lead to a worse prognosis,” Aurelia Viglione explained at the annual congress of the European College of Neuropsychopharmacology.
This originally was demonstrated by her senior coinvestigators in mouse studies, which showed increased brain plasticity and responsiveness of mood to living conditions in the presence of the serotonin boost provided by SSRIs. Now this animal research has been confirmed in a more clinically relevant fashion via a secondary analysis of the venerable National Institute of Mental Health–funded STAR*D (Sequenced Treatment Alternatives to Relieve Depression) data set, which Ms. Viglione presented at the ECNP congress.
This hypothesis, which she and her colleagues have dubbed the “undirected susceptibility to change” hypothesis of the mechanism of action of SSRIs, attempts to account for the drugs’ highly variable efficacy. As prescribing physicians and their often-frustrated patients with major depression are all too aware, the SSRIs – the most widely prescribed treatment for the disorder – induce remission in only 30%-40% of patients, while an additional 30%-40% fail to experience even a significant response. And despite much research effort, to date there is no reliable way to match the best antidepressant to a given individual in accord with the current priority to develop a personalized medicine approach.
The undirected susceptibility to change hypothesis provides a plausible explanation for these mixed clinical outcomes. The hypothesis posits that high serotonin levels lead to increased brain plasticity and consequent openness to change in mood, which can be for the better or worse – depending upon the quality of the surrounding environment.
Ms. Viglione, a PhD student in neuroscience at the University of Pisa (Italy), presented a study of a 591-patient subset of patients with major depression in STAR*D who received citalopram at 20 mg/day for 4 weeks, at which point the 40% of participants who weren’t showing a sufficiently favorable response trend had their citalopram increased to 40 mg/day. Patients’ living conditions were categorized as favorable or adverse based upon an amalgam of sociodemographic characteristics, including employment status, education, marital status, income, insurance status, ethnicity, drug abuse, and history of traumatic events. Treatment response was measured using the 16-item Quick Inventory of Depressive Symptomatology (QIDS-SR16) score.
. Patients were more likely to achieve remission as defined by a QIDS-SR16 score of 5 or less at week 6 if they had a college education, a job, higher income, and/or private health insurance than if those proxies for favorable living conditions were not present.
The impact of sociodemographic factors on change in mood was much larger in patients on citalopram at 40 mg/day than 20 mg/day. For example, having a college education rather than a high school education was independently associated with up to a 37-fold greater remission rate among patients on the higher dose of the SSRI, depending upon their sociodemographic status, compared with a 2-fold greater likelihood of remission in patients on citalopram at 20 mg/day.
“The correlation is very high. It’s a very huge effect,” Ms. Viglione commented.
On the other hand, the 40-mg dose also was associated with an increased likelihood of worsening in patients living in an unfavorable environment. For example, 35% of patients in the 40-mg group with only a high school degree showed worsening depression, compared with 21% on citalopram at 20 mg/day. And 40% of low-income patients on citalopram at 40 mg/day showed worsening depression, compared with 35% of low-income patients on 20 mg. These figures probably underestimate the true downside of higher-dose treatment in patients living in an adverse environment, because STAR*D guidelines called for treatment discontinuation in the setting of worsening depression, she noted.
The correlation between higher-dose SSRI therapy, sociodemographic environment, and change in depressive symptoms was not uniform across all symptom categories. Ms. Viglione and her colleagues grouped the elements of the QIDS-SR16 rating scale into three domains: core emotional symptoms, sleep/insomnia symptoms, and weight/appetite. They found that the impact of higher-dose therapy in combination with favorable living conditions was greatest on sleep/insomnia symptoms.
The next step for the Italian investigators will be to determine in a prospective study whether the undirected susceptibility to change hypothesis can predict which patients will benefit from SSRI therapy.
Ms. Viglione reported having no financial conflicts regarding her study, which was funded by the Italian Ministry of Health.
BARCELONA – New evidence from the landmark 12-year-old STAR*D clinical trial provides fresh support for a novel Italian hypothesis that the efficacy of selective serotonin reuptake inhibitors in major depressive disorder results from their capacity to amplify the influence of living conditions upon mood.
“This hypothesis claims that SSRIs do not affect mood by themselves, but by increasing brain plasticity to render an individual more susceptible to the influence of living conditions. So in a positive environment, treatment leads to a beneficial outcome, and in a stressful environment it may lead to a worse prognosis,” Aurelia Viglione explained at the annual congress of the European College of Neuropsychopharmacology.
This originally was demonstrated by her senior coinvestigators in mouse studies, which showed increased brain plasticity and responsiveness of mood to living conditions in the presence of the serotonin boost provided by SSRIs. Now this animal research has been confirmed in a more clinically relevant fashion via a secondary analysis of the venerable National Institute of Mental Health–funded STAR*D (Sequenced Treatment Alternatives to Relieve Depression) data set, which Ms. Viglione presented at the ECNP congress.
This hypothesis, which she and her colleagues have dubbed the “undirected susceptibility to change” hypothesis of the mechanism of action of SSRIs, attempts to account for the drugs’ highly variable efficacy. As prescribing physicians and their often-frustrated patients with major depression are all too aware, the SSRIs – the most widely prescribed treatment for the disorder – induce remission in only 30%-40% of patients, while an additional 30%-40% fail to experience even a significant response. And despite much research effort, to date there is no reliable way to match the best antidepressant to a given individual in accord with the current priority to develop a personalized medicine approach.
The undirected susceptibility to change hypothesis provides a plausible explanation for these mixed clinical outcomes. The hypothesis posits that high serotonin levels lead to increased brain plasticity and consequent openness to change in mood, which can be for the better or worse – depending upon the quality of the surrounding environment.
Ms. Viglione, a PhD student in neuroscience at the University of Pisa (Italy), presented a study of a 591-patient subset of patients with major depression in STAR*D who received citalopram at 20 mg/day for 4 weeks, at which point the 40% of participants who weren’t showing a sufficiently favorable response trend had their citalopram increased to 40 mg/day. Patients’ living conditions were categorized as favorable or adverse based upon an amalgam of sociodemographic characteristics, including employment status, education, marital status, income, insurance status, ethnicity, drug abuse, and history of traumatic events. Treatment response was measured using the 16-item Quick Inventory of Depressive Symptomatology (QIDS-SR16) score.
. Patients were more likely to achieve remission as defined by a QIDS-SR16 score of 5 or less at week 6 if they had a college education, a job, higher income, and/or private health insurance than if those proxies for favorable living conditions were not present.
The impact of sociodemographic factors on change in mood was much larger in patients on citalopram at 40 mg/day than 20 mg/day. For example, having a college education rather than a high school education was independently associated with up to a 37-fold greater remission rate among patients on the higher dose of the SSRI, depending upon their sociodemographic status, compared with a 2-fold greater likelihood of remission in patients on citalopram at 20 mg/day.
“The correlation is very high. It’s a very huge effect,” Ms. Viglione commented.
On the other hand, the 40-mg dose also was associated with an increased likelihood of worsening in patients living in an unfavorable environment. For example, 35% of patients in the 40-mg group with only a high school degree showed worsening depression, compared with 21% on citalopram at 20 mg/day. And 40% of low-income patients on citalopram at 40 mg/day showed worsening depression, compared with 35% of low-income patients on 20 mg. These figures probably underestimate the true downside of higher-dose treatment in patients living in an adverse environment, because STAR*D guidelines called for treatment discontinuation in the setting of worsening depression, she noted.
The correlation between higher-dose SSRI therapy, sociodemographic environment, and change in depressive symptoms was not uniform across all symptom categories. Ms. Viglione and her colleagues grouped the elements of the QIDS-SR16 rating scale into three domains: core emotional symptoms, sleep/insomnia symptoms, and weight/appetite. They found that the impact of higher-dose therapy in combination with favorable living conditions was greatest on sleep/insomnia symptoms.
The next step for the Italian investigators will be to determine in a prospective study whether the undirected susceptibility to change hypothesis can predict which patients will benefit from SSRI therapy.
Ms. Viglione reported having no financial conflicts regarding her study, which was funded by the Italian Ministry of Health.
BARCELONA – New evidence from the landmark 12-year-old STAR*D clinical trial provides fresh support for a novel Italian hypothesis that the efficacy of selective serotonin reuptake inhibitors in major depressive disorder results from their capacity to amplify the influence of living conditions upon mood.
“This hypothesis claims that SSRIs do not affect mood by themselves, but by increasing brain plasticity to render an individual more susceptible to the influence of living conditions. So in a positive environment, treatment leads to a beneficial outcome, and in a stressful environment it may lead to a worse prognosis,” Aurelia Viglione explained at the annual congress of the European College of Neuropsychopharmacology.
This originally was demonstrated by her senior coinvestigators in mouse studies, which showed increased brain plasticity and responsiveness of mood to living conditions in the presence of the serotonin boost provided by SSRIs. Now this animal research has been confirmed in a more clinically relevant fashion via a secondary analysis of the venerable National Institute of Mental Health–funded STAR*D (Sequenced Treatment Alternatives to Relieve Depression) data set, which Ms. Viglione presented at the ECNP congress.
This hypothesis, which she and her colleagues have dubbed the “undirected susceptibility to change” hypothesis of the mechanism of action of SSRIs, attempts to account for the drugs’ highly variable efficacy. As prescribing physicians and their often-frustrated patients with major depression are all too aware, the SSRIs – the most widely prescribed treatment for the disorder – induce remission in only 30%-40% of patients, while an additional 30%-40% fail to experience even a significant response. And despite much research effort, to date there is no reliable way to match the best antidepressant to a given individual in accord with the current priority to develop a personalized medicine approach.
The undirected susceptibility to change hypothesis provides a plausible explanation for these mixed clinical outcomes. The hypothesis posits that high serotonin levels lead to increased brain plasticity and consequent openness to change in mood, which can be for the better or worse – depending upon the quality of the surrounding environment.
Ms. Viglione, a PhD student in neuroscience at the University of Pisa (Italy), presented a study of a 591-patient subset of patients with major depression in STAR*D who received citalopram at 20 mg/day for 4 weeks, at which point the 40% of participants who weren’t showing a sufficiently favorable response trend had their citalopram increased to 40 mg/day. Patients’ living conditions were categorized as favorable or adverse based upon an amalgam of sociodemographic characteristics, including employment status, education, marital status, income, insurance status, ethnicity, drug abuse, and history of traumatic events. Treatment response was measured using the 16-item Quick Inventory of Depressive Symptomatology (QIDS-SR16) score.
. Patients were more likely to achieve remission as defined by a QIDS-SR16 score of 5 or less at week 6 if they had a college education, a job, higher income, and/or private health insurance than if those proxies for favorable living conditions were not present.
The impact of sociodemographic factors on change in mood was much larger in patients on citalopram at 40 mg/day than 20 mg/day. For example, having a college education rather than a high school education was independently associated with up to a 37-fold greater remission rate among patients on the higher dose of the SSRI, depending upon their sociodemographic status, compared with a 2-fold greater likelihood of remission in patients on citalopram at 20 mg/day.
“The correlation is very high. It’s a very huge effect,” Ms. Viglione commented.
On the other hand, the 40-mg dose also was associated with an increased likelihood of worsening in patients living in an unfavorable environment. For example, 35% of patients in the 40-mg group with only a high school degree showed worsening depression, compared with 21% on citalopram at 20 mg/day. And 40% of low-income patients on citalopram at 40 mg/day showed worsening depression, compared with 35% of low-income patients on 20 mg. These figures probably underestimate the true downside of higher-dose treatment in patients living in an adverse environment, because STAR*D guidelines called for treatment discontinuation in the setting of worsening depression, she noted.
The correlation between higher-dose SSRI therapy, sociodemographic environment, and change in depressive symptoms was not uniform across all symptom categories. Ms. Viglione and her colleagues grouped the elements of the QIDS-SR16 rating scale into three domains: core emotional symptoms, sleep/insomnia symptoms, and weight/appetite. They found that the impact of higher-dose therapy in combination with favorable living conditions was greatest on sleep/insomnia symptoms.
The next step for the Italian investigators will be to determine in a prospective study whether the undirected susceptibility to change hypothesis can predict which patients will benefit from SSRI therapy.
Ms. Viglione reported having no financial conflicts regarding her study, which was funded by the Italian Ministry of Health.
REPORTING FROM THE ECNP CONGRESS
Key clinical point: There’s a new hypothesis to explain the mixed results of selective serotonin reuptake inhibitors for major depression.
Major finding: Improvement in symptoms of major depression was strongly correlated with SSRI dose and patients’ living conditions.
Study details: This secondary analysis of the STAR*D data set included 591 patients who received citalopram for major depression.
Disclosures: The presenter reported having no financial conflicts regarding the study, funded by the Italian Ministry of Health.
Mood stabilizers protect bipolar patients from suicide
BARCELONA – Lithium and valproic acid are the treatments of choice for patients with bipolar disorder at increased risk for suicide, according to the findings of a large Finnish national study.
“We found that – surprise, surprise – lithium and valproic acid had the lowest risk of anyone committing suicide on them. And funnily enough, some antidepressants seemed to be correlated with a higher risk of committing suicide,” Markku Lähteenvuo, MD, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
However, he suspects that the correlation between antidepressant therapy and suicide in bipolar patients probably was caused by confounding by indication.
“I wouldn’t have as a take-home message that SSRI [selective serotonin reuptake inhibitor] antidepressants are bad for you; it’s probably rather that these are really bad cases, treatment gets initiated when someone gets worse, and the antidepressants just aren’t quick enough to really give an effect. It takes 3-4 weeks for an SSRI to really kick in, whereas mood stabilizers like lithium and valproic acid usually kick in almost immediately, within a couple of days to a week,” explained Dr. Lähteenvuo, a forensic psychiatrist at Niuvanniemi Hospital and the University of Eastern Finland, in Kuopio.
He presented a study of all suicides among the 18,018 Finnish patients hospitalized for bipolar disorder nationwide during 1996-2012. Using prospective national databases to follow patients for a mean of 7.2 years, he and his coinvestigators were able to determine what medications they were on when they committed suicide and the likelihood they were actually taking their prescribed medications at the time.
In a multivariate proportional hazards analysis adjusted for concomitant psychotropic medications, duration of bipolar illness, intervals of drug exposure and nonexposure, age, sex, and number of hospitalizations for suicidality within the prior 2 years, bipolar patients on lithium had a 67% lower risk of suicide than did those not on the drug. Those on valproic acid were 39% less likely to commit suicide than those not on that mood stabilizer. And bipolar patients on lithium were 42% less likely to die from suicide than those on valproic acid.
At the other extreme, patients on antidepressants were in aggregate 28% more likely to commit suicide than those who were not. This risk varied considerably according to the specific antidepressant: for example, escitalopram and mirtazapine were associated with 71% and 57% greater risks of suicide, respectively, than in patients not on those antidepressants, while patients on paroxetine, venlafaxine, or sertraline were not at increased risk.
Also, the use of sedatives was associated with a 52% greater likelihood of suicide, and benzodiazepines were linked to a 21% increase in risk. Again, as with antidepressants, these associations with increased risk of completed suicide could be attributable in part or in whole to confounding by indication, the psychiatrist noted. He and his colleagues are conducting further analyses examining that possibility.
Pending the outcome of that closer look, however, Dr. Lähteenvuo believes the study’s message to clinicians is clear: “The use of lithium is getting less and less common all the time because of side effects. That’s also true for valproic acid. Many bipolar patients are now prescribed only antidepressants, even though all the guidelines advise against it due to the risk of hypomania. We think that this is a bad trend and that lithium and valproic acid should be prescribed more. Also, as a safety precaution, anyone with bipolar disorder on antidepressants or benzodiazepines or sedatives should be followed especially closely for suicidal signals, because they could carry a really highly increased risk – up to 50% – due to the medication as well.”
He reported having no financial conflicts regarding the study, funded by the Finnish government.
Dr. Lähteenvuo also was first author of a recent study showing that among the various drugs prescribed for treatment of bipolar disorder, lithium was the clear winner for prevention of rehospitalization in the same national Finnish population of more than 18,000 bipolar patients (JAMA Psychiatry. 2018;75[4]:347-55). One expert named this among the top four studies of the year in the field of mood disorders.
BARCELONA – Lithium and valproic acid are the treatments of choice for patients with bipolar disorder at increased risk for suicide, according to the findings of a large Finnish national study.
“We found that – surprise, surprise – lithium and valproic acid had the lowest risk of anyone committing suicide on them. And funnily enough, some antidepressants seemed to be correlated with a higher risk of committing suicide,” Markku Lähteenvuo, MD, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
However, he suspects that the correlation between antidepressant therapy and suicide in bipolar patients probably was caused by confounding by indication.
“I wouldn’t have as a take-home message that SSRI [selective serotonin reuptake inhibitor] antidepressants are bad for you; it’s probably rather that these are really bad cases, treatment gets initiated when someone gets worse, and the antidepressants just aren’t quick enough to really give an effect. It takes 3-4 weeks for an SSRI to really kick in, whereas mood stabilizers like lithium and valproic acid usually kick in almost immediately, within a couple of days to a week,” explained Dr. Lähteenvuo, a forensic psychiatrist at Niuvanniemi Hospital and the University of Eastern Finland, in Kuopio.
He presented a study of all suicides among the 18,018 Finnish patients hospitalized for bipolar disorder nationwide during 1996-2012. Using prospective national databases to follow patients for a mean of 7.2 years, he and his coinvestigators were able to determine what medications they were on when they committed suicide and the likelihood they were actually taking their prescribed medications at the time.
In a multivariate proportional hazards analysis adjusted for concomitant psychotropic medications, duration of bipolar illness, intervals of drug exposure and nonexposure, age, sex, and number of hospitalizations for suicidality within the prior 2 years, bipolar patients on lithium had a 67% lower risk of suicide than did those not on the drug. Those on valproic acid were 39% less likely to commit suicide than those not on that mood stabilizer. And bipolar patients on lithium were 42% less likely to die from suicide than those on valproic acid.
At the other extreme, patients on antidepressants were in aggregate 28% more likely to commit suicide than those who were not. This risk varied considerably according to the specific antidepressant: for example, escitalopram and mirtazapine were associated with 71% and 57% greater risks of suicide, respectively, than in patients not on those antidepressants, while patients on paroxetine, venlafaxine, or sertraline were not at increased risk.
Also, the use of sedatives was associated with a 52% greater likelihood of suicide, and benzodiazepines were linked to a 21% increase in risk. Again, as with antidepressants, these associations with increased risk of completed suicide could be attributable in part or in whole to confounding by indication, the psychiatrist noted. He and his colleagues are conducting further analyses examining that possibility.
Pending the outcome of that closer look, however, Dr. Lähteenvuo believes the study’s message to clinicians is clear: “The use of lithium is getting less and less common all the time because of side effects. That’s also true for valproic acid. Many bipolar patients are now prescribed only antidepressants, even though all the guidelines advise against it due to the risk of hypomania. We think that this is a bad trend and that lithium and valproic acid should be prescribed more. Also, as a safety precaution, anyone with bipolar disorder on antidepressants or benzodiazepines or sedatives should be followed especially closely for suicidal signals, because they could carry a really highly increased risk – up to 50% – due to the medication as well.”
He reported having no financial conflicts regarding the study, funded by the Finnish government.
Dr. Lähteenvuo also was first author of a recent study showing that among the various drugs prescribed for treatment of bipolar disorder, lithium was the clear winner for prevention of rehospitalization in the same national Finnish population of more than 18,000 bipolar patients (JAMA Psychiatry. 2018;75[4]:347-55). One expert named this among the top four studies of the year in the field of mood disorders.
BARCELONA – Lithium and valproic acid are the treatments of choice for patients with bipolar disorder at increased risk for suicide, according to the findings of a large Finnish national study.
“We found that – surprise, surprise – lithium and valproic acid had the lowest risk of anyone committing suicide on them. And funnily enough, some antidepressants seemed to be correlated with a higher risk of committing suicide,” Markku Lähteenvuo, MD, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
However, he suspects that the correlation between antidepressant therapy and suicide in bipolar patients probably was caused by confounding by indication.
“I wouldn’t have as a take-home message that SSRI [selective serotonin reuptake inhibitor] antidepressants are bad for you; it’s probably rather that these are really bad cases, treatment gets initiated when someone gets worse, and the antidepressants just aren’t quick enough to really give an effect. It takes 3-4 weeks for an SSRI to really kick in, whereas mood stabilizers like lithium and valproic acid usually kick in almost immediately, within a couple of days to a week,” explained Dr. Lähteenvuo, a forensic psychiatrist at Niuvanniemi Hospital and the University of Eastern Finland, in Kuopio.
He presented a study of all suicides among the 18,018 Finnish patients hospitalized for bipolar disorder nationwide during 1996-2012. Using prospective national databases to follow patients for a mean of 7.2 years, he and his coinvestigators were able to determine what medications they were on when they committed suicide and the likelihood they were actually taking their prescribed medications at the time.
In a multivariate proportional hazards analysis adjusted for concomitant psychotropic medications, duration of bipolar illness, intervals of drug exposure and nonexposure, age, sex, and number of hospitalizations for suicidality within the prior 2 years, bipolar patients on lithium had a 67% lower risk of suicide than did those not on the drug. Those on valproic acid were 39% less likely to commit suicide than those not on that mood stabilizer. And bipolar patients on lithium were 42% less likely to die from suicide than those on valproic acid.
At the other extreme, patients on antidepressants were in aggregate 28% more likely to commit suicide than those who were not. This risk varied considerably according to the specific antidepressant: for example, escitalopram and mirtazapine were associated with 71% and 57% greater risks of suicide, respectively, than in patients not on those antidepressants, while patients on paroxetine, venlafaxine, or sertraline were not at increased risk.
Also, the use of sedatives was associated with a 52% greater likelihood of suicide, and benzodiazepines were linked to a 21% increase in risk. Again, as with antidepressants, these associations with increased risk of completed suicide could be attributable in part or in whole to confounding by indication, the psychiatrist noted. He and his colleagues are conducting further analyses examining that possibility.
Pending the outcome of that closer look, however, Dr. Lähteenvuo believes the study’s message to clinicians is clear: “The use of lithium is getting less and less common all the time because of side effects. That’s also true for valproic acid. Many bipolar patients are now prescribed only antidepressants, even though all the guidelines advise against it due to the risk of hypomania. We think that this is a bad trend and that lithium and valproic acid should be prescribed more. Also, as a safety precaution, anyone with bipolar disorder on antidepressants or benzodiazepines or sedatives should be followed especially closely for suicidal signals, because they could carry a really highly increased risk – up to 50% – due to the medication as well.”
He reported having no financial conflicts regarding the study, funded by the Finnish government.
Dr. Lähteenvuo also was first author of a recent study showing that among the various drugs prescribed for treatment of bipolar disorder, lithium was the clear winner for prevention of rehospitalization in the same national Finnish population of more than 18,000 bipolar patients (JAMA Psychiatry. 2018;75[4]:347-55). One expert named this among the top four studies of the year in the field of mood disorders.
REPORTING FROM THE ECNP CONGRESS
Key clinical point: Lithium and valproic acid protect patients with bipolar disorder from suicide.
Major finding: The likelihood of suicide was reduced by two-thirds in bipolar patients on lithium, compared with that of patients not on the mood stabilizer.
Study details: This was a retrospective study of more than 18,000 Finnish patients hospitalized at some point for bipolar disorder and the medications they were on when a subset of them committed suicide.
Disclosures: The presenter reported having no financial conflicts regarding the study, funded by the Finnish government.
Four predictors confirmed for treatment-resistant depression
BARCELONA – Four potent clinical predictors of treatment-resistant depression have been confirmed in a validation study that showed the four factors collectively have a predictive accuracy of 87%, Alexander Kautzky, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
The four predictors – all easily assessed – are symptom severity, which was independently associated with a 3.3-fold increased likelihood of treatment-resistant depression (TRD) in a study of 916 patients with major depression at 10 referral centers in eight European countries; suicidal risk, with a 1.74-fold increased risk; comorbid anxiety disorder, with a 1.68-fold increased risk; and the lifetime number of major depressive episodes, for which the associated TRD risk climbs by 15% per prior episode, according to Dr. Kautzky of the Medical University of Vienna.
When these four predictors were put to the test in an independent validation cohort of 314 patients with major depression, the four clinical markers of TRD exhibited a sensitivity of 86%, a specificity of 88%, and an overall predictive accuracy of 87%.
In these studies, Response was defined as at least a 50% drop in MADRS score to below 22 points.
The validation of these clinical predictors of TRD is a welcome development in psychiatry. The World Health Organization ranks major depressive disorder as the No. 4 cause of disease burden worldwide, and an impressive number of antidepressant medications are available, but up to 60% of patients do not respond sufficiently to their first round of antidepressant therapy. And there is a notable absence of biological markers to aid in selecting the best initial antidepressant for a given individual, Dr. Kautzky observed.
The validation study was supported by an unrestricted research grant from Lundbeck to a European research consortium known as the Group for the Study of Resistant Depression. Dr. Kautzky reported having no financial conflicts of interest.
BARCELONA – Four potent clinical predictors of treatment-resistant depression have been confirmed in a validation study that showed the four factors collectively have a predictive accuracy of 87%, Alexander Kautzky, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
The four predictors – all easily assessed – are symptom severity, which was independently associated with a 3.3-fold increased likelihood of treatment-resistant depression (TRD) in a study of 916 patients with major depression at 10 referral centers in eight European countries; suicidal risk, with a 1.74-fold increased risk; comorbid anxiety disorder, with a 1.68-fold increased risk; and the lifetime number of major depressive episodes, for which the associated TRD risk climbs by 15% per prior episode, according to Dr. Kautzky of the Medical University of Vienna.
When these four predictors were put to the test in an independent validation cohort of 314 patients with major depression, the four clinical markers of TRD exhibited a sensitivity of 86%, a specificity of 88%, and an overall predictive accuracy of 87%.
In these studies, Response was defined as at least a 50% drop in MADRS score to below 22 points.
The validation of these clinical predictors of TRD is a welcome development in psychiatry. The World Health Organization ranks major depressive disorder as the No. 4 cause of disease burden worldwide, and an impressive number of antidepressant medications are available, but up to 60% of patients do not respond sufficiently to their first round of antidepressant therapy. And there is a notable absence of biological markers to aid in selecting the best initial antidepressant for a given individual, Dr. Kautzky observed.
The validation study was supported by an unrestricted research grant from Lundbeck to a European research consortium known as the Group for the Study of Resistant Depression. Dr. Kautzky reported having no financial conflicts of interest.
BARCELONA – Four potent clinical predictors of treatment-resistant depression have been confirmed in a validation study that showed the four factors collectively have a predictive accuracy of 87%, Alexander Kautzky, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
The four predictors – all easily assessed – are symptom severity, which was independently associated with a 3.3-fold increased likelihood of treatment-resistant depression (TRD) in a study of 916 patients with major depression at 10 referral centers in eight European countries; suicidal risk, with a 1.74-fold increased risk; comorbid anxiety disorder, with a 1.68-fold increased risk; and the lifetime number of major depressive episodes, for which the associated TRD risk climbs by 15% per prior episode, according to Dr. Kautzky of the Medical University of Vienna.
When these four predictors were put to the test in an independent validation cohort of 314 patients with major depression, the four clinical markers of TRD exhibited a sensitivity of 86%, a specificity of 88%, and an overall predictive accuracy of 87%.
In these studies, Response was defined as at least a 50% drop in MADRS score to below 22 points.
The validation of these clinical predictors of TRD is a welcome development in psychiatry. The World Health Organization ranks major depressive disorder as the No. 4 cause of disease burden worldwide, and an impressive number of antidepressant medications are available, but up to 60% of patients do not respond sufficiently to their first round of antidepressant therapy. And there is a notable absence of biological markers to aid in selecting the best initial antidepressant for a given individual, Dr. Kautzky observed.
The validation study was supported by an unrestricted research grant from Lundbeck to a European research consortium known as the Group for the Study of Resistant Depression. Dr. Kautzky reported having no financial conflicts of interest.
REPORTING FROM THE ECNP CONGRESS
Key clinical point: Patients with major depressive disorder at high risk for treatment-resistant depression are now readily identifiable.
Major finding: Four easily collected clinical variables predict treatment-resistant depression with 87% accuracy.
Study details: This project identified clinical predictors of treatment-resistant depression in a cohort of 916 patients with major depressive disorder then validated the predictors in a separate cohort of 314 patients.
Disclosures: The presenter reported having no financial conflicts regarding this study, which was supported by an unrestricted research grant from Lundbeck.
Adult ADHD? Screen for hoarding symptoms
BARCELONA – Clinically meaningful hoarding symptoms are present in roughly one in four adults with attention-deficit/hyperactivity disorder, Sharon Morein-Zamir, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
Her message to her fellow clinicians: “Nobody tends to ask about hoarding problems in adult ADHD clinics. Ask your ADHD patients carefully and routinely about hoarding symptoms. Screen them for it, ask their family members about it, and see whether it could be a problem contributing to daily impairment,” urged Dr. Morein-Zamir, a senior lecturer in clinical psychology at Anglia Ruskin University in Cambridge, England.
The clinician must broach the subject, because hoarding often is characterized by lack of insight.
“Patients don’t complain about it. You’ll have family members complain about it, neighbors complain about it, maybe social services, but the individuals themselves often don’t think they have a problem. And if they acknowledge it, they don’t seek treatment for it. So you really need to actively ask about the issue. They won’t raise it themselves,” she said.
Hoarding disorder and ADHD are considered two separate entities. But her study demonstrated that they share a common link: inattention symptoms.
the psychologist continued.
Indeed, one of the reasons why hoarding disorder is no longer grouped with obsessive-compulsive disorder in diagnostic schema is that inattention symptoms are not characteristic of OCD.
Dr. Morein-Zamir presented a cross-sectional study of 50 patients in an adult ADHD clinic and 46 age- and sex-matched controls. A total of 22 of the ADHD patients were on methylphenidate, 15 on selective serotonin reuptake inhibitors, 6 on amphetamine, and 7 were unmedicated.
Participants were assessed for hoarding using two validated measures well-suited for screening in daily practice: the Saving Inventory–Revised (SIR) and the Clutter Image Rating (CIR). Clinically meaningful hoarding symptoms – a designation requiring both a score of at least 42 on the SIR and 12 on the CIR – were present in 11 of 50 adult ADHD patients and none of the controls.
The group with clinically meaningful hoarding symptoms differed from the 39 ADHD patients without hoarding most noticeably in their more pronounced inattention symptoms as scored on the Adult ADHD Self-Report Scale (ASRS): a mean score of 32.8, compared with 28.8 in ADHD patients without clinically important hoarding. In contrast, the two groups scored similarly for hyperactivity/impulsivity on the patient-completed 18-item ASRS, as well as for depression and anxiety on the Depression Anxiety Stress Scales (DASS).
Within the ADHD group, only inattention as measured on the ASRS predicted hoarding severity on the SIR. In a multivariate regression analysis controlling for age, sex, hyperactivity/impulsivity on the ASRS, and DASS scores, inattention correlated strongly with all of the key hoarding dimensions: clutter, excessive acquisition, and difficulty discarding. Hyperactivity/impulsivity showed a modest correlation with clutter but not with the other hoarding dimensions.
Dr. Morein-Zamir observed that, while the last 3 or so years have seen booming interest in the development of manualized cognitive-behavioral therapy strategies for hoarding disorder, it’s not yet known whether those tools will be effective for treating high-level hoarding symptoms in patients with ADHD.
She reported having no financial conflicts regarding her study, which was funded by the British Academy.
BARCELONA – Clinically meaningful hoarding symptoms are present in roughly one in four adults with attention-deficit/hyperactivity disorder, Sharon Morein-Zamir, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
Her message to her fellow clinicians: “Nobody tends to ask about hoarding problems in adult ADHD clinics. Ask your ADHD patients carefully and routinely about hoarding symptoms. Screen them for it, ask their family members about it, and see whether it could be a problem contributing to daily impairment,” urged Dr. Morein-Zamir, a senior lecturer in clinical psychology at Anglia Ruskin University in Cambridge, England.
The clinician must broach the subject, because hoarding often is characterized by lack of insight.
“Patients don’t complain about it. You’ll have family members complain about it, neighbors complain about it, maybe social services, but the individuals themselves often don’t think they have a problem. And if they acknowledge it, they don’t seek treatment for it. So you really need to actively ask about the issue. They won’t raise it themselves,” she said.
Hoarding disorder and ADHD are considered two separate entities. But her study demonstrated that they share a common link: inattention symptoms.
the psychologist continued.
Indeed, one of the reasons why hoarding disorder is no longer grouped with obsessive-compulsive disorder in diagnostic schema is that inattention symptoms are not characteristic of OCD.
Dr. Morein-Zamir presented a cross-sectional study of 50 patients in an adult ADHD clinic and 46 age- and sex-matched controls. A total of 22 of the ADHD patients were on methylphenidate, 15 on selective serotonin reuptake inhibitors, 6 on amphetamine, and 7 were unmedicated.
Participants were assessed for hoarding using two validated measures well-suited for screening in daily practice: the Saving Inventory–Revised (SIR) and the Clutter Image Rating (CIR). Clinically meaningful hoarding symptoms – a designation requiring both a score of at least 42 on the SIR and 12 on the CIR – were present in 11 of 50 adult ADHD patients and none of the controls.
The group with clinically meaningful hoarding symptoms differed from the 39 ADHD patients without hoarding most noticeably in their more pronounced inattention symptoms as scored on the Adult ADHD Self-Report Scale (ASRS): a mean score of 32.8, compared with 28.8 in ADHD patients without clinically important hoarding. In contrast, the two groups scored similarly for hyperactivity/impulsivity on the patient-completed 18-item ASRS, as well as for depression and anxiety on the Depression Anxiety Stress Scales (DASS).
Within the ADHD group, only inattention as measured on the ASRS predicted hoarding severity on the SIR. In a multivariate regression analysis controlling for age, sex, hyperactivity/impulsivity on the ASRS, and DASS scores, inattention correlated strongly with all of the key hoarding dimensions: clutter, excessive acquisition, and difficulty discarding. Hyperactivity/impulsivity showed a modest correlation with clutter but not with the other hoarding dimensions.
Dr. Morein-Zamir observed that, while the last 3 or so years have seen booming interest in the development of manualized cognitive-behavioral therapy strategies for hoarding disorder, it’s not yet known whether those tools will be effective for treating high-level hoarding symptoms in patients with ADHD.
She reported having no financial conflicts regarding her study, which was funded by the British Academy.
BARCELONA – Clinically meaningful hoarding symptoms are present in roughly one in four adults with attention-deficit/hyperactivity disorder, Sharon Morein-Zamir, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
Her message to her fellow clinicians: “Nobody tends to ask about hoarding problems in adult ADHD clinics. Ask your ADHD patients carefully and routinely about hoarding symptoms. Screen them for it, ask their family members about it, and see whether it could be a problem contributing to daily impairment,” urged Dr. Morein-Zamir, a senior lecturer in clinical psychology at Anglia Ruskin University in Cambridge, England.
The clinician must broach the subject, because hoarding often is characterized by lack of insight.
“Patients don’t complain about it. You’ll have family members complain about it, neighbors complain about it, maybe social services, but the individuals themselves often don’t think they have a problem. And if they acknowledge it, they don’t seek treatment for it. So you really need to actively ask about the issue. They won’t raise it themselves,” she said.
Hoarding disorder and ADHD are considered two separate entities. But her study demonstrated that they share a common link: inattention symptoms.
the psychologist continued.
Indeed, one of the reasons why hoarding disorder is no longer grouped with obsessive-compulsive disorder in diagnostic schema is that inattention symptoms are not characteristic of OCD.
Dr. Morein-Zamir presented a cross-sectional study of 50 patients in an adult ADHD clinic and 46 age- and sex-matched controls. A total of 22 of the ADHD patients were on methylphenidate, 15 on selective serotonin reuptake inhibitors, 6 on amphetamine, and 7 were unmedicated.
Participants were assessed for hoarding using two validated measures well-suited for screening in daily practice: the Saving Inventory–Revised (SIR) and the Clutter Image Rating (CIR). Clinically meaningful hoarding symptoms – a designation requiring both a score of at least 42 on the SIR and 12 on the CIR – were present in 11 of 50 adult ADHD patients and none of the controls.
The group with clinically meaningful hoarding symptoms differed from the 39 ADHD patients without hoarding most noticeably in their more pronounced inattention symptoms as scored on the Adult ADHD Self-Report Scale (ASRS): a mean score of 32.8, compared with 28.8 in ADHD patients without clinically important hoarding. In contrast, the two groups scored similarly for hyperactivity/impulsivity on the patient-completed 18-item ASRS, as well as for depression and anxiety on the Depression Anxiety Stress Scales (DASS).
Within the ADHD group, only inattention as measured on the ASRS predicted hoarding severity on the SIR. In a multivariate regression analysis controlling for age, sex, hyperactivity/impulsivity on the ASRS, and DASS scores, inattention correlated strongly with all of the key hoarding dimensions: clutter, excessive acquisition, and difficulty discarding. Hyperactivity/impulsivity showed a modest correlation with clutter but not with the other hoarding dimensions.
Dr. Morein-Zamir observed that, while the last 3 or so years have seen booming interest in the development of manualized cognitive-behavioral therapy strategies for hoarding disorder, it’s not yet known whether those tools will be effective for treating high-level hoarding symptoms in patients with ADHD.
She reported having no financial conflicts regarding her study, which was funded by the British Academy.
REPORTING FROM THE ECNP CONGRESS
Key clinical point: Routinely screen adults with ADHD for hoarding disorder.
Major finding: Eleven of 50 (22%) unselected adults with ADHD displayed clinically meaningful hoarding symptoms.
Study details: This cross-sectional study included 50 adult ADHD patients and 46 matched controls who were assessed for hoarding symptoms and inattention.
Disclosures: The presenter reported having no financial conflicts regarding this study, which was funded by the British Academy.
The year’s top studies in child/adolescent psychiatry
BARCELONA – Prenatal exposure to selective serotonin reuptake inhibitors late in pregnancy was associated with a significantly increased risk of anxious and/or depressed behaviors at 5 years of age in the prospective Norwegian Mother and Child Cohort Study.
Other than that specific red flag, however, the outcomes of in utero exposure to maternal SSRIs were reassuringly benign. Prenatal exposure during early- or mid-pregnancy was not associated with increased risk of anxious/depressed behaviors, compared with nonexposure; that adverse effect was restricted to exposure at week 29 of pregnancy or later. Nor did in utero exposure to maternal SSRIs during any time in pregnancy pose an increased risk for pediatric externalizing, emotional, or social problems in this observational study of 8,359 Norwegian mother-child dyads, Josefina Castro-Fornieles, MD, PhD, observed at the annual congress of the European College of Neuropsychopharmacology.
The huge Norwegian study was among what she considers the four most important studies in child/adolescent psychiatry published through the first three quarters of 2018. The others she highlighted were a large longitudinal observational study that demonstrated that persistent maternal postnatal depression was strongly associated with a variety of pediatric behavioral disturbances documented during assessments at ages 3.5, 16, and 18 years; a Philadelphia study showing that multiple traumatic stressful events or any assaultive trauma experienced by children or adolescents were independently associated with significant psychopathology and neurocognitive deficits; and a Dutch brain MRI study that pinpointed a reduction in gray matter volume in the anterior cingulate cortex as a potential key mediator of the neurobiologic aftereffects of childhood sexual abuse.
She selected those studies because they shared a common theme, one that constituted her key take-home message: “When recording antecedents during a clinical assessment, both with adults and children, it is clear that we have to ask in a more detailed way – using validated scales and interviews if possible – about the mother’s prenatal problems, including psychopharmacological treatment. That is something we often don’t do in a sufficiently detailed way in our clinical practice. And it’s also important to ask about life events; abuse during childhood and adolescence can be really important. We can modulate our treatment depending upon whether there is an influence of any of these aspects,” said Dr. Castro-Fornieles, director of the Clinical Institute of Neuroscience at the Hospital Clinic of Barcelona and a recent past-president of the Spanish Society for Child and Adolescent Psychiatry.
The following are her Top 4 studies:
The Norwegian Mother and Child Cohort Study
The increased risk of anxious and/or depressed behaviors in children exposed to selective serotonin reuptake inhibitors (SSRIs) late in pregnancy did not emerge until the year-5 assessment; it wasn’t evident at the 1.5- or 3-year evaluations.
The investigators emphasized a key lesson from their study: The importance of following children with late-pregnancy exposure to maternal SSRI therapy for development of symptoms of anxiety and/or depression (J Am Acad Child Adolesc Psychiatry. 2018 Mar;57[3]:200-8). Dr. Castro-Fornieles strongly endorsed that recommendation. However, she noted what she considers an important limitation to the study: even though the University of Oslo investigators adjusted for numerous potential confounders in their risk models – including maternal body mass index, parity, education, smoking, substance use, breastfeeding, folic acid use, and other medications used during pregnancy – it’s not possible in a study such as this to control for genetic and environmental risk factors, which she suspects also were at work.
The Avon Longitudinal Study of Parents and Children in the United Kingdom
Maternal postnatal depression is common, affecting roughly 10% of mothers. But it is not invariably associated with adverse mental health outcomes in their children. This study of nearly 10,000 mothers and their children sought to identify which children were at most risk. Using the Edinburgh Postnatal Depression Scale, the international team of investigators categorized maternal postnatal depression as moderate, marked, or severe. The affective disorder was deemed persistent if scores on the Edinburgh scale were elevated at both 2 and 8 months after delivery.
Postnatal depression, whether persistent or not, was associated with roughly a 2- to 2.4-fold increase for child behavioral disturbances when assessed at age 3.5 years using the Rutter Total Problems Scale. But postnatal depression that was persistent was the real difference maker: It carried a much higher risk of adverse behavioral outcomes and cognitive deficits than did the nonpersistent version. Indeed, persistent severe postnatal depression was associated a 4.8-fold increased risk of behavioral problems at age 3.5 years, a 2.65-fold greater risk of markedly lower grades in mathematics at age 16 years, and a 7.4-fold increased prevalence of depression at 18 years of age. The investigators advised screening mothers during the first postpartum year in order to identify those with persistent postpartum depression (JAMA Psychiatry. 2018 Mar 1;75[3]:247-53).
Dr. Castro-Fornieles said an important shortcoming of the Avon study was that it did not record paternal data.
“The study didn’t consider depression or other functional measures in the father, his commitment to childrearing, and whether the family was together or divorced. I feel this is an important limitation in many studies. For me, it’s really important to consider what’s happening with the fathers,” she said.
Traumatic stress load, psychopathology, and cognition
An eye-opening report from the Philadelphia Neurodevelopmental Cohort documented a surprisingly high level of lifetime exposure to traumatic events among 9,498 youth aged 8-21 years, and the stepwise manner by which a greater traumatic stress load was associated with increasing severity of psychopathology and cognitive deficits. Notably, the study participants were recruited from general pediatric clinics in the Children’s Hospital of Philadelphia health care network; they were not patients seeking psychiatric help. And yet, extensive structured psychiatric evaluation showed that 23% of them had a history of one traumatic stressful event, 12% had two, and 1% had three or more.
In analyses adjusted for lifetime history of depression or PTSD, a higher traumatic event load was associated with increased risk of externalizing behaviors, mood/anxiety disorders, psychosis spectrum, and fear. Moreover, a high trauma stress load was associated with a 5.3-fold increased risk of suicidal thoughts and a 3.2-fold increased likelihood of cannabis use, compared with youth who had never been exposed to a traumatic event. Increased stress load also was associated with worse cognitive performance on tests of executive functioning, social cognition, and complex reasoning.
A history of assaultive trauma – being badly beaten, threatened with a weapon, or sexually abused – was associated with more severe psychopathology than in subjects with a history of nonassaultive traumatic events (Psychol Med. 2018 Apr 15:1-10).
Session moderator Carmen Moreno, MD, a child and adolescent psychiatrist at Gregorio Marañón University Hospital in Madrid, commented, “It was striking to me that the prevalence of childhood traumatic events was so high in a pediatric community sample. Is the measure the investigators chose the right measure?”
Dr. Castro-Fornieles replied that it was a very sensitive measure, in that an event many would consider part of normal life – for example, seeing a relative’s body on display in a funeral home – was scored as a traumatic exposure.
“Only one exposure is not that important,” she said. “The impact increases as you increase the number of traumatic events. And also the assaultive ones.”
Sexual abuse leaves a fingerprint
Investigators at Leiden (the Netherlands) University performed neuroimaging that looked at numerous brain regions of interest in 21 adolescents with childhood sexual abuse–related PTSD and 25 matched healthy controls. The standout finding was that the dorsal gray matter volume of the anterior cingulate cortex was significantly smaller in the teens with PTSD and a history of childhood sexual abuse (Eur Neuropsychopharmacol. 2017 Nov;27[11]:1163-71).
The investigators wanted a pure sample of patients with PTSD after childhood sexual abuse, so they excluded individuals who had experienced childhood sexual abuse and had a diagnosis of attention-deficit/hyperactivity disorder, oppositional defiant disorder, obsessive-compulsive disorder, conduct disorder, pervasive developmental disorder, bipolar disorder, or a psychotic disorder. That is both a strength and a limitation of the study, in Dr. Castro-Fornieles’ view.
“To me, that excludes too many of the children we see in our clinical settings. This work needs to be corroborated in a bigger sample, including patients with other diagnoses,” she said.
She reported having no financial conflicts regarding her presentation.
BARCELONA – Prenatal exposure to selective serotonin reuptake inhibitors late in pregnancy was associated with a significantly increased risk of anxious and/or depressed behaviors at 5 years of age in the prospective Norwegian Mother and Child Cohort Study.
Other than that specific red flag, however, the outcomes of in utero exposure to maternal SSRIs were reassuringly benign. Prenatal exposure during early- or mid-pregnancy was not associated with increased risk of anxious/depressed behaviors, compared with nonexposure; that adverse effect was restricted to exposure at week 29 of pregnancy or later. Nor did in utero exposure to maternal SSRIs during any time in pregnancy pose an increased risk for pediatric externalizing, emotional, or social problems in this observational study of 8,359 Norwegian mother-child dyads, Josefina Castro-Fornieles, MD, PhD, observed at the annual congress of the European College of Neuropsychopharmacology.
The huge Norwegian study was among what she considers the four most important studies in child/adolescent psychiatry published through the first three quarters of 2018. The others she highlighted were a large longitudinal observational study that demonstrated that persistent maternal postnatal depression was strongly associated with a variety of pediatric behavioral disturbances documented during assessments at ages 3.5, 16, and 18 years; a Philadelphia study showing that multiple traumatic stressful events or any assaultive trauma experienced by children or adolescents were independently associated with significant psychopathology and neurocognitive deficits; and a Dutch brain MRI study that pinpointed a reduction in gray matter volume in the anterior cingulate cortex as a potential key mediator of the neurobiologic aftereffects of childhood sexual abuse.
She selected those studies because they shared a common theme, one that constituted her key take-home message: “When recording antecedents during a clinical assessment, both with adults and children, it is clear that we have to ask in a more detailed way – using validated scales and interviews if possible – about the mother’s prenatal problems, including psychopharmacological treatment. That is something we often don’t do in a sufficiently detailed way in our clinical practice. And it’s also important to ask about life events; abuse during childhood and adolescence can be really important. We can modulate our treatment depending upon whether there is an influence of any of these aspects,” said Dr. Castro-Fornieles, director of the Clinical Institute of Neuroscience at the Hospital Clinic of Barcelona and a recent past-president of the Spanish Society for Child and Adolescent Psychiatry.
The following are her Top 4 studies:
The Norwegian Mother and Child Cohort Study
The increased risk of anxious and/or depressed behaviors in children exposed to selective serotonin reuptake inhibitors (SSRIs) late in pregnancy did not emerge until the year-5 assessment; it wasn’t evident at the 1.5- or 3-year evaluations.
The investigators emphasized a key lesson from their study: The importance of following children with late-pregnancy exposure to maternal SSRI therapy for development of symptoms of anxiety and/or depression (J Am Acad Child Adolesc Psychiatry. 2018 Mar;57[3]:200-8). Dr. Castro-Fornieles strongly endorsed that recommendation. However, she noted what she considers an important limitation to the study: even though the University of Oslo investigators adjusted for numerous potential confounders in their risk models – including maternal body mass index, parity, education, smoking, substance use, breastfeeding, folic acid use, and other medications used during pregnancy – it’s not possible in a study such as this to control for genetic and environmental risk factors, which she suspects also were at work.
The Avon Longitudinal Study of Parents and Children in the United Kingdom
Maternal postnatal depression is common, affecting roughly 10% of mothers. But it is not invariably associated with adverse mental health outcomes in their children. This study of nearly 10,000 mothers and their children sought to identify which children were at most risk. Using the Edinburgh Postnatal Depression Scale, the international team of investigators categorized maternal postnatal depression as moderate, marked, or severe. The affective disorder was deemed persistent if scores on the Edinburgh scale were elevated at both 2 and 8 months after delivery.
Postnatal depression, whether persistent or not, was associated with roughly a 2- to 2.4-fold increase for child behavioral disturbances when assessed at age 3.5 years using the Rutter Total Problems Scale. But postnatal depression that was persistent was the real difference maker: It carried a much higher risk of adverse behavioral outcomes and cognitive deficits than did the nonpersistent version. Indeed, persistent severe postnatal depression was associated a 4.8-fold increased risk of behavioral problems at age 3.5 years, a 2.65-fold greater risk of markedly lower grades in mathematics at age 16 years, and a 7.4-fold increased prevalence of depression at 18 years of age. The investigators advised screening mothers during the first postpartum year in order to identify those with persistent postpartum depression (JAMA Psychiatry. 2018 Mar 1;75[3]:247-53).
Dr. Castro-Fornieles said an important shortcoming of the Avon study was that it did not record paternal data.
“The study didn’t consider depression or other functional measures in the father, his commitment to childrearing, and whether the family was together or divorced. I feel this is an important limitation in many studies. For me, it’s really important to consider what’s happening with the fathers,” she said.
Traumatic stress load, psychopathology, and cognition
An eye-opening report from the Philadelphia Neurodevelopmental Cohort documented a surprisingly high level of lifetime exposure to traumatic events among 9,498 youth aged 8-21 years, and the stepwise manner by which a greater traumatic stress load was associated with increasing severity of psychopathology and cognitive deficits. Notably, the study participants were recruited from general pediatric clinics in the Children’s Hospital of Philadelphia health care network; they were not patients seeking psychiatric help. And yet, extensive structured psychiatric evaluation showed that 23% of them had a history of one traumatic stressful event, 12% had two, and 1% had three or more.
In analyses adjusted for lifetime history of depression or PTSD, a higher traumatic event load was associated with increased risk of externalizing behaviors, mood/anxiety disorders, psychosis spectrum, and fear. Moreover, a high trauma stress load was associated with a 5.3-fold increased risk of suicidal thoughts and a 3.2-fold increased likelihood of cannabis use, compared with youth who had never been exposed to a traumatic event. Increased stress load also was associated with worse cognitive performance on tests of executive functioning, social cognition, and complex reasoning.
A history of assaultive trauma – being badly beaten, threatened with a weapon, or sexually abused – was associated with more severe psychopathology than in subjects with a history of nonassaultive traumatic events (Psychol Med. 2018 Apr 15:1-10).
Session moderator Carmen Moreno, MD, a child and adolescent psychiatrist at Gregorio Marañón University Hospital in Madrid, commented, “It was striking to me that the prevalence of childhood traumatic events was so high in a pediatric community sample. Is the measure the investigators chose the right measure?”
Dr. Castro-Fornieles replied that it was a very sensitive measure, in that an event many would consider part of normal life – for example, seeing a relative’s body on display in a funeral home – was scored as a traumatic exposure.
“Only one exposure is not that important,” she said. “The impact increases as you increase the number of traumatic events. And also the assaultive ones.”
Sexual abuse leaves a fingerprint
Investigators at Leiden (the Netherlands) University performed neuroimaging that looked at numerous brain regions of interest in 21 adolescents with childhood sexual abuse–related PTSD and 25 matched healthy controls. The standout finding was that the dorsal gray matter volume of the anterior cingulate cortex was significantly smaller in the teens with PTSD and a history of childhood sexual abuse (Eur Neuropsychopharmacol. 2017 Nov;27[11]:1163-71).
The investigators wanted a pure sample of patients with PTSD after childhood sexual abuse, so they excluded individuals who had experienced childhood sexual abuse and had a diagnosis of attention-deficit/hyperactivity disorder, oppositional defiant disorder, obsessive-compulsive disorder, conduct disorder, pervasive developmental disorder, bipolar disorder, or a psychotic disorder. That is both a strength and a limitation of the study, in Dr. Castro-Fornieles’ view.
“To me, that excludes too many of the children we see in our clinical settings. This work needs to be corroborated in a bigger sample, including patients with other diagnoses,” she said.
She reported having no financial conflicts regarding her presentation.
BARCELONA – Prenatal exposure to selective serotonin reuptake inhibitors late in pregnancy was associated with a significantly increased risk of anxious and/or depressed behaviors at 5 years of age in the prospective Norwegian Mother and Child Cohort Study.
Other than that specific red flag, however, the outcomes of in utero exposure to maternal SSRIs were reassuringly benign. Prenatal exposure during early- or mid-pregnancy was not associated with increased risk of anxious/depressed behaviors, compared with nonexposure; that adverse effect was restricted to exposure at week 29 of pregnancy or later. Nor did in utero exposure to maternal SSRIs during any time in pregnancy pose an increased risk for pediatric externalizing, emotional, or social problems in this observational study of 8,359 Norwegian mother-child dyads, Josefina Castro-Fornieles, MD, PhD, observed at the annual congress of the European College of Neuropsychopharmacology.
The huge Norwegian study was among what she considers the four most important studies in child/adolescent psychiatry published through the first three quarters of 2018. The others she highlighted were a large longitudinal observational study that demonstrated that persistent maternal postnatal depression was strongly associated with a variety of pediatric behavioral disturbances documented during assessments at ages 3.5, 16, and 18 years; a Philadelphia study showing that multiple traumatic stressful events or any assaultive trauma experienced by children or adolescents were independently associated with significant psychopathology and neurocognitive deficits; and a Dutch brain MRI study that pinpointed a reduction in gray matter volume in the anterior cingulate cortex as a potential key mediator of the neurobiologic aftereffects of childhood sexual abuse.
She selected those studies because they shared a common theme, one that constituted her key take-home message: “When recording antecedents during a clinical assessment, both with adults and children, it is clear that we have to ask in a more detailed way – using validated scales and interviews if possible – about the mother’s prenatal problems, including psychopharmacological treatment. That is something we often don’t do in a sufficiently detailed way in our clinical practice. And it’s also important to ask about life events; abuse during childhood and adolescence can be really important. We can modulate our treatment depending upon whether there is an influence of any of these aspects,” said Dr. Castro-Fornieles, director of the Clinical Institute of Neuroscience at the Hospital Clinic of Barcelona and a recent past-president of the Spanish Society for Child and Adolescent Psychiatry.
The following are her Top 4 studies:
The Norwegian Mother and Child Cohort Study
The increased risk of anxious and/or depressed behaviors in children exposed to selective serotonin reuptake inhibitors (SSRIs) late in pregnancy did not emerge until the year-5 assessment; it wasn’t evident at the 1.5- or 3-year evaluations.
The investigators emphasized a key lesson from their study: The importance of following children with late-pregnancy exposure to maternal SSRI therapy for development of symptoms of anxiety and/or depression (J Am Acad Child Adolesc Psychiatry. 2018 Mar;57[3]:200-8). Dr. Castro-Fornieles strongly endorsed that recommendation. However, she noted what she considers an important limitation to the study: even though the University of Oslo investigators adjusted for numerous potential confounders in their risk models – including maternal body mass index, parity, education, smoking, substance use, breastfeeding, folic acid use, and other medications used during pregnancy – it’s not possible in a study such as this to control for genetic and environmental risk factors, which she suspects also were at work.
The Avon Longitudinal Study of Parents and Children in the United Kingdom
Maternal postnatal depression is common, affecting roughly 10% of mothers. But it is not invariably associated with adverse mental health outcomes in their children. This study of nearly 10,000 mothers and their children sought to identify which children were at most risk. Using the Edinburgh Postnatal Depression Scale, the international team of investigators categorized maternal postnatal depression as moderate, marked, or severe. The affective disorder was deemed persistent if scores on the Edinburgh scale were elevated at both 2 and 8 months after delivery.
Postnatal depression, whether persistent or not, was associated with roughly a 2- to 2.4-fold increase for child behavioral disturbances when assessed at age 3.5 years using the Rutter Total Problems Scale. But postnatal depression that was persistent was the real difference maker: It carried a much higher risk of adverse behavioral outcomes and cognitive deficits than did the nonpersistent version. Indeed, persistent severe postnatal depression was associated a 4.8-fold increased risk of behavioral problems at age 3.5 years, a 2.65-fold greater risk of markedly lower grades in mathematics at age 16 years, and a 7.4-fold increased prevalence of depression at 18 years of age. The investigators advised screening mothers during the first postpartum year in order to identify those with persistent postpartum depression (JAMA Psychiatry. 2018 Mar 1;75[3]:247-53).
Dr. Castro-Fornieles said an important shortcoming of the Avon study was that it did not record paternal data.
“The study didn’t consider depression or other functional measures in the father, his commitment to childrearing, and whether the family was together or divorced. I feel this is an important limitation in many studies. For me, it’s really important to consider what’s happening with the fathers,” she said.
Traumatic stress load, psychopathology, and cognition
An eye-opening report from the Philadelphia Neurodevelopmental Cohort documented a surprisingly high level of lifetime exposure to traumatic events among 9,498 youth aged 8-21 years, and the stepwise manner by which a greater traumatic stress load was associated with increasing severity of psychopathology and cognitive deficits. Notably, the study participants were recruited from general pediatric clinics in the Children’s Hospital of Philadelphia health care network; they were not patients seeking psychiatric help. And yet, extensive structured psychiatric evaluation showed that 23% of them had a history of one traumatic stressful event, 12% had two, and 1% had three or more.
In analyses adjusted for lifetime history of depression or PTSD, a higher traumatic event load was associated with increased risk of externalizing behaviors, mood/anxiety disorders, psychosis spectrum, and fear. Moreover, a high trauma stress load was associated with a 5.3-fold increased risk of suicidal thoughts and a 3.2-fold increased likelihood of cannabis use, compared with youth who had never been exposed to a traumatic event. Increased stress load also was associated with worse cognitive performance on tests of executive functioning, social cognition, and complex reasoning.
A history of assaultive trauma – being badly beaten, threatened with a weapon, or sexually abused – was associated with more severe psychopathology than in subjects with a history of nonassaultive traumatic events (Psychol Med. 2018 Apr 15:1-10).
Session moderator Carmen Moreno, MD, a child and adolescent psychiatrist at Gregorio Marañón University Hospital in Madrid, commented, “It was striking to me that the prevalence of childhood traumatic events was so high in a pediatric community sample. Is the measure the investigators chose the right measure?”
Dr. Castro-Fornieles replied that it was a very sensitive measure, in that an event many would consider part of normal life – for example, seeing a relative’s body on display in a funeral home – was scored as a traumatic exposure.
“Only one exposure is not that important,” she said. “The impact increases as you increase the number of traumatic events. And also the assaultive ones.”
Sexual abuse leaves a fingerprint
Investigators at Leiden (the Netherlands) University performed neuroimaging that looked at numerous brain regions of interest in 21 adolescents with childhood sexual abuse–related PTSD and 25 matched healthy controls. The standout finding was that the dorsal gray matter volume of the anterior cingulate cortex was significantly smaller in the teens with PTSD and a history of childhood sexual abuse (Eur Neuropsychopharmacol. 2017 Nov;27[11]:1163-71).
The investigators wanted a pure sample of patients with PTSD after childhood sexual abuse, so they excluded individuals who had experienced childhood sexual abuse and had a diagnosis of attention-deficit/hyperactivity disorder, oppositional defiant disorder, obsessive-compulsive disorder, conduct disorder, pervasive developmental disorder, bipolar disorder, or a psychotic disorder. That is both a strength and a limitation of the study, in Dr. Castro-Fornieles’ view.
“To me, that excludes too many of the children we see in our clinical settings. This work needs to be corroborated in a bigger sample, including patients with other diagnoses,” she said.
She reported having no financial conflicts regarding her presentation.
REPORTING FROM THE ECNP CONGRESS



















