User login
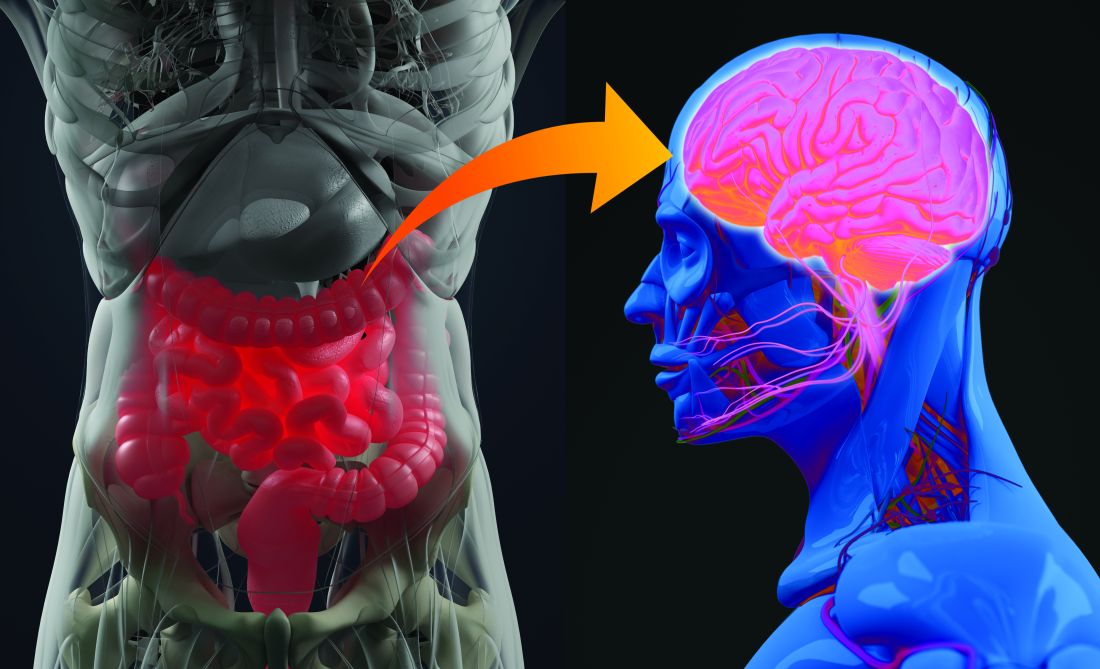
Divergent COVID-19 mental health impacts seen in Spain and China
Spain and China used very different public health responses to the COVID-19 crisis, and that has had significant consequences in terms of the mental health as well as physical health of the two countries’ citizens, Roger Ho, MD, reported at the virtual congress of the European College of Neuropsychopharmacology.
Dr. Ho, a psychiatrist at the National University of Singapore, presented a first-of-its-kind cross-cultural comparative study of the impact of the COVID-19 pandemic in two epicenters on opposite sides of the world. A total of 1,539 participants drawn from the general populations in the two countries completed the online National University of Singapore COVID-19 Questionnaire. The survey was conducted in late February/early March in China and in mid-April in Spain, times of intense disease activity in the countries.
The questionnaire assesses knowledge and concerns about COVID, precautionary measures taken in the last 14 days, contact history, and physical symptoms related to COVID in the last 14 days. The pandemic’s psychological impact was evaluated using the Impact of Event Scale–Revised (IES-R). Participants also completed the Depression, Anxiety, and Stress-21 Scale (DASS-21).
Of note, the pandemic has taken a vastly greater physical toll in Spain than China. As of May 5, there were 83,000 confirmed cases of COVID-19 in China, with a population of 1.39 billion, compared with 248,000 in Spain, with a population of 46.9 million. The Spanish case rate of 5,500 per 1 million population was 100 times greater than China’s; the Spanish mortality rate of 585 per million was 185-fold greater.
Mental health findings
Spaniards experienced significantly higher levels of stress and depression as reflected in DASS-21 subscale scores of 14.22 and 8.65, respectively, compared with 7.86 and 6.38, in Chinese respondents. Spanish subjects also reported greater anxiety levels than the Chinese on the DASS-21 anxiety subscale, although not to a statistically significant extent. Yet, counterintuitively, given the DASS-21 results, the pandemic had a greater adverse psychological impact on the Chinese subjects as reflected in their significantly higher average IES-D score of 30.76 versus 27.64 in Spain. Dr. Ho offered a hypothesis as to why: The survey documented that many Chinese respondents felt socially stigmatized, and that their nation had been discriminated against by the rest of the world because the pandemic started in China.
Satisfaction with the public health response
Spanish respondents reported less confidence in their COVID-related medical services.
“This could be due to the rising number of infected health care workers in Spain. In contrast, the Chinese had more confidence in their medical services, probably because the government quickly deployed medical personnel and treated COVID-19 patients at rapidly built hospitals,” according to Dr. Ho.
Spain and other European countries shared four shortcomings in their pandemic response, he continued: lack of personal protective equipment for health care workers, delay in developing response strategies, a shortage of hospital beds, and inability to protect vulnerable elderly individuals from infection in nursing homes.
Experiencing cough, shortness of breath, myalgia, or other physical symptoms potentially associated with COVID-19 within the past 14 days was associated with worse depression, anxiety, and stress scores in both China and Spain. This underscores from a mental health standpoint the importance of rapid and accurate testing for the infection, Dr. Ho said.
Significantly more Spanish respondents felt there was too much unnecessary worry about COVID-19, suggesting a need for better health education regarding the pandemic.
Use of face masks
Consistent use of face masks regardless of the presence or absence of symptoms was far more common in the Chinese epicenter, where, unlike in Spain, this precautionary measure was associated with significantly lower IES-R and DASS-21 scores.
but for the Spanish, wearing a face mask was associated with higher IES-R scores,” Dr. Ho said. “We understand that it is difficult for Europeans to accept the need to use masks for healthy people because mask-wearing suggests vulnerability to sickness and concealment of identity. The Chinese have a collective culture. They believe they should wear a face mask to protect their health and that of other people.”
Dr. Ho reported no financial conflicts regarding his study, conducted with coinvestigators at Huaibei (China) Normal University and Complutense University of Madrid.
SOURCE: Ho R. ECNP 2020, Session ISE01.
Spain and China used very different public health responses to the COVID-19 crisis, and that has had significant consequences in terms of the mental health as well as physical health of the two countries’ citizens, Roger Ho, MD, reported at the virtual congress of the European College of Neuropsychopharmacology.
Dr. Ho, a psychiatrist at the National University of Singapore, presented a first-of-its-kind cross-cultural comparative study of the impact of the COVID-19 pandemic in two epicenters on opposite sides of the world. A total of 1,539 participants drawn from the general populations in the two countries completed the online National University of Singapore COVID-19 Questionnaire. The survey was conducted in late February/early March in China and in mid-April in Spain, times of intense disease activity in the countries.
The questionnaire assesses knowledge and concerns about COVID, precautionary measures taken in the last 14 days, contact history, and physical symptoms related to COVID in the last 14 days. The pandemic’s psychological impact was evaluated using the Impact of Event Scale–Revised (IES-R). Participants also completed the Depression, Anxiety, and Stress-21 Scale (DASS-21).
Of note, the pandemic has taken a vastly greater physical toll in Spain than China. As of May 5, there were 83,000 confirmed cases of COVID-19 in China, with a population of 1.39 billion, compared with 248,000 in Spain, with a population of 46.9 million. The Spanish case rate of 5,500 per 1 million population was 100 times greater than China’s; the Spanish mortality rate of 585 per million was 185-fold greater.
Mental health findings
Spaniards experienced significantly higher levels of stress and depression as reflected in DASS-21 subscale scores of 14.22 and 8.65, respectively, compared with 7.86 and 6.38, in Chinese respondents. Spanish subjects also reported greater anxiety levels than the Chinese on the DASS-21 anxiety subscale, although not to a statistically significant extent. Yet, counterintuitively, given the DASS-21 results, the pandemic had a greater adverse psychological impact on the Chinese subjects as reflected in their significantly higher average IES-D score of 30.76 versus 27.64 in Spain. Dr. Ho offered a hypothesis as to why: The survey documented that many Chinese respondents felt socially stigmatized, and that their nation had been discriminated against by the rest of the world because the pandemic started in China.
Satisfaction with the public health response
Spanish respondents reported less confidence in their COVID-related medical services.
“This could be due to the rising number of infected health care workers in Spain. In contrast, the Chinese had more confidence in their medical services, probably because the government quickly deployed medical personnel and treated COVID-19 patients at rapidly built hospitals,” according to Dr. Ho.
Spain and other European countries shared four shortcomings in their pandemic response, he continued: lack of personal protective equipment for health care workers, delay in developing response strategies, a shortage of hospital beds, and inability to protect vulnerable elderly individuals from infection in nursing homes.
Experiencing cough, shortness of breath, myalgia, or other physical symptoms potentially associated with COVID-19 within the past 14 days was associated with worse depression, anxiety, and stress scores in both China and Spain. This underscores from a mental health standpoint the importance of rapid and accurate testing for the infection, Dr. Ho said.
Significantly more Spanish respondents felt there was too much unnecessary worry about COVID-19, suggesting a need for better health education regarding the pandemic.
Use of face masks
Consistent use of face masks regardless of the presence or absence of symptoms was far more common in the Chinese epicenter, where, unlike in Spain, this precautionary measure was associated with significantly lower IES-R and DASS-21 scores.
but for the Spanish, wearing a face mask was associated with higher IES-R scores,” Dr. Ho said. “We understand that it is difficult for Europeans to accept the need to use masks for healthy people because mask-wearing suggests vulnerability to sickness and concealment of identity. The Chinese have a collective culture. They believe they should wear a face mask to protect their health and that of other people.”
Dr. Ho reported no financial conflicts regarding his study, conducted with coinvestigators at Huaibei (China) Normal University and Complutense University of Madrid.
SOURCE: Ho R. ECNP 2020, Session ISE01.
Spain and China used very different public health responses to the COVID-19 crisis, and that has had significant consequences in terms of the mental health as well as physical health of the two countries’ citizens, Roger Ho, MD, reported at the virtual congress of the European College of Neuropsychopharmacology.
Dr. Ho, a psychiatrist at the National University of Singapore, presented a first-of-its-kind cross-cultural comparative study of the impact of the COVID-19 pandemic in two epicenters on opposite sides of the world. A total of 1,539 participants drawn from the general populations in the two countries completed the online National University of Singapore COVID-19 Questionnaire. The survey was conducted in late February/early March in China and in mid-April in Spain, times of intense disease activity in the countries.
The questionnaire assesses knowledge and concerns about COVID, precautionary measures taken in the last 14 days, contact history, and physical symptoms related to COVID in the last 14 days. The pandemic’s psychological impact was evaluated using the Impact of Event Scale–Revised (IES-R). Participants also completed the Depression, Anxiety, and Stress-21 Scale (DASS-21).
Of note, the pandemic has taken a vastly greater physical toll in Spain than China. As of May 5, there were 83,000 confirmed cases of COVID-19 in China, with a population of 1.39 billion, compared with 248,000 in Spain, with a population of 46.9 million. The Spanish case rate of 5,500 per 1 million population was 100 times greater than China’s; the Spanish mortality rate of 585 per million was 185-fold greater.
Mental health findings
Spaniards experienced significantly higher levels of stress and depression as reflected in DASS-21 subscale scores of 14.22 and 8.65, respectively, compared with 7.86 and 6.38, in Chinese respondents. Spanish subjects also reported greater anxiety levels than the Chinese on the DASS-21 anxiety subscale, although not to a statistically significant extent. Yet, counterintuitively, given the DASS-21 results, the pandemic had a greater adverse psychological impact on the Chinese subjects as reflected in their significantly higher average IES-D score of 30.76 versus 27.64 in Spain. Dr. Ho offered a hypothesis as to why: The survey documented that many Chinese respondents felt socially stigmatized, and that their nation had been discriminated against by the rest of the world because the pandemic started in China.
Satisfaction with the public health response
Spanish respondents reported less confidence in their COVID-related medical services.
“This could be due to the rising number of infected health care workers in Spain. In contrast, the Chinese had more confidence in their medical services, probably because the government quickly deployed medical personnel and treated COVID-19 patients at rapidly built hospitals,” according to Dr. Ho.
Spain and other European countries shared four shortcomings in their pandemic response, he continued: lack of personal protective equipment for health care workers, delay in developing response strategies, a shortage of hospital beds, and inability to protect vulnerable elderly individuals from infection in nursing homes.
Experiencing cough, shortness of breath, myalgia, or other physical symptoms potentially associated with COVID-19 within the past 14 days was associated with worse depression, anxiety, and stress scores in both China and Spain. This underscores from a mental health standpoint the importance of rapid and accurate testing for the infection, Dr. Ho said.
Significantly more Spanish respondents felt there was too much unnecessary worry about COVID-19, suggesting a need for better health education regarding the pandemic.
Use of face masks
Consistent use of face masks regardless of the presence or absence of symptoms was far more common in the Chinese epicenter, where, unlike in Spain, this precautionary measure was associated with significantly lower IES-R and DASS-21 scores.
but for the Spanish, wearing a face mask was associated with higher IES-R scores,” Dr. Ho said. “We understand that it is difficult for Europeans to accept the need to use masks for healthy people because mask-wearing suggests vulnerability to sickness and concealment of identity. The Chinese have a collective culture. They believe they should wear a face mask to protect their health and that of other people.”
Dr. Ho reported no financial conflicts regarding his study, conducted with coinvestigators at Huaibei (China) Normal University and Complutense University of Madrid.
SOURCE: Ho R. ECNP 2020, Session ISE01.
FROM ECNP 2020
Listening to Mozart helps tame epilepsy
Listening to Mozart’s piano music improves epilepsy, according to a meta-analysis presented at the virtual congress of the European College of Neuropsychopharmacology.
The results of the meta-analysis of 12 published studies of the so-called Mozart Effect that met rigorous Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines demonstrate that listening to Mozart results in significant reductions in both epileptic seizure frequency and interictal epileptiform discharges (IED), compared with baseline.
The benefits were apparent during and after even a single listening session, although the effect was greater with regular daily listening sessions, according to Gianluca Sesso, MD, a resident in child and adolescent psychiatry at the University of Pisa (Italy.)
“Obviously other music may have similar effects, but it may be that Mozart’s sonatas have distinctive rhythmic structures which are particularly suited to working on epilepsy,” he speculated, adding that the mechanism involved in the Mozart Effect on brain systems remains unclear.
“The highly consistent results of our meta-analysis strongly suggest that music-based neurostimulation may improve the clinical outcome in epilepsy by reducing seizures and IED, and thus deserves to be included in the set of nonpharmacologic complementary approaches for treating epilepsy,” Dr. Sesso added.
Four studies examined the effects of listening to Mozart’s Sonata for Two Pianos in D, K.448, the most-studied piece of music as a treatment for epilepsy. The data documented a 31% reduction in seizure frequency and 28% decrease in IED during a single listen, and a 79% reduction in IED after long-term Mozart music therapy. Similarly, studies demonstrated that listening to a set of Mozart’s compositions resulted in a 36% reduction in IED during and 38% decrease after a single listen, while regular listening in a prolonged treatment period resulted in a 66% reduction in seizure frequency from baseline.
Several studies compared the benefits of listening to K. 488 with those accrued through listening to Piano Sonata No. 16 in C major, K. 545. There was no significant difference between the two, according to Dr. Sesso.
He reported having no financial conflicts regarding his meta-analysis, carried out free of commercial support.
The full details of the meta-analysis were recently published in Clinical Neurophysiology.
Listening to Mozart’s piano music improves epilepsy, according to a meta-analysis presented at the virtual congress of the European College of Neuropsychopharmacology.
The results of the meta-analysis of 12 published studies of the so-called Mozart Effect that met rigorous Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines demonstrate that listening to Mozart results in significant reductions in both epileptic seizure frequency and interictal epileptiform discharges (IED), compared with baseline.
The benefits were apparent during and after even a single listening session, although the effect was greater with regular daily listening sessions, according to Gianluca Sesso, MD, a resident in child and adolescent psychiatry at the University of Pisa (Italy.)
“Obviously other music may have similar effects, but it may be that Mozart’s sonatas have distinctive rhythmic structures which are particularly suited to working on epilepsy,” he speculated, adding that the mechanism involved in the Mozart Effect on brain systems remains unclear.
“The highly consistent results of our meta-analysis strongly suggest that music-based neurostimulation may improve the clinical outcome in epilepsy by reducing seizures and IED, and thus deserves to be included in the set of nonpharmacologic complementary approaches for treating epilepsy,” Dr. Sesso added.
Four studies examined the effects of listening to Mozart’s Sonata for Two Pianos in D, K.448, the most-studied piece of music as a treatment for epilepsy. The data documented a 31% reduction in seizure frequency and 28% decrease in IED during a single listen, and a 79% reduction in IED after long-term Mozart music therapy. Similarly, studies demonstrated that listening to a set of Mozart’s compositions resulted in a 36% reduction in IED during and 38% decrease after a single listen, while regular listening in a prolonged treatment period resulted in a 66% reduction in seizure frequency from baseline.
Several studies compared the benefits of listening to K. 488 with those accrued through listening to Piano Sonata No. 16 in C major, K. 545. There was no significant difference between the two, according to Dr. Sesso.
He reported having no financial conflicts regarding his meta-analysis, carried out free of commercial support.
The full details of the meta-analysis were recently published in Clinical Neurophysiology.
Listening to Mozart’s piano music improves epilepsy, according to a meta-analysis presented at the virtual congress of the European College of Neuropsychopharmacology.
The results of the meta-analysis of 12 published studies of the so-called Mozart Effect that met rigorous Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines demonstrate that listening to Mozart results in significant reductions in both epileptic seizure frequency and interictal epileptiform discharges (IED), compared with baseline.
The benefits were apparent during and after even a single listening session, although the effect was greater with regular daily listening sessions, according to Gianluca Sesso, MD, a resident in child and adolescent psychiatry at the University of Pisa (Italy.)
“Obviously other music may have similar effects, but it may be that Mozart’s sonatas have distinctive rhythmic structures which are particularly suited to working on epilepsy,” he speculated, adding that the mechanism involved in the Mozart Effect on brain systems remains unclear.
“The highly consistent results of our meta-analysis strongly suggest that music-based neurostimulation may improve the clinical outcome in epilepsy by reducing seizures and IED, and thus deserves to be included in the set of nonpharmacologic complementary approaches for treating epilepsy,” Dr. Sesso added.
Four studies examined the effects of listening to Mozart’s Sonata for Two Pianos in D, K.448, the most-studied piece of music as a treatment for epilepsy. The data documented a 31% reduction in seizure frequency and 28% decrease in IED during a single listen, and a 79% reduction in IED after long-term Mozart music therapy. Similarly, studies demonstrated that listening to a set of Mozart’s compositions resulted in a 36% reduction in IED during and 38% decrease after a single listen, while regular listening in a prolonged treatment period resulted in a 66% reduction in seizure frequency from baseline.
Several studies compared the benefits of listening to K. 488 with those accrued through listening to Piano Sonata No. 16 in C major, K. 545. There was no significant difference between the two, according to Dr. Sesso.
He reported having no financial conflicts regarding his meta-analysis, carried out free of commercial support.
The full details of the meta-analysis were recently published in Clinical Neurophysiology.
FROM ECNP 2020
Cognitive impairments in major depression cluster in three patterns
Objective neuropsychological tests can be used to subclassify the cognitive symptoms present in patients with major depression into three patterns having implications for treatment responsiveness, Gitte Moos Knudsen, MD, reported at the virtual congress of the European College of Neuropsychopharmacology.
“Our data highlight the importance of assessing and targeting cognitive symptoms,” said Dr. Knudsen, the ECNP president and professor of neurology at the University of Copenhagen.
She was a coauthor of the Danish NeuroPharm study, in which 92 antidepressant-free patients with moderate or severe major depressive disorder and 103 healthy controls completed a comprehensive neuropsychological test battery. The testing included a validation study of the EMOTICOM test battery, a novel neuropsychological test battery developed specifically to assess what has been called “hot” cognition, such as emotion processing, social cognition, and affective verbal memory.
Overall, the depressed patients collectively showed moderate increases in measures of guilt and shame, moderate deficits in working and verbal memory, moderately slowed reaction time, and mild to moderate negative affective bias, compared with controls. No correlation was found between performance on any of the individual cognitive domains and depression severity as measured using the Hamilton Depression Rating Scale, underscoring the concept that cognitive impairment is a distinct component of depressive pathology rather than an extension of the classic mood and somatic symptoms of major depression.
Cluster analysis revealed three distinct patterns of cognitive impairment in the study population. Unlike the individual cognitive domains, these cognitive clusters did correlate with depression severity. The implication is that as they become available.
Investigators classified 38 of the 92 patients with major depressive disorder as falling within Cluster A. That is, they exhibited marked deficits in hot cognition expressed in a greatly impaired ability to accurately identify facial emotions on photographs, with resultant high scores for emotion recognition bias and emotion misattribution bias. This impairment in hot cognition was accompanied by minimal guilt and shame and little or no deficits in the cold cognitive domains of verbal and working memory.
Cluster B, composed of 28 patients, was characterized by generally good cognitive function, with positive biases in emotion processing, near-normal guilt and shame ratings, but moderate deficits across the cold cognition domains, making for a mirror image of Cluster A.
The 26 patients in Cluster C demonstrated large deficits in both the hot and cold cognition domains, with particularly pronounced guilt and shame scores.
The three clusters didn’t differ in terms of age or sex. However, patients in Cluster C had significantly more severe core depressive symptoms as measured by Hamilton scores than in Clusters A and B.
This analysis from the NeuroPharm study was cross-sectional. Dr. Knudsen cited a recent large Chinese longitudinal study to underscore how the prevalence of patient-reported cognitive deficits in major depressive disorder is high. And while those deficits decrease over time, they nonetheless remain substantial after 6 months on antidepressant therapy.
That study included 598 Chinese outpatients with major depressive disorder. At baseline, 77% had cognitive symptoms as evidenced by a total score of 21 or more on the self-rated Perceived Deficits Questionnaire–Depression (PDQ-D). One month after going on antidepressant monotherapy, the prevalence of cognitive symptoms had dropped to 59%. At 2 months, the rate was 45%. And at month 6, a PDQ-D score of 21 or greater was still present in 32.4% of patients. High baseline PDQ-D scores were associated with worse clinical outcomes, including a lower treatment response rate at 1 month and a lower remission rate at 2 months. Moreover, high PDQ-D scores at 2 months were associated with lower remission and higher relapse rates at 6 months.
Dr. Knudsen reported having no financial conflicts regarding the NeuroPharm study, which was conducted free of commercial support. She serves as an adviser to Sage Therapeutics and Sanos.
Objective neuropsychological tests can be used to subclassify the cognitive symptoms present in patients with major depression into three patterns having implications for treatment responsiveness, Gitte Moos Knudsen, MD, reported at the virtual congress of the European College of Neuropsychopharmacology.
“Our data highlight the importance of assessing and targeting cognitive symptoms,” said Dr. Knudsen, the ECNP president and professor of neurology at the University of Copenhagen.
She was a coauthor of the Danish NeuroPharm study, in which 92 antidepressant-free patients with moderate or severe major depressive disorder and 103 healthy controls completed a comprehensive neuropsychological test battery. The testing included a validation study of the EMOTICOM test battery, a novel neuropsychological test battery developed specifically to assess what has been called “hot” cognition, such as emotion processing, social cognition, and affective verbal memory.
Overall, the depressed patients collectively showed moderate increases in measures of guilt and shame, moderate deficits in working and verbal memory, moderately slowed reaction time, and mild to moderate negative affective bias, compared with controls. No correlation was found between performance on any of the individual cognitive domains and depression severity as measured using the Hamilton Depression Rating Scale, underscoring the concept that cognitive impairment is a distinct component of depressive pathology rather than an extension of the classic mood and somatic symptoms of major depression.
Cluster analysis revealed three distinct patterns of cognitive impairment in the study population. Unlike the individual cognitive domains, these cognitive clusters did correlate with depression severity. The implication is that as they become available.
Investigators classified 38 of the 92 patients with major depressive disorder as falling within Cluster A. That is, they exhibited marked deficits in hot cognition expressed in a greatly impaired ability to accurately identify facial emotions on photographs, with resultant high scores for emotion recognition bias and emotion misattribution bias. This impairment in hot cognition was accompanied by minimal guilt and shame and little or no deficits in the cold cognitive domains of verbal and working memory.
Cluster B, composed of 28 patients, was characterized by generally good cognitive function, with positive biases in emotion processing, near-normal guilt and shame ratings, but moderate deficits across the cold cognition domains, making for a mirror image of Cluster A.
The 26 patients in Cluster C demonstrated large deficits in both the hot and cold cognition domains, with particularly pronounced guilt and shame scores.
The three clusters didn’t differ in terms of age or sex. However, patients in Cluster C had significantly more severe core depressive symptoms as measured by Hamilton scores than in Clusters A and B.
This analysis from the NeuroPharm study was cross-sectional. Dr. Knudsen cited a recent large Chinese longitudinal study to underscore how the prevalence of patient-reported cognitive deficits in major depressive disorder is high. And while those deficits decrease over time, they nonetheless remain substantial after 6 months on antidepressant therapy.
That study included 598 Chinese outpatients with major depressive disorder. At baseline, 77% had cognitive symptoms as evidenced by a total score of 21 or more on the self-rated Perceived Deficits Questionnaire–Depression (PDQ-D). One month after going on antidepressant monotherapy, the prevalence of cognitive symptoms had dropped to 59%. At 2 months, the rate was 45%. And at month 6, a PDQ-D score of 21 or greater was still present in 32.4% of patients. High baseline PDQ-D scores were associated with worse clinical outcomes, including a lower treatment response rate at 1 month and a lower remission rate at 2 months. Moreover, high PDQ-D scores at 2 months were associated with lower remission and higher relapse rates at 6 months.
Dr. Knudsen reported having no financial conflicts regarding the NeuroPharm study, which was conducted free of commercial support. She serves as an adviser to Sage Therapeutics and Sanos.
Objective neuropsychological tests can be used to subclassify the cognitive symptoms present in patients with major depression into three patterns having implications for treatment responsiveness, Gitte Moos Knudsen, MD, reported at the virtual congress of the European College of Neuropsychopharmacology.
“Our data highlight the importance of assessing and targeting cognitive symptoms,” said Dr. Knudsen, the ECNP president and professor of neurology at the University of Copenhagen.
She was a coauthor of the Danish NeuroPharm study, in which 92 antidepressant-free patients with moderate or severe major depressive disorder and 103 healthy controls completed a comprehensive neuropsychological test battery. The testing included a validation study of the EMOTICOM test battery, a novel neuropsychological test battery developed specifically to assess what has been called “hot” cognition, such as emotion processing, social cognition, and affective verbal memory.
Overall, the depressed patients collectively showed moderate increases in measures of guilt and shame, moderate deficits in working and verbal memory, moderately slowed reaction time, and mild to moderate negative affective bias, compared with controls. No correlation was found between performance on any of the individual cognitive domains and depression severity as measured using the Hamilton Depression Rating Scale, underscoring the concept that cognitive impairment is a distinct component of depressive pathology rather than an extension of the classic mood and somatic symptoms of major depression.
Cluster analysis revealed three distinct patterns of cognitive impairment in the study population. Unlike the individual cognitive domains, these cognitive clusters did correlate with depression severity. The implication is that as they become available.
Investigators classified 38 of the 92 patients with major depressive disorder as falling within Cluster A. That is, they exhibited marked deficits in hot cognition expressed in a greatly impaired ability to accurately identify facial emotions on photographs, with resultant high scores for emotion recognition bias and emotion misattribution bias. This impairment in hot cognition was accompanied by minimal guilt and shame and little or no deficits in the cold cognitive domains of verbal and working memory.
Cluster B, composed of 28 patients, was characterized by generally good cognitive function, with positive biases in emotion processing, near-normal guilt and shame ratings, but moderate deficits across the cold cognition domains, making for a mirror image of Cluster A.
The 26 patients in Cluster C demonstrated large deficits in both the hot and cold cognition domains, with particularly pronounced guilt and shame scores.
The three clusters didn’t differ in terms of age or sex. However, patients in Cluster C had significantly more severe core depressive symptoms as measured by Hamilton scores than in Clusters A and B.
This analysis from the NeuroPharm study was cross-sectional. Dr. Knudsen cited a recent large Chinese longitudinal study to underscore how the prevalence of patient-reported cognitive deficits in major depressive disorder is high. And while those deficits decrease over time, they nonetheless remain substantial after 6 months on antidepressant therapy.
That study included 598 Chinese outpatients with major depressive disorder. At baseline, 77% had cognitive symptoms as evidenced by a total score of 21 or more on the self-rated Perceived Deficits Questionnaire–Depression (PDQ-D). One month after going on antidepressant monotherapy, the prevalence of cognitive symptoms had dropped to 59%. At 2 months, the rate was 45%. And at month 6, a PDQ-D score of 21 or greater was still present in 32.4% of patients. High baseline PDQ-D scores were associated with worse clinical outcomes, including a lower treatment response rate at 1 month and a lower remission rate at 2 months. Moreover, high PDQ-D scores at 2 months were associated with lower remission and higher relapse rates at 6 months.
Dr. Knudsen reported having no financial conflicts regarding the NeuroPharm study, which was conducted free of commercial support. She serves as an adviser to Sage Therapeutics and Sanos.
FROM ECNP 2020
Novel schizophrenia drugs advance through pipeline
Two oral agents with novel mechanisms of action in schizophrenia generated considerable audience interest after acing large phase 2 clinical trials presented at the virtual congress of the European College of Neuropsychopharmacology.
The two successful drugs moving on to definitive phase 3 studies after their performance at ECNP 2020 are KarXT, a proprietary combination of xanomeline and trospium chloride, and an inhibitor of glycine transporter 1 (Gly-T1) known for now as BI 425809.
Pimavanserin, an oral selective serotonin inverse agonist with a high affinity for 5-HT2A receptors and low affinity for 5-HT2C receptors, has taken a more convoluted path through the developmental pipeline for schizophrenia. It recently failed to outperform placebo as adjunctive treatment for schizophrenia on the primary endpoint of improvement in Positive And Negative Syndrome Scale (PANSS) total score in the 6-week, phase 3 ENHANCE (Efficacy and Safety of Adjunctive Pimavanserin for the Treatment of Schizophrenia) study. The drug did, however, show significant benefit on secondary endpoints involving negative symptoms.
And in the 400-patient, 26-week, placebo-controlled, phase 2 ADVANCE trial, adjunctive pimavanserin was positive for the primary endpoint of improvement in the Negative Symptom Assessment-16 (NSA-16) score. A phase 3 program evaluating the drug specifically for negative symptoms is underway.
Another novel therapy, an investigational selective estrogen receptor beta agonist, proved reassuringly safe but completely ineffective in men with schizophrenia in a study presented at ECNP 2020.
“The results, unfortunately, were disappointing. We saw no signal on cognition, no change on brain imaging with fMRI, and no improvement in negative symptoms or PANSS total score,” reported Alan Breier, MD, professor and vice chair of the department of psychiatry at Indiana University in Indianapolis.
Broad agreement exists that current antipsychotics targeting D2 dopamine and serotonin receptors in schizophrenia leave much to be desired. They’re ineffective for two of the three major symptom categories that define schizophrenia: cognitive impairment and negative symptoms, such as apathy and social withdrawal. And even for the current antipsychotics’ forte – treatment of positive symptoms, including hallucinations and delusions – effectiveness is often only modest to moderate and accompanied by limiting side effects.
KarXT
KarXT combines xanomeline, a selective M1/M4 muscarinic receptor agonist exclusively licensed from Lilly to Karuna Therapeutics, with trospium chloride, a muscarinic antagonist approved for more than a decade in the United States and Europe for treatment of overactive bladder. Xanomeline was synthesized in the 1990s. It showed promising evidence of antipsychotic efficacy in schizophrenia and Alzheimer’s disease in yearlong clinical trials totaling more than 800 patients, but interest in further developing the drug cooled because of limiting GI and other cholinergic adverse events. KarXT, Karuna’s lead product candidate, is designed to maintain the efficacy of xanomeline while trospium, which doesn’t cross the blood-brain barrier, cancels out its side effects, explained Stephen Brannan, MD, a psychiatrist and chief medical officer at the company.
He presented the results of the phase 2 study, a multicenter, randomized, double-blind, placebo-controlled, 5-week trial conducted with 182 schizophrenia inpatients experiencing an acute psychotic exacerbation. All other antipsychotics were washed out before randomization to KarXT at 50 mg xanomeline/20 mg trospium twice daily, titrated to 100/20 twice daily on days 3-7 and 100/20 b.i.d. thereafter, with an optional increase to 125/30 twice daily.
The primary study endpoint was change from baseline to week 5 in PANSS total score. The results were positive at the P < .0001 level, with a mean 17.4-point reduction in the KarXT group, compared with a 5.9-point improvement in placebo-treated controls. The between-group difference was significant by the first assessment at week 2.
Four of five prespecified secondary endpoints were also positive in rapid and sustained fashion: improvement in the PANSS positive subscore, PANSS negative subscore, Marder PANSS negative subscore, and Clinical Global Impressions-Severity. The fifth secondary endpoint – the proportion of patients with a Clinical Global Impressions rating of 1 or 2, meaning normal or only mildly mentally ill – wasn’t significantly different with KarXT, compared with placebo, but Dr. Brannan shrugged that off.
“In hindsight, it was probably a little overly optimistic to think that after 5 weeks [patients with schizophrenia] would be either well or almost well,” he quipped.
An exploratory analysis of participants’ before-and-after scores on a battery of six cognitive tests showed an encouraging trend: Patients on KarXT performed numerically better than did controls on five of the six tests, albeit not significantly so. Moreover, in a further analysis stratified by baseline impairment, patients in the most impaired tertile showed a larger, statistically significant benefit in response to KarXT.
“We’re interested enough that we plan to continue to look at this in our upcoming larger and longer-term trials,” Dr. Brannan said.
As for safety and tolerability, he continued: “We were pleasantly surprised. We certainly see more side effects with KarXT than with placebo, but not by that much, and they’re much, much better than with xanomeline alone.”
The side effects, mostly cholinergic and anticholinergic, occurred 2-4 times more frequently than in controls. Notably, the rates of nausea, vomiting, and dry mouth – three of the five most common treatment-related adverse events in patients on KarXT – decreased over time to levels similar to placebo by week 5. In contrast, rates of constipation and dyspepsia remained stable over time. All side effects were mild to moderate, and none led to study discontinuation.
A key point was that the KarXT-related side effects were not the same ones that are commonly problematic and limiting with current antipsychotics. There was no weight gain or other metabolic changes, sleepiness or sedation, or extrapyramidal symptoms.
“These results show KarXT has the potential to offer patients a novel mechanism-of-action antipsychotic with a different efficacy and/or tolerability profile than current antipsychotic medications,” Dr. Brannan said.
BI 425809
BI 425809 is a once-daily oral inhibitor of glycine transporter 1 (Gly-T1) specifically designed to alleviate cognitive impairment in people with schizophrenia. Underactivity by the NMDA (N-methyl-D-aspartic acid) receptor has been implicated in this cognitive dysfunction. Glycine is an NMDA cotransmitter. By blocking glutamatergic presynaptic and astrocyte reuptake of glycine, BI 425809 results in increased glycine levels in the synaptic cleft, facilitating neurotransmission, explained W. Wolfgang Fleischhacker, MD, president of the Medical University of Innsbruck (Austria), where he is also professor of psychiatry.
He presented the results of a phase 2, randomized, double-blind, 11-country study in which 509 adults with stable schizophrenia on no more than two antipsychotics were placed on add-on BI 425809 at 2, 5, 10, or 25 mg once daily or placebo for 12 weeks.
The primary endpoint was change from baseline to 12 weeks in the MATRICS (Measurement and Treatment Research to Improve Cognition in Schizophrenia) Consensus Cognitive Battery (MCCB) score. The results were strongly positive, with patients on the two top doses of BI 425809 – 10 and 25 mg/day – showing roughly a 2-point greater improvement in MCCB overall composite T-score compared with controls. Dr. Fleischhacker drew attention to the high study completion rates in the various study arms, ranging from a low of 91% to 97.6% in the 25 mg/day group.
“That’s a very nice but also an unusual finding for a trial of this length,” he observed.
The high study completion rate was a reflection of the drug’s high-level tolerability. Indeed, the rate of adverse events leading to treatment discontinuation was 0% with BI 425809 at 10 mg/day, 2.4% at 25 mg/day, and identical at 2.4% with placebo. No increase was found in psychiatric adverse events such as suicidal ideation or behavior.
“This is a first very promising result,” Dr. Fleischhacker concluded. “Basically, this is the first study that has really shown in a convincing fashion an effect of any novel compound on cognitive impairment in people suffering from schizophrenia.”
A separate ongoing phase 2 study is evaluating BI 425809 in combination with adjunctive computerized cognitive training in an effort to increase cognitive stimulation. The company is awaiting those study results before designing its phase 3 program.
Pimavanserin
It has been a busy year for pimavanserin, with both successes and disappointments in clinical trials addressing a range of psychotic disorders, according to Dragana Bugarski-Kirola, MD, MBA, MSc, vice president for clinical development at Acadia Pharmaceuticals in San Diego.
At present, pimavanserin is Food and Drug Administration–approved as Nuplazid only for treatment of hallucinations and delusions associated with Parkinson’s disease psychosis. But in July 2020, on the strength of the positive results of the pivotal phase 3 HARMONY trial, Acadia filed an application with the FDA for marketing approval of the drug for treatment of dementia-related psychosis. In HARMONY, patients on placebo proved to be 2.8-fold more likely to experience a relapse of delusions or hallucinations than with pimavanserin.
A big recent disappointment was that pimavanserin failed to meet its primary endpoint in the phase 3 CLARITY I and CLARITY II trials as adjunctive therapy for major depressive disorder inadequately responsive to a selective serotonin reuptake inhibitor or serotonin norepinephrine reuptake inhibitor. The change in Hamilton Depression Rating Scale–17 scores in patients on the atypical antipsychotic wasn’t significantly better than with placebo. However, pimavanserin did outperform placebo on the secondary endpoint of Clinical Global Impression–Severity. Additional clinical trials of the drug for treatment of major depression are planned, Dr. Bugarski-Kirola said.
LY500307
Although schizophrenia is equally common in men and women, the disease has a later onset and more benign course in women. This suggests a possible protective effect of estrogen, and indeed, extensive literature supports the use of exogenous estrogen in schizophrenia, where it reduces relapses and improves cognitive impairment and negative symptoms.
“We have no other agents that do that,” noted Dr. Breier, also chief of the psychotic disorders program and director of the Prevention and Recovery Center at Indiana University.
What he considers the best-executed clinical trial of estradiol in schizophrenia, an 8-week, double-blind, randomized study of a 200-mcg estradiol patch or placebo in 200 women aged 19-46 on antipsychotics, was published last year (JAMA Psychiatry. 2019 Jul 31;76[10]:1-9).
The results were impressive. However, estrogen may not be a viable treatment for men and premenopausal women because of its side effects, including feminization, and increased thrombotic and malignancy risks.
This was the impetus for the placebo-controlled randomized trial of LY500307, a highly selective estrogen receptor beta agonist originally developed by Eli Lilly as a potential treatment for benign prostatic hypertrophy, for which it proved ineffective. In animal models, estrogen receptor beta is responsible for a range of effects, including enhanced cognition, social behavior, and an anxiolytic action, whereas the alpha receptor affects the sex organs, skeletal and metabolic homeostasis, and is responsible for estrogen’s problematic side effects.
All three doses studied in the phase 2 randomized trial, which included 94 men with schizophrenia, proved safe, well-tolerated – and ineffective.
“I think one potential conclusion one could consider from these data is that estrogen receptor alpha engagement may be necessary for the estrogenic therapeutics in schizophrenia,” Dr. Breier said.
He reported having no financial conflicts regarding the trial, funded by Indiana University. Outside the scope of the study, he serves as a consultant to Karuna Therapeutics, BioXcel, and Perception Neuroscience.
Dr. Fleischhacker serves as a consultant to Boehringer Ingelheim, which sponsored the phase 2 study of BI 425809, as well as to Angelini, Richter, and Recordati.
SOURCE: ECNP 2020, Session S.12.
Two oral agents with novel mechanisms of action in schizophrenia generated considerable audience interest after acing large phase 2 clinical trials presented at the virtual congress of the European College of Neuropsychopharmacology.
The two successful drugs moving on to definitive phase 3 studies after their performance at ECNP 2020 are KarXT, a proprietary combination of xanomeline and trospium chloride, and an inhibitor of glycine transporter 1 (Gly-T1) known for now as BI 425809.
Pimavanserin, an oral selective serotonin inverse agonist with a high affinity for 5-HT2A receptors and low affinity for 5-HT2C receptors, has taken a more convoluted path through the developmental pipeline for schizophrenia. It recently failed to outperform placebo as adjunctive treatment for schizophrenia on the primary endpoint of improvement in Positive And Negative Syndrome Scale (PANSS) total score in the 6-week, phase 3 ENHANCE (Efficacy and Safety of Adjunctive Pimavanserin for the Treatment of Schizophrenia) study. The drug did, however, show significant benefit on secondary endpoints involving negative symptoms.
And in the 400-patient, 26-week, placebo-controlled, phase 2 ADVANCE trial, adjunctive pimavanserin was positive for the primary endpoint of improvement in the Negative Symptom Assessment-16 (NSA-16) score. A phase 3 program evaluating the drug specifically for negative symptoms is underway.
Another novel therapy, an investigational selective estrogen receptor beta agonist, proved reassuringly safe but completely ineffective in men with schizophrenia in a study presented at ECNP 2020.
“The results, unfortunately, were disappointing. We saw no signal on cognition, no change on brain imaging with fMRI, and no improvement in negative symptoms or PANSS total score,” reported Alan Breier, MD, professor and vice chair of the department of psychiatry at Indiana University in Indianapolis.
Broad agreement exists that current antipsychotics targeting D2 dopamine and serotonin receptors in schizophrenia leave much to be desired. They’re ineffective for two of the three major symptom categories that define schizophrenia: cognitive impairment and negative symptoms, such as apathy and social withdrawal. And even for the current antipsychotics’ forte – treatment of positive symptoms, including hallucinations and delusions – effectiveness is often only modest to moderate and accompanied by limiting side effects.
KarXT
KarXT combines xanomeline, a selective M1/M4 muscarinic receptor agonist exclusively licensed from Lilly to Karuna Therapeutics, with trospium chloride, a muscarinic antagonist approved for more than a decade in the United States and Europe for treatment of overactive bladder. Xanomeline was synthesized in the 1990s. It showed promising evidence of antipsychotic efficacy in schizophrenia and Alzheimer’s disease in yearlong clinical trials totaling more than 800 patients, but interest in further developing the drug cooled because of limiting GI and other cholinergic adverse events. KarXT, Karuna’s lead product candidate, is designed to maintain the efficacy of xanomeline while trospium, which doesn’t cross the blood-brain barrier, cancels out its side effects, explained Stephen Brannan, MD, a psychiatrist and chief medical officer at the company.
He presented the results of the phase 2 study, a multicenter, randomized, double-blind, placebo-controlled, 5-week trial conducted with 182 schizophrenia inpatients experiencing an acute psychotic exacerbation. All other antipsychotics were washed out before randomization to KarXT at 50 mg xanomeline/20 mg trospium twice daily, titrated to 100/20 twice daily on days 3-7 and 100/20 b.i.d. thereafter, with an optional increase to 125/30 twice daily.
The primary study endpoint was change from baseline to week 5 in PANSS total score. The results were positive at the P < .0001 level, with a mean 17.4-point reduction in the KarXT group, compared with a 5.9-point improvement in placebo-treated controls. The between-group difference was significant by the first assessment at week 2.
Four of five prespecified secondary endpoints were also positive in rapid and sustained fashion: improvement in the PANSS positive subscore, PANSS negative subscore, Marder PANSS negative subscore, and Clinical Global Impressions-Severity. The fifth secondary endpoint – the proportion of patients with a Clinical Global Impressions rating of 1 or 2, meaning normal or only mildly mentally ill – wasn’t significantly different with KarXT, compared with placebo, but Dr. Brannan shrugged that off.
“In hindsight, it was probably a little overly optimistic to think that after 5 weeks [patients with schizophrenia] would be either well or almost well,” he quipped.
An exploratory analysis of participants’ before-and-after scores on a battery of six cognitive tests showed an encouraging trend: Patients on KarXT performed numerically better than did controls on five of the six tests, albeit not significantly so. Moreover, in a further analysis stratified by baseline impairment, patients in the most impaired tertile showed a larger, statistically significant benefit in response to KarXT.
“We’re interested enough that we plan to continue to look at this in our upcoming larger and longer-term trials,” Dr. Brannan said.
As for safety and tolerability, he continued: “We were pleasantly surprised. We certainly see more side effects with KarXT than with placebo, but not by that much, and they’re much, much better than with xanomeline alone.”
The side effects, mostly cholinergic and anticholinergic, occurred 2-4 times more frequently than in controls. Notably, the rates of nausea, vomiting, and dry mouth – three of the five most common treatment-related adverse events in patients on KarXT – decreased over time to levels similar to placebo by week 5. In contrast, rates of constipation and dyspepsia remained stable over time. All side effects were mild to moderate, and none led to study discontinuation.
A key point was that the KarXT-related side effects were not the same ones that are commonly problematic and limiting with current antipsychotics. There was no weight gain or other metabolic changes, sleepiness or sedation, or extrapyramidal symptoms.
“These results show KarXT has the potential to offer patients a novel mechanism-of-action antipsychotic with a different efficacy and/or tolerability profile than current antipsychotic medications,” Dr. Brannan said.
BI 425809
BI 425809 is a once-daily oral inhibitor of glycine transporter 1 (Gly-T1) specifically designed to alleviate cognitive impairment in people with schizophrenia. Underactivity by the NMDA (N-methyl-D-aspartic acid) receptor has been implicated in this cognitive dysfunction. Glycine is an NMDA cotransmitter. By blocking glutamatergic presynaptic and astrocyte reuptake of glycine, BI 425809 results in increased glycine levels in the synaptic cleft, facilitating neurotransmission, explained W. Wolfgang Fleischhacker, MD, president of the Medical University of Innsbruck (Austria), where he is also professor of psychiatry.
He presented the results of a phase 2, randomized, double-blind, 11-country study in which 509 adults with stable schizophrenia on no more than two antipsychotics were placed on add-on BI 425809 at 2, 5, 10, or 25 mg once daily or placebo for 12 weeks.
The primary endpoint was change from baseline to 12 weeks in the MATRICS (Measurement and Treatment Research to Improve Cognition in Schizophrenia) Consensus Cognitive Battery (MCCB) score. The results were strongly positive, with patients on the two top doses of BI 425809 – 10 and 25 mg/day – showing roughly a 2-point greater improvement in MCCB overall composite T-score compared with controls. Dr. Fleischhacker drew attention to the high study completion rates in the various study arms, ranging from a low of 91% to 97.6% in the 25 mg/day group.
“That’s a very nice but also an unusual finding for a trial of this length,” he observed.
The high study completion rate was a reflection of the drug’s high-level tolerability. Indeed, the rate of adverse events leading to treatment discontinuation was 0% with BI 425809 at 10 mg/day, 2.4% at 25 mg/day, and identical at 2.4% with placebo. No increase was found in psychiatric adverse events such as suicidal ideation or behavior.
“This is a first very promising result,” Dr. Fleischhacker concluded. “Basically, this is the first study that has really shown in a convincing fashion an effect of any novel compound on cognitive impairment in people suffering from schizophrenia.”
A separate ongoing phase 2 study is evaluating BI 425809 in combination with adjunctive computerized cognitive training in an effort to increase cognitive stimulation. The company is awaiting those study results before designing its phase 3 program.
Pimavanserin
It has been a busy year for pimavanserin, with both successes and disappointments in clinical trials addressing a range of psychotic disorders, according to Dragana Bugarski-Kirola, MD, MBA, MSc, vice president for clinical development at Acadia Pharmaceuticals in San Diego.
At present, pimavanserin is Food and Drug Administration–approved as Nuplazid only for treatment of hallucinations and delusions associated with Parkinson’s disease psychosis. But in July 2020, on the strength of the positive results of the pivotal phase 3 HARMONY trial, Acadia filed an application with the FDA for marketing approval of the drug for treatment of dementia-related psychosis. In HARMONY, patients on placebo proved to be 2.8-fold more likely to experience a relapse of delusions or hallucinations than with pimavanserin.
A big recent disappointment was that pimavanserin failed to meet its primary endpoint in the phase 3 CLARITY I and CLARITY II trials as adjunctive therapy for major depressive disorder inadequately responsive to a selective serotonin reuptake inhibitor or serotonin norepinephrine reuptake inhibitor. The change in Hamilton Depression Rating Scale–17 scores in patients on the atypical antipsychotic wasn’t significantly better than with placebo. However, pimavanserin did outperform placebo on the secondary endpoint of Clinical Global Impression–Severity. Additional clinical trials of the drug for treatment of major depression are planned, Dr. Bugarski-Kirola said.
LY500307
Although schizophrenia is equally common in men and women, the disease has a later onset and more benign course in women. This suggests a possible protective effect of estrogen, and indeed, extensive literature supports the use of exogenous estrogen in schizophrenia, where it reduces relapses and improves cognitive impairment and negative symptoms.
“We have no other agents that do that,” noted Dr. Breier, also chief of the psychotic disorders program and director of the Prevention and Recovery Center at Indiana University.
What he considers the best-executed clinical trial of estradiol in schizophrenia, an 8-week, double-blind, randomized study of a 200-mcg estradiol patch or placebo in 200 women aged 19-46 on antipsychotics, was published last year (JAMA Psychiatry. 2019 Jul 31;76[10]:1-9).
The results were impressive. However, estrogen may not be a viable treatment for men and premenopausal women because of its side effects, including feminization, and increased thrombotic and malignancy risks.
This was the impetus for the placebo-controlled randomized trial of LY500307, a highly selective estrogen receptor beta agonist originally developed by Eli Lilly as a potential treatment for benign prostatic hypertrophy, for which it proved ineffective. In animal models, estrogen receptor beta is responsible for a range of effects, including enhanced cognition, social behavior, and an anxiolytic action, whereas the alpha receptor affects the sex organs, skeletal and metabolic homeostasis, and is responsible for estrogen’s problematic side effects.
All three doses studied in the phase 2 randomized trial, which included 94 men with schizophrenia, proved safe, well-tolerated – and ineffective.
“I think one potential conclusion one could consider from these data is that estrogen receptor alpha engagement may be necessary for the estrogenic therapeutics in schizophrenia,” Dr. Breier said.
He reported having no financial conflicts regarding the trial, funded by Indiana University. Outside the scope of the study, he serves as a consultant to Karuna Therapeutics, BioXcel, and Perception Neuroscience.
Dr. Fleischhacker serves as a consultant to Boehringer Ingelheim, which sponsored the phase 2 study of BI 425809, as well as to Angelini, Richter, and Recordati.
SOURCE: ECNP 2020, Session S.12.
Two oral agents with novel mechanisms of action in schizophrenia generated considerable audience interest after acing large phase 2 clinical trials presented at the virtual congress of the European College of Neuropsychopharmacology.
The two successful drugs moving on to definitive phase 3 studies after their performance at ECNP 2020 are KarXT, a proprietary combination of xanomeline and trospium chloride, and an inhibitor of glycine transporter 1 (Gly-T1) known for now as BI 425809.
Pimavanserin, an oral selective serotonin inverse agonist with a high affinity for 5-HT2A receptors and low affinity for 5-HT2C receptors, has taken a more convoluted path through the developmental pipeline for schizophrenia. It recently failed to outperform placebo as adjunctive treatment for schizophrenia on the primary endpoint of improvement in Positive And Negative Syndrome Scale (PANSS) total score in the 6-week, phase 3 ENHANCE (Efficacy and Safety of Adjunctive Pimavanserin for the Treatment of Schizophrenia) study. The drug did, however, show significant benefit on secondary endpoints involving negative symptoms.
And in the 400-patient, 26-week, placebo-controlled, phase 2 ADVANCE trial, adjunctive pimavanserin was positive for the primary endpoint of improvement in the Negative Symptom Assessment-16 (NSA-16) score. A phase 3 program evaluating the drug specifically for negative symptoms is underway.
Another novel therapy, an investigational selective estrogen receptor beta agonist, proved reassuringly safe but completely ineffective in men with schizophrenia in a study presented at ECNP 2020.
“The results, unfortunately, were disappointing. We saw no signal on cognition, no change on brain imaging with fMRI, and no improvement in negative symptoms or PANSS total score,” reported Alan Breier, MD, professor and vice chair of the department of psychiatry at Indiana University in Indianapolis.
Broad agreement exists that current antipsychotics targeting D2 dopamine and serotonin receptors in schizophrenia leave much to be desired. They’re ineffective for two of the three major symptom categories that define schizophrenia: cognitive impairment and negative symptoms, such as apathy and social withdrawal. And even for the current antipsychotics’ forte – treatment of positive symptoms, including hallucinations and delusions – effectiveness is often only modest to moderate and accompanied by limiting side effects.
KarXT
KarXT combines xanomeline, a selective M1/M4 muscarinic receptor agonist exclusively licensed from Lilly to Karuna Therapeutics, with trospium chloride, a muscarinic antagonist approved for more than a decade in the United States and Europe for treatment of overactive bladder. Xanomeline was synthesized in the 1990s. It showed promising evidence of antipsychotic efficacy in schizophrenia and Alzheimer’s disease in yearlong clinical trials totaling more than 800 patients, but interest in further developing the drug cooled because of limiting GI and other cholinergic adverse events. KarXT, Karuna’s lead product candidate, is designed to maintain the efficacy of xanomeline while trospium, which doesn’t cross the blood-brain barrier, cancels out its side effects, explained Stephen Brannan, MD, a psychiatrist and chief medical officer at the company.
He presented the results of the phase 2 study, a multicenter, randomized, double-blind, placebo-controlled, 5-week trial conducted with 182 schizophrenia inpatients experiencing an acute psychotic exacerbation. All other antipsychotics were washed out before randomization to KarXT at 50 mg xanomeline/20 mg trospium twice daily, titrated to 100/20 twice daily on days 3-7 and 100/20 b.i.d. thereafter, with an optional increase to 125/30 twice daily.
The primary study endpoint was change from baseline to week 5 in PANSS total score. The results were positive at the P < .0001 level, with a mean 17.4-point reduction in the KarXT group, compared with a 5.9-point improvement in placebo-treated controls. The between-group difference was significant by the first assessment at week 2.
Four of five prespecified secondary endpoints were also positive in rapid and sustained fashion: improvement in the PANSS positive subscore, PANSS negative subscore, Marder PANSS negative subscore, and Clinical Global Impressions-Severity. The fifth secondary endpoint – the proportion of patients with a Clinical Global Impressions rating of 1 or 2, meaning normal or only mildly mentally ill – wasn’t significantly different with KarXT, compared with placebo, but Dr. Brannan shrugged that off.
“In hindsight, it was probably a little overly optimistic to think that after 5 weeks [patients with schizophrenia] would be either well or almost well,” he quipped.
An exploratory analysis of participants’ before-and-after scores on a battery of six cognitive tests showed an encouraging trend: Patients on KarXT performed numerically better than did controls on five of the six tests, albeit not significantly so. Moreover, in a further analysis stratified by baseline impairment, patients in the most impaired tertile showed a larger, statistically significant benefit in response to KarXT.
“We’re interested enough that we plan to continue to look at this in our upcoming larger and longer-term trials,” Dr. Brannan said.
As for safety and tolerability, he continued: “We were pleasantly surprised. We certainly see more side effects with KarXT than with placebo, but not by that much, and they’re much, much better than with xanomeline alone.”
The side effects, mostly cholinergic and anticholinergic, occurred 2-4 times more frequently than in controls. Notably, the rates of nausea, vomiting, and dry mouth – three of the five most common treatment-related adverse events in patients on KarXT – decreased over time to levels similar to placebo by week 5. In contrast, rates of constipation and dyspepsia remained stable over time. All side effects were mild to moderate, and none led to study discontinuation.
A key point was that the KarXT-related side effects were not the same ones that are commonly problematic and limiting with current antipsychotics. There was no weight gain or other metabolic changes, sleepiness or sedation, or extrapyramidal symptoms.
“These results show KarXT has the potential to offer patients a novel mechanism-of-action antipsychotic with a different efficacy and/or tolerability profile than current antipsychotic medications,” Dr. Brannan said.
BI 425809
BI 425809 is a once-daily oral inhibitor of glycine transporter 1 (Gly-T1) specifically designed to alleviate cognitive impairment in people with schizophrenia. Underactivity by the NMDA (N-methyl-D-aspartic acid) receptor has been implicated in this cognitive dysfunction. Glycine is an NMDA cotransmitter. By blocking glutamatergic presynaptic and astrocyte reuptake of glycine, BI 425809 results in increased glycine levels in the synaptic cleft, facilitating neurotransmission, explained W. Wolfgang Fleischhacker, MD, president of the Medical University of Innsbruck (Austria), where he is also professor of psychiatry.
He presented the results of a phase 2, randomized, double-blind, 11-country study in which 509 adults with stable schizophrenia on no more than two antipsychotics were placed on add-on BI 425809 at 2, 5, 10, or 25 mg once daily or placebo for 12 weeks.
The primary endpoint was change from baseline to 12 weeks in the MATRICS (Measurement and Treatment Research to Improve Cognition in Schizophrenia) Consensus Cognitive Battery (MCCB) score. The results were strongly positive, with patients on the two top doses of BI 425809 – 10 and 25 mg/day – showing roughly a 2-point greater improvement in MCCB overall composite T-score compared with controls. Dr. Fleischhacker drew attention to the high study completion rates in the various study arms, ranging from a low of 91% to 97.6% in the 25 mg/day group.
“That’s a very nice but also an unusual finding for a trial of this length,” he observed.
The high study completion rate was a reflection of the drug’s high-level tolerability. Indeed, the rate of adverse events leading to treatment discontinuation was 0% with BI 425809 at 10 mg/day, 2.4% at 25 mg/day, and identical at 2.4% with placebo. No increase was found in psychiatric adverse events such as suicidal ideation or behavior.
“This is a first very promising result,” Dr. Fleischhacker concluded. “Basically, this is the first study that has really shown in a convincing fashion an effect of any novel compound on cognitive impairment in people suffering from schizophrenia.”
A separate ongoing phase 2 study is evaluating BI 425809 in combination with adjunctive computerized cognitive training in an effort to increase cognitive stimulation. The company is awaiting those study results before designing its phase 3 program.
Pimavanserin
It has been a busy year for pimavanserin, with both successes and disappointments in clinical trials addressing a range of psychotic disorders, according to Dragana Bugarski-Kirola, MD, MBA, MSc, vice president for clinical development at Acadia Pharmaceuticals in San Diego.
At present, pimavanserin is Food and Drug Administration–approved as Nuplazid only for treatment of hallucinations and delusions associated with Parkinson’s disease psychosis. But in July 2020, on the strength of the positive results of the pivotal phase 3 HARMONY trial, Acadia filed an application with the FDA for marketing approval of the drug for treatment of dementia-related psychosis. In HARMONY, patients on placebo proved to be 2.8-fold more likely to experience a relapse of delusions or hallucinations than with pimavanserin.
A big recent disappointment was that pimavanserin failed to meet its primary endpoint in the phase 3 CLARITY I and CLARITY II trials as adjunctive therapy for major depressive disorder inadequately responsive to a selective serotonin reuptake inhibitor or serotonin norepinephrine reuptake inhibitor. The change in Hamilton Depression Rating Scale–17 scores in patients on the atypical antipsychotic wasn’t significantly better than with placebo. However, pimavanserin did outperform placebo on the secondary endpoint of Clinical Global Impression–Severity. Additional clinical trials of the drug for treatment of major depression are planned, Dr. Bugarski-Kirola said.
LY500307
Although schizophrenia is equally common in men and women, the disease has a later onset and more benign course in women. This suggests a possible protective effect of estrogen, and indeed, extensive literature supports the use of exogenous estrogen in schizophrenia, where it reduces relapses and improves cognitive impairment and negative symptoms.
“We have no other agents that do that,” noted Dr. Breier, also chief of the psychotic disorders program and director of the Prevention and Recovery Center at Indiana University.
What he considers the best-executed clinical trial of estradiol in schizophrenia, an 8-week, double-blind, randomized study of a 200-mcg estradiol patch or placebo in 200 women aged 19-46 on antipsychotics, was published last year (JAMA Psychiatry. 2019 Jul 31;76[10]:1-9).
The results were impressive. However, estrogen may not be a viable treatment for men and premenopausal women because of its side effects, including feminization, and increased thrombotic and malignancy risks.
This was the impetus for the placebo-controlled randomized trial of LY500307, a highly selective estrogen receptor beta agonist originally developed by Eli Lilly as a potential treatment for benign prostatic hypertrophy, for which it proved ineffective. In animal models, estrogen receptor beta is responsible for a range of effects, including enhanced cognition, social behavior, and an anxiolytic action, whereas the alpha receptor affects the sex organs, skeletal and metabolic homeostasis, and is responsible for estrogen’s problematic side effects.
All three doses studied in the phase 2 randomized trial, which included 94 men with schizophrenia, proved safe, well-tolerated – and ineffective.
“I think one potential conclusion one could consider from these data is that estrogen receptor alpha engagement may be necessary for the estrogenic therapeutics in schizophrenia,” Dr. Breier said.
He reported having no financial conflicts regarding the trial, funded by Indiana University. Outside the scope of the study, he serves as a consultant to Karuna Therapeutics, BioXcel, and Perception Neuroscience.
Dr. Fleischhacker serves as a consultant to Boehringer Ingelheim, which sponsored the phase 2 study of BI 425809, as well as to Angelini, Richter, and Recordati.
SOURCE: ECNP 2020, Session S.12.
FROM ECNP 2020
Binge eating in ADHD may not be impulsivity-related
The disinhibited binge eating style often seen in individuals with high ADHD symptoms is attributable to a heightened neural reward response to food rather than to the impulsivity that’s a core feature of ADHD, Elizabeth Martin, MSc, reported at the virtual congress of the European College of Neuropsychopharmacology.
She presented a functional MRI brain-imaging study designed to help pin down the mechanism involved in the disordered eating patterns that often accompany ADHD.
“Determining the underlying mechanism between binge eating and ADHD may be helpful in developing novel therapies for both ADHD and binge eating disorder. Our research suggests that further investigation of the role of altered reward processing in ADHD may be an avenue for this,” said Ms. Martin, a doctoral researcher in the department of psychology at the University of Birmingham (England).
She and her coinvestigators recruited 31 university student volunteers with high ADHD symptoms as evidenced by their mean score of 29.3 on the 0-54 Conners’ Adult ADHD Rating Scale, and 27 others with low ADHD symptoms and a mean Conners’ score of 6.8. The two groups didn’t differ in age or BMI. However, not surprisingly, the high-ADHD group exhibited greater impulsivity, with a mean score of 72 on the Barratt Impulsiveness Scale, versus 56.5 in the low ADHD group.
A battery of eating disorder scales was applied to assess participants in terms of binge/disinhibited or restrictive eating patterns. The high- and low–ADHD symptom groups didn’t differ in terms of prevalence of a restrictive eating style, which was low, but the high-ADHD participants scored on average roughly 50% higher on the binge/disinhibited eating style measure, compared with the low-ADHD group.
Each study participant underwent a 1-hour BOLD (blood oxygen level dependent) functional MRI scan while performing two sets of tasks. One task entailed quickly looking at 120 photos of food items and an equal number of nonfood items and rating how appealing the pictures were. The other challenge was what psychologists call a go/no-go task, a computerized cognitive test used to assess inhibitory control based upon reaction times and error rates.
On the go/no-go task, there were no between-group differences in rates of errors of omission or commission or reaction time. Moreover, the MRI results indicated there were no between-group differences in neural circuitry activation during this task. The investigators therefore concluded that the tendency toward binge eating in the high–ADHD symptoms group was not tied to greater impulsivity as reflected in less effective inhibitory processes.
The food picture rating task told a different story. The MRIs demonstrated increased responses to food versus nonfood images in the high-ADHD subjects, compared with the low-ADHD subjects in reward-related brain areas, including the ventromedial prefrontal cortex, caudate nucleus, and ventral tegmental area.
in response to viewing food pictures,” according to Ms. Martin. “This suggests that enhanced responsiveness to food cues may be a mediating mechanism underlying overeating in ADHD.”
Of note, only one drug – lisdexamfetamine dimesylate (Vyvanse) is Food and Drug Administration-approved for the treatment of both ADHD and binge-eating disorder.
“Until now it’s been unclear how lisdexamfetamine dimesylate reduces binge eating, but our results suggest that one mechanism worthy of further investigation is the potential effect of the drug on food reward processes,” Ms. Martin said.
She reported having no financial conflicts regarding the study, which was supported by university funding.
SOURCE: Martin E. ECNP 2020. Abstr. P.041.
The disinhibited binge eating style often seen in individuals with high ADHD symptoms is attributable to a heightened neural reward response to food rather than to the impulsivity that’s a core feature of ADHD, Elizabeth Martin, MSc, reported at the virtual congress of the European College of Neuropsychopharmacology.
She presented a functional MRI brain-imaging study designed to help pin down the mechanism involved in the disordered eating patterns that often accompany ADHD.
“Determining the underlying mechanism between binge eating and ADHD may be helpful in developing novel therapies for both ADHD and binge eating disorder. Our research suggests that further investigation of the role of altered reward processing in ADHD may be an avenue for this,” said Ms. Martin, a doctoral researcher in the department of psychology at the University of Birmingham (England).
She and her coinvestigators recruited 31 university student volunteers with high ADHD symptoms as evidenced by their mean score of 29.3 on the 0-54 Conners’ Adult ADHD Rating Scale, and 27 others with low ADHD symptoms and a mean Conners’ score of 6.8. The two groups didn’t differ in age or BMI. However, not surprisingly, the high-ADHD group exhibited greater impulsivity, with a mean score of 72 on the Barratt Impulsiveness Scale, versus 56.5 in the low ADHD group.
A battery of eating disorder scales was applied to assess participants in terms of binge/disinhibited or restrictive eating patterns. The high- and low–ADHD symptom groups didn’t differ in terms of prevalence of a restrictive eating style, which was low, but the high-ADHD participants scored on average roughly 50% higher on the binge/disinhibited eating style measure, compared with the low-ADHD group.
Each study participant underwent a 1-hour BOLD (blood oxygen level dependent) functional MRI scan while performing two sets of tasks. One task entailed quickly looking at 120 photos of food items and an equal number of nonfood items and rating how appealing the pictures were. The other challenge was what psychologists call a go/no-go task, a computerized cognitive test used to assess inhibitory control based upon reaction times and error rates.
On the go/no-go task, there were no between-group differences in rates of errors of omission or commission or reaction time. Moreover, the MRI results indicated there were no between-group differences in neural circuitry activation during this task. The investigators therefore concluded that the tendency toward binge eating in the high–ADHD symptoms group was not tied to greater impulsivity as reflected in less effective inhibitory processes.
The food picture rating task told a different story. The MRIs demonstrated increased responses to food versus nonfood images in the high-ADHD subjects, compared with the low-ADHD subjects in reward-related brain areas, including the ventromedial prefrontal cortex, caudate nucleus, and ventral tegmental area.
in response to viewing food pictures,” according to Ms. Martin. “This suggests that enhanced responsiveness to food cues may be a mediating mechanism underlying overeating in ADHD.”
Of note, only one drug – lisdexamfetamine dimesylate (Vyvanse) is Food and Drug Administration-approved for the treatment of both ADHD and binge-eating disorder.
“Until now it’s been unclear how lisdexamfetamine dimesylate reduces binge eating, but our results suggest that one mechanism worthy of further investigation is the potential effect of the drug on food reward processes,” Ms. Martin said.
She reported having no financial conflicts regarding the study, which was supported by university funding.
SOURCE: Martin E. ECNP 2020. Abstr. P.041.
The disinhibited binge eating style often seen in individuals with high ADHD symptoms is attributable to a heightened neural reward response to food rather than to the impulsivity that’s a core feature of ADHD, Elizabeth Martin, MSc, reported at the virtual congress of the European College of Neuropsychopharmacology.
She presented a functional MRI brain-imaging study designed to help pin down the mechanism involved in the disordered eating patterns that often accompany ADHD.
“Determining the underlying mechanism between binge eating and ADHD may be helpful in developing novel therapies for both ADHD and binge eating disorder. Our research suggests that further investigation of the role of altered reward processing in ADHD may be an avenue for this,” said Ms. Martin, a doctoral researcher in the department of psychology at the University of Birmingham (England).
She and her coinvestigators recruited 31 university student volunteers with high ADHD symptoms as evidenced by their mean score of 29.3 on the 0-54 Conners’ Adult ADHD Rating Scale, and 27 others with low ADHD symptoms and a mean Conners’ score of 6.8. The two groups didn’t differ in age or BMI. However, not surprisingly, the high-ADHD group exhibited greater impulsivity, with a mean score of 72 on the Barratt Impulsiveness Scale, versus 56.5 in the low ADHD group.
A battery of eating disorder scales was applied to assess participants in terms of binge/disinhibited or restrictive eating patterns. The high- and low–ADHD symptom groups didn’t differ in terms of prevalence of a restrictive eating style, which was low, but the high-ADHD participants scored on average roughly 50% higher on the binge/disinhibited eating style measure, compared with the low-ADHD group.
Each study participant underwent a 1-hour BOLD (blood oxygen level dependent) functional MRI scan while performing two sets of tasks. One task entailed quickly looking at 120 photos of food items and an equal number of nonfood items and rating how appealing the pictures were. The other challenge was what psychologists call a go/no-go task, a computerized cognitive test used to assess inhibitory control based upon reaction times and error rates.
On the go/no-go task, there were no between-group differences in rates of errors of omission or commission or reaction time. Moreover, the MRI results indicated there were no between-group differences in neural circuitry activation during this task. The investigators therefore concluded that the tendency toward binge eating in the high–ADHD symptoms group was not tied to greater impulsivity as reflected in less effective inhibitory processes.
The food picture rating task told a different story. The MRIs demonstrated increased responses to food versus nonfood images in the high-ADHD subjects, compared with the low-ADHD subjects in reward-related brain areas, including the ventromedial prefrontal cortex, caudate nucleus, and ventral tegmental area.
in response to viewing food pictures,” according to Ms. Martin. “This suggests that enhanced responsiveness to food cues may be a mediating mechanism underlying overeating in ADHD.”
Of note, only one drug – lisdexamfetamine dimesylate (Vyvanse) is Food and Drug Administration-approved for the treatment of both ADHD and binge-eating disorder.
“Until now it’s been unclear how lisdexamfetamine dimesylate reduces binge eating, but our results suggest that one mechanism worthy of further investigation is the potential effect of the drug on food reward processes,” Ms. Martin said.
She reported having no financial conflicts regarding the study, which was supported by university funding.
SOURCE: Martin E. ECNP 2020. Abstr. P.041.
FROM ECNP 2020
Suicidality jumped in Israel during spring COVID-19 lockdown
Suicidality appears to have increased sharply in Israel during the initial nationwide lockdown implemented in response to the COVID-19 pandemic, Gil Zalsman, MD, MHA, reported at the virtual congress of the European College of Neuropsychopharmacology.
He presented highlights from a soon-to-be-published analysis of the content of online chat sessions fielded by a national crisis hotline (Sahar.org.il) during the first 6 months of 2020, compared with January through June 2019, in the pre-COVID-19 era.
It’s far too early to say whether actual deaths tied to suicide rose significantly during the spring lockdown, since medical examiners often take a long time before ruling suicide as cause of death. But this much is clear: The number of suicide-related chat sessions recorded at the volunteer-staffed national hotline during April 2020 was two-and-a-half times greater than in April 2019, and threefold greater in May 2020 than a year earlier, according to Dr. Zalsman, professor of psychiatry at Tel Aviv University and director of the Geha Mental Health Center in Petach Tikva, Israel, where he also directs an adolescent day unit.
The proportion of chats handled at the crisis hotline, many of them concerned with the standard topics – relationships, stress, fears, anxiety, and other non–suicide-related issues – was 48% greater in the first half of 2020, compared with a year earlier. Indeed, the pandemic is putting an enormous strain on crisis hotlines the world over.
“Everybody who is working hotlines knows that they’re falling apart. There are too many calls, too many chats. They need to multiply their volunteers,” Dr. Zalsman said.
The number of suicide-related online chats jumped the week of March 12, when schools closed across Israel and a partial lockdown began. The peak in suicide-related chats occurred beginning the week of April 17, when the forced total lockdown was declared.
“Everything was closed. You couldn’t go out or the police would arrest you,” Dr. Zalsman recalled.
The suicide-related chat count started to drop off in mid-May, when schools reopened, and continued to decline through the end of June.
Only a small percentage of suicide-related chats were deemed by crisis hotline volunteers and their supervisors to be truly life-threatening situations necessitating a call to the police. But the number of such exchanges was significantly greater in April and May 2020 than in January and February, or in April and May 2019.
Use of the crisis hotline is ordinarily skewed toward tech-savvy young people, or as Dr. Zalsman called them, “kids who live inside their computers.” He note that the psychological impact of the pandemic on children and adolescents is largely unexplored research territory to date.
“ You can kill your grandfather by coughing,” Dr. Zalsman said.
Older people also seek help
A finding that he and his coinvestigators didn’t anticipate was the significantly increased use of the service by individuals aged 65 and older during the pandemic. This underscores the increased vulnerability of older people, which stems in part from their heightened risk for severe infection and consequent need for prolonged physical isolation, he said.
The conventional thinking among suicidologists is that during times of crisis – wars, natural disasters – suicidality plunges, then rises quickly afterward.
“People withhold themselves. When there’s a big danger from outside they ignore the danger from inside. And once the danger from outside is gone, they’re left with emptiness, unemployment, economic crisis, and they start” taking their own lives, Dr. Zalsman explained. He expects suicidality to increase after the pandemic, or as the Israeli crisis hotline data suggest, perhaps even during it, for multiple reasons. Patients with preexisting psychiatric disorders are often going untreated. The prolonged physical isolation causes emotional difficulties for some people, especially when accompanied by social isolation and loneliness. There is grief over the loss of friends and relatives because of COVID-19. And there is an expectation of looming economic hardship, with mounting unemployment and bankruptcies.
Dr. Zalsman reported having no financial conflicts regarding his study, conducted free of commercial support.
SOURCE: Zalsman G. ECNP 2020, Session TP.06.
Suicidality appears to have increased sharply in Israel during the initial nationwide lockdown implemented in response to the COVID-19 pandemic, Gil Zalsman, MD, MHA, reported at the virtual congress of the European College of Neuropsychopharmacology.
He presented highlights from a soon-to-be-published analysis of the content of online chat sessions fielded by a national crisis hotline (Sahar.org.il) during the first 6 months of 2020, compared with January through June 2019, in the pre-COVID-19 era.
It’s far too early to say whether actual deaths tied to suicide rose significantly during the spring lockdown, since medical examiners often take a long time before ruling suicide as cause of death. But this much is clear: The number of suicide-related chat sessions recorded at the volunteer-staffed national hotline during April 2020 was two-and-a-half times greater than in April 2019, and threefold greater in May 2020 than a year earlier, according to Dr. Zalsman, professor of psychiatry at Tel Aviv University and director of the Geha Mental Health Center in Petach Tikva, Israel, where he also directs an adolescent day unit.
The proportion of chats handled at the crisis hotline, many of them concerned with the standard topics – relationships, stress, fears, anxiety, and other non–suicide-related issues – was 48% greater in the first half of 2020, compared with a year earlier. Indeed, the pandemic is putting an enormous strain on crisis hotlines the world over.
“Everybody who is working hotlines knows that they’re falling apart. There are too many calls, too many chats. They need to multiply their volunteers,” Dr. Zalsman said.
The number of suicide-related online chats jumped the week of March 12, when schools closed across Israel and a partial lockdown began. The peak in suicide-related chats occurred beginning the week of April 17, when the forced total lockdown was declared.
“Everything was closed. You couldn’t go out or the police would arrest you,” Dr. Zalsman recalled.
The suicide-related chat count started to drop off in mid-May, when schools reopened, and continued to decline through the end of June.
Only a small percentage of suicide-related chats were deemed by crisis hotline volunteers and their supervisors to be truly life-threatening situations necessitating a call to the police. But the number of such exchanges was significantly greater in April and May 2020 than in January and February, or in April and May 2019.
Use of the crisis hotline is ordinarily skewed toward tech-savvy young people, or as Dr. Zalsman called them, “kids who live inside their computers.” He note that the psychological impact of the pandemic on children and adolescents is largely unexplored research territory to date.
“ You can kill your grandfather by coughing,” Dr. Zalsman said.
Older people also seek help
A finding that he and his coinvestigators didn’t anticipate was the significantly increased use of the service by individuals aged 65 and older during the pandemic. This underscores the increased vulnerability of older people, which stems in part from their heightened risk for severe infection and consequent need for prolonged physical isolation, he said.
The conventional thinking among suicidologists is that during times of crisis – wars, natural disasters – suicidality plunges, then rises quickly afterward.
“People withhold themselves. When there’s a big danger from outside they ignore the danger from inside. And once the danger from outside is gone, they’re left with emptiness, unemployment, economic crisis, and they start” taking their own lives, Dr. Zalsman explained. He expects suicidality to increase after the pandemic, or as the Israeli crisis hotline data suggest, perhaps even during it, for multiple reasons. Patients with preexisting psychiatric disorders are often going untreated. The prolonged physical isolation causes emotional difficulties for some people, especially when accompanied by social isolation and loneliness. There is grief over the loss of friends and relatives because of COVID-19. And there is an expectation of looming economic hardship, with mounting unemployment and bankruptcies.
Dr. Zalsman reported having no financial conflicts regarding his study, conducted free of commercial support.
SOURCE: Zalsman G. ECNP 2020, Session TP.06.
Suicidality appears to have increased sharply in Israel during the initial nationwide lockdown implemented in response to the COVID-19 pandemic, Gil Zalsman, MD, MHA, reported at the virtual congress of the European College of Neuropsychopharmacology.
He presented highlights from a soon-to-be-published analysis of the content of online chat sessions fielded by a national crisis hotline (Sahar.org.il) during the first 6 months of 2020, compared with January through June 2019, in the pre-COVID-19 era.
It’s far too early to say whether actual deaths tied to suicide rose significantly during the spring lockdown, since medical examiners often take a long time before ruling suicide as cause of death. But this much is clear: The number of suicide-related chat sessions recorded at the volunteer-staffed national hotline during April 2020 was two-and-a-half times greater than in April 2019, and threefold greater in May 2020 than a year earlier, according to Dr. Zalsman, professor of psychiatry at Tel Aviv University and director of the Geha Mental Health Center in Petach Tikva, Israel, where he also directs an adolescent day unit.
The proportion of chats handled at the crisis hotline, many of them concerned with the standard topics – relationships, stress, fears, anxiety, and other non–suicide-related issues – was 48% greater in the first half of 2020, compared with a year earlier. Indeed, the pandemic is putting an enormous strain on crisis hotlines the world over.
“Everybody who is working hotlines knows that they’re falling apart. There are too many calls, too many chats. They need to multiply their volunteers,” Dr. Zalsman said.
The number of suicide-related online chats jumped the week of March 12, when schools closed across Israel and a partial lockdown began. The peak in suicide-related chats occurred beginning the week of April 17, when the forced total lockdown was declared.
“Everything was closed. You couldn’t go out or the police would arrest you,” Dr. Zalsman recalled.
The suicide-related chat count started to drop off in mid-May, when schools reopened, and continued to decline through the end of June.
Only a small percentage of suicide-related chats were deemed by crisis hotline volunteers and their supervisors to be truly life-threatening situations necessitating a call to the police. But the number of such exchanges was significantly greater in April and May 2020 than in January and February, or in April and May 2019.
Use of the crisis hotline is ordinarily skewed toward tech-savvy young people, or as Dr. Zalsman called them, “kids who live inside their computers.” He note that the psychological impact of the pandemic on children and adolescents is largely unexplored research territory to date.
“ You can kill your grandfather by coughing,” Dr. Zalsman said.
Older people also seek help
A finding that he and his coinvestigators didn’t anticipate was the significantly increased use of the service by individuals aged 65 and older during the pandemic. This underscores the increased vulnerability of older people, which stems in part from their heightened risk for severe infection and consequent need for prolonged physical isolation, he said.
The conventional thinking among suicidologists is that during times of crisis – wars, natural disasters – suicidality plunges, then rises quickly afterward.
“People withhold themselves. When there’s a big danger from outside they ignore the danger from inside. And once the danger from outside is gone, they’re left with emptiness, unemployment, economic crisis, and they start” taking their own lives, Dr. Zalsman explained. He expects suicidality to increase after the pandemic, or as the Israeli crisis hotline data suggest, perhaps even during it, for multiple reasons. Patients with preexisting psychiatric disorders are often going untreated. The prolonged physical isolation causes emotional difficulties for some people, especially when accompanied by social isolation and loneliness. There is grief over the loss of friends and relatives because of COVID-19. And there is an expectation of looming economic hardship, with mounting unemployment and bankruptcies.
Dr. Zalsman reported having no financial conflicts regarding his study, conducted free of commercial support.
SOURCE: Zalsman G. ECNP 2020, Session TP.06.
FROM ECNP 2020
Breathing enriched oxygen improves major depression
Maybe the hipsters patronizing trendy oxygen bars seeking elevation of mood back in the prepandemic era were actually onto something – because Israeli investigators have now shown in a pilot double-blind, placebo-controlled, randomized trial that breathing enriched oxygen on a nightly basis resulted in clinically meaningful symptomatic improvement in mild to moderate major depression.
“We saw a highly significant effect of normobaric hyperoxia therapy in lowering Hamilton Rating Scale for Depression scores,” R. Haim Belmaker, MD, reported at the virtual congress of the European College of Neuropsychopharmacology.
In addition, the patients on enriched oxygen also showed statistically significant and clinically meaningful improvements relative to sham-treated controls on the secondary endpoints of Clinical Global Impressions Scale, the World Health Organization–Five Well-Being Index, the Sheehan Disability Scale, and the Sense of Coherence Scale, added Dr. Belmaker, professor emeritus of psychiatry at the Ben Gurion University of the Negev in Beersheva, Israel.
Numerous PET imaging studies have documented diminished brain mitochondrial function in patients with depression or schizophrenia. And mitochondria need oxygen to do their work. Yet, the idea of administering enriched oxygen in an effort to boost mitochondrial energy metabolism has long been viewed with skepticism – even though it’s a simple and well-tolerated intervention – because of the fact that 90%-95% of the oxygen supply is carried bound to hemoglobin, and oxygen enrichment doesn’t further increase hemoglobin saturation in individuals with normal lung function. However, recently it has been shown that inspired enriched oxygen roughly doubles arterial oxygen tension, and while this doesn’t translate into anything close to a doubled oxygen supply to tissues, it may result in increased oxygen diffusion into brain tissue, the psychiatrist explained.
Normobaric hyperoxia therapy is not to be confused with hyperbaric oxygen therapy, which requires a special chamber to handle markedly increased atmospheric pressures and has some inherent dangers. Mobile bedside oxygen generator units for oxygen enrichment are commercially available over the counter. Those used in the Israeli study were about the size of a vacuum cleaner and weighed a little more than 40 lb. Much smaller, more convenient units are available as well, but are costlier.
Dr. Belmaker reported on 51 adults with mild or moderate symptoms of major depressive disorder and a mean 11-year disease history who were randomized double blind to breathe either 35% oxygen or normal air – that is, 21% oxygen – at 1 atm pressure delivered from an investigator-supplied oxygen generator through standard plastic nasal prongs at a flow rate of 5 L/min for 7 hours nightly for 1 month.
“Controls heard the same flow and felt the same feeling on the face but were receiving 21% oxygen,” he noted.
Oxygen generator units are capable of enriching air to more than 90% oxygen; however, the investigators wanted to be cautious in a pilot study of an untested therapy, and they found evidence from both animal and human studies that 40% oxygen is reassuringly safe. Study exclusion criteria included obesity, acute or chronic respiratory disease, psychosis, and suicidality.
The primary study endpoint was the change in Hamilton Rating Scale for Depression score at 1 month. From a mean baseline of 14.6, the score in the normobaric hyperoxia group dropped by more than 4 points while remaining unchanged in controls. In a subscale analysis, it was apparent that most of the improvement occurred in the anxiety and cognitive disturbance subscale domains, according to Dr. Belmaker.
Of note, all patients rated by blinded investigators as much improved or very much improved on the Clinical Global Impression scale came from the enriched oxygen group.
No treatment-related adverse events occurred in the study.
“We don’t know the mechanism of the benefit of oxygen on the brain. It’s complex. In stroke and acute MI we used to think oxygen was beneficial, but scientists now feel that it’s not,” the psychiatrist said. “This early data deserve replication with higher concentrations of oxygen, different time periods of application, and in different patient groups.”
He emphasized that, since individuals with physical illnesses – including sleep apnea and chronic obstructive pulmonary disease – were excluded from the study, it’s not possible to say whether normobaric hyperoxia therapy would have an antidepressant effect in such patients.
“I would be especially careful with the normobaric oxygen in any patients with any cardiovascular or hypertensive disease because the increased oxygen pressure can have the side effect of contracting cardiac capillaries as a reflex action. So I certainly cannot recommend applying this study in any patients with a physical disease at this point,” Dr. Belmaker emphasized.
He reported having no financial conflicts regarding the study, funded by a grant from the Brain and Behavior Research Foundation.
SOURCE: Belmaker RH. ECNP 2020, Session S.12.
Maybe the hipsters patronizing trendy oxygen bars seeking elevation of mood back in the prepandemic era were actually onto something – because Israeli investigators have now shown in a pilot double-blind, placebo-controlled, randomized trial that breathing enriched oxygen on a nightly basis resulted in clinically meaningful symptomatic improvement in mild to moderate major depression.
“We saw a highly significant effect of normobaric hyperoxia therapy in lowering Hamilton Rating Scale for Depression scores,” R. Haim Belmaker, MD, reported at the virtual congress of the European College of Neuropsychopharmacology.
In addition, the patients on enriched oxygen also showed statistically significant and clinically meaningful improvements relative to sham-treated controls on the secondary endpoints of Clinical Global Impressions Scale, the World Health Organization–Five Well-Being Index, the Sheehan Disability Scale, and the Sense of Coherence Scale, added Dr. Belmaker, professor emeritus of psychiatry at the Ben Gurion University of the Negev in Beersheva, Israel.
Numerous PET imaging studies have documented diminished brain mitochondrial function in patients with depression or schizophrenia. And mitochondria need oxygen to do their work. Yet, the idea of administering enriched oxygen in an effort to boost mitochondrial energy metabolism has long been viewed with skepticism – even though it’s a simple and well-tolerated intervention – because of the fact that 90%-95% of the oxygen supply is carried bound to hemoglobin, and oxygen enrichment doesn’t further increase hemoglobin saturation in individuals with normal lung function. However, recently it has been shown that inspired enriched oxygen roughly doubles arterial oxygen tension, and while this doesn’t translate into anything close to a doubled oxygen supply to tissues, it may result in increased oxygen diffusion into brain tissue, the psychiatrist explained.
Normobaric hyperoxia therapy is not to be confused with hyperbaric oxygen therapy, which requires a special chamber to handle markedly increased atmospheric pressures and has some inherent dangers. Mobile bedside oxygen generator units for oxygen enrichment are commercially available over the counter. Those used in the Israeli study were about the size of a vacuum cleaner and weighed a little more than 40 lb. Much smaller, more convenient units are available as well, but are costlier.
Dr. Belmaker reported on 51 adults with mild or moderate symptoms of major depressive disorder and a mean 11-year disease history who were randomized double blind to breathe either 35% oxygen or normal air – that is, 21% oxygen – at 1 atm pressure delivered from an investigator-supplied oxygen generator through standard plastic nasal prongs at a flow rate of 5 L/min for 7 hours nightly for 1 month.
“Controls heard the same flow and felt the same feeling on the face but were receiving 21% oxygen,” he noted.
Oxygen generator units are capable of enriching air to more than 90% oxygen; however, the investigators wanted to be cautious in a pilot study of an untested therapy, and they found evidence from both animal and human studies that 40% oxygen is reassuringly safe. Study exclusion criteria included obesity, acute or chronic respiratory disease, psychosis, and suicidality.
The primary study endpoint was the change in Hamilton Rating Scale for Depression score at 1 month. From a mean baseline of 14.6, the score in the normobaric hyperoxia group dropped by more than 4 points while remaining unchanged in controls. In a subscale analysis, it was apparent that most of the improvement occurred in the anxiety and cognitive disturbance subscale domains, according to Dr. Belmaker.
Of note, all patients rated by blinded investigators as much improved or very much improved on the Clinical Global Impression scale came from the enriched oxygen group.
No treatment-related adverse events occurred in the study.
“We don’t know the mechanism of the benefit of oxygen on the brain. It’s complex. In stroke and acute MI we used to think oxygen was beneficial, but scientists now feel that it’s not,” the psychiatrist said. “This early data deserve replication with higher concentrations of oxygen, different time periods of application, and in different patient groups.”
He emphasized that, since individuals with physical illnesses – including sleep apnea and chronic obstructive pulmonary disease – were excluded from the study, it’s not possible to say whether normobaric hyperoxia therapy would have an antidepressant effect in such patients.
“I would be especially careful with the normobaric oxygen in any patients with any cardiovascular or hypertensive disease because the increased oxygen pressure can have the side effect of contracting cardiac capillaries as a reflex action. So I certainly cannot recommend applying this study in any patients with a physical disease at this point,” Dr. Belmaker emphasized.
He reported having no financial conflicts regarding the study, funded by a grant from the Brain and Behavior Research Foundation.
SOURCE: Belmaker RH. ECNP 2020, Session S.12.
Maybe the hipsters patronizing trendy oxygen bars seeking elevation of mood back in the prepandemic era were actually onto something – because Israeli investigators have now shown in a pilot double-blind, placebo-controlled, randomized trial that breathing enriched oxygen on a nightly basis resulted in clinically meaningful symptomatic improvement in mild to moderate major depression.
“We saw a highly significant effect of normobaric hyperoxia therapy in lowering Hamilton Rating Scale for Depression scores,” R. Haim Belmaker, MD, reported at the virtual congress of the European College of Neuropsychopharmacology.
In addition, the patients on enriched oxygen also showed statistically significant and clinically meaningful improvements relative to sham-treated controls on the secondary endpoints of Clinical Global Impressions Scale, the World Health Organization–Five Well-Being Index, the Sheehan Disability Scale, and the Sense of Coherence Scale, added Dr. Belmaker, professor emeritus of psychiatry at the Ben Gurion University of the Negev in Beersheva, Israel.
Numerous PET imaging studies have documented diminished brain mitochondrial function in patients with depression or schizophrenia. And mitochondria need oxygen to do their work. Yet, the idea of administering enriched oxygen in an effort to boost mitochondrial energy metabolism has long been viewed with skepticism – even though it’s a simple and well-tolerated intervention – because of the fact that 90%-95% of the oxygen supply is carried bound to hemoglobin, and oxygen enrichment doesn’t further increase hemoglobin saturation in individuals with normal lung function. However, recently it has been shown that inspired enriched oxygen roughly doubles arterial oxygen tension, and while this doesn’t translate into anything close to a doubled oxygen supply to tissues, it may result in increased oxygen diffusion into brain tissue, the psychiatrist explained.
Normobaric hyperoxia therapy is not to be confused with hyperbaric oxygen therapy, which requires a special chamber to handle markedly increased atmospheric pressures and has some inherent dangers. Mobile bedside oxygen generator units for oxygen enrichment are commercially available over the counter. Those used in the Israeli study were about the size of a vacuum cleaner and weighed a little more than 40 lb. Much smaller, more convenient units are available as well, but are costlier.
Dr. Belmaker reported on 51 adults with mild or moderate symptoms of major depressive disorder and a mean 11-year disease history who were randomized double blind to breathe either 35% oxygen or normal air – that is, 21% oxygen – at 1 atm pressure delivered from an investigator-supplied oxygen generator through standard plastic nasal prongs at a flow rate of 5 L/min for 7 hours nightly for 1 month.
“Controls heard the same flow and felt the same feeling on the face but were receiving 21% oxygen,” he noted.
Oxygen generator units are capable of enriching air to more than 90% oxygen; however, the investigators wanted to be cautious in a pilot study of an untested therapy, and they found evidence from both animal and human studies that 40% oxygen is reassuringly safe. Study exclusion criteria included obesity, acute or chronic respiratory disease, psychosis, and suicidality.
The primary study endpoint was the change in Hamilton Rating Scale for Depression score at 1 month. From a mean baseline of 14.6, the score in the normobaric hyperoxia group dropped by more than 4 points while remaining unchanged in controls. In a subscale analysis, it was apparent that most of the improvement occurred in the anxiety and cognitive disturbance subscale domains, according to Dr. Belmaker.
Of note, all patients rated by blinded investigators as much improved or very much improved on the Clinical Global Impression scale came from the enriched oxygen group.
No treatment-related adverse events occurred in the study.
“We don’t know the mechanism of the benefit of oxygen on the brain. It’s complex. In stroke and acute MI we used to think oxygen was beneficial, but scientists now feel that it’s not,” the psychiatrist said. “This early data deserve replication with higher concentrations of oxygen, different time periods of application, and in different patient groups.”
He emphasized that, since individuals with physical illnesses – including sleep apnea and chronic obstructive pulmonary disease – were excluded from the study, it’s not possible to say whether normobaric hyperoxia therapy would have an antidepressant effect in such patients.
“I would be especially careful with the normobaric oxygen in any patients with any cardiovascular or hypertensive disease because the increased oxygen pressure can have the side effect of contracting cardiac capillaries as a reflex action. So I certainly cannot recommend applying this study in any patients with a physical disease at this point,” Dr. Belmaker emphasized.
He reported having no financial conflicts regarding the study, funded by a grant from the Brain and Behavior Research Foundation.
SOURCE: Belmaker RH. ECNP 2020, Session S.12.
FROM ECNP 2020
ECG promising for predicting major depression, treatment response
Individuals with major depressive disorder (MDD) have an increased heart rate – a finding that may have the potential to identify individuals at risk for the disorder and predict treatment response, early research suggests.
Using the rapid-action of the novel antidepressant ketamine and the latest wearable 24-hour electrocardiogram (ECG) technology, investigators found that heart rate could distinguish MDD patients from healthy individuals.
They also found that patients with MDD with the highest resting heart rate had a greater treatment response. In fact, on average, depressed patients had a heart rate that was roughly 10-15 beats per minute higher than healthy controls.
The innovative design of the proof-of-concept study “allowed us to see that average resting heart rate may change quite suddenly to reflect the change in mood,” lead investigator Carmen Schiweck, PhD, Goethe University, Frankfurt am Main, Germany, said in an interview.
These results could have “exciting implications for treatment selection,” and the researchers plan to assess the potential for heart rate to act as an early warning system for depression, they noted.
The findings were presented at the 33rd European College of Neuropsychopharmacology (ECNP) Congress, which was held online this year because of the COVID-19 pandemic.
Identifying trait markers
There have been recent attempts to assess heart rate or heart rate variability (HRV) in patients with MDD to identify trait markers, which are present regardless of the disease phase, or state markers, which are present only during a depressive phase.
However, heart rate and HRV are “highly variable” over a 24-hour cycle, a fact that has been ignored by recent classification efforts, the researchers noted. Moreover, most commonly used antidepressants have a long onset of action, which makes studying their impact on the heart rate challenging.
The researchers’ goal was to determine whether heart rate and HRV could be used as trait markers to distinguish MDD patients from healthy individuals and, through the use of ketamine, whether they can also act as state markers for depression.
For the study, 16 treatment-resistant patients with MDD and 16 age- and sex-matched healthy controls wore a portable ECG device for 4 consecutive days and 3 nights. Heart rate and HRV data were subsequently averaged to obtain a 24-hour ECG.
Participants then received a single infusion of intravenous ketamine for 40 minutes. After waiting for 1 hour, the patients resumed ECG recording for an additional 4 days, with changes in mood assessed using the Hamilton Rating Scale for Depression (HAM-D).
Results showed that, compared with the control group, patients with MDD had a significantly higher 24-hour heart rate (P < .001) and a significantly lower HRV, as measured by the root mean square of successive differences (P < .001).
The investigators also found a reduction in heart rate amplitude, which indicates “significant blunting of circadian rhythm variation throughout the day and less recovery at night.”
Ninety percent accuracy
Harmonic and binary regression showed that heart rate was able to identify those with MDD versus those in the control group, particularly using nighttime readings, with 90.6% accuracy. The data correctly identified 14 (87.5%) patients and 15 (93.8%) members of the control group.
Following treatment, heart rate decreased significantly among the MDD group (P < .001), but there was no significant change in HRV (P = .295).
There was a significant positive association between baseline heart rate and response to treatment on the HAM-D (r = .55; P = .046), which suggested better outcomes in patients with a higher heart rate.
Interestingly, while heart rate was positively correlated with depression severity before treatment (r = .59; P = .03), this relationship disappeared following treatment (r = –0.04; P = .90), suggesting heart rate changes were not linked to depression states.
While heart rate levels may be useful as a trait marker and, potentially, for predicting response to antidepressant treatment, they did not show potential as a state marker, the investigators noted.
They suggested that, while the results need to be confirmed in longitudinal studies, approval of a ketamine nasal spray “may open up new avenues to conceive treatment paradigms, as explored in this study.”
However, “this is a small proof-of-concept study,” the investigators acknowledged. They also point out that only six of the patients with MDD had a reduction in HAM-D scores of at least 30% in response to treatment.
Future research plans
Dr. Schiweck said in an interview that her team was able to identify differences in heart rate and HRV in MDD patients that were not observed in other studies because portable at-home devices allowed them to monitor heart rate continuously over days.
The use of ketamine may also have been advantageous because the Netherlands Study of Depression and Anxiety (NESDA), which was published in 2010, clearly showed that “traditional antidepressants,” in particular tricyclic antidepressants, have a strong influence on heart rate and HRV, Dr. Schiweck said.
because most of the recent studies “just assess patients who are remitted and patients who are currently depressed, but it’s a cross-sectional study design,” said Schiweck.
“If we can follow up the same patients over time then we might really know if it is possible to use heart rate as a state marker for depression,” she added. “That’s what we tried to do with ketamine, but our study was very, very small.”
She noted that the investigators would also like to assess individuals who are “very stressed” and may show some depressive symptoms but don’t yet have a diagnosis of depression.
Mind-body link
Commenting on the findings, Brenda W.J.H. Penninx, MD, PhD, professor of psychiatric epidemiology at the VU University Medical Center in Amsterdam, said the concept of higher heart rate and lower HRV in depression “is indicative of more sympathetic drive and less parasympathetic drive of the autonomic nervous system.”
“That fits with the overall thought that depression is a state with more continuous exposure to stress overactivation of the body, which can be reflected in the [hypothalamic-pituitary-adrenal] axis, leading to higher levels of cortisol stress hormone. But it can also be reflected in the parasympathetic and sympathetic activation of the autonomic nervous system,” she said.
Dr. Penninx, who was also the principal investigator of the NESDA study, was not involved with the current research.
She noted that the NESDA study showed that, when patients with depressive disorder are compared with a healthy controls group, they have a higher heart rate and lower HRV. “But if we then divide people into medicated and nonmedicated people ... then we see that these deviations are only seen in people using medication,” she added.
“Our findings indicate that at least the use of antidepressants is having quite a large impact on autonomic nervous system dysregulation,” Dr. Penninx said. The difference with the current study, she pointed out, is that “it examines the problem over a completely different time scale.”
Although this offers advantages, the current study did not have the large patient numbers that were included in the NESDA study and “they were not clearly able to distinguish the effect of disease from medication,” noted Dr. Penninx.
In addition, this is not an easy area to investigate because there are multiple factors that can mitigate results, including the psychiatric state of the patient, use of medications for both mental illness and cardiometabolic disease, and a patient’s age and gender.
Still, the study clearly illustrates the importance of the interplay between mental health and somatic health and that there is “a very clear indication that we don’t need to separate those,” Dr. Penninx said.
The study was funded by a TGO-IWT Grant from Belgium. The study authors and Dr. Penninx have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Individuals with major depressive disorder (MDD) have an increased heart rate – a finding that may have the potential to identify individuals at risk for the disorder and predict treatment response, early research suggests.
Using the rapid-action of the novel antidepressant ketamine and the latest wearable 24-hour electrocardiogram (ECG) technology, investigators found that heart rate could distinguish MDD patients from healthy individuals.
They also found that patients with MDD with the highest resting heart rate had a greater treatment response. In fact, on average, depressed patients had a heart rate that was roughly 10-15 beats per minute higher than healthy controls.
The innovative design of the proof-of-concept study “allowed us to see that average resting heart rate may change quite suddenly to reflect the change in mood,” lead investigator Carmen Schiweck, PhD, Goethe University, Frankfurt am Main, Germany, said in an interview.
These results could have “exciting implications for treatment selection,” and the researchers plan to assess the potential for heart rate to act as an early warning system for depression, they noted.
The findings were presented at the 33rd European College of Neuropsychopharmacology (ECNP) Congress, which was held online this year because of the COVID-19 pandemic.
Identifying trait markers
There have been recent attempts to assess heart rate or heart rate variability (HRV) in patients with MDD to identify trait markers, which are present regardless of the disease phase, or state markers, which are present only during a depressive phase.
However, heart rate and HRV are “highly variable” over a 24-hour cycle, a fact that has been ignored by recent classification efforts, the researchers noted. Moreover, most commonly used antidepressants have a long onset of action, which makes studying their impact on the heart rate challenging.
The researchers’ goal was to determine whether heart rate and HRV could be used as trait markers to distinguish MDD patients from healthy individuals and, through the use of ketamine, whether they can also act as state markers for depression.
For the study, 16 treatment-resistant patients with MDD and 16 age- and sex-matched healthy controls wore a portable ECG device for 4 consecutive days and 3 nights. Heart rate and HRV data were subsequently averaged to obtain a 24-hour ECG.
Participants then received a single infusion of intravenous ketamine for 40 minutes. After waiting for 1 hour, the patients resumed ECG recording for an additional 4 days, with changes in mood assessed using the Hamilton Rating Scale for Depression (HAM-D).
Results showed that, compared with the control group, patients with MDD had a significantly higher 24-hour heart rate (P < .001) and a significantly lower HRV, as measured by the root mean square of successive differences (P < .001).
The investigators also found a reduction in heart rate amplitude, which indicates “significant blunting of circadian rhythm variation throughout the day and less recovery at night.”
Ninety percent accuracy
Harmonic and binary regression showed that heart rate was able to identify those with MDD versus those in the control group, particularly using nighttime readings, with 90.6% accuracy. The data correctly identified 14 (87.5%) patients and 15 (93.8%) members of the control group.
Following treatment, heart rate decreased significantly among the MDD group (P < .001), but there was no significant change in HRV (P = .295).
There was a significant positive association between baseline heart rate and response to treatment on the HAM-D (r = .55; P = .046), which suggested better outcomes in patients with a higher heart rate.
Interestingly, while heart rate was positively correlated with depression severity before treatment (r = .59; P = .03), this relationship disappeared following treatment (r = –0.04; P = .90), suggesting heart rate changes were not linked to depression states.
While heart rate levels may be useful as a trait marker and, potentially, for predicting response to antidepressant treatment, they did not show potential as a state marker, the investigators noted.
They suggested that, while the results need to be confirmed in longitudinal studies, approval of a ketamine nasal spray “may open up new avenues to conceive treatment paradigms, as explored in this study.”
However, “this is a small proof-of-concept study,” the investigators acknowledged. They also point out that only six of the patients with MDD had a reduction in HAM-D scores of at least 30% in response to treatment.
Future research plans
Dr. Schiweck said in an interview that her team was able to identify differences in heart rate and HRV in MDD patients that were not observed in other studies because portable at-home devices allowed them to monitor heart rate continuously over days.
The use of ketamine may also have been advantageous because the Netherlands Study of Depression and Anxiety (NESDA), which was published in 2010, clearly showed that “traditional antidepressants,” in particular tricyclic antidepressants, have a strong influence on heart rate and HRV, Dr. Schiweck said.
because most of the recent studies “just assess patients who are remitted and patients who are currently depressed, but it’s a cross-sectional study design,” said Schiweck.
“If we can follow up the same patients over time then we might really know if it is possible to use heart rate as a state marker for depression,” she added. “That’s what we tried to do with ketamine, but our study was very, very small.”
She noted that the investigators would also like to assess individuals who are “very stressed” and may show some depressive symptoms but don’t yet have a diagnosis of depression.
Mind-body link
Commenting on the findings, Brenda W.J.H. Penninx, MD, PhD, professor of psychiatric epidemiology at the VU University Medical Center in Amsterdam, said the concept of higher heart rate and lower HRV in depression “is indicative of more sympathetic drive and less parasympathetic drive of the autonomic nervous system.”
“That fits with the overall thought that depression is a state with more continuous exposure to stress overactivation of the body, which can be reflected in the [hypothalamic-pituitary-adrenal] axis, leading to higher levels of cortisol stress hormone. But it can also be reflected in the parasympathetic and sympathetic activation of the autonomic nervous system,” she said.
Dr. Penninx, who was also the principal investigator of the NESDA study, was not involved with the current research.
She noted that the NESDA study showed that, when patients with depressive disorder are compared with a healthy controls group, they have a higher heart rate and lower HRV. “But if we then divide people into medicated and nonmedicated people ... then we see that these deviations are only seen in people using medication,” she added.
“Our findings indicate that at least the use of antidepressants is having quite a large impact on autonomic nervous system dysregulation,” Dr. Penninx said. The difference with the current study, she pointed out, is that “it examines the problem over a completely different time scale.”
Although this offers advantages, the current study did not have the large patient numbers that were included in the NESDA study and “they were not clearly able to distinguish the effect of disease from medication,” noted Dr. Penninx.
In addition, this is not an easy area to investigate because there are multiple factors that can mitigate results, including the psychiatric state of the patient, use of medications for both mental illness and cardiometabolic disease, and a patient’s age and gender.
Still, the study clearly illustrates the importance of the interplay between mental health and somatic health and that there is “a very clear indication that we don’t need to separate those,” Dr. Penninx said.
The study was funded by a TGO-IWT Grant from Belgium. The study authors and Dr. Penninx have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Individuals with major depressive disorder (MDD) have an increased heart rate – a finding that may have the potential to identify individuals at risk for the disorder and predict treatment response, early research suggests.
Using the rapid-action of the novel antidepressant ketamine and the latest wearable 24-hour electrocardiogram (ECG) technology, investigators found that heart rate could distinguish MDD patients from healthy individuals.
They also found that patients with MDD with the highest resting heart rate had a greater treatment response. In fact, on average, depressed patients had a heart rate that was roughly 10-15 beats per minute higher than healthy controls.
The innovative design of the proof-of-concept study “allowed us to see that average resting heart rate may change quite suddenly to reflect the change in mood,” lead investigator Carmen Schiweck, PhD, Goethe University, Frankfurt am Main, Germany, said in an interview.
These results could have “exciting implications for treatment selection,” and the researchers plan to assess the potential for heart rate to act as an early warning system for depression, they noted.
The findings were presented at the 33rd European College of Neuropsychopharmacology (ECNP) Congress, which was held online this year because of the COVID-19 pandemic.
Identifying trait markers
There have been recent attempts to assess heart rate or heart rate variability (HRV) in patients with MDD to identify trait markers, which are present regardless of the disease phase, or state markers, which are present only during a depressive phase.
However, heart rate and HRV are “highly variable” over a 24-hour cycle, a fact that has been ignored by recent classification efforts, the researchers noted. Moreover, most commonly used antidepressants have a long onset of action, which makes studying their impact on the heart rate challenging.
The researchers’ goal was to determine whether heart rate and HRV could be used as trait markers to distinguish MDD patients from healthy individuals and, through the use of ketamine, whether they can also act as state markers for depression.
For the study, 16 treatment-resistant patients with MDD and 16 age- and sex-matched healthy controls wore a portable ECG device for 4 consecutive days and 3 nights. Heart rate and HRV data were subsequently averaged to obtain a 24-hour ECG.
Participants then received a single infusion of intravenous ketamine for 40 minutes. After waiting for 1 hour, the patients resumed ECG recording for an additional 4 days, with changes in mood assessed using the Hamilton Rating Scale for Depression (HAM-D).
Results showed that, compared with the control group, patients with MDD had a significantly higher 24-hour heart rate (P < .001) and a significantly lower HRV, as measured by the root mean square of successive differences (P < .001).
The investigators also found a reduction in heart rate amplitude, which indicates “significant blunting of circadian rhythm variation throughout the day and less recovery at night.”
Ninety percent accuracy
Harmonic and binary regression showed that heart rate was able to identify those with MDD versus those in the control group, particularly using nighttime readings, with 90.6% accuracy. The data correctly identified 14 (87.5%) patients and 15 (93.8%) members of the control group.
Following treatment, heart rate decreased significantly among the MDD group (P < .001), but there was no significant change in HRV (P = .295).
There was a significant positive association between baseline heart rate and response to treatment on the HAM-D (r = .55; P = .046), which suggested better outcomes in patients with a higher heart rate.
Interestingly, while heart rate was positively correlated with depression severity before treatment (r = .59; P = .03), this relationship disappeared following treatment (r = –0.04; P = .90), suggesting heart rate changes were not linked to depression states.
While heart rate levels may be useful as a trait marker and, potentially, for predicting response to antidepressant treatment, they did not show potential as a state marker, the investigators noted.
They suggested that, while the results need to be confirmed in longitudinal studies, approval of a ketamine nasal spray “may open up new avenues to conceive treatment paradigms, as explored in this study.”
However, “this is a small proof-of-concept study,” the investigators acknowledged. They also point out that only six of the patients with MDD had a reduction in HAM-D scores of at least 30% in response to treatment.
Future research plans
Dr. Schiweck said in an interview that her team was able to identify differences in heart rate and HRV in MDD patients that were not observed in other studies because portable at-home devices allowed them to monitor heart rate continuously over days.
The use of ketamine may also have been advantageous because the Netherlands Study of Depression and Anxiety (NESDA), which was published in 2010, clearly showed that “traditional antidepressants,” in particular tricyclic antidepressants, have a strong influence on heart rate and HRV, Dr. Schiweck said.
because most of the recent studies “just assess patients who are remitted and patients who are currently depressed, but it’s a cross-sectional study design,” said Schiweck.
“If we can follow up the same patients over time then we might really know if it is possible to use heart rate as a state marker for depression,” she added. “That’s what we tried to do with ketamine, but our study was very, very small.”
She noted that the investigators would also like to assess individuals who are “very stressed” and may show some depressive symptoms but don’t yet have a diagnosis of depression.
Mind-body link
Commenting on the findings, Brenda W.J.H. Penninx, MD, PhD, professor of psychiatric epidemiology at the VU University Medical Center in Amsterdam, said the concept of higher heart rate and lower HRV in depression “is indicative of more sympathetic drive and less parasympathetic drive of the autonomic nervous system.”
“That fits with the overall thought that depression is a state with more continuous exposure to stress overactivation of the body, which can be reflected in the [hypothalamic-pituitary-adrenal] axis, leading to higher levels of cortisol stress hormone. But it can also be reflected in the parasympathetic and sympathetic activation of the autonomic nervous system,” she said.
Dr. Penninx, who was also the principal investigator of the NESDA study, was not involved with the current research.
She noted that the NESDA study showed that, when patients with depressive disorder are compared with a healthy controls group, they have a higher heart rate and lower HRV. “But if we then divide people into medicated and nonmedicated people ... then we see that these deviations are only seen in people using medication,” she added.
“Our findings indicate that at least the use of antidepressants is having quite a large impact on autonomic nervous system dysregulation,” Dr. Penninx said. The difference with the current study, she pointed out, is that “it examines the problem over a completely different time scale.”
Although this offers advantages, the current study did not have the large patient numbers that were included in the NESDA study and “they were not clearly able to distinguish the effect of disease from medication,” noted Dr. Penninx.
In addition, this is not an easy area to investigate because there are multiple factors that can mitigate results, including the psychiatric state of the patient, use of medications for both mental illness and cardiometabolic disease, and a patient’s age and gender.
Still, the study clearly illustrates the importance of the interplay between mental health and somatic health and that there is “a very clear indication that we don’t need to separate those,” Dr. Penninx said.
The study was funded by a TGO-IWT Grant from Belgium. The study authors and Dr. Penninx have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The gut a new therapeutic target for major depression?
The gut microbiota differs significantly between patients with major depressive disorder (MDD) and healthy individuals and may be modifiable with a probiotic diet to improve stress and depression scores, two new studies suggest.
In one study, investigators compared stool samples between patients with MDD and healthy controls. They found significant differences in bacterial profiles between the two groups, as well as between patients who responded vs those who were resistant to treatment.
“This finding further supports the relevance of an altered composition of the gut microbiota in the etiopathogenesis of MDD and suggests a role in response to antidepressants,” coinvestigator Andrea Fontana, MSc, Fondazione IRCCS Casa Sollievo della Sofferenza, San Giovanni Rotondo, Italy, said in an interview.
Results from the second study showed significant improvements in self-reported stress, anxiety, and depression scores in healthy individuals following a “psychobiotic” diet (using probiotics or prebiotics to manipulate the microbiota to improve mental health) that was rich in fruit, vegetables, and fermented foods vs. those who received dietary advice alone.
The investigators, led by Kirsten Berding, PhD, APC Microbiome Ireland, University College Cork, Ireland, now plan on testing their psychobiotic diet in patients with MDD and hope the findings could be helpful in “the development of adjuvant therapeutic opportunities” where pharmacologic treatment is not effective.
Both studies were presented at the virtual congress of the European College of Neuropsychopharmacology, held online this year because of the COVID-19 pandemic.
A “hallmark” of major depression
Mr. Fontana and colleagues note that the mostly suboptimal response to pharmacologic treatments among patients with MDD is one of the factors that “contributes to the large socioeconomic burden” of the disease.
Previous research shows patients with MDD have gut dysbiosis, or an imbalance in the natural flora; that antidepressants have antimicrobial properties; and that probiotics have an antibiotic effect. However, the correlation between the composition of the gut microbiota and antidepressant response is poorly understood.
The investigators recruited 34 patients with MDD (aged 18-70 years) who were in a euthymic phase and who did not have comorbid conditions that could affect the gut microbiota.
Eight patients were treatment resistant, defined as a poor response to at least two adequate trials of different antidepressant classes, while 19 were treatment responsive and seven were treatment naive.
The researchers also recruited 20 healthy individuals via word of mouth to act as the control group. There were no significant differences between patients and the control group in terms of baseline characteristics.
Genomic sequencing of bacteria obtained from stool samples showed that it was possible to distinguish between patients with MDD and the healthy individuals, especially at the family, genus, and species levels.
In particular, there were significant differences in the Paenibacillaceae and Flavobacteriaceaea families, for the genus Fenollaria, and the species Flintibacter butyricus, Christensenella timonensis, and Eisenbergiella massiliensis, among others.
Results also showed that the phyla Proteobacteria, Tenericutes, and the family Peptostreptococcaceae were more common in patients with treatment-resistant MDD, whereas the phylum Actinobacteria was more abundant in treatment responders.
Moreover, several bacteria were found only in the microbiota of patients with treatment-resistant MDD, while others were seen only in treatment-responsive patients. This made it possible to discriminate not only between treatment-resistant and -responsive patients but also between those two patient groups and healthy controls.
“The results of our study confirm that gut dysbiosis is a hallmark of MDD, and suggests that the gut microbiota of patients with treatment-resistant MDD significantly differs from responders to antidepressants,” Mr. Fontana said.
Psychobiotic diet
For the second study, Dr. Berding and colleagues note that “psychobiotics” has previously achieved “promising results.”
In addition, diet is both “one of the most influential modifying factors” for the gut microbiota and an easily accessible strategy, they wrote. However, there is also a paucity of studies in this area, they added.
The researchers randomly assigned healthy volunteers with relatively poor dietary habits to either a 4-week psychobiotic diet group (n = 21) or a control group (n = 19).
Individuals in the psychobiotic group were told to eat a diet rich in prebiotics, such as fruit and vegetables, fiber including whole grains and legumes, and fermented foods. The control group was educated on Irish healthy-eating guidelines.
Stool and saliva samples were collected and the participants completed several self-reported mental health questionnaires, as well as a 7-day food diary. They also took the socially evaluated cold-pressor test (SECPT) to measure acute stress responses.
Results showed that total daily energy intake decreased significantly in both the diet and control groups over the study period (P = .04 for both) but did not differ significantly between the groups.
In contrast, dietary fiber intake increased significantly in the diet group (P < .001) and was significantly higher than in the control group at the end of the intervention (P = .03).
Individuals in the diet group showed significant decreases in scores on the Perceived Stress Scale (P = .002) and the Beck Depression Inventory (P = .007) during the study, an effect that was not found in the control group.
Dietary intervention
There were no significant effects of diet on the acute stress response, but both groups showed improvements in self-concept, or perceived ability to cope, on the Primary Appraisal, Secondary Appraisal index (P = .03 for the diet group, P = .04 for the control group).
The results show that a dietary intervention targeted at the microbiota “can improve subjective feelings of stress and depression in a healthy population,” the investigators wrote.
However, elucidating the “contribution of the microbiota-gut-brain axis on the signaling response to dietary interventions” will require further studies on microbiota sequencing and biological measures of stress, they added.
This will “contribute to the understanding of the benefits of a psychobiotic diet on stress and anxiety,” wrote the researchers.
Dr. Berding said in an interview that while the consumption of dietary fiber changed the most in the diet group, “it would not be the only nutrient” that had an impact on the results, with fermented foods a likely candidate.
She said the next step is to test the dietary intervention in patients with MDD; however, “doing nutritional interventions in diseased populations is always difficult.”
Dr. Berding suggested that the best approach would be to study inpatients in a clinic, as “we would be able to provide every meal and only provide foods that are part of the dietary intervention.”
Although another option would be to conduct the study in outpatients, she noted that assessing inpatients “would give us the best control over compliance.”
“Brilliant ideas”
Commenting on the findings, Sergueï Fetissov, MD, PhD, professor of physiology at Rouen University, Mont-Saint-Aignan, France, said that although both studies bring attention to a possible role for the gut microbiota in MDD, neither “provide any experimental evidence of a causative nature.”
Dr. Fetissov, who was not involved in either study, noted that this topic has been the subject of clinical nutritional research for many years.
However, “we still need some strong evidence to prove that some bacteria can influence the regulation of mood and anxiety and stress,” he said.
In addition, researchers currently do not know what actually causes MDD. “How we can say the gut bacteria regulates something if we don’t know what really causes the altered mood?” said Dr. Fetissov.
He noted that over the last 50 years, there have been great advances in the development of drugs that alleviate depression and anxiety by regulating dopamine, serotonin, and other neurotransmitters. However, it is still unknown whether these reflect primary or secondary aspects of mood disorders.
Furthermore, it is not clear “how probiotics to bacteria can influence these neuronal pathways,” he said.
The research by Dr. Berding and colleagues is funded by a postdoctoral fellowship grant from the Irish Research Council. The study authors and Dr. Fetissov have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The gut microbiota differs significantly between patients with major depressive disorder (MDD) and healthy individuals and may be modifiable with a probiotic diet to improve stress and depression scores, two new studies suggest.
In one study, investigators compared stool samples between patients with MDD and healthy controls. They found significant differences in bacterial profiles between the two groups, as well as between patients who responded vs those who were resistant to treatment.
“This finding further supports the relevance of an altered composition of the gut microbiota in the etiopathogenesis of MDD and suggests a role in response to antidepressants,” coinvestigator Andrea Fontana, MSc, Fondazione IRCCS Casa Sollievo della Sofferenza, San Giovanni Rotondo, Italy, said in an interview.
Results from the second study showed significant improvements in self-reported stress, anxiety, and depression scores in healthy individuals following a “psychobiotic” diet (using probiotics or prebiotics to manipulate the microbiota to improve mental health) that was rich in fruit, vegetables, and fermented foods vs. those who received dietary advice alone.
The investigators, led by Kirsten Berding, PhD, APC Microbiome Ireland, University College Cork, Ireland, now plan on testing their psychobiotic diet in patients with MDD and hope the findings could be helpful in “the development of adjuvant therapeutic opportunities” where pharmacologic treatment is not effective.
Both studies were presented at the virtual congress of the European College of Neuropsychopharmacology, held online this year because of the COVID-19 pandemic.
A “hallmark” of major depression
Mr. Fontana and colleagues note that the mostly suboptimal response to pharmacologic treatments among patients with MDD is one of the factors that “contributes to the large socioeconomic burden” of the disease.
Previous research shows patients with MDD have gut dysbiosis, or an imbalance in the natural flora; that antidepressants have antimicrobial properties; and that probiotics have an antibiotic effect. However, the correlation between the composition of the gut microbiota and antidepressant response is poorly understood.
The investigators recruited 34 patients with MDD (aged 18-70 years) who were in a euthymic phase and who did not have comorbid conditions that could affect the gut microbiota.
Eight patients were treatment resistant, defined as a poor response to at least two adequate trials of different antidepressant classes, while 19 were treatment responsive and seven were treatment naive.
The researchers also recruited 20 healthy individuals via word of mouth to act as the control group. There were no significant differences between patients and the control group in terms of baseline characteristics.
Genomic sequencing of bacteria obtained from stool samples showed that it was possible to distinguish between patients with MDD and the healthy individuals, especially at the family, genus, and species levels.
In particular, there were significant differences in the Paenibacillaceae and Flavobacteriaceaea families, for the genus Fenollaria, and the species Flintibacter butyricus, Christensenella timonensis, and Eisenbergiella massiliensis, among others.
Results also showed that the phyla Proteobacteria, Tenericutes, and the family Peptostreptococcaceae were more common in patients with treatment-resistant MDD, whereas the phylum Actinobacteria was more abundant in treatment responders.
Moreover, several bacteria were found only in the microbiota of patients with treatment-resistant MDD, while others were seen only in treatment-responsive patients. This made it possible to discriminate not only between treatment-resistant and -responsive patients but also between those two patient groups and healthy controls.
“The results of our study confirm that gut dysbiosis is a hallmark of MDD, and suggests that the gut microbiota of patients with treatment-resistant MDD significantly differs from responders to antidepressants,” Mr. Fontana said.
Psychobiotic diet
For the second study, Dr. Berding and colleagues note that “psychobiotics” has previously achieved “promising results.”
In addition, diet is both “one of the most influential modifying factors” for the gut microbiota and an easily accessible strategy, they wrote. However, there is also a paucity of studies in this area, they added.
The researchers randomly assigned healthy volunteers with relatively poor dietary habits to either a 4-week psychobiotic diet group (n = 21) or a control group (n = 19).
Individuals in the psychobiotic group were told to eat a diet rich in prebiotics, such as fruit and vegetables, fiber including whole grains and legumes, and fermented foods. The control group was educated on Irish healthy-eating guidelines.
Stool and saliva samples were collected and the participants completed several self-reported mental health questionnaires, as well as a 7-day food diary. They also took the socially evaluated cold-pressor test (SECPT) to measure acute stress responses.
Results showed that total daily energy intake decreased significantly in both the diet and control groups over the study period (P = .04 for both) but did not differ significantly between the groups.
In contrast, dietary fiber intake increased significantly in the diet group (P < .001) and was significantly higher than in the control group at the end of the intervention (P = .03).
Individuals in the diet group showed significant decreases in scores on the Perceived Stress Scale (P = .002) and the Beck Depression Inventory (P = .007) during the study, an effect that was not found in the control group.
Dietary intervention
There were no significant effects of diet on the acute stress response, but both groups showed improvements in self-concept, or perceived ability to cope, on the Primary Appraisal, Secondary Appraisal index (P = .03 for the diet group, P = .04 for the control group).
The results show that a dietary intervention targeted at the microbiota “can improve subjective feelings of stress and depression in a healthy population,” the investigators wrote.
However, elucidating the “contribution of the microbiota-gut-brain axis on the signaling response to dietary interventions” will require further studies on microbiota sequencing and biological measures of stress, they added.
This will “contribute to the understanding of the benefits of a psychobiotic diet on stress and anxiety,” wrote the researchers.
Dr. Berding said in an interview that while the consumption of dietary fiber changed the most in the diet group, “it would not be the only nutrient” that had an impact on the results, with fermented foods a likely candidate.
She said the next step is to test the dietary intervention in patients with MDD; however, “doing nutritional interventions in diseased populations is always difficult.”
Dr. Berding suggested that the best approach would be to study inpatients in a clinic, as “we would be able to provide every meal and only provide foods that are part of the dietary intervention.”
Although another option would be to conduct the study in outpatients, she noted that assessing inpatients “would give us the best control over compliance.”
“Brilliant ideas”
Commenting on the findings, Sergueï Fetissov, MD, PhD, professor of physiology at Rouen University, Mont-Saint-Aignan, France, said that although both studies bring attention to a possible role for the gut microbiota in MDD, neither “provide any experimental evidence of a causative nature.”
Dr. Fetissov, who was not involved in either study, noted that this topic has been the subject of clinical nutritional research for many years.
However, “we still need some strong evidence to prove that some bacteria can influence the regulation of mood and anxiety and stress,” he said.
In addition, researchers currently do not know what actually causes MDD. “How we can say the gut bacteria regulates something if we don’t know what really causes the altered mood?” said Dr. Fetissov.
He noted that over the last 50 years, there have been great advances in the development of drugs that alleviate depression and anxiety by regulating dopamine, serotonin, and other neurotransmitters. However, it is still unknown whether these reflect primary or secondary aspects of mood disorders.
Furthermore, it is not clear “how probiotics to bacteria can influence these neuronal pathways,” he said.
The research by Dr. Berding and colleagues is funded by a postdoctoral fellowship grant from the Irish Research Council. The study authors and Dr. Fetissov have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The gut microbiota differs significantly between patients with major depressive disorder (MDD) and healthy individuals and may be modifiable with a probiotic diet to improve stress and depression scores, two new studies suggest.
In one study, investigators compared stool samples between patients with MDD and healthy controls. They found significant differences in bacterial profiles between the two groups, as well as between patients who responded vs those who were resistant to treatment.
“This finding further supports the relevance of an altered composition of the gut microbiota in the etiopathogenesis of MDD and suggests a role in response to antidepressants,” coinvestigator Andrea Fontana, MSc, Fondazione IRCCS Casa Sollievo della Sofferenza, San Giovanni Rotondo, Italy, said in an interview.
Results from the second study showed significant improvements in self-reported stress, anxiety, and depression scores in healthy individuals following a “psychobiotic” diet (using probiotics or prebiotics to manipulate the microbiota to improve mental health) that was rich in fruit, vegetables, and fermented foods vs. those who received dietary advice alone.
The investigators, led by Kirsten Berding, PhD, APC Microbiome Ireland, University College Cork, Ireland, now plan on testing their psychobiotic diet in patients with MDD and hope the findings could be helpful in “the development of adjuvant therapeutic opportunities” where pharmacologic treatment is not effective.
Both studies were presented at the virtual congress of the European College of Neuropsychopharmacology, held online this year because of the COVID-19 pandemic.
A “hallmark” of major depression
Mr. Fontana and colleagues note that the mostly suboptimal response to pharmacologic treatments among patients with MDD is one of the factors that “contributes to the large socioeconomic burden” of the disease.
Previous research shows patients with MDD have gut dysbiosis, or an imbalance in the natural flora; that antidepressants have antimicrobial properties; and that probiotics have an antibiotic effect. However, the correlation between the composition of the gut microbiota and antidepressant response is poorly understood.
The investigators recruited 34 patients with MDD (aged 18-70 years) who were in a euthymic phase and who did not have comorbid conditions that could affect the gut microbiota.
Eight patients were treatment resistant, defined as a poor response to at least two adequate trials of different antidepressant classes, while 19 were treatment responsive and seven were treatment naive.
The researchers also recruited 20 healthy individuals via word of mouth to act as the control group. There were no significant differences between patients and the control group in terms of baseline characteristics.
Genomic sequencing of bacteria obtained from stool samples showed that it was possible to distinguish between patients with MDD and the healthy individuals, especially at the family, genus, and species levels.
In particular, there were significant differences in the Paenibacillaceae and Flavobacteriaceaea families, for the genus Fenollaria, and the species Flintibacter butyricus, Christensenella timonensis, and Eisenbergiella massiliensis, among others.
Results also showed that the phyla Proteobacteria, Tenericutes, and the family Peptostreptococcaceae were more common in patients with treatment-resistant MDD, whereas the phylum Actinobacteria was more abundant in treatment responders.
Moreover, several bacteria were found only in the microbiota of patients with treatment-resistant MDD, while others were seen only in treatment-responsive patients. This made it possible to discriminate not only between treatment-resistant and -responsive patients but also between those two patient groups and healthy controls.
“The results of our study confirm that gut dysbiosis is a hallmark of MDD, and suggests that the gut microbiota of patients with treatment-resistant MDD significantly differs from responders to antidepressants,” Mr. Fontana said.
Psychobiotic diet
For the second study, Dr. Berding and colleagues note that “psychobiotics” has previously achieved “promising results.”
In addition, diet is both “one of the most influential modifying factors” for the gut microbiota and an easily accessible strategy, they wrote. However, there is also a paucity of studies in this area, they added.
The researchers randomly assigned healthy volunteers with relatively poor dietary habits to either a 4-week psychobiotic diet group (n = 21) or a control group (n = 19).
Individuals in the psychobiotic group were told to eat a diet rich in prebiotics, such as fruit and vegetables, fiber including whole grains and legumes, and fermented foods. The control group was educated on Irish healthy-eating guidelines.
Stool and saliva samples were collected and the participants completed several self-reported mental health questionnaires, as well as a 7-day food diary. They also took the socially evaluated cold-pressor test (SECPT) to measure acute stress responses.
Results showed that total daily energy intake decreased significantly in both the diet and control groups over the study period (P = .04 for both) but did not differ significantly between the groups.
In contrast, dietary fiber intake increased significantly in the diet group (P < .001) and was significantly higher than in the control group at the end of the intervention (P = .03).
Individuals in the diet group showed significant decreases in scores on the Perceived Stress Scale (P = .002) and the Beck Depression Inventory (P = .007) during the study, an effect that was not found in the control group.
Dietary intervention
There were no significant effects of diet on the acute stress response, but both groups showed improvements in self-concept, or perceived ability to cope, on the Primary Appraisal, Secondary Appraisal index (P = .03 for the diet group, P = .04 for the control group).
The results show that a dietary intervention targeted at the microbiota “can improve subjective feelings of stress and depression in a healthy population,” the investigators wrote.
However, elucidating the “contribution of the microbiota-gut-brain axis on the signaling response to dietary interventions” will require further studies on microbiota sequencing and biological measures of stress, they added.
This will “contribute to the understanding of the benefits of a psychobiotic diet on stress and anxiety,” wrote the researchers.
Dr. Berding said in an interview that while the consumption of dietary fiber changed the most in the diet group, “it would not be the only nutrient” that had an impact on the results, with fermented foods a likely candidate.
She said the next step is to test the dietary intervention in patients with MDD; however, “doing nutritional interventions in diseased populations is always difficult.”
Dr. Berding suggested that the best approach would be to study inpatients in a clinic, as “we would be able to provide every meal and only provide foods that are part of the dietary intervention.”
Although another option would be to conduct the study in outpatients, she noted that assessing inpatients “would give us the best control over compliance.”
“Brilliant ideas”
Commenting on the findings, Sergueï Fetissov, MD, PhD, professor of physiology at Rouen University, Mont-Saint-Aignan, France, said that although both studies bring attention to a possible role for the gut microbiota in MDD, neither “provide any experimental evidence of a causative nature.”
Dr. Fetissov, who was not involved in either study, noted that this topic has been the subject of clinical nutritional research for many years.
However, “we still need some strong evidence to prove that some bacteria can influence the regulation of mood and anxiety and stress,” he said.
In addition, researchers currently do not know what actually causes MDD. “How we can say the gut bacteria regulates something if we don’t know what really causes the altered mood?” said Dr. Fetissov.
He noted that over the last 50 years, there have been great advances in the development of drugs that alleviate depression and anxiety by regulating dopamine, serotonin, and other neurotransmitters. However, it is still unknown whether these reflect primary or secondary aspects of mood disorders.
Furthermore, it is not clear “how probiotics to bacteria can influence these neuronal pathways,” he said.
The research by Dr. Berding and colleagues is funded by a postdoctoral fellowship grant from the Irish Research Council. The study authors and Dr. Fetissov have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
ECT reduces all-cause mortality in Danish study
The two top developments of the year in the field of neurostimulation involve the oldest form of the therapy: electroconvulsive therapy, Alexander Sartorius, MD, said at the virtual congress of the European College of Neuropsychopharmacology.
One of these studies shot down the longstanding notion that ECT causes brain damage. The other, a Danish national registry study, demonstrated that ECT is associated with lower all-cause mortality than in patients with similarly severe depression who don’t undergo ECT.
“The take-home messages are that ECT does not lead to brain damage but rather to a profound gray matter increase. And secondly, ECT lowers all-cause mortality in patients with depression,” said Dr. Sartorius, a psychiatrist at the Central Institute of Mental Health in Mannheim, Germany.
The year has been less fruitful in terms of research involving deep brain stimulation using implantable electrodes, he continued.
“Basically, one can state that there is no breaking news in the field of deep brain stimulation. DBS remains a highly experimental form of therapy,” Dr. Sartorius said.
ECT-induced brain changes
Investigators participating in the international multicenter Global ECT-MRI Research Collaboration (GEMRIC) reported on the longitudinal effects of ECT on gray matter, white matter, and ventricular volumes in 328 patients who underwent imaging before and after ECT for a major depressive episode, as well as in 95 nondepressed controls.
The key finding was that ECT induced a widespread, nonspecific global increase in gray matter volume. Indeed, the volumetric increase was documented in 79 of 84 gray matter regions of interest. Subcortical gray matter volume increased by a mean of 1.47% in ECT-treated patients, and total cortical volume rose by 1.04%. Total white matter volume remained unchanged.
“The gray matter increase induced by ECT looks quite similar to the gray matter increase seen with physical activity,” Dr. Sartorius noted.
The size of the gray matter volume increase rose with the number of ECT sessions. However, gray matter enlargement in response to ECT showed no relationship with clinical response, indicating that this finding on brain imaging doesn’t have a promising future as a potential biomarker of treatment effectiveness (Biol Psychiatry. 2020 Mar 1;87[5]:451-61).
“The good news from this study is there is no gray matter decrease,” Dr. Sartorius said. “This study enhances the existing evidence falsifying the old idea or dogma that ECT induces brain damage. I would claim that the opposite is clearly the case.”
ECT and mortality
The same group of investigators at the University of Copenhagen who several years ago harnessed Danish national patient registries data to report that ECT was associated with an unadjusted 32% and adjusted 23% reduction in the risk of developing dementia in patients aged 70 years and older (Lancet Psychiatry. 2018 Apr;5[4]:348-56) have recently concluded that ECT was also associated with a 19% reduction in all-cause mortality, compared with that of patients hospitalized for major depression who didn’t receive ECT.
The national registries study included 5,004 patients who were treated with ECT and nearly 88,000 others who were hospitalized for major depression during 2005-2016 but didn’t receive ECT. During up to 11.3 years of follow-up, the risk of all-cause mortality was 8% lower with ECT than with no ECT in patients categorized as having mild depression, 17% lower with a history of ECT for moderate depression, 4% less with severe depression without psychotic features, and 30% less with ECT than no ECT for severe depression with psychotic features (J Psychopharmacol. 2020 Mar;34[3]:273-9).
ECT was associated with an adjusted 6.99-fold increased risk of suicide in patients with mild depression. At first look that’s unsettling, Dr. Sartorius said, but he pointed out that the size of the ECT-associated suicide risk lessened with increasing severity of depression, and in fact, there was no increased suicide risk with ECT in severely depressed patients with psychotic features. ECT was also associated with significantly higher rates of psychiatric rehospitalization, emergency department visits, and suicidal behavior in patients classified as having mild or moderate depression. Dr. Sartorius suspects these findings reflect diagnostic uncertainty surrounding the milder forms of depression, coupled with the reality that ECT is generally reserved for the most unstable, treatment-resistant patients.
Deep transcranial magnetic stimulation
A Taiwanese meta-analysis has helped clarify the role of deep transcranial magnetic stimulation (dTMS) for treatment-resistant depression. The meta-analysis included 198 patients with treatment-resistant depression who underwent dTMS and 219 who received sham treatment in a total of 15 studies, only three of which were randomized controlled trials.
Active treatment was associated with a 2.06-fold increased likelihood of remission of depressive symptoms; however, the effect was statistically significant only in the subgroup of patients on concurrent antidepressant medication. Moreover, the therapeutic benefit of dTMS was significantly greater in the nonrandomized studies, where the odds ratio for remission was 3.8-fold greater than with sham therapy. In contrast, the odds ratio for remission with dTMS dropped to 1.37 in the randomized trials (Prog Neuropsychopharmacol Biol Psychiatry. 2020 Apr 20;99:109850. doi: 10.1016/j.pnpbp.2019.109850). “dTMS has quite a bit of antidepressant potential in treatment-resistant depression, but as an augmentation strategy. More randomized trials are needed, with a particular focus on which classes of antidepressants might be most effective as concurrent therapy,” Dr. Sartorius concluded.
He reported having no financial conflicts regarding his presentation.
SOURCE: Sartorius A. ECNP 2020, Session TP.03.
The two top developments of the year in the field of neurostimulation involve the oldest form of the therapy: electroconvulsive therapy, Alexander Sartorius, MD, said at the virtual congress of the European College of Neuropsychopharmacology.
One of these studies shot down the longstanding notion that ECT causes brain damage. The other, a Danish national registry study, demonstrated that ECT is associated with lower all-cause mortality than in patients with similarly severe depression who don’t undergo ECT.
“The take-home messages are that ECT does not lead to brain damage but rather to a profound gray matter increase. And secondly, ECT lowers all-cause mortality in patients with depression,” said Dr. Sartorius, a psychiatrist at the Central Institute of Mental Health in Mannheim, Germany.
The year has been less fruitful in terms of research involving deep brain stimulation using implantable electrodes, he continued.
“Basically, one can state that there is no breaking news in the field of deep brain stimulation. DBS remains a highly experimental form of therapy,” Dr. Sartorius said.
ECT-induced brain changes
Investigators participating in the international multicenter Global ECT-MRI Research Collaboration (GEMRIC) reported on the longitudinal effects of ECT on gray matter, white matter, and ventricular volumes in 328 patients who underwent imaging before and after ECT for a major depressive episode, as well as in 95 nondepressed controls.
The key finding was that ECT induced a widespread, nonspecific global increase in gray matter volume. Indeed, the volumetric increase was documented in 79 of 84 gray matter regions of interest. Subcortical gray matter volume increased by a mean of 1.47% in ECT-treated patients, and total cortical volume rose by 1.04%. Total white matter volume remained unchanged.
“The gray matter increase induced by ECT looks quite similar to the gray matter increase seen with physical activity,” Dr. Sartorius noted.
The size of the gray matter volume increase rose with the number of ECT sessions. However, gray matter enlargement in response to ECT showed no relationship with clinical response, indicating that this finding on brain imaging doesn’t have a promising future as a potential biomarker of treatment effectiveness (Biol Psychiatry. 2020 Mar 1;87[5]:451-61).
“The good news from this study is there is no gray matter decrease,” Dr. Sartorius said. “This study enhances the existing evidence falsifying the old idea or dogma that ECT induces brain damage. I would claim that the opposite is clearly the case.”
ECT and mortality
The same group of investigators at the University of Copenhagen who several years ago harnessed Danish national patient registries data to report that ECT was associated with an unadjusted 32% and adjusted 23% reduction in the risk of developing dementia in patients aged 70 years and older (Lancet Psychiatry. 2018 Apr;5[4]:348-56) have recently concluded that ECT was also associated with a 19% reduction in all-cause mortality, compared with that of patients hospitalized for major depression who didn’t receive ECT.
The national registries study included 5,004 patients who were treated with ECT and nearly 88,000 others who were hospitalized for major depression during 2005-2016 but didn’t receive ECT. During up to 11.3 years of follow-up, the risk of all-cause mortality was 8% lower with ECT than with no ECT in patients categorized as having mild depression, 17% lower with a history of ECT for moderate depression, 4% less with severe depression without psychotic features, and 30% less with ECT than no ECT for severe depression with psychotic features (J Psychopharmacol. 2020 Mar;34[3]:273-9).
ECT was associated with an adjusted 6.99-fold increased risk of suicide in patients with mild depression. At first look that’s unsettling, Dr. Sartorius said, but he pointed out that the size of the ECT-associated suicide risk lessened with increasing severity of depression, and in fact, there was no increased suicide risk with ECT in severely depressed patients with psychotic features. ECT was also associated with significantly higher rates of psychiatric rehospitalization, emergency department visits, and suicidal behavior in patients classified as having mild or moderate depression. Dr. Sartorius suspects these findings reflect diagnostic uncertainty surrounding the milder forms of depression, coupled with the reality that ECT is generally reserved for the most unstable, treatment-resistant patients.
Deep transcranial magnetic stimulation
A Taiwanese meta-analysis has helped clarify the role of deep transcranial magnetic stimulation (dTMS) for treatment-resistant depression. The meta-analysis included 198 patients with treatment-resistant depression who underwent dTMS and 219 who received sham treatment in a total of 15 studies, only three of which were randomized controlled trials.
Active treatment was associated with a 2.06-fold increased likelihood of remission of depressive symptoms; however, the effect was statistically significant only in the subgroup of patients on concurrent antidepressant medication. Moreover, the therapeutic benefit of dTMS was significantly greater in the nonrandomized studies, where the odds ratio for remission was 3.8-fold greater than with sham therapy. In contrast, the odds ratio for remission with dTMS dropped to 1.37 in the randomized trials (Prog Neuropsychopharmacol Biol Psychiatry. 2020 Apr 20;99:109850. doi: 10.1016/j.pnpbp.2019.109850). “dTMS has quite a bit of antidepressant potential in treatment-resistant depression, but as an augmentation strategy. More randomized trials are needed, with a particular focus on which classes of antidepressants might be most effective as concurrent therapy,” Dr. Sartorius concluded.
He reported having no financial conflicts regarding his presentation.
SOURCE: Sartorius A. ECNP 2020, Session TP.03.
The two top developments of the year in the field of neurostimulation involve the oldest form of the therapy: electroconvulsive therapy, Alexander Sartorius, MD, said at the virtual congress of the European College of Neuropsychopharmacology.
One of these studies shot down the longstanding notion that ECT causes brain damage. The other, a Danish national registry study, demonstrated that ECT is associated with lower all-cause mortality than in patients with similarly severe depression who don’t undergo ECT.
“The take-home messages are that ECT does not lead to brain damage but rather to a profound gray matter increase. And secondly, ECT lowers all-cause mortality in patients with depression,” said Dr. Sartorius, a psychiatrist at the Central Institute of Mental Health in Mannheim, Germany.
The year has been less fruitful in terms of research involving deep brain stimulation using implantable electrodes, he continued.
“Basically, one can state that there is no breaking news in the field of deep brain stimulation. DBS remains a highly experimental form of therapy,” Dr. Sartorius said.
ECT-induced brain changes
Investigators participating in the international multicenter Global ECT-MRI Research Collaboration (GEMRIC) reported on the longitudinal effects of ECT on gray matter, white matter, and ventricular volumes in 328 patients who underwent imaging before and after ECT for a major depressive episode, as well as in 95 nondepressed controls.
The key finding was that ECT induced a widespread, nonspecific global increase in gray matter volume. Indeed, the volumetric increase was documented in 79 of 84 gray matter regions of interest. Subcortical gray matter volume increased by a mean of 1.47% in ECT-treated patients, and total cortical volume rose by 1.04%. Total white matter volume remained unchanged.
“The gray matter increase induced by ECT looks quite similar to the gray matter increase seen with physical activity,” Dr. Sartorius noted.
The size of the gray matter volume increase rose with the number of ECT sessions. However, gray matter enlargement in response to ECT showed no relationship with clinical response, indicating that this finding on brain imaging doesn’t have a promising future as a potential biomarker of treatment effectiveness (Biol Psychiatry. 2020 Mar 1;87[5]:451-61).
“The good news from this study is there is no gray matter decrease,” Dr. Sartorius said. “This study enhances the existing evidence falsifying the old idea or dogma that ECT induces brain damage. I would claim that the opposite is clearly the case.”
ECT and mortality
The same group of investigators at the University of Copenhagen who several years ago harnessed Danish national patient registries data to report that ECT was associated with an unadjusted 32% and adjusted 23% reduction in the risk of developing dementia in patients aged 70 years and older (Lancet Psychiatry. 2018 Apr;5[4]:348-56) have recently concluded that ECT was also associated with a 19% reduction in all-cause mortality, compared with that of patients hospitalized for major depression who didn’t receive ECT.
The national registries study included 5,004 patients who were treated with ECT and nearly 88,000 others who were hospitalized for major depression during 2005-2016 but didn’t receive ECT. During up to 11.3 years of follow-up, the risk of all-cause mortality was 8% lower with ECT than with no ECT in patients categorized as having mild depression, 17% lower with a history of ECT for moderate depression, 4% less with severe depression without psychotic features, and 30% less with ECT than no ECT for severe depression with psychotic features (J Psychopharmacol. 2020 Mar;34[3]:273-9).
ECT was associated with an adjusted 6.99-fold increased risk of suicide in patients with mild depression. At first look that’s unsettling, Dr. Sartorius said, but he pointed out that the size of the ECT-associated suicide risk lessened with increasing severity of depression, and in fact, there was no increased suicide risk with ECT in severely depressed patients with psychotic features. ECT was also associated with significantly higher rates of psychiatric rehospitalization, emergency department visits, and suicidal behavior in patients classified as having mild or moderate depression. Dr. Sartorius suspects these findings reflect diagnostic uncertainty surrounding the milder forms of depression, coupled with the reality that ECT is generally reserved for the most unstable, treatment-resistant patients.
Deep transcranial magnetic stimulation
A Taiwanese meta-analysis has helped clarify the role of deep transcranial magnetic stimulation (dTMS) for treatment-resistant depression. The meta-analysis included 198 patients with treatment-resistant depression who underwent dTMS and 219 who received sham treatment in a total of 15 studies, only three of which were randomized controlled trials.
Active treatment was associated with a 2.06-fold increased likelihood of remission of depressive symptoms; however, the effect was statistically significant only in the subgroup of patients on concurrent antidepressant medication. Moreover, the therapeutic benefit of dTMS was significantly greater in the nonrandomized studies, where the odds ratio for remission was 3.8-fold greater than with sham therapy. In contrast, the odds ratio for remission with dTMS dropped to 1.37 in the randomized trials (Prog Neuropsychopharmacol Biol Psychiatry. 2020 Apr 20;99:109850. doi: 10.1016/j.pnpbp.2019.109850). “dTMS has quite a bit of antidepressant potential in treatment-resistant depression, but as an augmentation strategy. More randomized trials are needed, with a particular focus on which classes of antidepressants might be most effective as concurrent therapy,” Dr. Sartorius concluded.
He reported having no financial conflicts regarding his presentation.
SOURCE: Sartorius A. ECNP 2020, Session TP.03.
FROM ECNP 2020