User login
RA expert updates latest pathologic findings from Accelerating Medicines Partnership
Macrophages are among the most important inflammatory cells in the synovium of patients with rheumatoid arthritis, according to research discussed at the Canadian Arthritis Research Conference: Research with Impact.
Work conducted as part of the Accelerating Medicines Partnership (AMP) Rheumatoid Arthritis and Systemic Lupus Erythematosus (RA/SLE) Network suggests that not only do macrophages play an inflammatory role, but there may also be a subset of macrophages that have a predominantly anti-inflammatory effect.
“These are cells that are really activated and can produce a lot of proinflammatory cytokines, including TNF [tumor necrosis factor],” said Jennifer Howitt Anolik, MD, PhD, associate professor of medicine at the University of Rochester (N.Y.) and cochair of the AMP RA/SLE Network.
“In addition to inflammatory mediators there’s an anti-inflammatory population which may control the disease,” she added, at the virtual meeting, which was sponsored by The Arthritis Society, the Canadian Rheumatology Association, and Canada’s Institute of Health and Arthritis.
There are up to 15 different populations of macrophages found so far as part of a project by Fan Zhang of Brigham and Women’s Hospital and Harvard Medical School in Boston, Dr. Anolik revealed. Of these, three have been shown to be proinflammatory and five have been shown to be anti-inflammatory – including one of particular interest that expresses MERTK, which recent work suggests are lacking in people with RA, compared with a control population of people with osteoarthritis (OA).
Clearly, Dr. Anolik said, there is “lots more work to do to understand how those anti-inflammatory monocytes might work, understand the relationship to treatment response and treatment failure, and how to target them.”
AMP RA/SLE Network: Examining RA synovial tissue
What’s unique about the AMP’s work is that it is involving single-cell analytics in which individual cells derived from patients with RA are subjected to an array of RNA sequencing and molecular classification methods.
“If we’re able to define the cells that are driving the disease at the tissue level, this may lead to better therapeutics and more of like a precision medicine approach,” Dr. Anolik said. An important feature of the AMP’s work is that it is based on the use of existing and thus “very informative cohorts” for whom we know a lot about disease characteristics, she said.
The AMP RA/SLE Network officially formed in 2014 and is a public–private partnership between the National Institutes of Health, the Food and Drug Administration, several biopharmaceutical companies, and nonprofit organizations. The task was to try to accelerate discoveries that would lead to better patient care.
“The initial phase [Research Phase 0], was really about developing the procedures in a standardized way,” Dr. Anolik said. “Because we’re looking at patient joint tissue samples, we needed to access that tissue and that required developing needle biopsy approaches.” Synovial biopsy had been pioneered in the United Kingdom and become fairly standard to perform, she added, but this was not an approach that was routinely being used in the United States at the time.
In the next step, Research Phase I, researchers looked at the expression profiles of RA synovial cells in a small group of patients. In all, around 5,000 cells from the joints of up to 20 patients with RA were analyzed. What was apparent was that while there were fibroblasts, monocytes, T cells, and B cells all present to some degree, there was substantial heterogeneity among those subtypes.
“Within all the different immune cells and stromal cells, we found 18 different populations overall,” Dr. Anolik said, giving some of the top-level findings. Both single-cell RNA sequencing and mass cytometry revealed that there were greatly (16-fold) increased numbers of a population of sublining fibroblasts and a 3.3-fold increase in interleukin-1-beta-expressing proinflammatory monocytes. There was a threefold increase in a subset of B cells expressing CD11/T-bet, and a 2.4-fold increase in certain peripheral T cells.
“Interestingly, we were able to pinpoint which cells are making which kinds of inflammatory mediators like inflammatory cytokines,” Dr. Anolik said. Notably, one of the fibroblast populations and one of the B cells were prominent producers of interleukin-6.
The AMP RA/SLE Network is now in Research Phase II, looking at much greater numbers of cells (>5,000) in more than 100 samples from individual patients. It’s a “very comprehensive, big data look at RA,” according to Dr. Anolik.
Research Phase II will also see more rigorous groups of patients being examined, including those who have not had any or much exposure to disease-modifying antirheumatic drugs and those who have inadequately responded to methotrexate or anti-TNF drugs.
Recent AMP RA/SLE Network findings
Recent work by the AMP RA/SLE Network has shown that stromal fibroblasts can become highly inflammatory in RA.
“What’s becoming clear is that these are more than just lining of the joint or structure of the joint, they actually play an active role in the disease,” Dr. Anolik said.
There is a lot of diversity in these fibroblasts but they broadly fall into lining or sublining subtypes. Those that are proinflammatory tend to express markers such as HLA-DR and CD90, and one that is of notable interest is a subgroup of sublining fibroblasts that express Notch3. Indeed, it has been shown that the higher the number of Notch3-expressing fibroblasts there are in the joint, the greater the level of inflammation. Also, mice lacking Notch3 seem to get less arthritis than those with Notch3. This makes Notch3 an interesting potential target that no one had thought of before.
Dr. Anolik noted that some evolving concepts about T cells include evidence showing CD8-postive T cells are more abundant in the joint tissue than previously thought and, together with natural killer (NK) cells, are an important producer of interferon-gamma.
“There are some very interesting CD4 T-cell populations, including an expansion of T peripheral helper cells that may be very important in driving B-cell activation,” Dr. Anolik said. There are also many other clusters of T cells and NK cells that have unknown roles.
Over the past years, Dr. Anolik’s research had focused on the role B cells play in autoimmune disease, and one of the cells of interest are known as age-related B cells, or ABCs. High percentages of ABCs have been found in the RA synovium, and these seem to be related to disease activity as measured by the Disease Activity Score in 28 joints (DAS28). These cells also seem to cluster with some of the T helper cell populations found in the joint. Another interesting target could be B cells expressing a transcription factor known as T-bet. Work in mice suggests that the absence of T-bet B cells could be associated with reduced levels of arthritis.
“One of the things that we’re really interested in about B cells, in addition to their production of autoantibodies, is that they may be important for some of the structural damage that occurs with rheumatoid arthritis,” she said.
T-bet B cells seem to have an effect on both osteoclasts and osteoblasts – activating one while inhibiting the other to have a negative effect on bone overall, she explained. However, knocking out T-bet seems to resolve this, again suggesting that T-bet B cells may be another interesting subpopulation to target.
“Overall, the AMP has been a really interesting approach. This is a massive data set. We are putting the data together now to publish, and it will be available in the public domain,” Dr. Anolik said.
Members of the AMP RA/SLE Network include: AbbVie, the Arthritis Foundation, Bristol‐Myers Squibb, the Foundation for the NIH, the Lupus Foundation of America, the Lupus Research Alliance, Merck Sharp & Dohme, the National Institute of Allergy and Infectious Diseases, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Pfizer, the Rheumatology Research Foundation, Sanofi, and Takeda Pharmaceuticals International.
Dr. Anolik had no disclosures.
Macrophages are among the most important inflammatory cells in the synovium of patients with rheumatoid arthritis, according to research discussed at the Canadian Arthritis Research Conference: Research with Impact.
Work conducted as part of the Accelerating Medicines Partnership (AMP) Rheumatoid Arthritis and Systemic Lupus Erythematosus (RA/SLE) Network suggests that not only do macrophages play an inflammatory role, but there may also be a subset of macrophages that have a predominantly anti-inflammatory effect.
“These are cells that are really activated and can produce a lot of proinflammatory cytokines, including TNF [tumor necrosis factor],” said Jennifer Howitt Anolik, MD, PhD, associate professor of medicine at the University of Rochester (N.Y.) and cochair of the AMP RA/SLE Network.
“In addition to inflammatory mediators there’s an anti-inflammatory population which may control the disease,” she added, at the virtual meeting, which was sponsored by The Arthritis Society, the Canadian Rheumatology Association, and Canada’s Institute of Health and Arthritis.
There are up to 15 different populations of macrophages found so far as part of a project by Fan Zhang of Brigham and Women’s Hospital and Harvard Medical School in Boston, Dr. Anolik revealed. Of these, three have been shown to be proinflammatory and five have been shown to be anti-inflammatory – including one of particular interest that expresses MERTK, which recent work suggests are lacking in people with RA, compared with a control population of people with osteoarthritis (OA).
Clearly, Dr. Anolik said, there is “lots more work to do to understand how those anti-inflammatory monocytes might work, understand the relationship to treatment response and treatment failure, and how to target them.”
AMP RA/SLE Network: Examining RA synovial tissue
What’s unique about the AMP’s work is that it is involving single-cell analytics in which individual cells derived from patients with RA are subjected to an array of RNA sequencing and molecular classification methods.
“If we’re able to define the cells that are driving the disease at the tissue level, this may lead to better therapeutics and more of like a precision medicine approach,” Dr. Anolik said. An important feature of the AMP’s work is that it is based on the use of existing and thus “very informative cohorts” for whom we know a lot about disease characteristics, she said.
The AMP RA/SLE Network officially formed in 2014 and is a public–private partnership between the National Institutes of Health, the Food and Drug Administration, several biopharmaceutical companies, and nonprofit organizations. The task was to try to accelerate discoveries that would lead to better patient care.
“The initial phase [Research Phase 0], was really about developing the procedures in a standardized way,” Dr. Anolik said. “Because we’re looking at patient joint tissue samples, we needed to access that tissue and that required developing needle biopsy approaches.” Synovial biopsy had been pioneered in the United Kingdom and become fairly standard to perform, she added, but this was not an approach that was routinely being used in the United States at the time.
In the next step, Research Phase I, researchers looked at the expression profiles of RA synovial cells in a small group of patients. In all, around 5,000 cells from the joints of up to 20 patients with RA were analyzed. What was apparent was that while there were fibroblasts, monocytes, T cells, and B cells all present to some degree, there was substantial heterogeneity among those subtypes.
“Within all the different immune cells and stromal cells, we found 18 different populations overall,” Dr. Anolik said, giving some of the top-level findings. Both single-cell RNA sequencing and mass cytometry revealed that there were greatly (16-fold) increased numbers of a population of sublining fibroblasts and a 3.3-fold increase in interleukin-1-beta-expressing proinflammatory monocytes. There was a threefold increase in a subset of B cells expressing CD11/T-bet, and a 2.4-fold increase in certain peripheral T cells.
“Interestingly, we were able to pinpoint which cells are making which kinds of inflammatory mediators like inflammatory cytokines,” Dr. Anolik said. Notably, one of the fibroblast populations and one of the B cells were prominent producers of interleukin-6.
The AMP RA/SLE Network is now in Research Phase II, looking at much greater numbers of cells (>5,000) in more than 100 samples from individual patients. It’s a “very comprehensive, big data look at RA,” according to Dr. Anolik.
Research Phase II will also see more rigorous groups of patients being examined, including those who have not had any or much exposure to disease-modifying antirheumatic drugs and those who have inadequately responded to methotrexate or anti-TNF drugs.
Recent AMP RA/SLE Network findings
Recent work by the AMP RA/SLE Network has shown that stromal fibroblasts can become highly inflammatory in RA.
“What’s becoming clear is that these are more than just lining of the joint or structure of the joint, they actually play an active role in the disease,” Dr. Anolik said.
There is a lot of diversity in these fibroblasts but they broadly fall into lining or sublining subtypes. Those that are proinflammatory tend to express markers such as HLA-DR and CD90, and one that is of notable interest is a subgroup of sublining fibroblasts that express Notch3. Indeed, it has been shown that the higher the number of Notch3-expressing fibroblasts there are in the joint, the greater the level of inflammation. Also, mice lacking Notch3 seem to get less arthritis than those with Notch3. This makes Notch3 an interesting potential target that no one had thought of before.
Dr. Anolik noted that some evolving concepts about T cells include evidence showing CD8-postive T cells are more abundant in the joint tissue than previously thought and, together with natural killer (NK) cells, are an important producer of interferon-gamma.
“There are some very interesting CD4 T-cell populations, including an expansion of T peripheral helper cells that may be very important in driving B-cell activation,” Dr. Anolik said. There are also many other clusters of T cells and NK cells that have unknown roles.
Over the past years, Dr. Anolik’s research had focused on the role B cells play in autoimmune disease, and one of the cells of interest are known as age-related B cells, or ABCs. High percentages of ABCs have been found in the RA synovium, and these seem to be related to disease activity as measured by the Disease Activity Score in 28 joints (DAS28). These cells also seem to cluster with some of the T helper cell populations found in the joint. Another interesting target could be B cells expressing a transcription factor known as T-bet. Work in mice suggests that the absence of T-bet B cells could be associated with reduced levels of arthritis.
“One of the things that we’re really interested in about B cells, in addition to their production of autoantibodies, is that they may be important for some of the structural damage that occurs with rheumatoid arthritis,” she said.
T-bet B cells seem to have an effect on both osteoclasts and osteoblasts – activating one while inhibiting the other to have a negative effect on bone overall, she explained. However, knocking out T-bet seems to resolve this, again suggesting that T-bet B cells may be another interesting subpopulation to target.
“Overall, the AMP has been a really interesting approach. This is a massive data set. We are putting the data together now to publish, and it will be available in the public domain,” Dr. Anolik said.
Members of the AMP RA/SLE Network include: AbbVie, the Arthritis Foundation, Bristol‐Myers Squibb, the Foundation for the NIH, the Lupus Foundation of America, the Lupus Research Alliance, Merck Sharp & Dohme, the National Institute of Allergy and Infectious Diseases, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Pfizer, the Rheumatology Research Foundation, Sanofi, and Takeda Pharmaceuticals International.
Dr. Anolik had no disclosures.
Macrophages are among the most important inflammatory cells in the synovium of patients with rheumatoid arthritis, according to research discussed at the Canadian Arthritis Research Conference: Research with Impact.
Work conducted as part of the Accelerating Medicines Partnership (AMP) Rheumatoid Arthritis and Systemic Lupus Erythematosus (RA/SLE) Network suggests that not only do macrophages play an inflammatory role, but there may also be a subset of macrophages that have a predominantly anti-inflammatory effect.
“These are cells that are really activated and can produce a lot of proinflammatory cytokines, including TNF [tumor necrosis factor],” said Jennifer Howitt Anolik, MD, PhD, associate professor of medicine at the University of Rochester (N.Y.) and cochair of the AMP RA/SLE Network.
“In addition to inflammatory mediators there’s an anti-inflammatory population which may control the disease,” she added, at the virtual meeting, which was sponsored by The Arthritis Society, the Canadian Rheumatology Association, and Canada’s Institute of Health and Arthritis.
There are up to 15 different populations of macrophages found so far as part of a project by Fan Zhang of Brigham and Women’s Hospital and Harvard Medical School in Boston, Dr. Anolik revealed. Of these, three have been shown to be proinflammatory and five have been shown to be anti-inflammatory – including one of particular interest that expresses MERTK, which recent work suggests are lacking in people with RA, compared with a control population of people with osteoarthritis (OA).
Clearly, Dr. Anolik said, there is “lots more work to do to understand how those anti-inflammatory monocytes might work, understand the relationship to treatment response and treatment failure, and how to target them.”
AMP RA/SLE Network: Examining RA synovial tissue
What’s unique about the AMP’s work is that it is involving single-cell analytics in which individual cells derived from patients with RA are subjected to an array of RNA sequencing and molecular classification methods.
“If we’re able to define the cells that are driving the disease at the tissue level, this may lead to better therapeutics and more of like a precision medicine approach,” Dr. Anolik said. An important feature of the AMP’s work is that it is based on the use of existing and thus “very informative cohorts” for whom we know a lot about disease characteristics, she said.
The AMP RA/SLE Network officially formed in 2014 and is a public–private partnership between the National Institutes of Health, the Food and Drug Administration, several biopharmaceutical companies, and nonprofit organizations. The task was to try to accelerate discoveries that would lead to better patient care.
“The initial phase [Research Phase 0], was really about developing the procedures in a standardized way,” Dr. Anolik said. “Because we’re looking at patient joint tissue samples, we needed to access that tissue and that required developing needle biopsy approaches.” Synovial biopsy had been pioneered in the United Kingdom and become fairly standard to perform, she added, but this was not an approach that was routinely being used in the United States at the time.
In the next step, Research Phase I, researchers looked at the expression profiles of RA synovial cells in a small group of patients. In all, around 5,000 cells from the joints of up to 20 patients with RA were analyzed. What was apparent was that while there were fibroblasts, monocytes, T cells, and B cells all present to some degree, there was substantial heterogeneity among those subtypes.
“Within all the different immune cells and stromal cells, we found 18 different populations overall,” Dr. Anolik said, giving some of the top-level findings. Both single-cell RNA sequencing and mass cytometry revealed that there were greatly (16-fold) increased numbers of a population of sublining fibroblasts and a 3.3-fold increase in interleukin-1-beta-expressing proinflammatory monocytes. There was a threefold increase in a subset of B cells expressing CD11/T-bet, and a 2.4-fold increase in certain peripheral T cells.
“Interestingly, we were able to pinpoint which cells are making which kinds of inflammatory mediators like inflammatory cytokines,” Dr. Anolik said. Notably, one of the fibroblast populations and one of the B cells were prominent producers of interleukin-6.
The AMP RA/SLE Network is now in Research Phase II, looking at much greater numbers of cells (>5,000) in more than 100 samples from individual patients. It’s a “very comprehensive, big data look at RA,” according to Dr. Anolik.
Research Phase II will also see more rigorous groups of patients being examined, including those who have not had any or much exposure to disease-modifying antirheumatic drugs and those who have inadequately responded to methotrexate or anti-TNF drugs.
Recent AMP RA/SLE Network findings
Recent work by the AMP RA/SLE Network has shown that stromal fibroblasts can become highly inflammatory in RA.
“What’s becoming clear is that these are more than just lining of the joint or structure of the joint, they actually play an active role in the disease,” Dr. Anolik said.
There is a lot of diversity in these fibroblasts but they broadly fall into lining or sublining subtypes. Those that are proinflammatory tend to express markers such as HLA-DR and CD90, and one that is of notable interest is a subgroup of sublining fibroblasts that express Notch3. Indeed, it has been shown that the higher the number of Notch3-expressing fibroblasts there are in the joint, the greater the level of inflammation. Also, mice lacking Notch3 seem to get less arthritis than those with Notch3. This makes Notch3 an interesting potential target that no one had thought of before.
Dr. Anolik noted that some evolving concepts about T cells include evidence showing CD8-postive T cells are more abundant in the joint tissue than previously thought and, together with natural killer (NK) cells, are an important producer of interferon-gamma.
“There are some very interesting CD4 T-cell populations, including an expansion of T peripheral helper cells that may be very important in driving B-cell activation,” Dr. Anolik said. There are also many other clusters of T cells and NK cells that have unknown roles.
Over the past years, Dr. Anolik’s research had focused on the role B cells play in autoimmune disease, and one of the cells of interest are known as age-related B cells, or ABCs. High percentages of ABCs have been found in the RA synovium, and these seem to be related to disease activity as measured by the Disease Activity Score in 28 joints (DAS28). These cells also seem to cluster with some of the T helper cell populations found in the joint. Another interesting target could be B cells expressing a transcription factor known as T-bet. Work in mice suggests that the absence of T-bet B cells could be associated with reduced levels of arthritis.
“One of the things that we’re really interested in about B cells, in addition to their production of autoantibodies, is that they may be important for some of the structural damage that occurs with rheumatoid arthritis,” she said.
T-bet B cells seem to have an effect on both osteoclasts and osteoblasts – activating one while inhibiting the other to have a negative effect on bone overall, she explained. However, knocking out T-bet seems to resolve this, again suggesting that T-bet B cells may be another interesting subpopulation to target.
“Overall, the AMP has been a really interesting approach. This is a massive data set. We are putting the data together now to publish, and it will be available in the public domain,” Dr. Anolik said.
Members of the AMP RA/SLE Network include: AbbVie, the Arthritis Foundation, Bristol‐Myers Squibb, the Foundation for the NIH, the Lupus Foundation of America, the Lupus Research Alliance, Merck Sharp & Dohme, the National Institute of Allergy and Infectious Diseases, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Pfizer, the Rheumatology Research Foundation, Sanofi, and Takeda Pharmaceuticals International.
Dr. Anolik had no disclosures.
FROM CARC 2021
OA risk-reduction program targets injured knees
A novel educational and personalized physical therapy program is showing signs that it may help people to mitigate their risk of developing knee osteoarthritis after an injury.
Speaking at the Canadian Arthritis Research Conference: Research with Impact, Jackie Whittaker, PhD, observed that initial work from the Stop Osteoarthritis (SOAR) program showed that meaningful improvements in knee-related quality of life and improvement in participants’ perceived self-management could be achieved.
Further feasibility work is ongoing and a proof-of-concept and phase 3 study need to follow, but the research suggests the approach could potentially help to reduce the substantial burden of managing people who develop posttraumatic OA (PTOA) of the knee.
Understanding the post–knee injury period
“Despite the progress that we’ve made in preventing injuries, and reducing disability in people with osteoarthritis, we lack good evidence about what should be done in the period between joint injury and the onset of osteoarthritis to delay or halt that onset,” Dr. Whittaker said at the virtual meeting, which was sponsored by the Arthritis Society, the Canadian Rheumatology Association, and Canada’s Institute of Musculoskeletal Health and Arthritis.
That’s where the SOAR program comes in. For the past 8 years, Dr. Whittaker, an assistant professor in the department of physical therapy at the University of British Columbia in Vancouver and affiliated to Arthritis Research Canada, and collaborators have been looking into the post–knee injury period with the aim of developing an intervention that could potentially reduce the risk of OA further down the line.
Much work has gone into understanding the burden and risk factors for PTOA of the knee in order to know who exactly to target with the intervention and what the risk factors may be for the subsequent development of OA .
This research suggests that knee injuries are most commonly seen in people aged between 15 and 35 years who participated in sporting or other physical activities, so this is the target population for the SOAR intervention.
Broadly speaking, sustaining any knee injury is associated with a sixfold increased risk for subsequent PTOA, Dr. Whittaker observed.
“Despite the fact that ACL [anterior cruciate ligament] and meniscal tears get all the press, collateral ligament injury are still associated with about a fivefold increased risk of osteoarthritis, and therefore maybe shouldn’t be so easily dismissed as an important target,” Dr. Whittaker said.
Postinjury risk factors for OA
“Basically, what all prevention comes down to is our understanding of risk factors and our ability to be able to modify them,” she said.
Previous joint injury is one of the strongest and most established modifiable risk factors for developing knee OA, and Dr. Whittaker and associates have performed two small but “mighty” cohort studies comparing people who have and have not had a knee injury. These two studies have looked at different time periods following injury to see if they could first identify the risk factors for developing OA some 3-10 years later, and then to look more closely at some of those risk factors in first 2 years after injury with a view to targeting these with an intervention.
Data analysis of the latter study is still ongoing but have shown that, among injured subjects, there is a fear of movement and reinjury, knee strength is weaker in both injured and uninjured knees, and they are perhaps less physically active than those who have not been injured.
“Going into those two studies, we knew that this group of people already [had an] increased risk for osteoarthritis because they had an injury. However, what we found is that it looks like this risk may be compounded through adiposity [and] deficits in muscle strength and physical inactivity, which are associated with pain, stiffness, lack of confidence, and at times, unrealistic expectations and poor pacing,” Dr. Whittaker said.
She added: “It also looks like some of these additional factors and particular adiposity or fat gain may develop after injury, which would then give us a concrete target for delaying or halting the onset of osteoarthritis in the segment of the population.”
SOAR program components
The SOAR program intervention is an 8-week, physiotherapist-led program that targets people aged 15-35 years who have had a sport-related knee injury and received formal care. All of this is conducted via videoconferencing software and starts off with a 2-hour group education session or “knee camp.” This is followed by a one-on-one assessment with a physiotherapist and setting exercise and physical activity goals for the week.
Participants then undertake their personalized exercise and physical activity programs at home and track their progress using an activity monitor. They can participate in an optional weekly group exercise class and receive weekly one-on-one physiotherapy counseling where goals can be modified and any issues participants might be experiencing solved.
According to Dr. Whittaker, “this program really aims to increase participants capacity to manage their elevated risk for osteoarthritis, and we’re doing this by also optimizing their knee muscle function and their physical activity participation.”
While the knee camp enables a therapeutic alliance to be formed between participants and their physiotherapists, the weekly group classes provide social support and an opportunity to interact with others.
“Brief action planning builds self-efficacy [and] promotes autonomous health behaviors, while goal setting and tracking provide accountability, feedback about progress, and facilitated adherence,” she said.
And finally, regular communication with a physiotherapist in the program ensures timely support to learn how to navigate obstacles and helps participants to learn how to deal with their own knee health.
Testing the feasibility of the SOAR program intervention
“Currently we are smack in the middle of our feasibility study,” Dr. Whittaker said. So far, four physiotherapists have been trained to deliver an abridged, 4-week version of the program, and 25 of a planned 30 participants have been enrolled.
Results seem promising so far. No participants have dropped out of the program to date and attendance is at 100%.
“Based on data from the first 12 participants who completed the program, we are meeting all of our ‘a priori’ program benchmarks,” Dr. Whittaker said.
“It is very early days,” she emphasized, but “we are excited to see clinically important improvements in both knee-related quality of life and perceived self-management.
“This gives us some confidence that maybe all this time that we’ve put into developing our intervention is paying off, but obviously time will tell if we’re headed in the right direction,” she said. “Perhaps in time, we may be able to look at whether or not the individuals that participated in that program have fewer symptoms of OA disease. But that will obviously take us a few years before we’ll be able to get to that point.”
Dr. Whittaker acknowledged receiving funding for the SOAR program from the Arthritis Society, the Michael Smith Foundation for Health Research, BC SUPPORT Unit, and the Canadian Musculoskeletal Rehab Network.
A novel educational and personalized physical therapy program is showing signs that it may help people to mitigate their risk of developing knee osteoarthritis after an injury.
Speaking at the Canadian Arthritis Research Conference: Research with Impact, Jackie Whittaker, PhD, observed that initial work from the Stop Osteoarthritis (SOAR) program showed that meaningful improvements in knee-related quality of life and improvement in participants’ perceived self-management could be achieved.
Further feasibility work is ongoing and a proof-of-concept and phase 3 study need to follow, but the research suggests the approach could potentially help to reduce the substantial burden of managing people who develop posttraumatic OA (PTOA) of the knee.
Understanding the post–knee injury period
“Despite the progress that we’ve made in preventing injuries, and reducing disability in people with osteoarthritis, we lack good evidence about what should be done in the period between joint injury and the onset of osteoarthritis to delay or halt that onset,” Dr. Whittaker said at the virtual meeting, which was sponsored by the Arthritis Society, the Canadian Rheumatology Association, and Canada’s Institute of Musculoskeletal Health and Arthritis.
That’s where the SOAR program comes in. For the past 8 years, Dr. Whittaker, an assistant professor in the department of physical therapy at the University of British Columbia in Vancouver and affiliated to Arthritis Research Canada, and collaborators have been looking into the post–knee injury period with the aim of developing an intervention that could potentially reduce the risk of OA further down the line.
Much work has gone into understanding the burden and risk factors for PTOA of the knee in order to know who exactly to target with the intervention and what the risk factors may be for the subsequent development of OA .
This research suggests that knee injuries are most commonly seen in people aged between 15 and 35 years who participated in sporting or other physical activities, so this is the target population for the SOAR intervention.
Broadly speaking, sustaining any knee injury is associated with a sixfold increased risk for subsequent PTOA, Dr. Whittaker observed.
“Despite the fact that ACL [anterior cruciate ligament] and meniscal tears get all the press, collateral ligament injury are still associated with about a fivefold increased risk of osteoarthritis, and therefore maybe shouldn’t be so easily dismissed as an important target,” Dr. Whittaker said.
Postinjury risk factors for OA
“Basically, what all prevention comes down to is our understanding of risk factors and our ability to be able to modify them,” she said.
Previous joint injury is one of the strongest and most established modifiable risk factors for developing knee OA, and Dr. Whittaker and associates have performed two small but “mighty” cohort studies comparing people who have and have not had a knee injury. These two studies have looked at different time periods following injury to see if they could first identify the risk factors for developing OA some 3-10 years later, and then to look more closely at some of those risk factors in first 2 years after injury with a view to targeting these with an intervention.
Data analysis of the latter study is still ongoing but have shown that, among injured subjects, there is a fear of movement and reinjury, knee strength is weaker in both injured and uninjured knees, and they are perhaps less physically active than those who have not been injured.
“Going into those two studies, we knew that this group of people already [had an] increased risk for osteoarthritis because they had an injury. However, what we found is that it looks like this risk may be compounded through adiposity [and] deficits in muscle strength and physical inactivity, which are associated with pain, stiffness, lack of confidence, and at times, unrealistic expectations and poor pacing,” Dr. Whittaker said.
She added: “It also looks like some of these additional factors and particular adiposity or fat gain may develop after injury, which would then give us a concrete target for delaying or halting the onset of osteoarthritis in the segment of the population.”
SOAR program components
The SOAR program intervention is an 8-week, physiotherapist-led program that targets people aged 15-35 years who have had a sport-related knee injury and received formal care. All of this is conducted via videoconferencing software and starts off with a 2-hour group education session or “knee camp.” This is followed by a one-on-one assessment with a physiotherapist and setting exercise and physical activity goals for the week.
Participants then undertake their personalized exercise and physical activity programs at home and track their progress using an activity monitor. They can participate in an optional weekly group exercise class and receive weekly one-on-one physiotherapy counseling where goals can be modified and any issues participants might be experiencing solved.
According to Dr. Whittaker, “this program really aims to increase participants capacity to manage their elevated risk for osteoarthritis, and we’re doing this by also optimizing their knee muscle function and their physical activity participation.”
While the knee camp enables a therapeutic alliance to be formed between participants and their physiotherapists, the weekly group classes provide social support and an opportunity to interact with others.
“Brief action planning builds self-efficacy [and] promotes autonomous health behaviors, while goal setting and tracking provide accountability, feedback about progress, and facilitated adherence,” she said.
And finally, regular communication with a physiotherapist in the program ensures timely support to learn how to navigate obstacles and helps participants to learn how to deal with their own knee health.
Testing the feasibility of the SOAR program intervention
“Currently we are smack in the middle of our feasibility study,” Dr. Whittaker said. So far, four physiotherapists have been trained to deliver an abridged, 4-week version of the program, and 25 of a planned 30 participants have been enrolled.
Results seem promising so far. No participants have dropped out of the program to date and attendance is at 100%.
“Based on data from the first 12 participants who completed the program, we are meeting all of our ‘a priori’ program benchmarks,” Dr. Whittaker said.
“It is very early days,” she emphasized, but “we are excited to see clinically important improvements in both knee-related quality of life and perceived self-management.
“This gives us some confidence that maybe all this time that we’ve put into developing our intervention is paying off, but obviously time will tell if we’re headed in the right direction,” she said. “Perhaps in time, we may be able to look at whether or not the individuals that participated in that program have fewer symptoms of OA disease. But that will obviously take us a few years before we’ll be able to get to that point.”
Dr. Whittaker acknowledged receiving funding for the SOAR program from the Arthritis Society, the Michael Smith Foundation for Health Research, BC SUPPORT Unit, and the Canadian Musculoskeletal Rehab Network.
A novel educational and personalized physical therapy program is showing signs that it may help people to mitigate their risk of developing knee osteoarthritis after an injury.
Speaking at the Canadian Arthritis Research Conference: Research with Impact, Jackie Whittaker, PhD, observed that initial work from the Stop Osteoarthritis (SOAR) program showed that meaningful improvements in knee-related quality of life and improvement in participants’ perceived self-management could be achieved.
Further feasibility work is ongoing and a proof-of-concept and phase 3 study need to follow, but the research suggests the approach could potentially help to reduce the substantial burden of managing people who develop posttraumatic OA (PTOA) of the knee.
Understanding the post–knee injury period
“Despite the progress that we’ve made in preventing injuries, and reducing disability in people with osteoarthritis, we lack good evidence about what should be done in the period between joint injury and the onset of osteoarthritis to delay or halt that onset,” Dr. Whittaker said at the virtual meeting, which was sponsored by the Arthritis Society, the Canadian Rheumatology Association, and Canada’s Institute of Musculoskeletal Health and Arthritis.
That’s where the SOAR program comes in. For the past 8 years, Dr. Whittaker, an assistant professor in the department of physical therapy at the University of British Columbia in Vancouver and affiliated to Arthritis Research Canada, and collaborators have been looking into the post–knee injury period with the aim of developing an intervention that could potentially reduce the risk of OA further down the line.
Much work has gone into understanding the burden and risk factors for PTOA of the knee in order to know who exactly to target with the intervention and what the risk factors may be for the subsequent development of OA .
This research suggests that knee injuries are most commonly seen in people aged between 15 and 35 years who participated in sporting or other physical activities, so this is the target population for the SOAR intervention.
Broadly speaking, sustaining any knee injury is associated with a sixfold increased risk for subsequent PTOA, Dr. Whittaker observed.
“Despite the fact that ACL [anterior cruciate ligament] and meniscal tears get all the press, collateral ligament injury are still associated with about a fivefold increased risk of osteoarthritis, and therefore maybe shouldn’t be so easily dismissed as an important target,” Dr. Whittaker said.
Postinjury risk factors for OA
“Basically, what all prevention comes down to is our understanding of risk factors and our ability to be able to modify them,” she said.
Previous joint injury is one of the strongest and most established modifiable risk factors for developing knee OA, and Dr. Whittaker and associates have performed two small but “mighty” cohort studies comparing people who have and have not had a knee injury. These two studies have looked at different time periods following injury to see if they could first identify the risk factors for developing OA some 3-10 years later, and then to look more closely at some of those risk factors in first 2 years after injury with a view to targeting these with an intervention.
Data analysis of the latter study is still ongoing but have shown that, among injured subjects, there is a fear of movement and reinjury, knee strength is weaker in both injured and uninjured knees, and they are perhaps less physically active than those who have not been injured.
“Going into those two studies, we knew that this group of people already [had an] increased risk for osteoarthritis because they had an injury. However, what we found is that it looks like this risk may be compounded through adiposity [and] deficits in muscle strength and physical inactivity, which are associated with pain, stiffness, lack of confidence, and at times, unrealistic expectations and poor pacing,” Dr. Whittaker said.
She added: “It also looks like some of these additional factors and particular adiposity or fat gain may develop after injury, which would then give us a concrete target for delaying or halting the onset of osteoarthritis in the segment of the population.”
SOAR program components
The SOAR program intervention is an 8-week, physiotherapist-led program that targets people aged 15-35 years who have had a sport-related knee injury and received formal care. All of this is conducted via videoconferencing software and starts off with a 2-hour group education session or “knee camp.” This is followed by a one-on-one assessment with a physiotherapist and setting exercise and physical activity goals for the week.
Participants then undertake their personalized exercise and physical activity programs at home and track their progress using an activity monitor. They can participate in an optional weekly group exercise class and receive weekly one-on-one physiotherapy counseling where goals can be modified and any issues participants might be experiencing solved.
According to Dr. Whittaker, “this program really aims to increase participants capacity to manage their elevated risk for osteoarthritis, and we’re doing this by also optimizing their knee muscle function and their physical activity participation.”
While the knee camp enables a therapeutic alliance to be formed between participants and their physiotherapists, the weekly group classes provide social support and an opportunity to interact with others.
“Brief action planning builds self-efficacy [and] promotes autonomous health behaviors, while goal setting and tracking provide accountability, feedback about progress, and facilitated adherence,” she said.
And finally, regular communication with a physiotherapist in the program ensures timely support to learn how to navigate obstacles and helps participants to learn how to deal with their own knee health.
Testing the feasibility of the SOAR program intervention
“Currently we are smack in the middle of our feasibility study,” Dr. Whittaker said. So far, four physiotherapists have been trained to deliver an abridged, 4-week version of the program, and 25 of a planned 30 participants have been enrolled.
Results seem promising so far. No participants have dropped out of the program to date and attendance is at 100%.
“Based on data from the first 12 participants who completed the program, we are meeting all of our ‘a priori’ program benchmarks,” Dr. Whittaker said.
“It is very early days,” she emphasized, but “we are excited to see clinically important improvements in both knee-related quality of life and perceived self-management.
“This gives us some confidence that maybe all this time that we’ve put into developing our intervention is paying off, but obviously time will tell if we’re headed in the right direction,” she said. “Perhaps in time, we may be able to look at whether or not the individuals that participated in that program have fewer symptoms of OA disease. But that will obviously take us a few years before we’ll be able to get to that point.”
Dr. Whittaker acknowledged receiving funding for the SOAR program from the Arthritis Society, the Michael Smith Foundation for Health Research, BC SUPPORT Unit, and the Canadian Musculoskeletal Rehab Network.
FROM CARC 2021
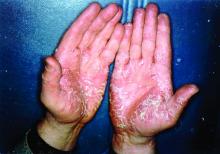
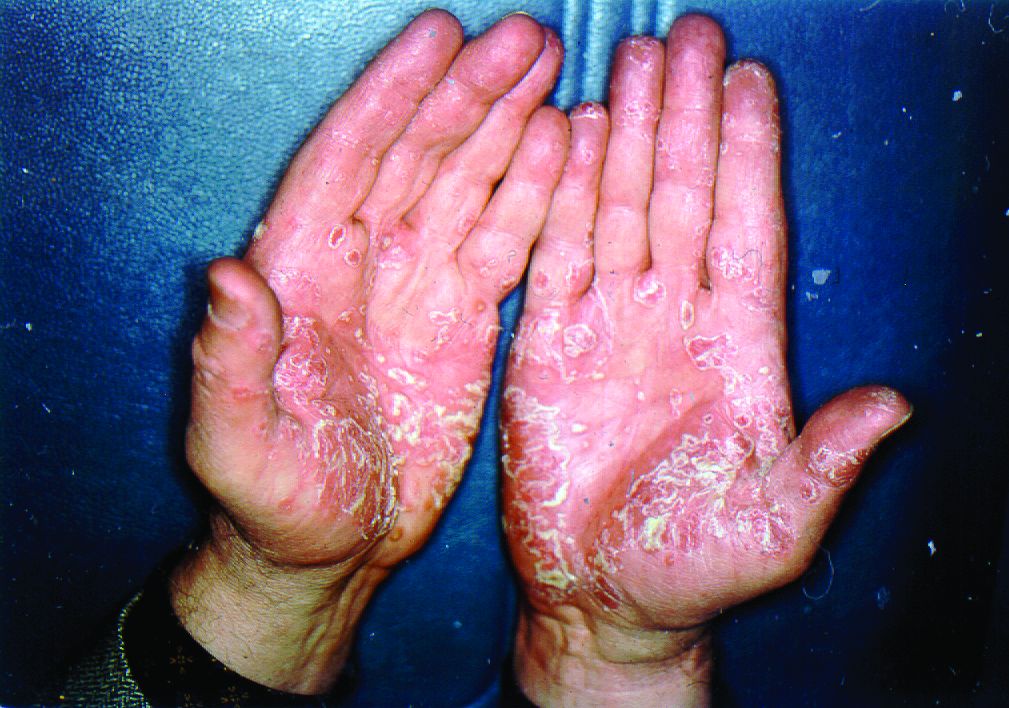
Pandemic puts patients with psoriatic disease off seeking medical help
More than half of respondents to a recent survey looking at how the COVID-19 pandemic has affected people with psoriasis or psoriatic arthritis (PsA) said that they had avoided seeking medical care in person with a doctor or at a hospital.
Moreover, around a quarter had their appointment with a rheumatologist canceled, rescheduled, or conducted virtually. Another 1 in 10 had their treatment plan disrupted, and 6% had to change or stop treatment entirely.
The mental health impact of living with these conditions during the pandemic was also notable, said Rachael Manion, the executive director of the Canadian Association of Psoriasis Patients (CAPP), which conducted the survey in collaboration with the Canadian Psoriasis Network (CPN) and Unmasking Psoriasis.
“It’s important to know that there have been a lot of different impacts of the pandemic on people living with psoriatic arthritis and psoriasis. Mental health in particular has had a really big hit as a result,” she said at the Canadian Arthritis Research Conference: Research with Impact.
“About half of the people who responded to our survey noted that their mental health was ‘worse’ or ‘much worse’ during the pandemic,” she said at the meeting, which was sponsored by the Arthritis Society, the Canadian Rheumatology Association, and Canada’s Institute of Musculoskeletal Health and Arthritis. Anxiety and feelings of isolation were reported by a respective 57% and 58% of respondents, and 40% reported depression.
“We can compare that to our earlier information around depression,” Ms. Manion said, which showed that, prior to the pandemic, 24% of people with psoriasis and 23% of those with PsA had said they experienced depression.
“What I found alarming looking at these results was that about a third of people were experiencing despair. Now that’s a really big, scary, overwhelming emotion that has a lot of burden on your mental health,” Ms. Manion said.
Despite the substantial effects on mental health, only 29% of respondents said they had been able to access mental health services during the pandemic.
To look at the impact of the COVID-19 pandemic on the psoriasis and PsA community in Canada, three patient advocacy groups – CAPP, CPN, and Unmasking Psoriasis – codeveloped a survey to look at the disease experience before and after the start of the COVID-19 pandemic. The survey was performed once, with 830 respondents providing information on their lives with psoriasis or PsA in the months before the start of the pandemic and at the time they were surveyed in September and October 2020.
Most of the survey respondents lived in Ontario, Quebec, British Columbia, or Alberta, although other provinces or territories were represented. Almost all respondents (96%) had psoriasis, and 60% also had PsA.
Pre-COVID, nearly half (49%) of patients said that they had not been seen by a rheumatologist, and 39% had not seen a dermatologist for treatment. Asked why, 56% and 27%, respectively, had not been referred, 9% and 15% said they had no specialist located nearby, and 7% and 10% stated that the wait list was too long.
“This tells us that there’s a lot more work that can be done and a lot more education of general practitioners and family medicine professionals about the benefits and the value of specialized care for psoriatic arthritis,” Ms. Manion suggested.
Before the pandemic, joint pain was occurring in 88% of patients, stiffness in 71%, and joint swelling in 67%. Disease flares or sudden periods of worsening occurred on a daily basis for 17%, and around one in five (21%) experienced multiple flares every month.
Prepandemic data also highlighted the negative impact that living with psoriasis or PsA has on people’s ability to sleep, interactions and intimacy with others, and on their school or work lives.
During the pandemic, around a quarter (26%) of respondents said they had worse or much worse access to employment, as well as its benefits such as a stable income (24%). A minority of respondent also described worse access to prescription medication (15%) and over-the-counter medication (13%).
“There are all kinds of things going on for patients in our community: changes to their work, changes to their drug coverage, their ability to sleep and sleep well, their mental health, and their ability to access care and treatments as part of their disease management,” Ms. Manion said.
Her final message to health care professionals was: “I just want to encourage you to continue to check in with your patients about what their experiences have been during the pandemic, and to really consider those impacts as you’re working with them to manage their disease.”
The survey received funding support from AbbVie, Bausch Health, Boehringer Ingelheim, Janssen, LEO Pharma, and Novartis.
More than half of respondents to a recent survey looking at how the COVID-19 pandemic has affected people with psoriasis or psoriatic arthritis (PsA) said that they had avoided seeking medical care in person with a doctor or at a hospital.
Moreover, around a quarter had their appointment with a rheumatologist canceled, rescheduled, or conducted virtually. Another 1 in 10 had their treatment plan disrupted, and 6% had to change or stop treatment entirely.
The mental health impact of living with these conditions during the pandemic was also notable, said Rachael Manion, the executive director of the Canadian Association of Psoriasis Patients (CAPP), which conducted the survey in collaboration with the Canadian Psoriasis Network (CPN) and Unmasking Psoriasis.
“It’s important to know that there have been a lot of different impacts of the pandemic on people living with psoriatic arthritis and psoriasis. Mental health in particular has had a really big hit as a result,” she said at the Canadian Arthritis Research Conference: Research with Impact.
“About half of the people who responded to our survey noted that their mental health was ‘worse’ or ‘much worse’ during the pandemic,” she said at the meeting, which was sponsored by the Arthritis Society, the Canadian Rheumatology Association, and Canada’s Institute of Musculoskeletal Health and Arthritis. Anxiety and feelings of isolation were reported by a respective 57% and 58% of respondents, and 40% reported depression.
“We can compare that to our earlier information around depression,” Ms. Manion said, which showed that, prior to the pandemic, 24% of people with psoriasis and 23% of those with PsA had said they experienced depression.
“What I found alarming looking at these results was that about a third of people were experiencing despair. Now that’s a really big, scary, overwhelming emotion that has a lot of burden on your mental health,” Ms. Manion said.
Despite the substantial effects on mental health, only 29% of respondents said they had been able to access mental health services during the pandemic.
To look at the impact of the COVID-19 pandemic on the psoriasis and PsA community in Canada, three patient advocacy groups – CAPP, CPN, and Unmasking Psoriasis – codeveloped a survey to look at the disease experience before and after the start of the COVID-19 pandemic. The survey was performed once, with 830 respondents providing information on their lives with psoriasis or PsA in the months before the start of the pandemic and at the time they were surveyed in September and October 2020.
Most of the survey respondents lived in Ontario, Quebec, British Columbia, or Alberta, although other provinces or territories were represented. Almost all respondents (96%) had psoriasis, and 60% also had PsA.
Pre-COVID, nearly half (49%) of patients said that they had not been seen by a rheumatologist, and 39% had not seen a dermatologist for treatment. Asked why, 56% and 27%, respectively, had not been referred, 9% and 15% said they had no specialist located nearby, and 7% and 10% stated that the wait list was too long.
“This tells us that there’s a lot more work that can be done and a lot more education of general practitioners and family medicine professionals about the benefits and the value of specialized care for psoriatic arthritis,” Ms. Manion suggested.
Before the pandemic, joint pain was occurring in 88% of patients, stiffness in 71%, and joint swelling in 67%. Disease flares or sudden periods of worsening occurred on a daily basis for 17%, and around one in five (21%) experienced multiple flares every month.
Prepandemic data also highlighted the negative impact that living with psoriasis or PsA has on people’s ability to sleep, interactions and intimacy with others, and on their school or work lives.
During the pandemic, around a quarter (26%) of respondents said they had worse or much worse access to employment, as well as its benefits such as a stable income (24%). A minority of respondent also described worse access to prescription medication (15%) and over-the-counter medication (13%).
“There are all kinds of things going on for patients in our community: changes to their work, changes to their drug coverage, their ability to sleep and sleep well, their mental health, and their ability to access care and treatments as part of their disease management,” Ms. Manion said.
Her final message to health care professionals was: “I just want to encourage you to continue to check in with your patients about what their experiences have been during the pandemic, and to really consider those impacts as you’re working with them to manage their disease.”
The survey received funding support from AbbVie, Bausch Health, Boehringer Ingelheim, Janssen, LEO Pharma, and Novartis.
More than half of respondents to a recent survey looking at how the COVID-19 pandemic has affected people with psoriasis or psoriatic arthritis (PsA) said that they had avoided seeking medical care in person with a doctor or at a hospital.
Moreover, around a quarter had their appointment with a rheumatologist canceled, rescheduled, or conducted virtually. Another 1 in 10 had their treatment plan disrupted, and 6% had to change or stop treatment entirely.
The mental health impact of living with these conditions during the pandemic was also notable, said Rachael Manion, the executive director of the Canadian Association of Psoriasis Patients (CAPP), which conducted the survey in collaboration with the Canadian Psoriasis Network (CPN) and Unmasking Psoriasis.
“It’s important to know that there have been a lot of different impacts of the pandemic on people living with psoriatic arthritis and psoriasis. Mental health in particular has had a really big hit as a result,” she said at the Canadian Arthritis Research Conference: Research with Impact.
“About half of the people who responded to our survey noted that their mental health was ‘worse’ or ‘much worse’ during the pandemic,” she said at the meeting, which was sponsored by the Arthritis Society, the Canadian Rheumatology Association, and Canada’s Institute of Musculoskeletal Health and Arthritis. Anxiety and feelings of isolation were reported by a respective 57% and 58% of respondents, and 40% reported depression.
“We can compare that to our earlier information around depression,” Ms. Manion said, which showed that, prior to the pandemic, 24% of people with psoriasis and 23% of those with PsA had said they experienced depression.
“What I found alarming looking at these results was that about a third of people were experiencing despair. Now that’s a really big, scary, overwhelming emotion that has a lot of burden on your mental health,” Ms. Manion said.
Despite the substantial effects on mental health, only 29% of respondents said they had been able to access mental health services during the pandemic.
To look at the impact of the COVID-19 pandemic on the psoriasis and PsA community in Canada, three patient advocacy groups – CAPP, CPN, and Unmasking Psoriasis – codeveloped a survey to look at the disease experience before and after the start of the COVID-19 pandemic. The survey was performed once, with 830 respondents providing information on their lives with psoriasis or PsA in the months before the start of the pandemic and at the time they were surveyed in September and October 2020.
Most of the survey respondents lived in Ontario, Quebec, British Columbia, or Alberta, although other provinces or territories were represented. Almost all respondents (96%) had psoriasis, and 60% also had PsA.
Pre-COVID, nearly half (49%) of patients said that they had not been seen by a rheumatologist, and 39% had not seen a dermatologist for treatment. Asked why, 56% and 27%, respectively, had not been referred, 9% and 15% said they had no specialist located nearby, and 7% and 10% stated that the wait list was too long.
“This tells us that there’s a lot more work that can be done and a lot more education of general practitioners and family medicine professionals about the benefits and the value of specialized care for psoriatic arthritis,” Ms. Manion suggested.
Before the pandemic, joint pain was occurring in 88% of patients, stiffness in 71%, and joint swelling in 67%. Disease flares or sudden periods of worsening occurred on a daily basis for 17%, and around one in five (21%) experienced multiple flares every month.
Prepandemic data also highlighted the negative impact that living with psoriasis or PsA has on people’s ability to sleep, interactions and intimacy with others, and on their school or work lives.
During the pandemic, around a quarter (26%) of respondents said they had worse or much worse access to employment, as well as its benefits such as a stable income (24%). A minority of respondent also described worse access to prescription medication (15%) and over-the-counter medication (13%).
“There are all kinds of things going on for patients in our community: changes to their work, changes to their drug coverage, their ability to sleep and sleep well, their mental health, and their ability to access care and treatments as part of their disease management,” Ms. Manion said.
Her final message to health care professionals was: “I just want to encourage you to continue to check in with your patients about what their experiences have been during the pandemic, and to really consider those impacts as you’re working with them to manage their disease.”
The survey received funding support from AbbVie, Bausch Health, Boehringer Ingelheim, Janssen, LEO Pharma, and Novartis.
FROM CARC 2021