News


EXPERT ANALYSIS FROM THE 2016 AAAAI ANNUAL MEETING
LOS ANGELES – For patients with severe atopic dermatitis and their families and treating physicians, there is big news: finally, there is light at the end of the tunnel, Dr. Lisa A. Beck declared in a plenary lecture at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Better drugs are on the way. The new agents in the developmental pipeline target specific immunologic pathways that appear to be central to atopic dermatitis. Moreover, exciting recent evidence indicates it’s possible to noninvasively identify children at high risk for atopic dermatitis and intervene preventively to reduce the likelihood of actually developing the disease, according to Dr. Beck, professor of dermatology and medicine at the University of Rochester (N.Y.).
“Many pharmaceutical companies have now turned their attention to atopic dermatitis and aren’t just focusing on asthma anymore. The biggest pipeline appears to involve drugs that might target the Th2 [T helper 2 cells] pathway, either by trying to eliminate alarmins such as TSLP [thymic stromal lymphopoietin], or reverse the effects of the Th2 cytokines interleukin-4 and -13, either alone or together, or prevent the recruitment of activated T cells,” she said.
Dr. Beck presented an update on three such promising investigational approaches on the horizon: the IL-4 and IL-13 inhibitor dupilumab; oral and topical Janus associated kinase (JAK) inhibitors; and anti-IgE therapies.
Dupilumab: This fully human monoclonal antibody that blocks IL-4 and IL-13 is also being developed as a treatment for eosinophilic asthma. Dr. Beck was first author of a report on a series of four phase II randomized trials of dupilumab for moderate to severe atopic dermatitis in adults. The publication caused a stir, with dupilumab-treated patients showing marked and rapid improvement to a degree previously unseen in the treatment of this disease (N Engl J Med. 2014;371[2]:130-9).
In the 12-week study, for example, 85% of dupilumab-treated patients achieved at least a 50% improvement in the Eczema Area and Severity Index (EASI) score, compared with 35% of placebo-treated controls, with a significant between-group difference seen in the first week. Maximum improvement – a 75%-80% reduction in EASI scores – was noted at 6-8 weeks. Forty percent of dupilumab-treated patients achieved clear or near-clear skin by investigator’s global assessment, compared with just 7% of controls.
Itching decreased markedly beginning in the first week, too. The investigational agent’s side effect profile was similar to placebo. Phase III clinical trials in atopic dermatitis are ongoing.
A study by other investigators found that dupilumab resulted in rapid improvement in the molecular signature of atopic dermatitis in skin biopsy specimens (J Allergy Clin Immunol. 2014 Dec;134[6]:1293-300). The observed changes in gene expression suggest that dupilumab might have a beneficial effect on the dysfunctional skin barrier that is a hallmark of atopic dermatitis. Further studies are now being planned to take a closer look at that possibility.
JAK inhibitors: “We’re all really excited about this approach because dogs, too, get allergic dermatitis, and in 2013 a JAK 1 and 3 inhibitor [oclacitinib, Apoquel] was approved as a veterinary medicine therapy. It has resulted in dramatic improvement in itch within 1 week of administration, as well as significant improvement in the dermatitis,” Dr. Beck said.
Three JAK inhibitors are now in phase II clinical trials for atopic dermatitis in humans: the JAK 1 and 3 inhibitor tofacitinib (Xeljanz), both as a topical ointment and the familiar oral formulation; baricitinib, an oral JAK 1 and 2 inhibitor; and an agent known for now as PF-04965842, which is an oral inhibitor specifically of JAK 1.
“JAK inhibitors have been quite effective in treating a number of other inflammatory conditions, as well as cancers. I think they will have a role in the treatment of atopic dermatitis. The biggest concerns will be the off-target effects,” she predicted.
Anti-IgE agents: Omalizumab (Xolair), a humanized monoclonal antibody that binds to IgE, has gotten mixed reviews as an investigational treatment for atopic dermatitis. The best study to date, a randomized, single-center, placebo-controlled, double-blind, 16-week clinical trial, found that omalizumab depleted IgE but didn’t improve the clinical course of atopic dermatitis (J Dtsch Dermatol Ges. 2010 Dec;8[12]:990-8). Nonetheless, a phase II trial of omalizumab is ongoing. Plus, ligelizumab, an anti-IgE monoclonal antibody with a higher affinity for IgE than omalizumab, is also in a phase II trial for adult atopic dermatitis.
“Anti-IgE therapy, I think, is still not dead in atopic dermatitis. I look forward to seeing whether omalizumab will work in unique subsets of patients, or whether a more potent anti-IgE molecule will be more beneficial,” Dr. Beck commented.


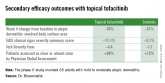
Topical tofacitinib, a Janus kinase inhibitor, may provide a novel safe and effective therapy for atopic dermatitis.
