Retreatment with tofacitinib after a period of treatment interruption was well tolerated and effective in patients with ulcerative colitis who had shown a previous response to tofacitinib induction, according to an analysis of data from the OCTAVE extension trial.
“Clinical response was recaptured in most patients by month 2, and about half of patients by month 36, irrespective of prior anti–[tumor necrosis factor] status,” said lead researcher Edward V. Loftus Jr, MD, from the Mayo Medical School, Rochester, Minn.
A temporary suspension of treatment with the oral, small-molecule Janus kinase (JAK) inhibitor might be necessary for a number of reasons, such as if a patient has to undergo surgery, experiences adverse events, or becomes pregnant.
For their study, Dr. Loftus and colleagues set out to assess the safety and efficacy of retreatment after a period of interruption.
“The population we’re interested in are patients who received tofacitinib during induction and placebo during maintenance” in the original OCTAVE trials, said Dr. Loftus. “They then either completed the trial or flared and rolled over to the open-label extension.”
The researchers looked at the 100 patients who had achieved a clinical response after 8 weeks of treatment with tofacitinib 10 mg twice-daily in the OCTAVE Induction 1 and OCTAVE Induction 2 trials and then received placebo in the OCTAVE Sustain trial and experienced treatment failure between week 8 and week 52. These patients went on to receive tofacitinib 10 mg twice daily as part of the ongoing, open-label, long-term extension OCTAVE Open trial.
Treatment failure was defined as an increase of at least 3 points from the baseline total Mayo score achieved in OCTAVE Sustain, plus an increase of at least 1 point in rectal bleeding and endoscopic subscores and an absolute endoscopic subscore of at least 2 points after at least 8 weeks of treatment. Efficacy was evaluated for up to 36 months in the open-label extension; adverse events were assessed throughout the study period.
The median time to treatment failure was 135 days, Dr. Loftus reported during his award-winning presentation at the virtual annual meeting of the American College of Gastroenterology.
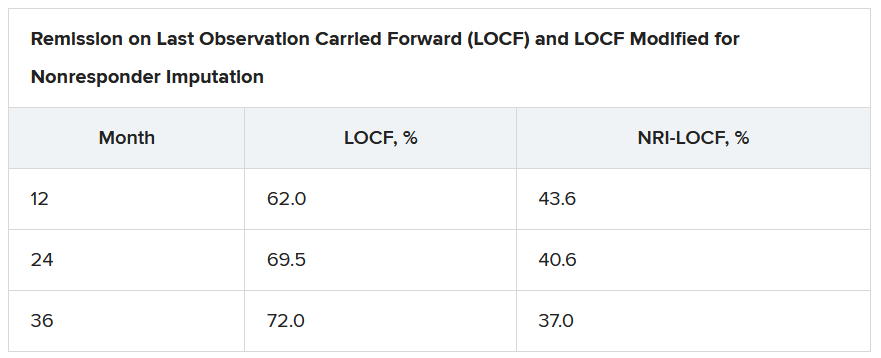
On last observation carried forward (LOCF) analysis, or observed data, 85.2% of the patients had recaptured clinical response by month 2. That rate fell to 74.3% when the analysis was modified for nonresponder imputation (NRI).
“The truth lies somewhere in between,” Dr. Loftus said.
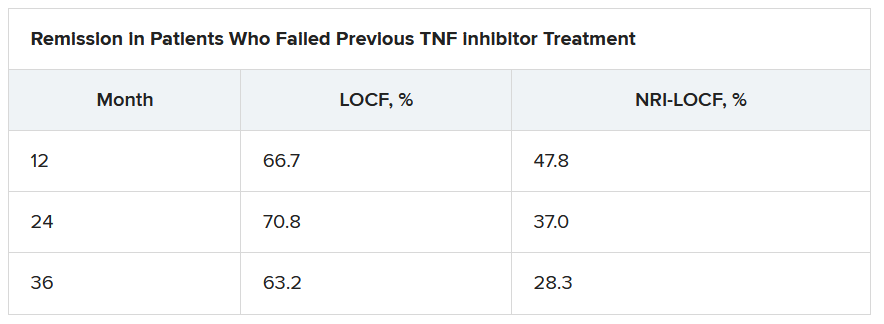
Of interest, a clinical response to tofacitinib retreatment at month 2 was achieved by 92.5% (observed data) and 80.4% (NRI-LOCF) of patients who experienced treatment failure after tumor necrosis factor inhibitor therapy.
“Many patients were able to regain response with tofacitinib and then maintain that over time,” said Dr. Loftus.
Study supports retreatment, which is good news for patients
Incidence rates of adverse events were comparable in the retreatment population and in the overall extension cohort. “There are no signals jumping out, saying that safety events were higher or more frequent in this retreatment population, which is reassuring,” Dr. Loftus added.
Findings such as these are to be expected given the mechanism of action and pharmacologic features of tofacitinib, said Gionata Fiorino, MD, from Humanitas University in Milan, who was not involved in the study.
“I think this is important for patients who need to stop therapy for several reasons – pregnancy, adverse events that do not require permanent withdrawal of the drug, or surgical interventions – and experience a flare after drug withdrawal,” he said in an interview.
“There are several other therapeutic options for these patients, but I have experienced many patients who do not respond to other mechanisms of action apart from JAK [inhibitors],” he added. “And, in the case of a patient who has stopped the drug after having achieved remission, this study clearly supports retreatment, which is good news, especially for patients.”
This study was funded by Pfizer. Dr. Loftus reported financial relationships with AbbVie, Allergan, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Celltrion, Eli Lilly, Exact Sciences, Genentech, Gilead, Janssen, Pfizer, Robarts Clinical Trials, Takeda, and UCB. Dr. Fiorino reports financial relationships with MSD, Takeda, AbbVie, Janssen, Pfizer, Celltrion, Sandoz, AlfaSigma, Samsung, Amgen, Roche, and Ferring.
A version of this article originally appeared on Medscape.com.