Selective estrogen receptor modulators (SERMs) are unique synthetic compounds that bind to the estrogen receptor and initiate either estrogenic agonistic or antagonistic activity, depending on the confirmational change they produce on binding to the receptor. Many SERMs have come to market, others have not. Unlike estrogens, which regardless of dose or route of administration all carry risks as a boxed warning on the label, referred to as class labeling,1 various SERMs exert various effects in some tissues (uterus, vagina) while they have apparent class properties in others (bone, breast).2
The first SERM, for all practical purposes, was tamoxifen (although clomiphene citrate is often considered a SERM). Tamoxifen was approved by the US Food and Drug Administration (FDA) in 1978 for the treatment of breast cancer and, subsequently, for breast cancer risk reduction. It became the most widely prescribed anticancer drug worldwide.
Subsequently, when data showed that tamoxifen could produce a small number of endometrial cancers and a larger number of endometrial polyps,3,4 there was renewed interest in raloxifene. In preclinical animal studies, raloxifene behaved differently than tamoxifen in the uterus. After clinical trials with raloxifene showed uterine safety,5 the drug was FDA approved for prevention of osteoporosis in 1997, for treatment of osteoporosis in 1999, and for breast cancer risk reduction in 2009. Most clinicians are familiar with these 2 SERMs, which have been in clinical use for more than 4 and 2 decades, respectively.
Ospemifene: A third-generation SERM and its indications
Hormone deficiency from menopause causes vulvovaginal and urogenital changes as well as a multitude of symptoms and signs, including vulvar and vaginal thinning, loss of rugal folds, diminished elasticity, increased pH, and most notably dyspareunia. The nomenclature that previously described vulvovaginal atrophy (VVA) has been expanded to include genitourinary syndrome of menopause (GSM).6 Unfortunately, many health care providers do not ask patients about GSM symptoms, and few women report their symptoms to their clinician.7 Furthermore, although low-dose local estrogens applied vaginally have been the mainstay of therapy for VVA/GSM, only 7% of symptomatic women use any pharmacologic agent,8 mainly because of fear of estrogens due to the class labeling mentioned above.
Ospemifene, a newer SERM, improved superficial cells and reduced parabasal cells as seen on a maturation index compared with placebo, according to results of multiple phase 3 clinical trials9,10; it also lowered vaginal pH and improved most bothersome symptoms (original studies were for dyspareunia). As a result, the FDA approved ospemifene for treatment of moderate to severe dyspareunia from VVA of menopause.
Subsequent studies allowed for a broadened indication to include treatment of moderate to severe dryness due to menopause.11 The ospemifene label contains a boxed warning that states, “In the endometrium, [ospemifene] has estrogen agonistic effects.”12 Although ospemifene is not an estrogen (it’s a SERM), the label goes on to state, “There is an increased risk of endometrial cancer in a woman with a uterus who uses unopposed estrogens.” This statement caused The Medical Letter to initially suggest that patients who receive ospemifene also should receive a progestational agent—a suggestion they later retracted.13,14
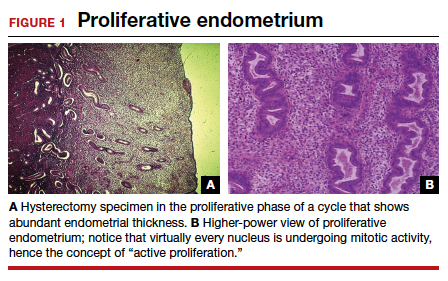
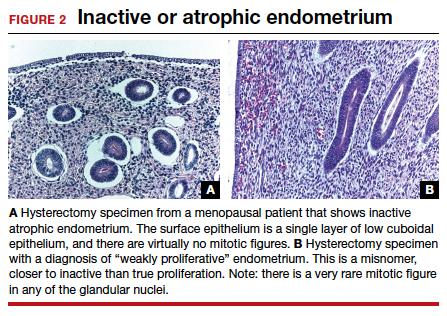
To understand why the ospemifene labeling might be worded in such a way, one must review the data regarding the poorly named entity “weakly proliferative endometrium.” The package labeling combines any proliferative endometrium (“weakly” plus “actively” plus “disordered”) that occurred in the clinical trial. Thus, 86.1 per 1,000 of the ospemifene-treated patients (vs 13.3 per 1,000 of those taking placebo) had any one of the proliferative types. The problem is that “actively proliferative” endometrial glands will have mitotic activity in virtually every nucleus of the gland as well as abundant glandular progression (FIGURE 1), whereas “weakly proliferative” is actually closer to inactive or atrophic endometrium with an occasional mitotic figure in only a few nuclei of each gland (FIGURE 2).
In addition, at 1 year, the incidence of active proliferation with ospemifene was 1%.15 In examining the uterine safety study for raloxifene, both doses of that agent had an active proliferation incidence of 3% at 1 year.5 Furthermore, that study had an estrogen-only arm in which, at end point, the incidence of endometrial proliferation was 39%, and hyperplasia, 23%!5 It therefore is evident that, in the endometrium, ospemifene is much more like the SERM raloxifene than it is like estrogen. The American College of Obstetricians and Gynecologists (ACOG) endorsed ospemifene (level A evidence) as a first-line therapy for dyspareunia, noting absent endometrial stimulation.16
Continue to: Ospemifene effects on breast and bone...