To the Editor:
Immune checkpoint inhibitors such as anti–programmed cell death protein 1 (anti–PD-1) and anticytotoxic T lymphocyte–associated protein 4 therapies are a promising class of cancer therapeutics. However, they are associated with a variety of immune-related adverse events (irAEs), including cutaneous toxicity.1 The PD-1/programmed death ligand 1 (PD-L1) pathway is important for the maintenance of immune tolerance, and a blockade has been shown to lead to development of various autoimmune diseases.2 We present the case of a patient who developed new-onset localized scleroderma during treatment with the PD-1 inhibitor nivolumab.
A 65-year-old woman presented with a rash on the left thigh that was associated with pruritus, pain, and a pulling sensation. She had a history of stage IV lung adenocarcinoma, with a mass in the right upper lobe with metastatic foci to the left femur, right humerus, right hilar, and pretracheal lymph nodes. She received palliative radiation to the left femur and was started on carboplatin and pemetrexed. Metastasis to the liver was noted after completion of 6 cycles of therapy, and the patient’s treatment was changed to nivolumab. After 17 months on nivolumab therapy (2 years after initial diagnosis and 20 months after radiation therapy), she presented to our dermatology clinic with a cutaneous eruption on the buttocks that spread to the left thigh. The rash failed to improve after 1 month of treatment with emollients and triamcinolone cream 0.1%.
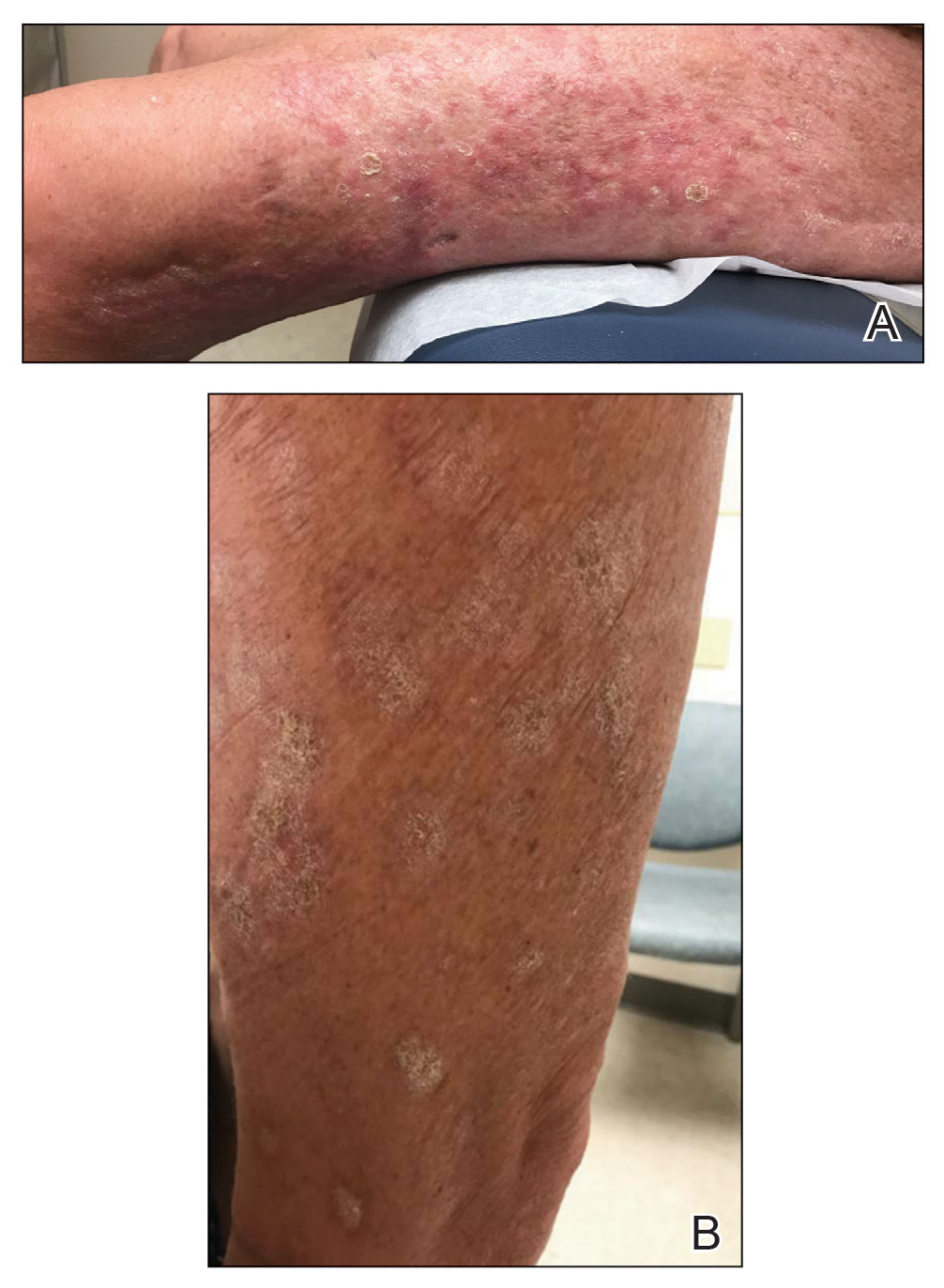
At the current presentation, which was 2 months after she initially presented to our clinic, dermatologic examination revealed erythematous and sclerotic plaques on the left lateral thigh (Figure 1A). Betamethasone cream 0.05% was prescribed, and nivolumab was discontinued due to progression of cutaneous symptoms. A punch biopsy from the left thigh demonstrated superficial dermal sclerosis that was suggestive of chronic radiation dermatitis; direct immunofluorescence testing was negative. The patient was started on prednisone 50 mg daily, which resulted in mild improvement in symptoms.
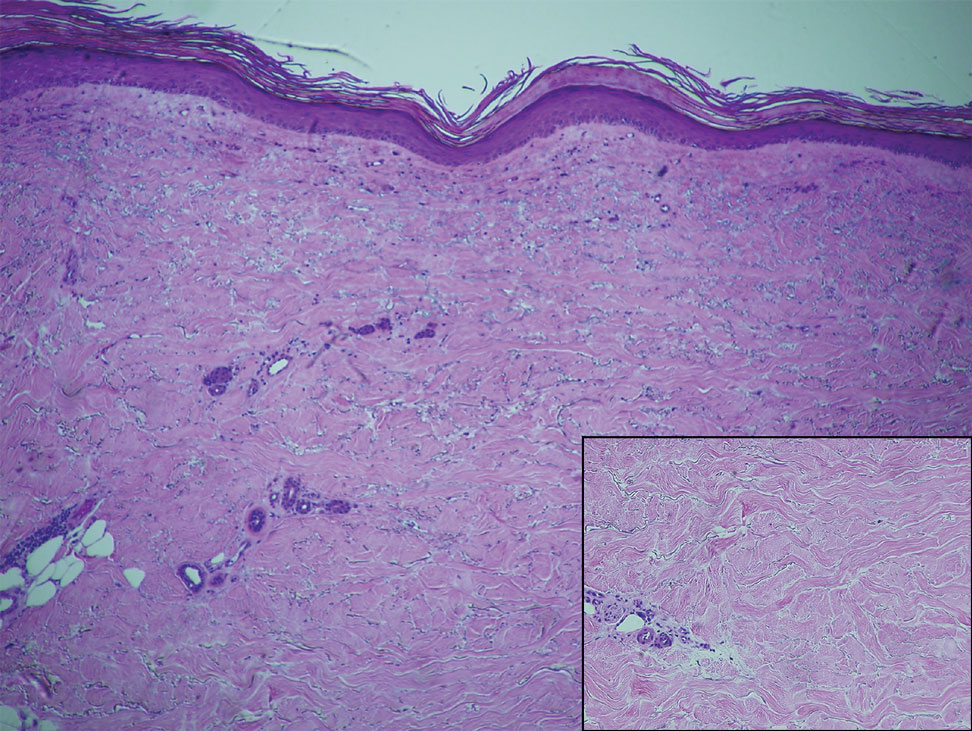
Within 6 months, new sclerotic plaques developed on the patient’s back and right thigh (Figure 1B). Because the lesions were located outside the radiation field of the left femur, a second biopsy was obtained from the right thigh. Histopathology revealed extensive dermal sclerosis and a perivascular lymphoplasmacytic infiltrate (Figure 2). An antinuclear antibody test was weakly positive (1:40, nucleolar pattern) with a negative extractable nuclear antigen panel result. Anti–double-stranded DNA, anti–topoisomerase 1, anti-Smith, antiribonucleoprotein, anti–Sjögren syndrome type A, anti–Sjögren syndrome type B, and anticentromere serology test results were negative. The patient denied decreased oral aperture, difficulty swallowing, or Raynaud phenomenon. Due to the atypical clinical presentation in the setting of PD-1 inhibitor therapy, the etiology of the eruption was potentially attributable to nivolumab. She was started on treatment with methotrexate 20 mg weekly and clobetasol cream 0.05% twice daily; she continued taking prednisone 5 mg daily. The cutaneous manifestations on the patient’s back completely resolved, and the legs continued to gradually improve on this regimen. Immunotherapy continued to be held due to skin toxicity.
Localized scleroderma is an autoimmune disorder characterized by inflammation and skin thickening. Overactive fibroblasts produce excess collagen, leading to the clinical symptoms of skin thickening, hardening, and discoloration.3 Lesions frequently develop on the arms, face, or legs and can present as patches or linear bands. Unlike systemic sclerosis, the internal organs typically are uninvolved; however, sclerotic lesions can be disfiguring and cause notable disability if they impede joint movement.
The PD-1/PD-L1 pathway is a negative regulator of the immune response that inactivates T cells and helps maintain self-tolerance. Modulation of the PD-1/PD-L1 pathway and overexpression of PD-L1 are seen in various cancers as a mechanism to help malignant cells avoid immune destruction.4 Conversely, inhibition of this pathway can be used to stimulate an antitumor immune response. This checkpoint inhibition strategy has been highly successful for the treatment of various cancers including melanoma and non–small cell lung carcinoma. There are several checkpoint inhibitors approved in the United States that are used for cancer therapy and target the PD-1/PD-L1 pathway, such as nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.4 A downside of checkpoint inhibitor treatment is that uncontrolled T-cell activation can lead to irAEs, including cutaneous eruptions, pruritus, diarrhea, colitis, hepatitis, endocrinopathies, pneumonitis, and renal insufficiency.5 These toxicities are reversible if treated appropriately but can cause notable morbidity and mortality if left unrecognized. Cutaneous eruption is one of the most common irAEs associated with anti–PD-1 and anti–PD-L1 therapies and can limit therapeutic efficacy, as the drug may need to be held or discontinued due to the severity of the eruption.6 Mid-potency to high-potency topical corticosteroids and systemic antihistamines are first-line treatments of grades 1 and 2 skin toxicities associated with PD-1 inhibitor therapy. For eruptions classified as grades 3 or 4 or refractory grade 2, discontinuation of the drug and systemic corticosteroids is recommended.7
The cutaneous eruption in immunotherapy-mediated dermatitis is thought to be largely mediated by activated T cells infiltrating the dermis.8 In localized scleroderma, increased tumor necrosis factor α, IFN-γ, IFN-γ–induced protein 10, and granulocyte macrophage colony stimulating factor activity have been shown to correlate with disease activity.9,10 Interestingly, increased tumor necrosis factor α and IFN-γ correlate with better response and increased overall survival in PD-1 inhibition therapy, suggesting a correlation between PD-1 inhibition and T helper activation as noted by the etiology of sclerosis in our patient.11 Additionally, history of radiation was a confounding factor in the diagnosis of our patient, as both sclerodermoid reactions and chronic radiation dermatitis can present with dermal sclerosis. However, the progression of disease outside of the radiation field excluded this etiology. Although new-onset sclerodermoid reactions have been reported with PD-1 inhibitors, they have been described secondary to sclerodermoid reactions from treatment with pembrolizumab.12,13 One case series reported a case of diffuse sclerodermoid reaction and a limited reaction in response to pembrolizumab treatment, while another case report described a relapse of generalized morphea in response to pembrolizumab treatment.12,13 One case of relapsing morphea in response to nivolumab treatment for stage IV lung adenocarcinoma also has been reported.14
Cutaneous toxicities are one of the most common irAEs associated with checkpoint inhibitors and are seen in more than one-third of treated patients. Most frequently, these irAEs manifest as spongiotic dermatitis on histopathology, but a broad spectrum of cutaneous reactions have been observed.15 Although sclerodermoid reactions have been reported with PD-1 inhibitors, most are described secondary to sclerodermoid reactions with pembrolizumab and involve relapse of previously diagnosed morphea rather than new-onset disease.12-14
Our case highlights new-onset localized scleroderma in the setting of nivolumab therapy that showed clinical improvement with methotrexate and topical and systemic steroids. This reaction pattern should be considered in all patients who develop cutaneous eruptions when treated with a PD-1 inhibitor. There should be a high index of suspicion for the potential occurrence of irAEs to ensure early recognition and treatment to minimize morbidity and maximize adherence to therapy for the underlying malignancy.