In 2023, ESPEN (the European Society for Clinical Nutrition and Metabolism) published consensus recommendations highlighting the importance of regular monitoring and treatment of nutrient deficiencies in patients with inflammatory bowel disease (IBD) for improved prognosis, mortality, and quality of life.1 Suboptimal nutrition in patients with IBD predominantly results from inflammation of the gastrointestinal (GI) tract leading to malabsorption; however, medications commonly used to manage IBD also can contribute to malnutrition.2,3 Additionally, patients may develop nausea and food avoidance due to medication or the disease itself, leading to nutritional withdrawal and eventual deficiency.4 Even with the development of diets focused on balancing nutritional needs and decreasing inflammation,5 offsetting this aversion to food can be difficult to overcome.2
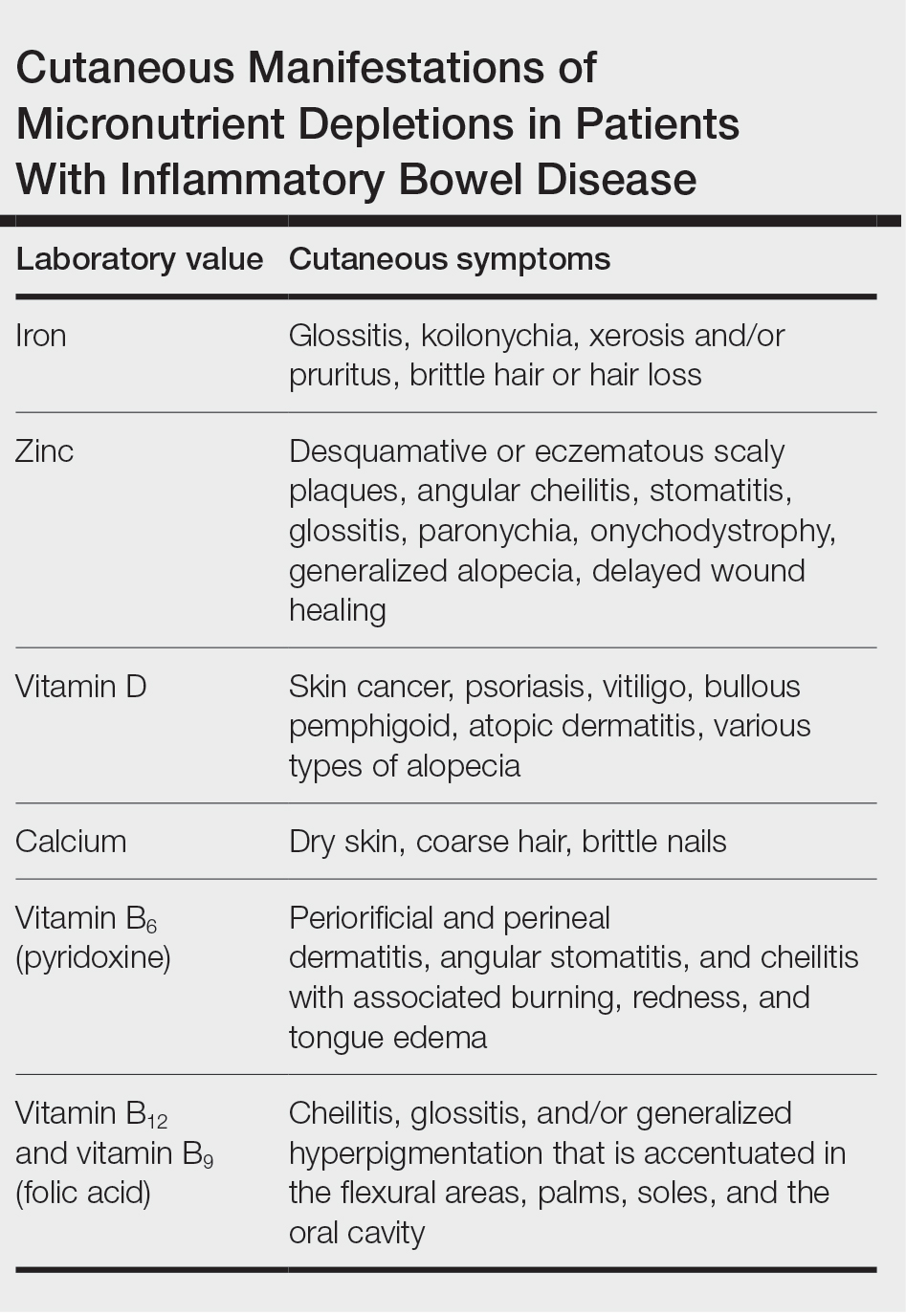
Cutaneous manifestations of IBD are multifaceted and can be secondary to the disease, reactive to or associated with IBD, or effects from nutritional deficiencies. The most common vitamin and nutrient deficiencies in patients with IBD include iron; zinc; calcium; vitamin D; and vitamins B6 (pyridoxine), B9 (folic acid), and B12.6 Malnutrition may manifest with cutaneous disease, and dermatologists can be the first to identify and assess for nutritional deficiencies. In this article, we review the mechanisms of these micronutrient depletions in the context of IBD, their subsequent dermatologic manifestations (Table), and treatment and monitoring guidelines for each deficiency.
Iron
A systematic review conducted from 2007 to 2012 in European patients with IBD (N=2192) found the overall prevalence of anemia in this population to be 24% (95% CI, 18%-31%), with 57% of patients with anemia experiencing iron deficiency.7 Anemia is observed more commonly in patients hospitalized with IBD and is common in patients with both Crohn disease and ulcerative colitis.8
Pathophysiology—Iron is critically important in oxygen transportation throughout the body as a major component of hemoglobin. Physiologically, the low pH of the duodenum and proximal jejunum allows divalent metal transporter 1 to transfer dietary Fe3+ into enterocytes, where it is reduced to the transportable Fe2+.9,10 Distribution of Fe2+ ions from enterocytes relies on ferroportin, an iron-transporting protein, which is heavily regulated by the protein hepcidin.11 Hepcidin, a known acute phase reactant, will increase in the setting of active IBD, causing a depletion of ferroportin and an inability of the body to utilize the stored iron in enterocytes.12 This poor utilization of iron stores combined with blood loss caused by inflammation in the GI tract is the proposed primary mechanism of iron-deficiency anemia observed in patients with IBD.13
Cutaneous Manifestations—From a dermatologic perspective, iron-deficiency anemia can manifest with a wide range of symptoms including glossitis, koilonychia, xerosis and/or pruritus, and brittle hair or hair loss.14,15 Although the underlying pathophysiology of these cutaneous manifestations is not fully understood, there are several theories assessing the mechanisms behind the skin findings of iron deficiency.
Atrophic glossitis has been observed in many patients with iron deficiency and is thought to manifest due to low iron concentrations in the blood, thereby decreasing oxygen delivery to the papillae of the dorsal tongue with resultant atrophy.16,17 Similarly, decreased oxygen delivery to the nail bed capillaries may cause deformities in the nail called koilonychia (or “spoon nails”).18 Iron is a key co-factor in collagen lysyl hydroxylase that promotes collagen binding; iron deficiency may lead to disruptions in the epidermal barrier that can cause pruritus and xerosis.19 An observational study of 200 healthy patients with a primary concern of pruritus found a correlation between low serum ferritin and a higher degree of pruritus (r=−0.768; P<.00001).20
Evidence for iron’s role in hair growth comes from a mouse model study with a mutation in the serine protease TMPRSS6—a protein that regulates hepcidin and iron absorption—which caused an increase in hepcidin production and subsequent systemic iron deficiency. Mice at 4 weeks of age were devoid of all body hair but had substantial regrowth after initiation of a 2-week iron-rich diet, which suggests a connection between iron repletion and hair growth in mice with iron deficiency.21 Additionally, a meta-analysis analyzing the comorbidities of patients with alopecia areata found them to have higher odds (odds ratio [OR]=2.78; 95% CI, 1.23-6.29) of iron-deficiency anemia but no association with IBD (OR=1.48; 95% CI, 0.32-6.82).22
Diagnosis and Monitoring—The American Gastroenterological Association recommends a complete blood cell count (CBC), serum ferritin, transferrin saturation (TfS), and C-reactive protein (CRP) as standard evaluations for iron deficiency in patients with IBD. Patients with active IBD should be screened every 3 months,and patients with inactive disease should be screened every 6 to 12 months.23
Although ferritin and TfS often are used as markers for iron status in healthy individuals, they are positive and negative acute phase reactants, respectively. Using them to assess iron status in patients with IBD may inaccurately represent iron status in the setting of inflammation from the disease.24 The European Crohn’s and Colitis Organisation (ECCO) produced guidelines to define iron deficiency as a TfS less than 20% or a ferritin level less than 30 µg/L in patients without evidence of active IBD and a ferritin level less than 100 µg/L for patients with active inflammation.25
A 2020 multicenter observational study of 202 patients with diagnosed IBD found that the ECCO guideline of ferritin less than 30 µg/L had an area under the receiver operating characteristic (AUROC) curve of 0.69, a sensitivity of 0.43, and a specificity of 0.95 in their population.26 In a sensitivity analysis stratifying patients by CRP level (<10 or ≥10 mg/L), the authors found that for patients with ulcerative colitis and a CRP less than 10 mg/L, a cut-off value of ferritin less than 65 µg/L (AUROC=0.78) had a sensitivity of 0.78 and specificity of 0.76, and a TfS value of less than 16% (AUROC=0.88) had a sensitivity of 0.79 and a specificity of 0.9. In patients with a CRP of 10 mg/L or greater, a cut-off value of ferritin 80 µg/L (AUROC=0.76) had a sensitivity of 0.75 and a specificity of 0.82, and a TfS value of less than 11% (AUROC=0.69) had a sensitivity of 0.79 and a specificity of 0.88. There were no ferritin cut-off values associated with good diagnostic performance (defined as both sensitivity and specificity >0.70) for iron deficiency in patients with Crohn disease.26
The authors recommended using an alternative iron measurement such as soluble transferrin receptor (sTfR)/log ferritin ratio (TfR-F) that is not influenced by active inflammation and has a good correlation with ferritin values (TfR-F: r=0.66; P<.001).26 However, both sTfR and TfR-F have high costs and intermethod variability as well as differences in their reference ranges depending on which laboratory performs the analysis, limiting the accessibility and practicality of easily obtaining these tests.27 Although there may be inaccuracies for standard ferritin or TfS under ECCO guidelines, proposed alternatives have their own limitations, which may make ferritin and TfS the most reasonable evaluations of iron status as long as disease activity status at the time of testing is taken into consideration.
Treatment—Treatment of underlying iron deficiency in patients with IBD requires reversing the cause of the deficiency and supplementing iron. In patients with IBD, the options to supplement iron may be limited by active disease, making oral intake less effective. Oral iron supplementation also is associated with notable GI adverse effects that may be exacerbated in patients with IBD. A systematic review of 43 randomized controlled trials (RCTs) evaluating GI adverse effects (eg, nausea, abdominal pain, diarrhea, constipation, and black or tarry stools) of oral ferrous sulfate compared with placebo or intravenous (IV) iron supplementation in healthy nonanemic individuals found a significant increase in GI adverse effects with oral supplementation (placebo: OR=2.32; P<.0001; IV: OR=3.05; P<.0001).28
Therefore, IV iron repletion may be necessary in patients with IBD and may require numerous infusions depending on the formulation of iron. In an RCT conducted in 2011, patients with iron-deficiency anemia with quiescent or mild to moderate IBD were treated with either IV iron sulfate or ferric carboxymaltose.29 With a primary end point of hemoglobin response greater than 2 g/dL, the authors found that 150 of 240 patients responded to ferric carboxymaltose vs 118 of 235 treated with iron sulfate (P=.004). The dosing for ferric carboxymaltose was 1 to 3 infusions of 500 to 1000 mg of iron and for iron sulfate up to 11 infusions of 200 mg of iron.29