AMSTERDAM – Twice-weekly proactive maintenance therapy with 0.1% tacrolimus ointment in adults with moderate to severe atopic dermatitis resulted in dramatic improvement in self-reported quality of life in a 12-month observational study.
This prospective study was designed to assess patients’ own experiences in real-world clinical practice. For example, patients had to pay for their own medication, unlike in randomized clinical trials, where they get it for free. Study participants also didn’t check in for regularly scheduled office visits or receive pep talks to boost medication adherence. They simply reported their outcomes to investigators through mailed questionnaires instead, Dr. Elisabeth A. Holm explained at the annual congress of the European Academy of Dermatology and Venereology.
A previous clinical trial by other investigators showed that twice-weekly maintenance therapy with topical tacrolimus kept adult atopic dermatitis in remission for extended periods and reduced the incidence of eczema exacerbations (Allergy 2008;63:742-50). But Dr. Holm, a dermatologist at Copenhagen University, wondered what happens when maintenance topical tacrolimus is employed as a proactive treatment strategy in real daily life, particularly in light of a Danish study that found that dermatology patients never filled one in three prescriptions for new medications (J. Am. Acad. Dermatol. 2008;59:27-33).
The current observational study included 214 adults with moderate to severe atopic dermatitis previously treated with corticosteroids who presented to 41 sites in Denmark and Sweden. They were given a prescription for 0.1% tacrolimus ointment (Protopic) with instructions to apply it twice daily until their eczema was cleared or almost cleared, then switch to twice weekly maintenance therapy, shifting back to BID therapy as needed to combat flares.
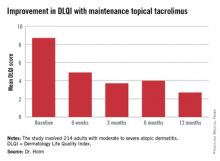
The primary endpoint was change in health-related quality of life during 12 months of followup. This was assessed using the Dermatology Life Quality Index (DLQI), which was administered at baseline, 6 weeks, and 3, 6, and 12 months. Scores improved dramatically.
At baseline, 35% of patients had a DLQI of 6-10, indicative of a moderate adverse impact of their skin disease on daily life. Another 27% had a DLQI of 11-20, reflecting a very large adverse effect. At 12 months, however, DLQI scores had undergone a big shift in the favorable direction: 14% of subjects had a DLQI of 6-10, a mere 4% scored 11-20, and 52% of patients had a DLQI of 0 or 1, meaning they felt their atopic dermatitis had no effect upon their quality of life.
Continue for other outcomes >>