Osteoid osteoma (OO) is one of the most common benign tumors of bone, representing roughly 10% of all benign bone-forming tumors and 5% of all primary bone tumors.1 The majority of cases occur in individuals under age 20 years and more frequently in males (2:1).2 These lesions tend to be cortically based and most often located about the hip and in the diaphysis of long bones. They typically are characterized radiographically by a nidus less than 2 cm in diameter surrounded by dense, reactive bone of variable thickness.
The classic presentation of OO is localized, dull, aching pain that is worse at night and that is relieved with use of salicylates or other nonsteroidal anti-inflammatory drugs (NSAIDs).3 The diagnosis is made by patient history and plain radiographs, often supported by computed tomography (CT) or magnetic resonance imaging for appropriate identification of the tumor nidus. Despite effective pain relief with NSAIDs as well as evidence suggesting that the natural history of these tumors is self-limited, most patients forgo medical management in favor of elective surgical treatment.4,5
Initially, treatment for OO focused on either symptom management or en bloc surgical resection of the tumor nidus. Several different minimally invasive therapies have since been developed, and good results reported.6-8 More recently, use of percutaneous radiofrequency ablation (RFA) has increased, as this method has demonstrated high efficacy and minimal morbidity.9-11 RFA for OO traditionally has been performed by radiologists under CT guidance in the radiology suite, but advances in intraoperative imaging techniques now allow orthopedic oncologists to perform image-guided RFA in the operating room.
To our knowledge, there have been no reports documenting use of intraoperative CT for localization of OO and use of RFA in the treatment of this lesion. In this article, we report the results of a series of 28 patients with OO treated with intraoperative CT-guided RFA by a single surgeon. We also provide a brief description of this novel technique.
Materials and Methods
The protocol used was approved by our institutional review board. All patients and/or their legal guardians provided informed consent to participate in the study and were informed at the time consent was obtained that case-related data would be submitted for publication.
Patients
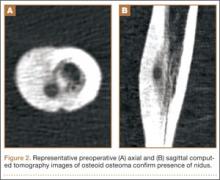
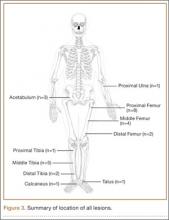
Between September 2004 and December 2008, 28 patients (19 males, 9 females) with OO underwent intraoperative percutaneous image-guided RFA at a university hospital. Mean age was 19.5 years, median age was 16 years (range, 7-54 years). Patients were referred for RFA if they had clinical and radiographic features of OO (Figures 1, 2) and wanted to forgo continued medical management. As we selected only patients with lesions that we thought were amenable to percutaneous RFA—lesions involving the long and short bones of the upper or lower extremity and selected flat bones—en bloc surgical resection was not offered to these patients. Lesions were located in the upper extremity (n = 1), lower extremity (n = 24), and pelvis (n = 3) (Figure 3). Twenty-seven procedures were performed for initial tumor treatment and 1 for recurrence after previous open excision. Two additional procedures were later performed on separate patients with recurrent symptoms after the index procedure. All procedures were performed by the senior author (DML).
Procedure
With each patient, all options were discussed, including continued medical management versus surgical treatment, and informed consent was obtained. All procedures were performed with the patient under general anesthesia in the operating room. RFA for an upper extremity lesion was performed with the patient in the supine position with the ipsilateral extremity draped over a hand table. The 2 procedures for lesions in the talus or calcaneus were performed with the patient in the supine position using a standard table with the bottom of the table flexed down 90° to allow the nonaffected leg to hang over the end of the table. The affected extremity in each case was then positioned in a well-padded leg holder to allow the foot and ankle to be draped free for 360° imaging.
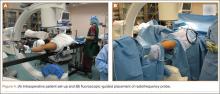
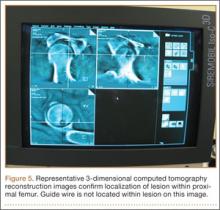
All other procedures for lower extremity diaphyseal or pelvic lesions were performed with a fracture table. After successful induction of general anesthesia, the patient was positioned supine on the table with the contralateral lower extremity abducted and externally rotated in a well-leg holder. The ipsilateral leg was held in the traction apparatus without traction applied and was prepared and draped accordingly (Figure 4). With use of the Siemens Siremobil ISO-C3D fluoroscopic C-arm (Siemens Medical Solutions, Malvern, Pennsylvania), a radiograph was taken of the affected area to identify the lesion. Local anesthetic was infiltrated into the surgical site down to the periosteum. A stab incision was made, and, with fluoroscopic guidance, a 0.062-mm Kirschner wire (K-wire) was placed into the lesion. Location within the tumor nidus was confirmed with biplanar fluoroscopic imaging. A Bonopty cannula (AprioMed, Uppsala, Sweden) was then passed over the K-wire. After the wire was removed, a 5-mm radiofrequency probe (Radionics, Burlington, Massachusetts) was placed through the cannula, and positioning within the nidus was confirmed with 3-dimensional (3-D) CT reconstructions in the sagittal, coronal, and axial planes (Figure 5). A radiofrequency generator (Radionics) was used to heat the lesion at 93°C for 7 minutes. The probe and trocar were then removed. Steri-strips and a sterile dressing were used to cover the wound, and the patient was taken to the recovery area after extubation. All patients were discharged home the day of the procedure.