BY CHARLES E. MILLER, MD
Chronic pelvic pain is described as the presence of lower abdominal or pelvic pain for longer than 6 months. It is believed to affect approximately one in six women and 12%-15% of women of reproductive age. The diagnosis and treatment of chronic pelvic pain adds as much as a $2 billion burden to our health system annually.
It was first described clinically in the literature in 1857, while the existence of pelvic varicosities wasn’t documented for nearly another 100 years. Pelvic congestion syndrome (PCS) accounts for 30%-70% of cases presenting with chronic pelvic pain. PCS can be due to pelvic venous insufficiency, characterized by reflux into pelvic veins leading to pelvic varicosities or alternative venous pathways secondary to varicose veins of the leg.
Other etiologies of PCS include nutcracker syndrome (left renal vein compressed between the aorta and the superior mesenteric artery), May-Thurner syndrome (compression of the left common iliac vein by the right common iliac artery) or, less likely, tumor thrombosis of the inferior vena cava, portal vein thrombosis, renal cell carcinoma, left renal thrombosis, or left kidney arterial-venous fistula.
While there appears to be significant literature indicating a long-term success rate of greater than 80% in patients treated by percutaneous endovascular procedures (embolization, stenting), there is far less information on the postsurgical success of blocking the varicose gonadal vein. Nevertheless, our long-term results with gonadal vein clipping is virtually the same as that of our radiological colleagues.
It is a pleasure to welcome Courtney Steller, DO, to this edition of the Master Class in Gynecologic Surgery to discuss the diagnosis and treatment of PCS, with an emphasis on surgical correction.
Dr. Steller is a recent graduate of the AAGL/SRS Fellowship in Minimally Invasive Gynecologic Surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. She is currently in private practice and is an associate at the Family Health Centers of San Diego, Calif.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS Fellowship in Minimally Invasive Gynecologic Surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported having no financial disclosures relevant to this column. Email him at obnews@frontlinemedcom.com.
Pelvic congestion syndrome: A treatable cause of pain
BY COURTNEY STELLER, DO
Pelvic congestion syndrome is a poorly understood and underdiagnosed disease. Yet, over the last decade, the syndrome has become less controversial as the etiology has become better understood and as the diagnostic approach has become more specific. Through these advances, treatments have also become increasingly more successful.
This is an important shift, because the chronic pelvic pain experienced by patients with pelvic congestion significantly impacts their quality of life and well-being. As the pain persists, it can become exceedingly difficult to manage. Many patients we have ultimately treated for pelvic congestion syndrome have had years of various work-ups, significant diagnostic investigations, and trials of different treatments without having any cause of their pain identified or achieving any lasting symptom relief.
The pelvic pain in patients with pelvic congestion syndrome (PCS) can be noncyclical or cyclical. It is present most of the time but tends to get worse at the end of the day and after long periods of standing and/or sitting. The pain also may worsen with intercourse, largely afterward. The syndrome tends to occur in premenopausal and multiparous women, but it’s important to appreciate that this is not always the case; we have diagnosed and treated PCS in several young, nulliparous patients as well.
Features and diagnosis
PCS is a disorder of pelvic venous circulation that predominantly affects the ovarian veins. It is sometimes referred to as pelvic vein incompetence or pelvic vascular dysfunction. Just as veins in the legs can enlarge and become varicose, the ovarian veins – and sometimes the internal iliac veins – can become incompetent and unable to effectively return blood back to the heart.
Pregnancy may predispose patients to developing the abnormally dilated and refluxing veins that characterize PCS, as the increase in pelvic vein capacity and uterine compression can lead to significant stasis of blood in the pelvis and subsequent damage to the veins and the venous valves. There also is believed to be an estrogen component to the development of PCS, because estrogen is known to act as a vasodilator. Moreover, a congenital absence and incompetence of venous valves in some cases has been reported.
In a recent study looking at pelvic vein incompetence and symptoms of chronic pelvic pain, these women were reported to have a distinctive symptom profile, with the “most notable” features being the presence of dull pelvic pain that radiates to the upper thighs and is aggravated by prolonged standing and walking – symptoms that are similar to the leg symptoms experienced by patients with severe varicose veins (Eur J Obstet Gynecol Reprod Biol. 2016 Jan;196:21-5).
Other investigators have similarly described the pelvic pain related to PCS as a dull ache or heaviness sensation that is most severe at the end of the day and that is lessened with supine positioning (though not necessarily immediately) and often exacerbated with sexual intercourse, especially post coitus. These descriptions are in line with my experience with PCS. There is usually exquisite tenderness on pelvic exam, especially localized to the adnexa. Patients will often have varicose veins on their upper legs or labia.
Interestingly, it has been repeatedly shown that many women have dilated and incompetent pelvic veins without also having such pathognomonic pain. We therefore cannot treat women based solely on the finding of abnormal veins.
On the other hand we must determine which patients with chronic pelvic pain have PCS. The differential diagnosis for PCS includes endometriosis, adenomyosis chronic pelvic inflammatory disease, adhesive disease, adnexal masses, adnexal torsion, and several nongynecologic diseases including interstitial cystitis and irritable bowel syndrome.
Venography has become the gold standard for diagnosing pelvic congestion. The procedure involves catheterization of the ovarian veins through a femoral or jugular approach. In our experience, the common femoral vein is the more frequently used access point. Using a contrast injection, the interventional radiologist can assess the degree of venous dilation and reflux in the pelvis.

There currently is no consensus on a cutoff for vein diameter or on any validated measures for congestion. According to one report on PCS authored by interventional radiologists, the diagnosis of PCS is confirmed with the venographic findings of ovarian vein diameter greater than 6 mm, retrograde ovarian or pelvic venous flow, presence of several tortuous collateral pelvic venous pathways, and delayed or stagnant clearance on contrast (Semin Intervent Radiol. 2008 Dec;25[4]:361-8).
The criteria vary, however. A recent literature review on pelvic congestion syndrome by Chiara Borghi, MD, and Lucio Dell’Atti, MD, states that incompetent pelvic veins are defined as more than 5-10 mm in diameter (Arch Gynecol Obstet. 2016 Feb;293[2]:291-301).
To more accurately diagnose PCS, our patients undergo tilt-table venography. The patient is placed into a reverse-Trendelenburg upright or semi-upright position to potentially exacerbate any venous reflux or dilation.
Other methods of identifying and diagnosing pelvic congestion have included transabdominal and transvaginal ultrasound, CT, and MRI. While CT and MRI both offer an overview of the pelvic vasculature and are helpful for ruling out other causes of chronic pelvic pain, they have low specificity for pelvic varices, according to the Italian review.
Sonography performed in the supine position, on the other hand, appears to be increasingly viewed as an acceptable screening tool for determining which patients may ultimately benefit from venography. It is also important in evaluation to rule out other pathologies not yet excluded. However, it should not be used for diagnosis of PCS.
Treating PCS
There are two main approaches to treating PCS: venous ligation (a gynecologic surgical approach) and percutaneous transcatheter embolization (performed by interventional radiologists).
The literature and evidence base is still in its infancy, but is growing. In our experience, both approaches lead to good resolution of symptoms over time in the majority of patients, and appear superior to the medical therapies that have been proposed for treating PCS, such as progestins and gonadotropin-releasing hormone agonists. Success rates with medical therapy are more variable and appear to be more short lived.
A review published this year on the effectiveness of embolization of pelvic veins for reducing chronic pelvic pain showed that 75% of women undergoing embolization had symptomatic relief that generally increased over time and was sustained. The authors concluded that embolization appears to be effective for the majority of women, and is safe, although they also noted that the quality of the evidence is low (J Vasc Interv Radiol. 2016 Oct;27[10]:1478-86.e8). Their review was based almost entirely on prospective case series.
Dr. Borghi and Dr. Dell’Atti offered a similar assessment of embolization for PCS, stating in their review article that clinical success has been reported in 70%-85% of patients. They also report nearly equivalent success rates of up to 75% with treatment via surgical ligation of ovarian and/or pelvic vasculature. These findings are from mostly observational data and case series.
Decisions about which approach to take should be individualized. If there are no differences with respect to insurance coverage for the patient, then embolization may be the preferred approach because it is the most minimally invasive technique and can potentially be performed at the time of diagnostic venography, negating the need for a second procedure. A skilled interventional radiologist familiar with the disease and the treatment is necessary. Various embolic agents are utilized, including coils, glues, foams, and other agents that cause sclerosis of the abnormal veins.
In other cases, venous ligation is preferred, especially when an additional gynecologic surgery, such as a cystectomy or myomectomy, is required.
Surgical ligation of ovarian veins was initially performed via laparotomy using a traditional retroperitoneal approach. The surgical goal is to isolate the ovarian vein significantly above the pelvic brim and before the vein becomes substantially dilated. Laparotomy therefore requires a vertical mid-line incision to provide adequate access to the appropriate portion of the ovarian vessels, leading to potentially high morbidity and poor cosmesis.
More recently, gynecologic surgeons skilled in laparoscopy have successfully managed PCS transperitoneally. A few small series of bilateral laparoscopic transperitoneal ligation of ovarian veins have been reported, including one by Tigellio Gargiulo, MD, who clipped both veins in their upper third, near their distal ends at the inferior vena cava (right) and the renal vein (left) (J Am Assoc Gynecol Laparosc. 2003 Nov;10[4]:501-4).
We prefer a robot-assisted laparoscopic approach for most of our patients. Not only does the improved dexterity help while working with sensitive vasculature, but more importantly we are able to use Firefly fluorescence.
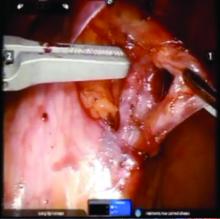
The procedure generally is as follows. The uterine adnexa on the affected side is grasped and placed on tension so that the infundibulopelvic (IP) ligament can be visualized as it courses up and above the pelvic brim. The peritoneum immediately over the IP ligament is gently grasped and tented upward, and a small incision is made into the peritoneum, providing access into the retroperitoneum. The ureter should be visualized medial to this dissection.
The peritoneal tissue is then gently dissected off the ovarian vessels. Once the vessels are freed from the peritoneal tissue, the dilated ovarian vein is often clearly visualized. It is important to note that if no venous dilation is seen during laparoscopy, the procedure should not be aborted. Due to the Trendelenburg position that is utilized in gynecologic – and especially laparoscopic – surgery, the venous system sometimes appears falsely “normal” at this time.
Once the ovarian vessels have been isolated, the arteries must be separated from the veins. The adventitial tissue is dissected until the vessels are separated. Great care should be taken to ensure that all movements run parallel to the vessels and not perpendicular, therefore decreasing the risk of bleeding.
This process can be challenging. The surgeon is working with delicate vasculature. Often there are several branches from the vein that have formed due to the abnormal venous system. The best way to approach it is to identify planes and separate those planes in order to isolate individual vessels. If difficulties are still encountered, the surgeon should restart the dissection higher.
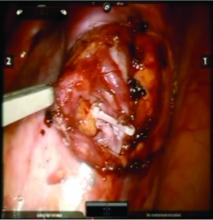
Once the dilated ovarian vein is isolated, one to two clips are placed.
Usually the artery is clearly distinct from the vein as it is smaller, more elastic, and can be seen pulsing. However, occasionally it is difficult to distinguish. In these cases, assistance with the da Vinci surgical system is useful: Indocyanine green (ICG) dye can be injected intravenously and visualized with a near-infrared light on the da Vinci platform. The dye is then seen glowing green as it first courses through the artery and then the vein.
For patients who have been found on venography to have bilateral disease, we perform the ligation procedure bilaterally. Once ligation is complete, the more competent collateral veins in the pelvis will assume more of the venous circulation.
In our experience, patients have ultimately noted substantial pain relief after these procedures, both with the endoscopic embolization and the surgical ligation. Patients are counseled that it can take several months to notice a relief in the pain.
In rare cases, pelvic congestion is related to extrinsic compression. For instance, the left renal vein can become compressed between the aorta and the superior mesenteric artery (the nutcracker syndrome), or the left common iliac vein can be compressed between the overlying right internal iliac artery and the underlying vertebral body (May-Thurner syndrome). Both of these conditions can lead to secondary PCS.
Such complex conditions are usually treated by vascular surgeons. May-Thurner syndrome is treated via stenting, while nutcracker syndrome can be treated with stenting or transposition of the renal vein to the distal vena cava.
Dr. Steller is an associate at the Family Health Centers of San Diego. She reported having no relevant financial disclosures.