Surgical site infections are a major source of patient morbidity. They are also an important quality metric for surgeons and hospital systems, and are increasingly being linked to reimbursement.
They occur in approximately 2% of the 600,000 women undergoing hysterectomy in the United States each year. The U.S. Centers for Disease Control and Prevention defines surgical site infection (SSI) as an infection that occurs within 30 days of a procedure in the part of the body where the surgery took place. Most SSIs are superficial incisional, but they also include deep incisional or organ or space infections.
Classification
The incidence of SSI varies according to the classification of the wound, as defined by the National Academy of Sciences.1 Most hysterectomies are classified as clean-contaminated wounds because they involve entry into the mucosa of the genitourinary tract. However, hysterectomy with contamination of bowel flora, or in the setting of acute infection (such as suppurative pelvic inflammatory disease) are considered a contaminated wound class, and are associated with even higher rates of SSI.
The main causative organisms of SSIs associated with hysterectomy include the gram-negative rods, enterococci, and anaerobes associated with the genitourinary tract, but can also include skin flora, such as Staphylococcus aureus and coagulase-negative staphylococci.2
Risk factors
The risk factors associated with SSI are both modifiable and unmodifiable. Broadly speaking, they include increased risk to endogenous flora (e.g., wound classification), increased exposure to exogenous flora (e.g., inadequate protection of a wound from external pathogens), and impairment of the body’s immune mechanisms to prevent and overcome infection (e.g., hypothermia and hypoglycemia).
Unmodifiable risk factors include increasing age, a history of radiation exposure, vascular disease, and a history of prior SSIs. Modifiable risk factors include obesity, tobacco use, immunosuppressive medications, hypoalbuminemia, route of hysterectomy, hair removal, preoperative infections (such as bacterial vaginosis), surgical scrub, skin and vaginal preparation, antimicrobial prophylaxis (inappropriate choice or timing, inadequate dosing or redosing), operative time, blood transfusion, surgical skill, and operating room characteristics (ventilation, increased OR traffic, and sterilization of surgical equipment).
Antimicrobial prophylaxis
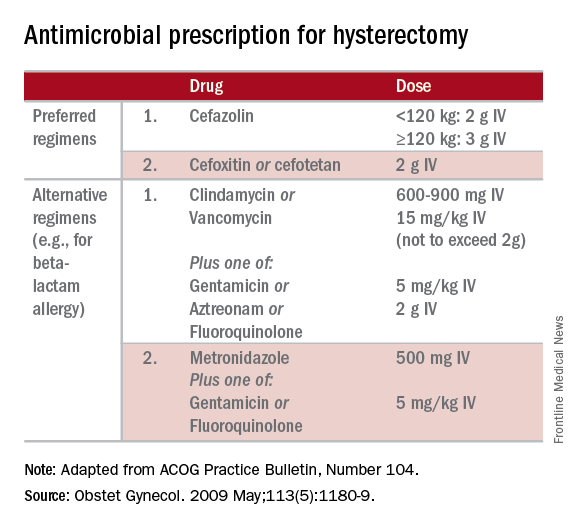
The CDC and the American College of Obstetricians and Gynecologists (ACOG) have provided clear guidelines regarding methods to reduce SSI in hysterectomy.3,4 There is strong evidence for using antimicrobial prophylaxis for hysterectomy.
It is important that physicians confirm the validity of beta-lactam allergies with patients because there are higher rates of SSI with the use of non–beta-lactam regimens, even those endorsed by the CDC and ACOG.5
Antibiotics should be administered within 1 hour of skin incision, and ideally within 30 minutes. They should be discontinued within 24 hours. Dosing should be adjusted to weight, and antimicrobials should be redosed for long procedures (at intervals of two half-lives), and for increased blood loss.
Skin preparation
Hair removal should be avoided unless necessary for technical reasons. If it is required, it should be performed outside of the operative space using clippers, not razors. For patients colonized with methicillin-resistant S. aureus, there is supporting evidence for pretreatment with mupirocin ointment to the nares, and chlorhexidine showers for 5-10 days. Patients who have bacterial vaginosis should be treated before surgery to decrease the rate of vaginal cuff SSI.
Preparation of the incision site and vagina is important in SSI prevention. The optimal skin preparation is an alcohol-containing preparation combined with either 4% chlorhexidine gluconate or iodine. Alcohol-containing preparations should be avoided for vaginal preparation. The manufacturer’s instructions warn against use of chlorhexidine gluconate as a vaginal preparation agent because of the potential for irritation; however, it has been shown to be effective and well tolerated.6 It may be superior to povidone iodine with respect to reducing SSIs when used as a vaginal prep. It also has a longer duration of action and is not inactivated by the presence of blood.If there is a planned or potential gastrointestinal procedure as part of the hysterectomy, the surgeon should consider using an impervious plastic wound protector in place of, or in addition to, other retractors. Preoperative oral antimicrobials with mechanical bowel preparation have been associated with decreased SSIs; however, this benefit is not observed with mechanical bowel preparation alone.
Wound closure
Surgical technique and wound closure techniques also impact SSI. Minimally invasive and vaginal hysterectomy routes are preferred, as these are associated with the lowest rates of SSI. Antimicrobial-impregnated suture materials appear to be unnecessary. Surgeons should ensure that there is delicate handling of tissues and closure of dead spaces. If the subcutaneous fat space depth measures more than 2.5 cm, it should be reapproximated with a rapidly-absorbing suture material.
Use of electrosurgery versus a scalpel when creating the incision does not appear to influence infection rates, nor does use of staples versus subcuticular suture during closure.7
Using a dilute iodine lavage in the subcutaneous space, opening a sterile closing tray, and having surgeons change gloves prior to skin closure should be considered. The CDC recommends keeping the skin dressing in place for 24 hours postoperatively.
Other strategies
Hyperglycemia is associated with impaired neutrophil response, and therefore blood glucose should be controlled before surgery (hemoglobin A1c levels of less than 7% preoperatively) and immediately postoperatively (less than 180 mg/dL within 18-24 hours after the end of anesthesia).
It is also important to minimize perioperative hypothermia (less than 35.5° F), as this also impairs the body’s immune response. Keeping operative room ambient temperatures higher, minimizing incision size, warming CO2 gas in minimally invasive procedures, warming fluids, and using extrinsic body warmers can help achieve this.
Excessive blood loss should be minimized because blood transfusion is associated with impaired macrophage function and increased risk for SSI.
In addition to teamwide (including nonsurgeon) strict adherence to hand hygiene, OR personnel should avoid unnecessary operating room traffic. Hospital officials should ensure that the facility’s ventilator systems are well maintained and that there is care and maintenance of air handlers.
Many strategies can be employed perioperatively to decrease SSI rates for hysterectomy. We advocate for a protocol-based approach (known as “bundling” strategies) to achieve consistency of practice and to maximize surgeon and institutional improvements in SSI rates. This is similar to the approach outlined in a recent consensus statement from the Council on Patient Safety in Women’s Health Care.8
A comprehensive multidisciplinary approach throughout the perioperative period is necessary. It is imperative that good communication exist with patients regarding SSIs after hysterectomy and how patients, surgeons, and hospitals can together minimize the risks of SSIs.
References
1. Altemeier WA. “Manual on Control of Infection in Surgical Patients” (Philadelphia: Lippincott Williams & Wilkins, 1984).
2. Rev Infect Dis. 1991 Sep-Oct;13(Suppl 10):S821-41.
3. Infect Control Hosp Epidemiol. 2014 Jun;35(6):605-27.
4. Obstet Gynecol. 2009 May;113(5):1180-9.
5. Obstet Gynecol. 2016 Feb;127(2):321-9.
6. Am J Obstet Gynecol. 2005 Feb;192(2):422-5.
7. J Gastrointest Surg. 2016 Dec;20(12):2083-92.
8. Obstet Gynecol. 2016 Dec 7. doi: 10.1097/AOG.0000000000001751.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Jackson-Moore is an associate professor in gynecologic oncology at UNC. They reported having no relevant financial disclosures.