A standardized approach is needed
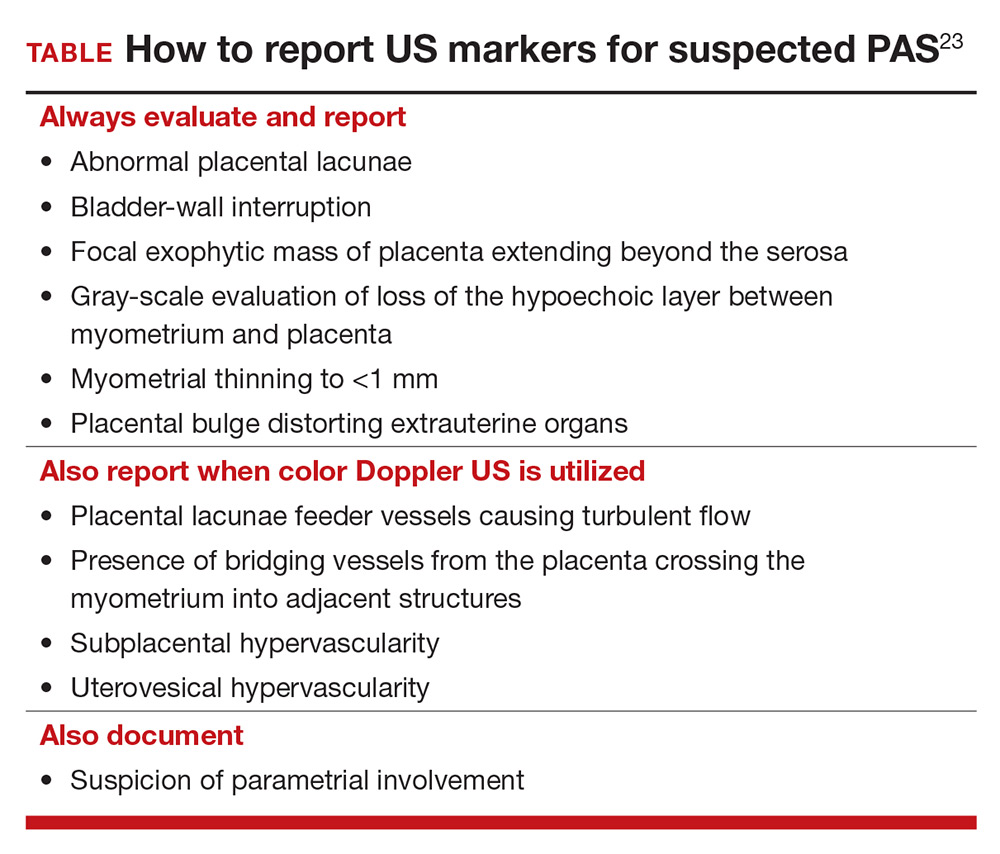
To decrease variability and improve the US diagnosis of PAS, it is important to define and standardize the diagnosis of each sonographic marker for PAS.4 In 2016, the European Working Group on Abnormally Invasive Placenta (EW-AIP) proposed a set of US markers that always should be reported when performing an US examination for suspected abnormal placentation (TABLE).23 Despite this effort by the EW-AIP, ambiguity remains over sonographic definitions of several PAS markers. For example, what determines a placental lacuna on US? And what constitutes an abnormal uterovesical interface? There is a need for a more objective definition of US markers of PAS and a standardized approach to the US examination in at-risk pregnancies.
The Society for Maternal-Fetal Medicine is coordinating a multi-society task force to address the need to define and standardize the US diagnosis of PAS.
Observations on other PAS diagnostic modalities
Magnetic resonance imaging
Adjunctive role. Magnetic resonance imaging (MRI) is often used as an adjunctive diagnostic modality in cases of suspected PAS. Several markers for PAS have been described on MRI, including15:
- intraplacental T2-weighted dark bands
- abnormal intraplacental vascularity
- heterogeneous intraplacental signal intensity
- focal interruption of the myometrium by the placenta
- uterine bulging.
- Assess a priori risk for the patient before initiating the US exam
- In the presence of a placenta previa, or low-lying placenta, we strongly recommend a transvaginal, in addition to transabdominal, US to further assess for the presence of placenta accreta spectrum (PAS) markers
- Until prospective studies clearly define the diagnostic accuracy of PAS sonographic markers and their performance in high-risk and low-risk pregnancies, we recommend that US findings be reported as a risk profile—that is, high, moderate, and low risk of PAS
- Be especially cautious with patients who are at substantially increased risk for PAS, such as those with placenta previa and prior multiple CDs. In this setting, a low-risk report for PAS only should be provided when none of the PAS markers are seen on transabdominal and transvaginal US examinations
- While awaiting national guidelines that 1) standardize the approach to the US examination and 2) define PAS US markers, we encourage US laboratories to develop local protocols to standardize the sonographic evaluation of the placenta and ensure uniform and complete placental assessment
Based on a recent meta-analysis, overall sensitivity of MRI for detecting PAS is 86% to 95%, with specificity of 80% to 95%. Although this is comparable to the sensitivity and specificity of US,27 studies of MRI in PAS are smaller and more prone to bias than in studies of US, because MRI typically is used only in patients at highest risk for PAS. Few studies comparing US to MRI for PAS have been performed; all are small and lack statistical power.
Complementary role. MRI can be complementary to US in cases in which the placenta is posterior or located laterally28 but, importantly, rarely changes decisions about surgical management when used in conjunction with US to assess patients for the diagnosis of PAS. (An exception might lie in the ability of MRI to assess the degree or depth of invasion of the placenta and discerning placenta percreta from placenta accreta.15)
Enhancement with contrast. Addition of gadolinium-based contrast might improve the ability of MRI to make a diagnosis of PAS, but gadolinium crosses the placenta barrier. Although fetal effects of gadolinium have not been observed, American College of Radiology guidelines recommend avoiding this contrast agent during pregnancy unless absolutely essential.29
Specific indications. MRI without contrast should be considered 1) when US is inconclusive and 2) to further evaluate a posterior placenta suspicious for invasion, to define the precise topography of extrauterine placental invasion. The additional information offered by MRI might alter surgical planning.15
Overall, based on current literature, gray-scale US appears to be an excellent tool for prenatal diagnosis of PAS in women at risk: Sensitivity has been reported in the range of 80% to 90%; specificity, 91% to 98%; positive predictive value, 65% to 93%; and negative predictive value, 98%.5,6
However, these values might overestimate the true ability of prenatal US to predict PAS. Why? Early studies that assessed the accuracy of US prediction of PAS might have been biased by inclusion of single-expert observations, high suspicion of placenta accreta, and prior knowledge of patients’ risk factors. In addition, small sample size, retrospective design, and wide variability in the definition of PAS and inclusion criteria led to inconsistency in performance and skewed sensitivity.7
In fact, when experienced providers, reviewing the same US images, were blinded to patients’ clinical history, the accuracy of US diagnosis of PAS decreased in regard to sensitivity (to 54%), specificity (88%), positive (82%) and negative (65%) predictive value, and accuracy (65%).4 Investigators also found wide inter-observer variability in the interpretation of markers of PAS.4 Furthermore, there is evidence that several PAS US markers are commonly seen in low-risk normal pregnancy.
Although studies have yielded variable findings of the precise sensitivity and positive predictive value of US in the diagnosis of PAS, there is a general agreement that US should be the primary imaging modality for this purpose, and can be used exclusively in most cases.
References
- Comstock CH, Bronsteen RA. The antenatal diagnosis of placenta accreta. BJOG. 2014;121:171-181.
- D’Antonio F, Iacovella C, Bhide A. Prenatal identification of invasive placentation using ultrasound: systematic review and metaanalysis. Ultrasound Obstet Gynecol. 2013;42:509-517.
- Comstock CH, Love JJ Jr, Bronsteen RA, et al. Sonographic detection of placenta accreta in the second and third trimesters of pregnancy. Am J Obstet Gynecol. 2004;190:1135-1140.
- Bowman ZS, Eller AG, Kennedy AM, et al. Interobserver variability of sonography for prediction of placenta accreta. J Ultrasound Med. 2014;33:2153-2158.
Biomarkers
Multiple serum biomarkers have been proposed to predict PAS in high-risk women. PAS might be associated with increased levels of first-trimester pregnancy-associated plasma protein A, second-trimester maternal serum alpha fetoprotein, and human chorionic gonadotropin, but studies of the utility of these biomarkers have yielded contradictory results.30,31 Biomarkers are of interest and have significant clinical applicability, but none of the ones identified to date have high sensitivity or specificity for predicting PAS prenatally. Research is ongoing to identify markers of PAS that have sufficient predictive power.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.